Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of E-Waste Samples
2.2. Treatment of the E-Waste for Metal Content Analysis
2.3. Screening of Microorganisms for E-Waste Remediation
- Procurement of microbial cultures: Seven microbial cultures, viz. Lentinus edodes, Pleurotus florida, Ganoderma lucidum, Aspergillus niger, Trametes versicolor, Streptomyces spp., and Pseudomonas spp., were analyzed for their biological remediation potential. These microbial cultures were procured from the culture repository of the Department of Microbiology, Punjab Agricultural University (PAU), Ludhiana. All the experiments were performed at Dr. H.S. Garcha Mushroom Laboratories, PAU, Ludhiana.
- Preparation of the culture media: The PCB samples were cut into small pieces (25.0 × 15.0 × 1.1 mm). A known weight of the PCB (5.0 g) was washed 4–5 times with distilled water, followed by ethanol washing, and then it was placed in liquid minimal broth (mushroom minimal media (for fungal cultures) and bacterial minimal media (M9 media; for bacterial cultures)). The composition of the mushroom minimal media (in g L−1) was: L-asparagine (1.60), D-glucose (20.0), magnesium sulfate heptahydrate (0.5), di-potassium hydrogen phosphate (1.0), potassium dihydrogen ortho-phosphate (0.46), and thiamine hydrochloride (0.125); pH 6.5. The bacterial minimal media composition had the following components (in g L−1): glucose (20), disodium hydrogen phosphate heptahydrate (33.9), potassium dihydrogen ortho-phosphate (15.0), ammonium chloride (5.0), and sodium chloride (2.5); pH 7.2. The pH of the media used for the growth of the bacterial and fungal cultures were kept at 6.5 and 7.2, respectively. The media were autoclaved at a temperature of 121 °C using steam under pressure (15 pounds per inch square) for 20 min duration.
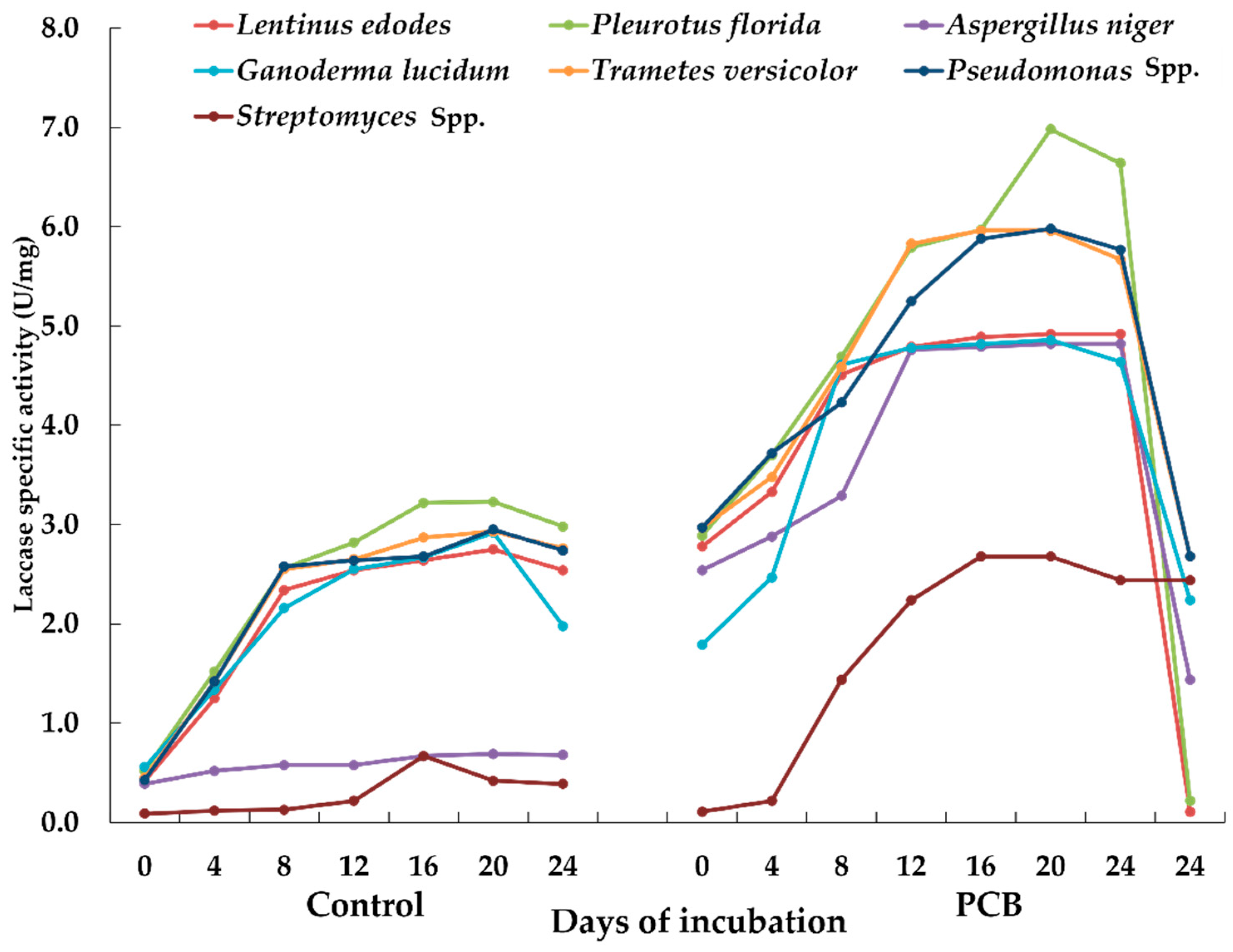
- Laccase activity: The sterilized broth containing the known quantity of the E-waste (5 g L−1 of the broth media) was inoculated with different microorganisms and incubated in a rotary shaker incubator at an appropriate temperature (fungal cultures 25 ± 2 °C; bacterial cultures 27 ± 2 °C). Control broth (without E-waste) was also inoculated with the microbial cultures. The specific activity of the laccase enzyme was performed using buffered guaiacol and measured at intervals of 4 days up to 24 days of incubation [31].
2.4. Biosorption and Bioleaching Experiment
2.5. Desorption Experiment
2.6. Morphological and Protein Profiling Alterations of PCBs Post Microbial Incubation for Screened Microbial Isolates
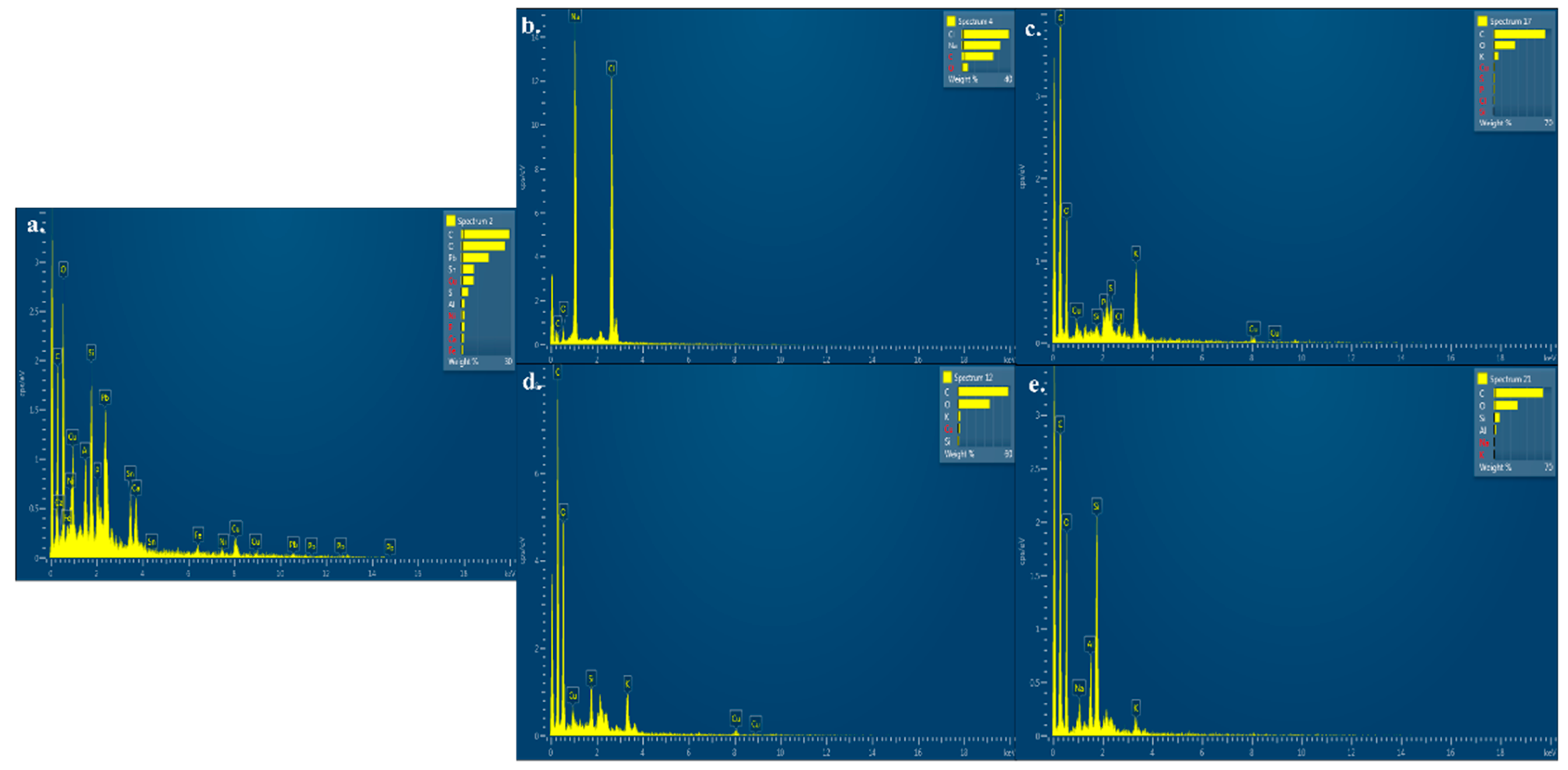
- Electron microscopy analysis: The scanning electron microscopy (SEM) analysis of the PCB samples in minimal broth inoculated with different microbial cultures involving Pleurotus florida, Trametes versicolor, Aspergillus niger, and Pseudomonas spp. was performed after 20 days of incubation. Post 20 days of incubation, the PCB samples were removed from the minimal broth, washed three times with phosphate buffer saline (PBS) to remove any loosely adhering substances, soaked in 4% glutaraldehyde for 2 h, immersed in 3% paraformaldehyde for 1 h, successively dehydrated in ethanol series, and dried out overnight. The processed samples were analyzed using Environmental SEM (Quanta-200, FEI, Hilsboro, Oregon, US) at 10 kV accelerating voltage to obtain SE micrographs.
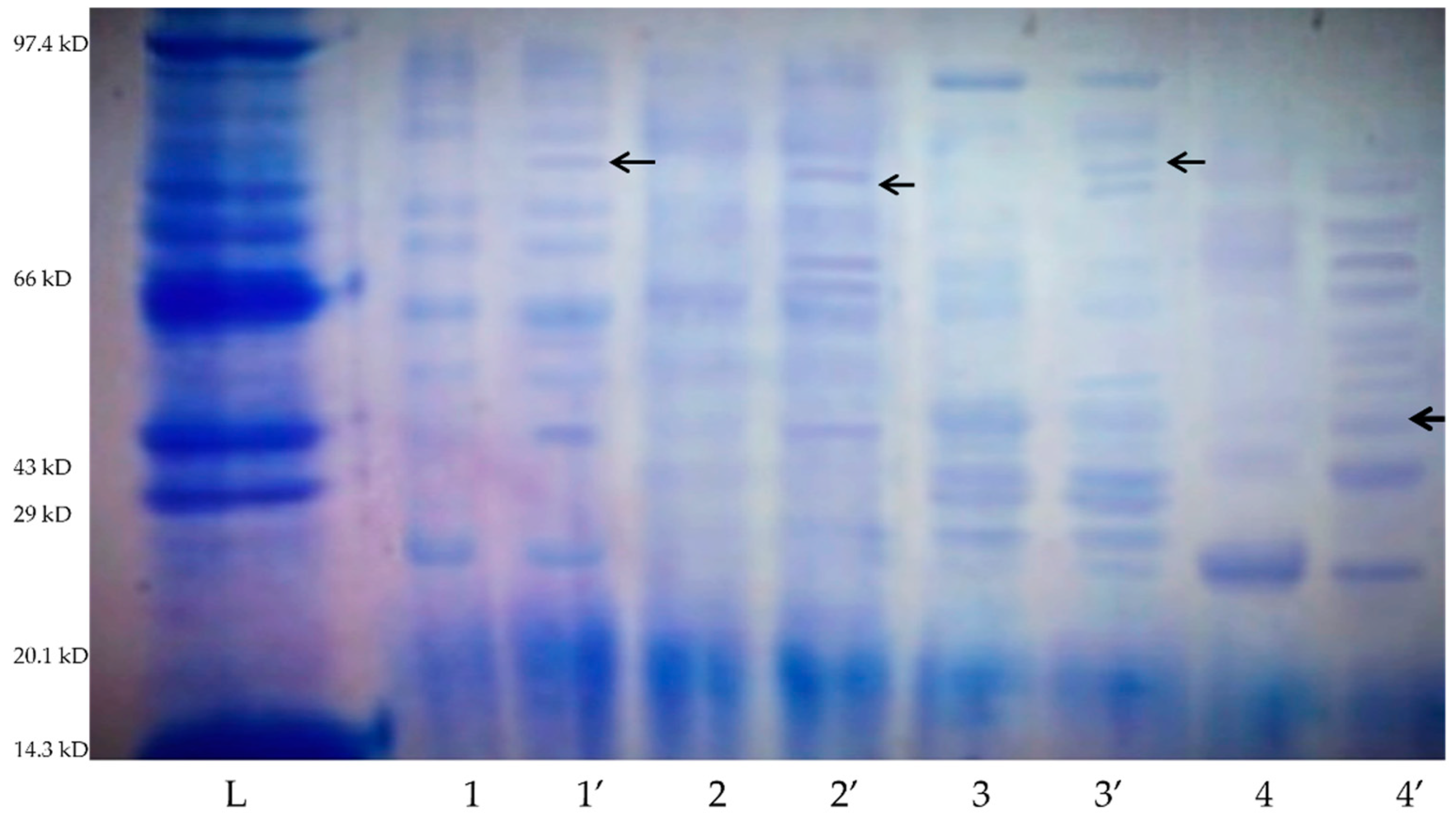
- Protein profiling of microbial cultures through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE): Based on the AAS results, the best four screened cultures were used for protein profiling via SDS-PAGE. These cultures were inoculated in the liquid medium with E-waste and without E-waste (as control). In the case of bacteria on the 20th day, the microbial biomass was separated via centrifuging the culture contents at 6000 rpm for 30 min. For fungal cultures, the biomass was collected via centrifuging the contents at 3000 rpm for 20 min. The SDS-PAGE was performed according to the method described by Laemmli for estimating the expression of proteins with a 12% polyacrylamide gel [33].
2.7. Statistical Analysis
3. Results
3.1. Metal Content Analysis of PCB
3.2. Screening for Identification of Potential Microbial Cultures
3.2.1. Laccase Activity
3.2.2. Biosorption and Bioleaching Assay
3.2.3. Desorption of Copper and Iron Ions by Different Microbial Cultures
3.3. Morphological and Protein Profile Alterations in the Screened Microbial Genera
3.3.1. Scanning Electron Microscopy Analysis
3.3.2. SEM–Energy-Dispersive X-ray Spectroscopy (SEM-EDX) Analysis
3.3.3. Protein Profiling through SDS-PAGE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, K.D.; Kang, H.; Ilankoon, I.M.S.K.; Chong, C.Y. Electronic waste collection systems using Internet of Things (IoT): Household electronic waste management in Malaysia. J. Clean. Prod. 2020, 252, 119801. [Google Scholar] [CrossRef]
- Lang, E.; Gonser, A.; Zadrazil, F. Influence of incubation temperature on activity of ligninolytic enzymes in sterile soil by Pleurotus sp. and Dichomitus squalens. J. Basic Microbiol. 2000, 40, 33–39. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Manna, A.; Sundaram, E.; Amutha, C.; Vasantha, V.S. Efficient removal of cadmium using edible fungus and its quantitative fluorimetric estimation using (Z)-2-(4 H-1, 2, 4-Triazol-4-yl) iminomethylphenol. ACS Omega 2018, 3, 6243–6250. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W.L. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Kalia, P.; Zia, A.; Mladenović, D. Examining country development indicators and e-waste under the moderating effect of country development levels and e-waste policy. Int. J. Qual. Reliab. Manag. 2022. [Google Scholar] [CrossRef]
- Ariffin, N.; Adullah, M.M.A.B.; Mohd Arif Zainol, M.R.R.; Murshed, M.F.; Hariz-Zain; Faris, M.A.; Bayuaji, R. Review on adsorption of heavy metals in wastewater by using geopolymer. Proc. MATEC Web Conf. 2017, 97, 01093. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Parga, J.R.; Valenzuela, J.L.; Cepeda, F. Pressure cynanide leaching for precious metal recovery. JOM 2007, 10, 43–47. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Khan, M.R.; Ng, K.H.; Wongsakulphasatch, S.; Cheng, C.K. Harnessing renewable hydrogen-rich syngas from valonization of palm oil mill effluent (POME) using steam reforming technique. Ren. Eng. 2019, 138, 1114–1126. [Google Scholar] [CrossRef]
- Sameera, V.; Naga Deepthi, C.H.; Srinu Bahu, G.; Ravi Teja, V. Role of biosorption in environmental cleanup. J. Microbial. Biochem. Technol. 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on past decade. J. Chem. Eng. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A review of the latest advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Endeshaw, A.; Birhanu, G.; Zerihun, T.; Misganaw, W. Application of microorganisms in bioremediation-review. J. Environ. Microb. 2017, 1, 2–9. [Google Scholar]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 18, 2568038. [Google Scholar] [CrossRef] [PubMed]
- Fourest, E.; Roux, J.C. Heavy metal biosorption by fungal mycelial by-products: Mechanisms and influence of pH. Appl. Microbiol. Biotechnol. 1992, 37, 399–403. [Google Scholar] [CrossRef]
- Kalia, A.; Singh, S. Myco-decontamination of azo dyes: Nano-augmentation technologies. 3-Biotech 2020, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Thiele, D.J. Oxidative stress induced heat shock factor phosphorylation and HSF-dependent activation of yeast metallothionein gene transcription. Genes. Dev. 1996, 10, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kuca, K.; Kalia, A. Alterations in growth and morphology of Ganoderma lucidum and Volvariella volvaceae in response to nanoparticle supplementation. Mycobiology 2020, 48, 383–391. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Zhu, M.; Tan, W. Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron–sulfur-oxidizing bacteria. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef]
- Trivedi, A.; Hait, S. Efficacy of metal extraction from discarded printed circuit board using Aspergillus tubingensis. In Bioresource Utilization and Bioprocess; Ghosh, S., Sen, R., Chanakya, H., Pariatamby, A., Eds.; Springer: Singapore, 2020; pp. 167–175. [Google Scholar]
- Palmieri, G.; Cennamo, G.; Faraco, V.; Amoresano, A.; Sannia, G.; Giardina, P. A typical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzym. Microb. Technol. 2003, 33, 220–230. [Google Scholar] [CrossRef]
- Xia, L.; Xu, X.; Zhu, W.; Huang, Q.; Chen, W. A comparative study on the biosorption of Cd2+ onto Paecilomyces lilacinus XLA and Mucoromycote sp. XLC. Int. J. Mol. Sci. 2015, 16, 15670–15687. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Q.; Li, S.; Zhu, H.Y.; Jiang, R.; Yin, L.F. Biosorption of copper (II) from aqueous solution by mycelial pellets of Rhizopus oryzae. Afr. J. Biotechnol. 2012, 11, 1403–1411. [Google Scholar]
- Manavalan, A.; Manavalan, T.; Murugesan, K.; Kutzner, A.; Kalaichelvan, P.T.; Heese, K. Characterization of a solvent, surfactant and temperature-tolerant laccase from Pleurotus sp. MAK-II and its dye decolorizing property. Biotechnol. Lett. 2015, 37, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry what prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Mecucci, A.; Scott, K. Leaching and electrochemical recovery of copper, lead and tin from scarp printed circuit boards. J. Chem. Technol. Biotechnol. 2002, 77, 449–457. [Google Scholar] [CrossRef]
- Muammer, K. Recovery of metals and non-metals from electronic wastes by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Jadhav, U.; Su, C.; Hocheng, H. Leaching of metals from printed circuit board powder by an Aspergillus niger culture supernatant and hydrogen peroxide. RSC Adv. 2016, 6, 43442–43452. [Google Scholar] [CrossRef]
- Turner, E.M. Phenoloxidase activity in relation to substrate and development stage in the mushroom, Agaricus bisporus. Trans. Br. Mycol. Soc. 1974, 63, 541–547. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, T.; Du, B. Effect of organic matter and calcium carbonate on behaviors of cadmium adsorption–desorption on/from purple paddy soils. Chemosphere 2014, 99, 41–48. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Khatri, B.R.; Sodha, A.B.; Shah, M.B.; Tipre, D.R.; Dave, S.R. Chemical and microbial leaching of base metals from obsolete cell-phone printed circuit boards. Sustain. Environ. Res. 2018, 28, 333–339. [Google Scholar] [CrossRef]
- Waldir, A.B.; Renata, A.F.; Valdelis, F.A. Characterization of printed circuit boards for metal and energy recovery after milling and mechanical separation. Materials 2014, 7, 4555–4566. [Google Scholar] [CrossRef]
- Shah, M.; Tipre, D.; Dave, S. Chemical and biological processes for multi-metal extraction from waste printed circuit boards of computers and mobile phones. Waste Manag. Res. 2014, 32, 1134–1141. [Google Scholar] [CrossRef]
- Szałatkiewicz, J. Metals content in printed circuit board waste. Pol. J. Environ. Stud. 2014, 23, 2365–2369. [Google Scholar]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. 2020. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014, 2014, 1632421. [Google Scholar] [CrossRef] [PubMed]
- Rautela, R.; Arya, S.; Vishwakarma, S.; Lee, J.; Kim, K.H.; Kumar, S. E-waste management and its effects on the environment and human health. Sci. Total Environ. 2021, 773, 145623. [Google Scholar] [CrossRef] [PubMed]
- Argumedo-Delira, R.; Gómez-Martínez, M.J.; Uribe-Kaffure, R. Fungal tolerance: An alternative for the selection of fungi with potential for the biological recovery of precious metals. Appl. Sci. 2020, 10, 8096. [Google Scholar] [CrossRef]
- Anaya-Garzon, J.; Hubau, A.; Joulian, C.; Guezennec, A.G. Bioleaching of E-waste: Influence of printed circuit boards on the activity of acidophilic iron-oxidizing bacteria. Front. Microbiol. 2021, 12, 669738. [Google Scholar] [CrossRef]
- Baldrian, P.; Gabriel, J. Copper and cadmium increase laccase activity in Pleurotus ostreatus. FEMS Microbiol. Lett. 2002, 206, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bao, G.; Huang, S. Optimization of laccase production in the white-rot fungus Pleurotus ostreatus (ACCC 52857) induced through yeast extract and copper. Biotechnol. Biotechnol. Equip. 2016, 30, 270–276. [Google Scholar] [CrossRef]
- Muthukumarasamy, N.P.; Jackson, B.; Joseph Raj, A.; Sevanan, M. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochem. Res. Int. 2015, 2015, 765190. [Google Scholar] [CrossRef]
- Neifar, M.; Jaouani, A.; Ellouze-Ghorbel, R.; Ellouze-Chaabouni, S.; Penninckx, M.J. Effect of culturing processes and copper addition on laccase production by the white-rot fungus Fomes fomentarius MUCL 35117. Lett. Appl. Microbiol. 2009, 49, 73–78. [Google Scholar] [CrossRef]
- Vrsanska, M.; Voberkova, S.; Langer, V.; Palovcikova, D.; Moulick, A.; Adam, V.; Kopel, P. Induction of laccase, lignin peroxidase and manganese peroxidase activities in white-rot fungi using copper complexes. Molecules 2016, 21, 1553. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Molina, M.; Antonella, B.; Silvana, F.; Tejerina, M.R.; Velázquez, J.E.; Sadañoski, M.A.; Zapata, P.D. Copper improves the production of laccase by Pleurotus sajor-caju with ability to grow on effluents of the citrus industry. Rev. Int. Contam. Ambient. 2020, 36, 105–114. [Google Scholar] [CrossRef]
- Xin, F.; Geng, A. Utilization of horticultural waste for laccase production by Trametes versicolor under solid-state fermentation. Appl. Biochem. Biotechnol. 2011, 163, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Blair, K.M.; Salama, N.R. Staying in shape: The impact of cell shape on bacterial survival in diverse environments. Microbiol. Mol. Biol. Rev. 2016, 80, 187–203. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 16, 832. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free. Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Meshkini, M.; Masoomeh, S.; Mehdi, I.; Amir, A. Bioleaching of copper oxide ore by Pseudomonas aeruginosa. Int. J. Miner. Metall. Mater. 2013, 20, 1130–1133. [Google Scholar]
- Kaliyaraj, D.; Rajendran, M.; Angamuthu, V.; Antony, A.R.; Kaari, M.; Thangavel, S.; Venugopal, G.; Joseph, J.; Manikkam, R. Bioleaching of heavy metals from printed circuit board (PCB) by Streptomyces albidoflavus TN10 isolated from insect nest. Bioresour. Bioprocess. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Cortés, S.; Soto, E.E.; Ordóñez, J.I. Recovery of copper from leached tailing solutions by biosorption. Minerals 2020, 10, 158. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Bektaş, S.; Arıca, M.Y. Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J. Hazard. Mater. 2003, 101, 285–300. [Google Scholar] [CrossRef]
- Jha, S.; Chauhan, R.; Dikshit, S.N. Fungal biomass as biosorbent for removal of heavy metal from industrial waste water effluent. Asian J. Plant Sci. 2014, 13, 93–97. [Google Scholar] [CrossRef]
- Fathollahi, A.; Khasteganan, N.; Coupe, S.J.; Newman, A.P. A meta-analysis of metal biosorption by suspended bacteria from three phyla. Chemosphere 2021, 268, 129290. [Google Scholar] [CrossRef] [PubMed]
- Tunali, S.; Çabuk, A.; Akar, T. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem. Eng. J. 2006, 115, 203–211. [Google Scholar] [CrossRef]
- Bueno, B.Y.M.; Torem, M.L.; Molina, F.A.L.M.S.; De Mesquita, L.M.S. Biosorption of lead (II), chromium (III) and copper (II) by R. opacus: Equilibrium and kinetic studies. Miner. Eng. 2008, 21, 65–75. [Google Scholar] [CrossRef]
- Vishan, I.; Sivaprakasam, S.; Kalamdhad, A. Biosorption of lead using Bacillus badius AK strain isolated from compost of green waste (water hyacinth). Environ. Technol. 2017, 38, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Marappa, N.; Dhanasekaran, D.; Vinothini, G.; Nooruddin, T. Extraction and recovery of precious metals from electronic waste printed circuit boards by bioleaching acidophilic fungi. Int. J. Environ. Sci. Technol. 2017, 15, 10. [Google Scholar] [CrossRef]
- Xie, C.; Hu, L.; Yang, Y.; Liao, D.; Yang, X. Accumulation and tolerance to cadmium heavy metal ions and induction of 14-3-3 gene expression in response to cadmium exposure in Coprinus atramentarius. Microbiol. Res. 2017, 196, 1–6. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, Q.; Zhang, X.; Zhao, L.; Li, H. Biosorption of Cr (VI) from aqueous solution using dormant spores of Aspergillus niger. RSC Adv. 2018, 8, 38157–38165. [Google Scholar] [CrossRef]
- Kamarudzaman, A.N.; Chay, T.C.; Amir, A.; Talib, S.A. Biosorption of Mn (II) ions from aqueous solution by Pleurotus spent mushroom compost in a fixed-bed column. Procedia-Soc. Behav. Sci. 2015, 195, 2709–2716. [Google Scholar] [CrossRef]
- Kolenčík, M.; Urík, M.; Čerňanský, S.; Molnárová, M.; Matúš, P. Leaching of zinc, cadmium, lead and copper from electronic scrap using organic acids and the Aspergillus niger strain. Fresenius Environ. Bull 2013, 22, 3673–3679. [Google Scholar]
- Štyriaková, I.; Štyriak, I.; Nandakumar, M.P.; Mattiasson, B. Bacterial destruction of mica during bioleaching of Kaolin and Quartz sands by Bacillus cereus. World J. Microbiol. Biotechnol. 2003, 19, 583–590. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process-a review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef]
- Galhaup, C.; Wagner, H.; Hinterstoisser, B.; Haltrich, D. Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzym. Microb. Technol. 2002, 30, 529–536. [Google Scholar] [CrossRef]
- Michalak, I.; Mironiuk, M.; Marycz, K. A comprehensive analysis of biosorption of metal ions by macroalgae using ICP-OES, SEM-EDX and FTIR techniques. PLoS ONE 2018, 13, e0205590. [Google Scholar] [CrossRef]
- Mungasavalli, D.P.; Viraraghavan, T.; Jin, Y.-C. Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: Batch and column studies. Colloids Surfaces A Physicochem. Eng. Aspects 2007, 301, 214–223. [Google Scholar] [CrossRef]
- Xing, S.C.; Chen, J.Y.; Lv, N.; Mi, J.D.; Chen, W.L.; Liang, J.B.; Liao, X.D. Biosorption of lead (Pb2+) by the vegetative and decay cells and spores of Bacillus coagulans R11 isolated from lead mine soil. Chemosphere 2018, 211, 804–816. [Google Scholar] [CrossRef]
- Park, K.M.; Park, S.S. Purification and characterization of laccase from basidiomycete Fomitella fraxinea. J. Microb. Biotechnol. 2008, 18, 670–675. [Google Scholar]
- Tamai, K.T.; Liu, X.; Silar, P.; Sosinowski, T.; Thiele, D.J. Heat shock transcription factor activates yeast metallothionein gene expression in response to heat and glucose starvation via distinct signaling pathways. Mol. Cell. Biol. 1994, 14, 8155–8165. [Google Scholar] [CrossRef] [PubMed]
- Chairin, T.; Nitheranont, T.; Watanabe, A.; Asada, Y.; Khanongnuch, C.; Lumyong, S. Biodegradation of bisphenol A and decolorization of synthetic dyes by laccase from white-rot fungus Trametes polyzona. Appl. Biochem. Biotechnol. 2013, 169, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.N.; Turkovskaya, O.V.; Yudina, E.N.; Rodakiewicz-Nowak, Y. Yellow laccase from the fungus Pleurotus ostreatus D1: Purification and characterization. Appl. Biochem. Microbiol. 2006, 42, 56–61. [Google Scholar] [CrossRef]
- Shaikh, S.M.; Shaikh, R.U.; Ade, A.B. Protein profiling of Thiobacillus ferrooxidans and Pseudomonas fluorescens mutants during metal extraction. J. Sci. Res. 2018, 10, 61–66. [Google Scholar] [CrossRef][Green Version]

| Metals | Khatri et al. [34] | Waldir et al. [35] | Shah et al. [36] | Szałatkiewicz [37] | Present Study |
|---|---|---|---|---|---|
| Cu | 275.5 | - | 360 | 14.6 | 268.6 ± 10.2 |
| Zn | 17.85 | - | 7.96 | - | 7.5 ± 2.2 |
| Ni | 19.55 | - | 8.55 | 1.65 | 10.20 ± 0.0 |
| Fe | 63.7 | - | 10.50 | 4.79 | 179.4 ± 16.8 |
| Cd | 0.02 | 22.0 | - | - | ND |
| Au | 0.08 | - | 0.10 | 0.0205 | ND |
| Ag | 0.08 | 0.02 | 0.28 | 0.045 | ND |
| Co | 0.42 | - | - | - | 0.13 ± 0.02 |
| Pb | 0.88 | 133.0 | 12.07 | 2.96 | ND |
| Pd | 0.08 | - | 0.64 | 0.022 | ND |
| Elements | Microorganisms | Bioleaching (mg L−1) (Broth Filtrate on 4th Day) | Biosorption (mg kg−1, Microbial Growth on 20th Day) |
|---|---|---|---|
| Copper | Lentinus edodes | 12.46 ± 1.12 e | 56.80 ± 0.96 d |
| Pleurotus florida | 44.30 ± 0.74 a | 97.26 ± 0.98 a | |
| Ganoderma lucidum | 20.93 ± 0.4 d | 49.40 ± 0.56 e | |
| Aspergillus niger | 36.13 ± 0.02 c | 73.66 ± 0.02 c | |
| Trametes versicolor | 41.26 ± 0.82 b | 76.66 ± 0.02 b | |
| Streptomyces spp. | 11.30 ± 0.26 f | 47.50 ± 1.34 e | |
| Pseudomonas spp. | 42.06 ± 0.20 b | 96.20 ± 0.55 a | |
| Iron | Lentinus edodes | 21.60 ± 0.26 e | 62.76 ± 0.08 e |
| Pleurotus florida | 43.13 ± 0.88 b | 94.13 ± 0.98 b | |
| Ganoderma lucidum | 13.96 ± 0.4 g | 57.86 ± 0.74 f | |
| Aspergillus niger | 34.10 ± 0.02 c | 70.93 ± 0.18 d | |
| Trametes versicolor | 32.10 ± 0.17 d | 82.13 ± 0.02 c | |
| Streptomyces spp. | 18.06 ± 0.66 f | 43.96 ± 0.12 g | |
| Pseudomonas spp. | 46.33 ± 0.52 a | 98.00 ± 0.08 a |
| Microorganisms | Rate of Desorption of Elements (%) | |
|---|---|---|
| Copper | Iron | |
| Lentinus edodes | 10.5 | 9.0 |
| Pleurotus florida | 18.0 | 12.4 |
| Ganoderma lucidum | 14.6 | 10.1 |
| Aspergillus niger | 15.2 | 11.2 |
| Trametes versicolor | 13.2 | 12.0 |
| Streptomyces spp. | 15.0 | 9.4 |
| Pseudomonas spp. | 16.6 | 13.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, P.; Sharma, S.; Albarakaty, F.M.; Kalia, A.; Hassan, M.M.; Abd-Elsalam, K.A. Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera. Sustainability 2022, 14, 935. https://doi.org/10.3390/su14020935
Kaur P, Sharma S, Albarakaty FM, Kalia A, Hassan MM, Abd-Elsalam KA. Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera. Sustainability. 2022; 14(2):935. https://doi.org/10.3390/su14020935
Chicago/Turabian StyleKaur, Preetiman, Shivani Sharma, Fawziah M. Albarakaty, Anu Kalia, Mohamed M. Hassan, and Kamel A. Abd-Elsalam. 2022. "Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera" Sustainability 14, no. 2: 935. https://doi.org/10.3390/su14020935
APA StyleKaur, P., Sharma, S., Albarakaty, F. M., Kalia, A., Hassan, M. M., & Abd-Elsalam, K. A. (2022). Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera. Sustainability, 14(2), 935. https://doi.org/10.3390/su14020935








