Abstract
Polymer composites display the synergistic property of the polymer (matrix) and inorganic particles (filler material), when their combination is properly utilized. In the present work, polymer composites possessing a superhydrophobic property are fabricated by imposing the combination of both surface free energy and surface roughness. Polybenzoxazine (Pbz) is a choice of low surface free energy material and TiO2 particles contribute to create surface roughness. Thus, Pbz/TiO2 composites were fabricated by varying TiO2 contents to produce superhydrophobicity. The hydrophobicity increased from 94° for Pbz to 140° for Pbz/T5. The advantage of molecular design flexibility is also utilized to synthesize benzoxazine monomer (Bzo), which then undergoes thermally induced self-polymerization with different contents of TiO2 to produce Pbz-TiO2 composites. The structure analysis and curing behavior of the Bzo monomer was examined using FT-IR, NMR and DSC techniques. Whereas the properties of the Pbz/TiO2 composites were analyzed by WCA, SEM, DMA, TGA, and dielectric techniques.
1. Introduction
Superhydrophobicity, also known as water repellency of a surface is governed by two important factors, viz., chemical composition and geometrical surface structure. These superhydrophobic surfaces possessing a water contact angle greater than 150° have attracted research efforts in various applications, including multifunctional coatings that can be used as antifouling paints; corrosion resistant; water proof clothes; and as intelligent membranes with self-cleaning properties that can be used for oil/water separation, and so on. In general terms, for a material to exhibit superhydrophobic property, it requires a proper balance of low surface energy and high surface roughness [1,2,3,4,5]. It is well known that polybenzoxazine (Pbz), a class of thermosetting resins, is a material of interest possessing low surface free energy. Interestingly, these materials are non-fluorine, non-silicon polymeric materials considered as an advanced form of phenol-formaldehyde resins. These polybenzoxazine possess several advantages, including one step synthesis of their monomer, i.e., benzoxazine; self-polymerization by thermal curing method; no release of volatiles during polymerization; producing void-free products with zero shrinkage, and so on. Additionally, one of their appreciable properties that broadens their application in various diverse fields, is their ‘molecular design flexibility’. According to the final use of the product, their precursors can be synthesized by choosing the appropriate amine and phenol. Moreover, Pbz possess low surface-free energy: PBA, synthesized from BA monomer (from bisphenol-A and aniline) possess a surface free energy of 16.4 mJ/m2, which is lower than that of Teflon (21 mJ/m2).
It is well known that semiconductor oxide materials, including TiO2, ZnO, CeO2, and V2O5, being hydrophilic in nature, can also exhibit superhydrophobic property, if their surface is roughened by modification with other hydrophobic material. Among these materials, TiO2 is the most preferred due to its renowned properties, including optical properties, chemical stability, biocompatibility, and self-cleaning properties. Several research works have been performed related to the superhydrophobic property of polymer/TiO2 based composites [6,7,8,9,10,11]. Wang et al. prepared UV-durable superhydrophobic coating using ZnO nanowire coated with ultrathin SiO2 shell. The work showed that the UV durability was improved greatly in addition to superhydrophobic property [6]. Xu et al. fabricated nanocomposite surfaces using multifunctional TiO2 coated high density polyethylene [7]. These surfaces possess reversible wettability on exposure to UV and heat. Kamegawa et al. applied a co-deposition technique and produced superhydrophobic surfaces using TiO2 and polytetrafluoroethylene with self-cleaning properties [9]. To cite a few, Qing et al., prepared superhydrophobic thin films from PDMS/TiO2 nanoparticles. They found that the heat treatment of the films at 120 °C was beneficial in restoring their superhydrophobic property [12]. Many superhydrophobic TiO2 surfaces have been fabricated by treating TiO2 with fluorosilane or other hydrophobic polymers, such as polytetrafluoroethylene (PTFE) and polydimethylsiloxane (PDMS). Even though, fabricating superhydrophobic TiO2 surfaces based on surface roughness alone is quite challenging, a proper balance of surface free energy and surface roughness is required to permanently induce superhydrophobicity to the material [13,14,15,16].
Considering all of these factors, in this work we fabricated superhydrophobic Pbz/TiO2 surfaces in which benzoxazine monomer was synthesized from a Mannich condensation reaction using the synthesized diamine (APTA) and synthesized phenol (PAP). The concept of molecular design flexibility has taken an active role in the synthesis of novel benzoxazine monomer. Different weight ratios of TiO2 particles have been added to the benzoxazine monomer, which undergoes thermally induced self-polymerization to form Pbz/TiO2 surfaces. The benefit of both surface free-energy and surface roughness has been utilized in this work, through fabricating Pbz/TiO2 surfaces. The synergistic effect of both Pbz and TiO2 has been brought about by finding the appropriate ratio of Pbz:TiO2 mixtures. The characterization results pertaining to the study have been examined and explained in detail.
2. Materials and Methods
Aniline, phenol, terephthaloyl chloride, 4-nitroaniline, Pd/C, paraformaldehyde, hydrazine hydrate, and dichlorodimethyl silane were purchased from Sigma-Aldrich (Burlington MA, USA). Sodium nitrite, HCl, NaOH, K2CO3, DMF, ethanol, DMSO, and THF were purchased from Duksen Chemicals Co., Ltd., Ansan-si, Gyeonggi-do, Korea. All chemicals were used without further purification.
2.1. Synthesis of Bzo-TA
The benzoxazine monomer was synthesized effortlessly in a single step by following the Mannich condensation reaction. The synthesized diamine (APTA) and the synthesized phenol (PAP) (the synthesis procedure for APTA and PAP is given in Supplementary Materials) were taken in a 1:2 ratio and were reacted in the presence of DMSO solvent at 120 °C for 5 h. After completion of the reaction, the reaction mixture was precipitated in 1 N NaOH solution, leaving behind the unreactants in the NaOH solution. The precipitated benzoxazine monomer was washed several times with DI water, filtered, and vacuum dried at 60 °C to afford Bzo-TA with 85% yield (Scheme 1).
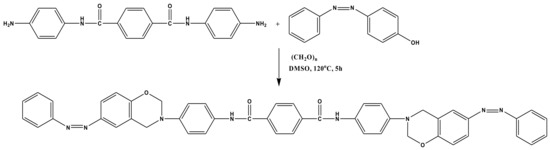
Scheme 1.
Synthesis of bis(6-phenyl diazenyl-3,4-dihydro-2H-1,3-benzoxazinyl) terephthalamide [Bzo-TA].
2.2. Fabrication of Pbz-TA/TiO2 Composites
The Pbz-TA/TiO2 composites were prepared by varying the content of TiO2 in Bzo. The fabrication process for one composition of Pbz-TA/TiO2 is shown here, as a similar procedure is followed for other compositions. For fabrication of Pbz-TA/T1 composites, Bzo-TA (1 g) and TiO2 (0.01 g) (1 wt% of Bzo-TA) were mixed with a very small amount of THF (~5 mL) to form a homogeneous solution; this was then poured into a petridish (pretreated with dichlordimethyl silane) and cured at 250 °C for 3 h to obtain Pbz-TA/T1 composites. Similarly, Pbz-TA/T3 and Pbz-TA/T5 composites were fabricated by varying TiO2 ratio w.r.t. the benzoxazine monomer. For comparison, Pbz without any TiO2 was also prepared and denoted as Pbz-TA/T0 [17,18,19,20].
3. Results
3.1. Structure Analysis of Bzo-TA
Figure 1 reveals the FTIR spectrum of the synthesized benzoxazine monomer. The bands at high frequency region between 3400 and 3200 cm−1 correspond to the asymmetric and symmetric stretching vibrations of the –N-H group, and the bands between 2900 and 2800 cm−1 correspond to the asymmetric and symmetric stretching vibrations of the –C-H group. The oxazine ring vibrations gave its vibrational bands at medium and low frequency regions. The middle and low frequency regions show their bands at 1231 and 1019 cm−1, due to the asymmetric and symmetric stretching vibrations of C-O-C; at 1151 cm−1 due to the stretching vibrations of C-N-C; and at 936 cm−1 due to the –CH2 vibrations of the oxazine ring. All these vibrational bands indicate the formation of benzoxazine ring through the Mannich reaction. To further elucidate the structure of Bzo-TA, it was subjected to NMR analysis. In the 1H-NMR spectrum (Figure 2), the presence of two singlets of equal intensities at 5.4 and 4.6 ppm, corresponds to the oxazine ring protons, viz., O-CH2-N and Ar-CH2-N, respectively. In addition, another singlet at 7.2 ppm, corresponds to the –NH protons of the amide group. All other aromatic protons resonate between 6.7 and 8.0 ppm. Similar to this, in 13C-NMR spectrum (Figure 3), the oxazine ring carbons gave peaks at 79 and 48 ppm, ascribed to the O-CH2-N and Ar-CH2-N carbons, respectively. Whereas the carbonyl of the amide group gave a peak at 167 ppm. All other aromatic carbons resonate between 115–156 ppm. Thus, the structure of Bzo-TA is verified by FT-IR and confirmed by NMR spectroscopic techniques.
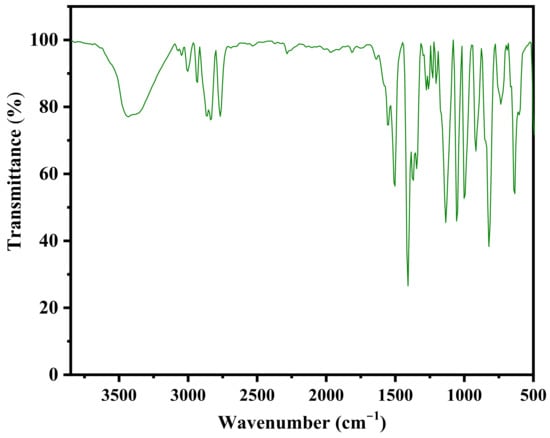
Figure 1.
FT-IR spectrum of Bzo-TA.
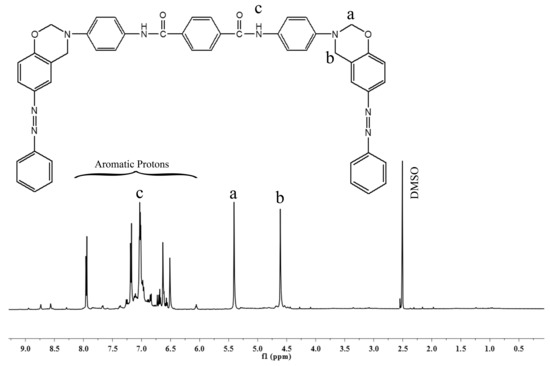
Figure 2.
1H-NMR spectrum of Bzo-TA.
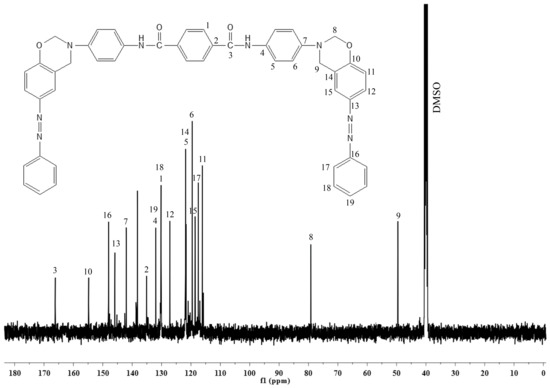
Figure 3.
13C-NMR spectrum of Bzo-TA.
3.2. Curing Behavior of Bzo-TA Blends
DSC analysis was done to study the curing behavior of Bzo-TA and Bzo-TA/TiO2 composites. The samples for DSC analysis were prepared as follows. A few grams of Bzo-TA were taken and to it different amounts of TiO2 particles were added and mixed well. Three different weights of TiO2 (1, 3 and 5 wt%) were added, according to the weight of Bzo-TA. Neat Bzo-TA without any TiO2 particles was also analyzed for comparison.
Figure 4 depicts the DSC thermograms of Bzo-TA and Bzo-TA/TiO2 blends, and Table 1 shows the data obtained from the thermograms. All thermograms show an endothermic and exothermic peak. The endotherm at 100 °C, attributes to the melting of the Bzo-TA monomer. Whereas the exothermic curves between 170–180 °C, attributes to the curing process of Bzo-TA. This shows that TiO2 does not alter the melting or curing of the benzoxine monomer, but as the polymerization proceeds, the produced –OH groups interact with the –OH groups of TiO2, which is indicated by a large area under the exothermic curves for the blends. These observations suggest that there is interaction between TiO2 particles and Pbz [21,22,23].
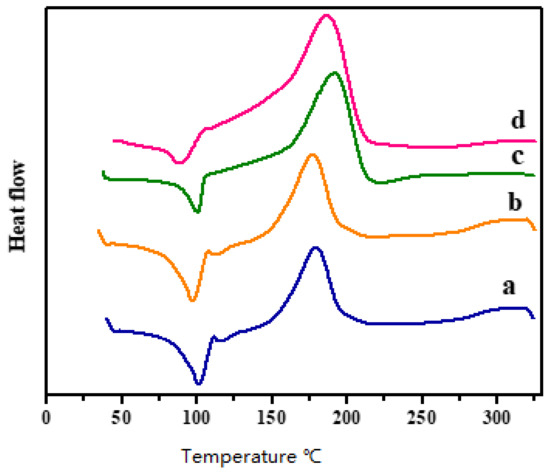
Figure 4.
DSC thermograms of (a) Bzo-TA/T0, (b) Bzo-TA/T1, (c) Bzo-TA/T3 and (d) Bzo-TA/T5.

Table 1.
Data obtained from DSC thermograms.
3.3. Morphological Studies
The morphology of the fabricated Pbz-TA/TiO2 composites was analyzed by SEM and AFM analyses. Figure 5 shows the top-view SEM images of Pbz-TA/TiO2 composites. As seen from the images, it is clearly observed that Pbz-TA has a smooth homogeneous surface, whereas the surface of Pbz-TA/TiO2 loses its smoothness and becomes rough. This is attributed to the fact that, when both Bzo-TA and TiO2 are dispersed in a solvent, Bzo-TA due to its low viscosity pushes the TiO2 particles to the surface during fabrication, forming a heterogenous surface. The dark region contributes to the Pbz-TA surface, whereas the grey region contributes to TiO2 particles. With increased TiO2 content in the composites, the grey region increases linearly. Moreover, with increased content of TiO2 (5 wt%) in the Pbz matrix, there happens to be a phase separation, due to the improper dispersion of TiO2 in Pbz, which leads to agglomeration. This could also be clearly observed by SEM images. In addition, the surface roughness of Pbz-TA and Pbz-TA/TiO2 was analyzed by AFM analysis. The neat Pbz-TA surface shows a smooth surface without any roughness (Figure 6). Whereas the Pbz-TA/TiO2 composites show a roughened surface, and the roughness increases with increased TiO2 content. Thus, the formation of a heterogeneous surface substantiates the inclusion of TiO2 particles in Pbz matrix [24].
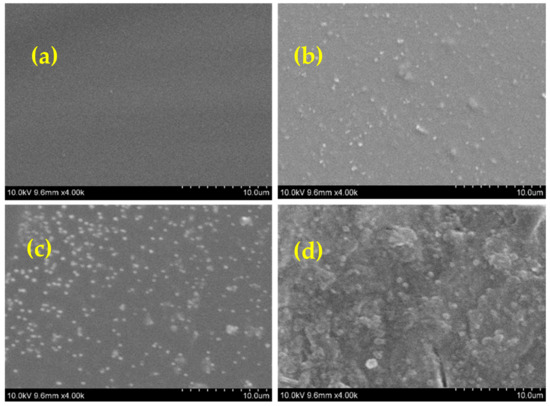
Figure 5.
SEM images of (a) Pbz-TA/T0, (b) Pbz-TA/T1, (c) Pbz-TA/T3 and (d) Pbz-TA/T5.
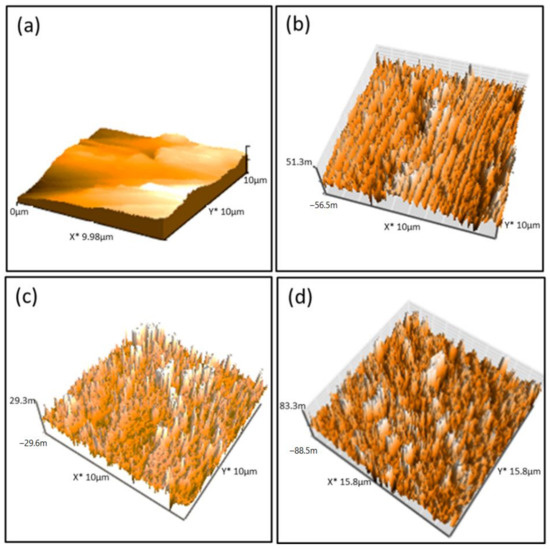
Figure 6.
AFM images of (a) Pbz-TA/T0, (b) Pbz-TA/T1, (c) Pbz-TA/T3 and (d) Pbz-TA/T5.
3.4. Water Contact Angle Analysis
The phenomenon lying behind the wetting behavior of a surface when it is in contact with water droplets is explained by Wenzel and Cassie-Baxter. To understand the concept, it can be explained in such a way that when a drop of water is placed on the surface of a material, the surface can either be completely wetted by the liquid or it can sit on the surface without wetting. The wetting behavior is explained by the Wenzel state, where the air between the liquid and material’s surface gets displaced completely by the liquid, leading to wettability exhibiting superhydrophilicity, whereas the non-wetting behavior is explained by the Cassie-Baxter state, where the liquid sits on the surface along with air. Such surfaces show the rolling behavior of liquid even when it is tilted, showing non-wetting behavior or superhydrophobicity [25,26,27,28].
Figure 7 depicts the water contact angle (WCA) images of neat Pbz-TA and Pbz-TA/TiO2 composites, providing a contact angle of 94° for Pbz-TA; 120° for Pbz-TA/T1; 134° for Pbz-TA/T3; and 140° for Pbz-TA/T5. It can be explained that the Pbz-TA surface, being homogeneous in nature, exhibits hydrophobic behavior. Yet, as the surface becomes heterogeneous with the incorporation of TiO2, the hydrophobicity increased linearly with increasing TiO2 contents. The air trapped in the cavities of these heterogeneous rough surface is the main reason for the superhydrophobic behavior [29].
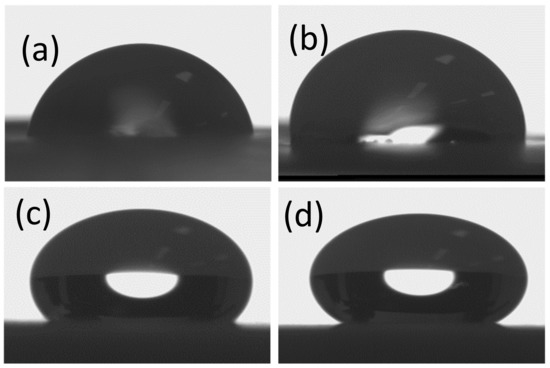
Figure 7.
WCA images of (a) Pbz-TA/T0, (b) Pbz-TA/T1, (c) Pbz-TA/T3 and (d) Pbz-TA/T5.
3.5. Thermo-Mechanical Behavior of Pbz-TA/TiO2 Composites
In a general concept, the surfaces with superhydrophobic behavior must also exhibit high durability. However, it is believed that materials with a rough surface are expected to possess poorer mechanical and thermal stability than materials with a smooth surface. The mechanical properties of Pbz-TA/TiO2 composites is explained by analyzing their storage modulus and loss modulus values. As seen from Figure 8A, the storage modulus of Pbz-TA/TiO2 composites increased from 2.62 GPa for Pbz-TA to 2.83 GPa for Pbz-TA/T5. In addition, their cross-link density (CLD) also increased from 3.4 × 105 mol/m3 for Pbz-TA to 3.7 × 105 mol/m3 for Pbz-TA/T5 (Figure 8B and Table 2). Similarly, their TGA thermograms in Figure 9 showed single step degradation profiles for all Pbz-TA/TiO2 composites. Their initial, 5% and 10% degradation temperature (Ti, T5 and T10) increased linearly with increased TiO2 content in the composites. Moreover, all the prepared composites deliver high char yield values and high limiting oxygen index (LOI) values, exhibiting flame retardant properties (Table 3). Even though a heterogeneous rough surface could exhibit poor mechanical properties, the enhanced properties in the present work are attributed to the formation of hydrogen bonding interactions between the phenolic –OH groups of Pbz-TA and –OH groups of TiO2 particles.
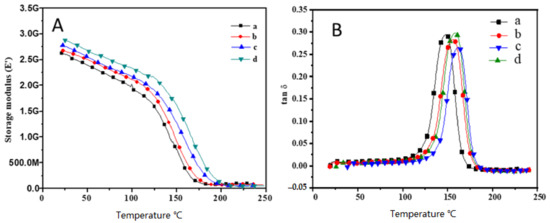
Figure 8.
Storage modulus (A) and loss modulus (B) of (a) Pbz-TA/T0, (b) Pbz-TA/T1, (c) Pbz-TA/T3 and (d) Pbz-TA/T5.

Table 2.
Data obtained from DMA analysis.
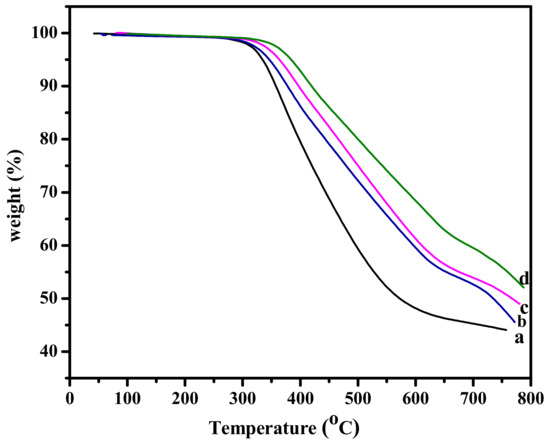
Figure 9.
TGA thermograms of (a) Pbz-TA/T0, (b) Pbz-TA/T1, (c) Pbz-TA/T3 and (d) Pbz-TA/T5.

Table 3.
Data obtained from TGA analysis.
3.6. Dielectric Behavior of Pbz-TA/TiO2 Composites
The dielectric behavior of Pbz-TA/TiO2 composites is explained by their dielectric constant and dielectric loss, as depicted in Figure 10A,B, whose values are shown in Table 4. The dielectric constant is directly related to the chemical structure of a material, in particular the extent to which the material can polarize. The dielectric constant values for the Pbz-TA/TiO2 composites decreases from 3.3 to 2.6, whereas their dielectric loss values decrease from 1.32 to 0.99. This could be explained in such a way that the TiO2 particles disperse the charge carriers by reducing their mobility and hence decreasing their dielectric constant and loss values [30].
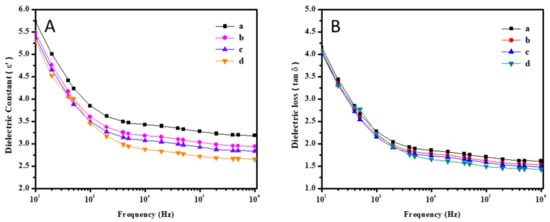
Figure 10.
Dielectric constant (A) and dielectric loss (B) of (a) Pbz-TA/T0, (b) Pbz-TA/T1, (c) Pbz-TA/T3 and, (d) Pbz-TA/T5.

Table 4.
Data obtained from impedance analysis.
4. Conclusions
Pbz/TiO2 composites have been fabricated successfully through thermal self-polymerization techniques. We have used a simple reaction process, adopting Mannich condensation, to synthesize the benzoxazine monomer, i.e., Bzo-TA. The curing behavior, as analyzed by DSC, shows that the presence of amide functional group (-CONH) helps to reduce the curing temperature. The onset of curing starts early (at 130 °C), which is much earlier when compared with BA-a monomer (~200 °C). It is to be noted that in the formation of polymer composites, the functional groups present in the polymer and TiO2 particles are connected through hydrogen bonding interactions. These HB interactions pave the way for enhanced properties of Pbz-TA/TiO2 composites. One composition among the fabricated composites (Pbz-TA/T5) exhibits superhydrophobic property with a WCA of 140°. Therefore, fabrication of such composites allowing for water roll-off properties can be made useful for creating self-cleaning materials. Even though phase separation occurs at 5 wt% loading of TiO2, the composites manage to withstand excellent thermal and mechanical properties (Pbz-TA/T5: storage modulus = 2.83 GPa; T10 = 394 °C) attributed to the presence of HB interactions. These kinds of superhydrophobic polymer composites with enhanced durability and the techniques used for their fabrication find potential application in diverse fields.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su142013401/s1. The details regarding the Instrumentation; synthesis procedure of precursors (APTA and PAP); structure analysis of precursors; Scheme S1: Synthesis of N, N’-bis(4-aminophenyl) terephthalamide [APTA]; Scheme S2: Synthesis of 4-phenyl diazenyl phenol [PAP]; Figure S1: FT-IR spectrum of APTA; Figure S2: FT-IR spectrum of PAP; Figure S3: 1H-NMR spectrum of APTA; Figure S4: 13C-NMR spectrum of APTA; Figure S5: 1H-NMR spectrum of PAP; Figure S6: 13C-NMR spectrum of PAP; Figure S7: Schematic representation showing the hydrogen bonding interactions.
Author Contributions
Conceptualization, S.P.A. and T.P.; methodology, C.J.R.; software, V.R.; validation, S.P.A., T.P. and C.J.R.; formal analysis, S.P.A.; investigation, S.P.A.; resources, T.P.; data curation, V.R.; writing—original draft preparation, S.P.A. and T.P.; writing—review and editing, C.J.R.; visualization, V.R.; supervision, S.C.K.; project administration, S.C.K.; funding acquisition, S.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2020R1I1A3052258). In addition, the work was also supported by the Technology Development Program (S3060516), funded by the Ministry of SMEs and Startups (MSS, Korea) in 2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.-K.; Wang, J.-H.; Fan, S.-K.; Chang, J.-Y. Reversible Hydrophobic/Hydrophilic Adhesive of PS-b-PNIPAAm Copolymer Brush Nanopillar Arrays for Mimicking the Climbing Aptitude of Geckos. J. Phys. Chem. C 2012, 116, 6980–6992. [Google Scholar] [CrossRef]
- Chapman, J.; Regan, F. Nanofunctionalized Superhydrophobic Antifouling Coatings for Environmental Sensor Applications-Advancing Deployment with Answers from Nature. Adv. Eng. Mater. 2012, 14, B175–B184. [Google Scholar] [CrossRef]
- Chen, J.-K.; Wang, J.-H.; Cheng, C.-C.; Chang, J.-Y. Reversibly Thermoswitchable Two-Dimensional Periodic Gratings Prepared from Tethered Poly(N-isopropylacrylamide) on Silicon Surfaces. ACS Appl. Mater. Interfaces 2013, 5, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Lin, S.; Tu, Y.; Liu, G.; Hu, J.; Li, F.; Miao, L.; Zhang, G.; Luo, H.; Liu, F.; et al. Simple approach towards fabrication of highly durable and robust superhydrophobic cotton fabric from functional diblock copolymer. J. Mater. Chem. A 2013, 1, 11246. [Google Scholar] [CrossRef]
- Kommireddy, D.S.; Patel, A.A.; Shutava, T.G.; Mills, D.K.; Lvov, Y.M. Layer-by-Layer Assembly of TiO2 Nanoparticles for Stable Hydrophilic Biocompatible Coatings. J. Nanosci. Nanotechnol. 2005, 5, 1081–1087. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Fu, Y.; Li, B.; Liu, Y. Bioinspired Preparation of Ultrathin SiO2 Shell on ZnO Nanowire Array for Ultraviolet-Durable Superhydrophobicity. Langmuir 2009, 25, 13619–13624. [Google Scholar] [CrossRef]
- Xu, Q.F.; Liu, Y.; Lin, F.-J.; Mondal, B.; Lyons, A.M. Superhydrophobic TiO2–Polymer Nanocomposite Surface with UV-Induced Reversible Wettability and Self-Cleaning Properties. ACS Appl. Mater. Interfaces 2013, 5, 8915–8924. [Google Scholar] [CrossRef]
- Fu, Q.; Rao, G.V.R.; Basame, S.B.; Keller, D.J.; Artyushkova, K.; Fulghum, J.E.; López, G.P. Reversible Control of Free Energy and Topography of Nanostructured Surfaces. J. Am. Chem. Soc. 2004, 126, 8904–8905. [Google Scholar] [CrossRef]
- Kamegawa, T.; Shimizu, Y.; Yamashita, H. Superhydrophobic Surfaces with Photocatalytic Self-Cleaning Properties by Nanocomposite Coating of TiO2 and Polytetrafluoroethylene. Adv. Mater. 2012, 24, 3697–3700. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Li, M.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Carbon-Doped ZnO Hybridized Homogeneously with Graphitic Carbon Nitride Nanocomposites for Photocatalysis. J. Phys. Chem. C 2014, 118, 10963–10971. [Google Scholar] [CrossRef]
- Feng, X.; Zhai, J.; Jiang, L. The Fabrication and Switchable Superhydrophobicity of TiO2 Nanorod Films. Angew. Chem. Int. Ed. 2005, 44, 5115–5118. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L.; Liu, C. Superhydrophobic TiO2/polyvinylidene fluoride composite surface with reversible wettability switching and corrosion resistance. Chem. Eng. J. 2016, 290, 37–44. [Google Scholar] [CrossRef]
- Lai, Y.; Pan, F.; Xu, C.; Fuchs, H.; Chi, L. In Situ Surface-Modification-Induced Superhydrophobic Patterns with Reversible Wettability and Adhesion. Adv. Mater. 2013, 25, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. High-performance impedimetric genosensor based on biocompatible TiO2 nanoparticles supported carbon ionic liquid electrode. Sens. Actuators B Chem. 2013, 176, 386–389. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S. Synthesis of TiO2/Al-MCM-41 Composites with Coal-Measure Kaolin and Performance in Its Photocatalysis. Mater. Sci. Appl. 2011, 2, 14–19. [Google Scholar]
- Nakata, K.; Kimura, H.; Sakai, M.; Ochiai, T.; Sakai, H.; Murakami, T.; Abe, M.; Fujishima, A. UV/Thermally Driven Rewritable Wettability Patterns on TiO2−PDMS Composite Films. ACS Appl. Mater. Interfaces 2010, 2, 2485–2488. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila, A.; Muthusamy, S. Synthesis and characterization of novel bio-based benzoxazines from eugenol. RSC Adv. 2014, 4, 7959–7966. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis and Copolymerization of Fully Biobased Benzoxazines from Renewable Resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Muthusamy, S. New benzoxazines containing polyhedral oligomeric silsesquioxane from eugenol, guaiacol and vanillin. New J. Chem. 2015, 39, 1691–1702. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Kim, S.C. Functionalized MWCNTs, an efficient reinforcement for the preparation of eugenol based high performance PBz/BMI/CNT nanocomposites exhibiting outstanding thermo-mechanical properties. New J. Chem. 2017, 41, 6607–6615. [Google Scholar]
- Agag, T.; Takeichi, T. Synthesis and Characterization of Novel Benzoxazine Monomers Containing Allyl Groups and Their High Performance Thermosets. Macromolecules 2003, 36, 6010–6017. [Google Scholar] [CrossRef]
- Wang, C.F.; Su, Y.; Kuo, S.; Huang, C.; Sheen, Y.; Chang, F. Low-Surface-Free-Energy Materials Based on Polybenzoxazines. Angew. Chem. Int. Ed. 2006, 45, 2248–2251. [Google Scholar] [CrossRef]
- Liao, C.-S.; Wang, C.-F.; Lin, H.-C.; Chou, H.-Y.; Chang, F.-C. Fabrication of Patterned Superhydrophobic Polybenzoxazine Hybrid Surfaces. Langmuir 2009, 25, 3359–3362. [Google Scholar] [CrossRef] [PubMed]
- Isimjan, T.T.; Wang, T.; Rohani, S. A novel method to prepare superhydrophobic, UV resistance and anti-corrosion steel surface. Chem. Eng. J. 2012, 210, 182–187. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Murakami, D.; Jinnai, H.; Takahara, A. Wetting Transition from the Cassie–Baxter State to the Wenzel State on Textured Polymer Surfaces. Langmuir 2014, 30, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-S.; Wang, C.-F.; Lin, H.-C.; Chou, H.-Y.; Chang, F.-C. Tuning the Surface Free Energy of Polybenzoxazine Thin Films. J. Phys. Chem. C 2008, 112, 16189–16191. [Google Scholar] [CrossRef]
- Schmitt, M. Synthesis and testing of ZnO nanoparticles for photo-initiation: Experimental observation of two different non-migration initiators for bulk polymerization. Nanoscale 2015, 7, 9532–9544. [Google Scholar] [CrossRef]
- Parveen, A.S.; Thirukumaran, P.; Sarojadevi, M. Low dielectric materials from fluorinated polybenzoxazines. Polym. Adv. Technol. 2014, 25, 1538–1545. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).