Red to Blue Light Ratio and Iron Nutrition Influence Growth, Metabolic Response, and Mineral Nutrients of Spinach Grown Indoors
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Lighting Treatments and Iron Biofortification
2.3. Measurements of Growth Parameters and Relative Chlorophyll Content
2.4. The Determination of Mineral Nutrients
2.5. The Determination of Soluble Sugars
2.6. The Determination of Antioxidant Capacity and Total Phenolic Content
2.7. Statistical Analyses
3. Results
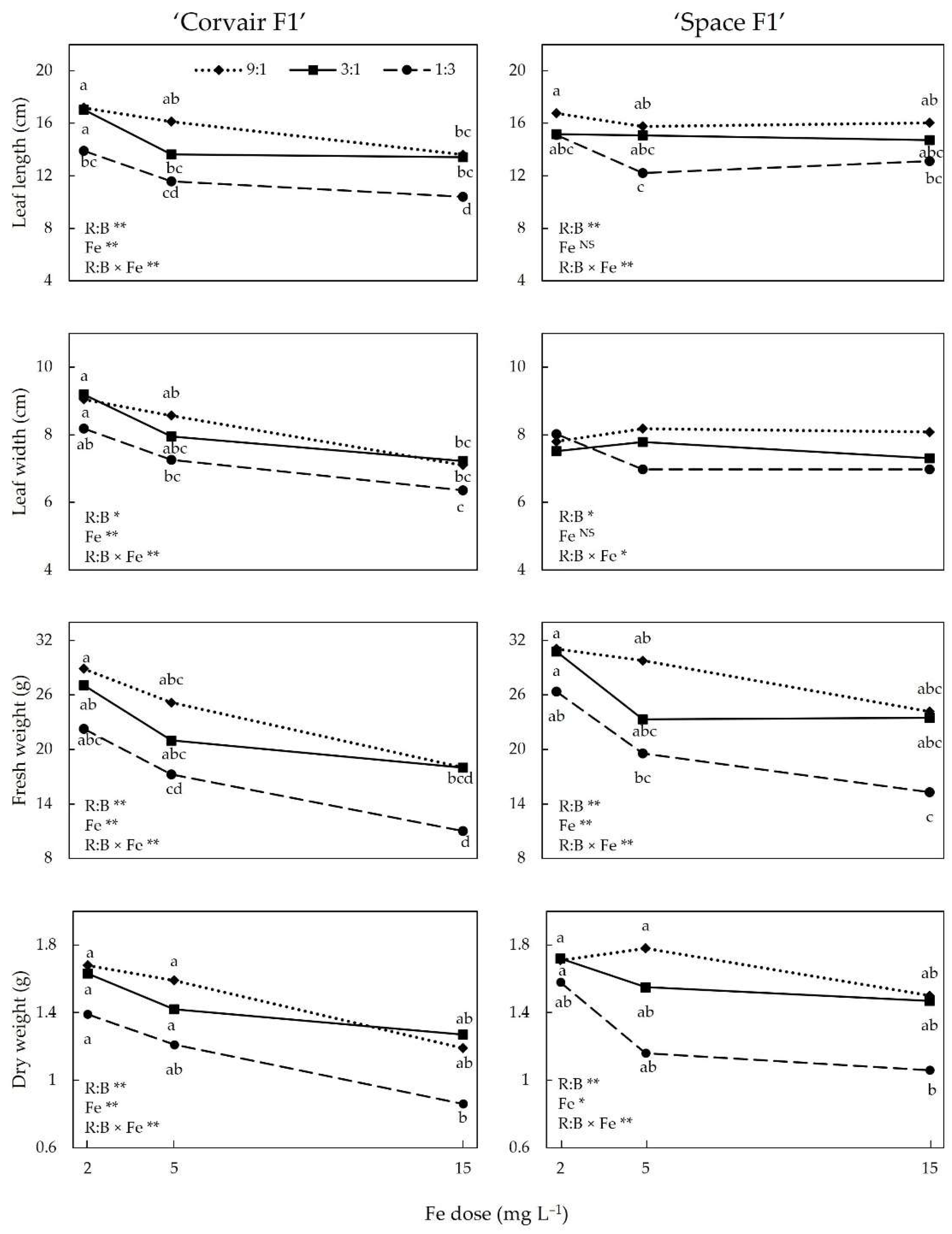
3.1. SPAD Index and Growth Indices
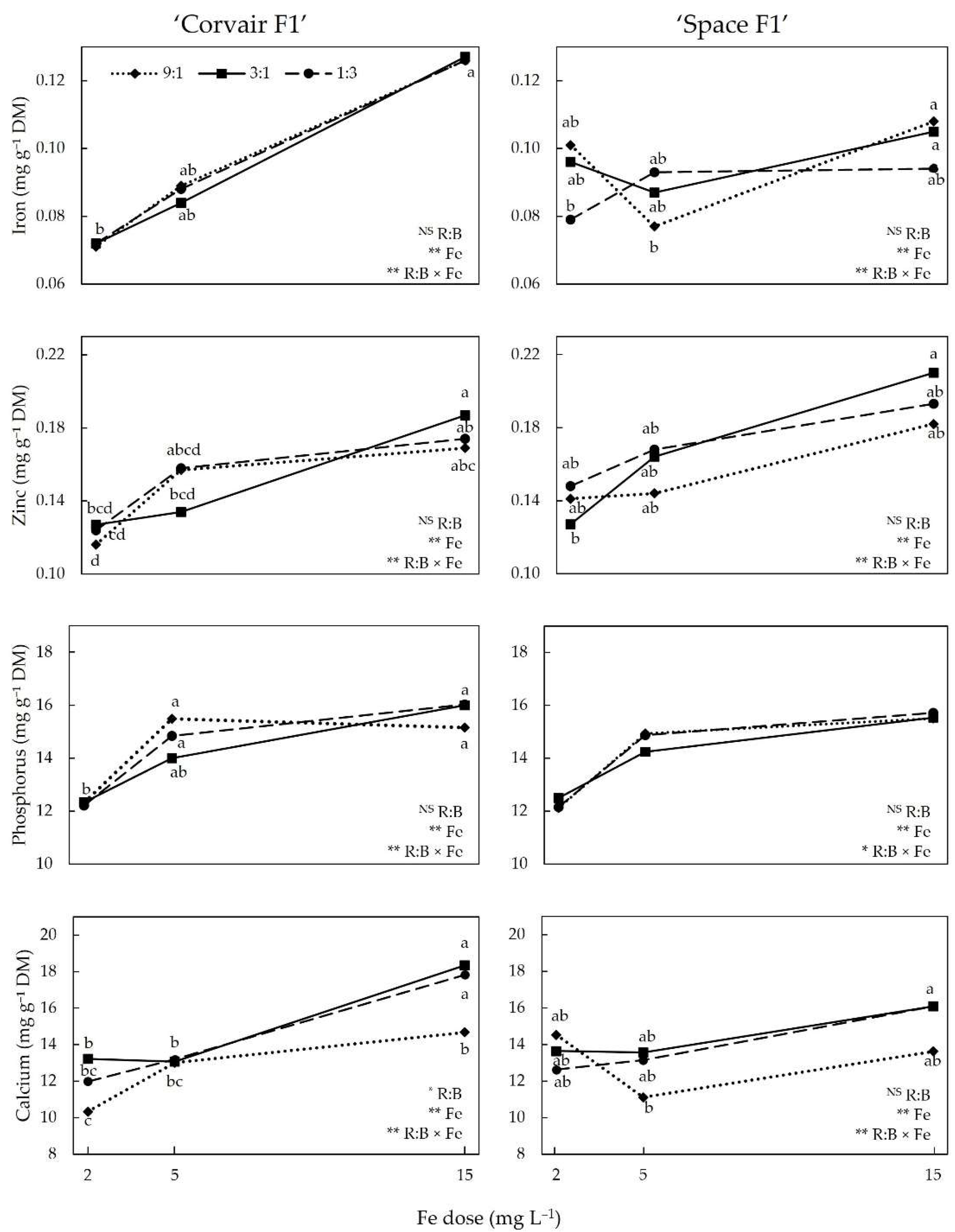
3.2. Mineral Nutrients
3.3. Soluble Sugars
3.4. Total Phenolic Content and Antiradical Activity
4. Discussion
4.1. Growth
4.2. Chlorophylls
4.3. Mineral Nutrients
4.4. Soluble Sugars
4.5. Antioxidant Capacity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roberts, J.L.; Moreau, R. Functional Properties of Spinach (Spinacia oleracea L.) Phytochemicals and Bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef]
- Dsouza, A.; Price, G.W.; Dixon, M.; Graham, T. Sustainability A Conceptual Framework for Incorporation of Composting in Closed-Loop Urban Controlled Environment Agriculture. Sustainability 2021, 13, 2471. [Google Scholar] [CrossRef]
- Mrinmoy Majumder, A.S. Suitable Substrate for Crop Growth under Protected Farms—An Assessment. J. Sci. Agric. 2018, 2, 62. [Google Scholar] [CrossRef][Green Version]
- Zou, T.; Huang, C.; Wu, P.; Ge, L.; Xu, Y. Optimization of Artificial Light for Spinach Growth in Plant Factory Based on Orthogonal Test. Plants 2020, 9, 490. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Wang Deng, X. Phytochrome Signaling Mechanisms. Arab. Book 2011, 9, e0148. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Zhao, C.; Pepper, M.; Lin, C. The Action Mechanisms of Plant Cryptochromes. Trends Plant Sci. 2011, 16, 684–691. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving Spinach, Radish, and Lettuce Growth under Red Light-Emitting Diodes (LEDs) with Blue Light Supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Sago, Y.; Watanabe, N.; Minami, Y. Zinc Biofortification of Hydroponic Baby Leaf Lettuce Grown under Artificial Lighting with Elevated Wind Speed and Root Zone Temperature. J. Agric. Meteorol. 2018, 74, 173–177. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė, V.; Viršilė, A.; Jankauskienė, J.; Samuolienė, G.; Sakalauskienė, S.; Novičkovas, A.; Miliauskienė, J.; Duchovskis, P. Changes in Mineral Element Content of Microgreens Cultivated under Different Lighting Conditions in a Greenhouse. Acta Hortic. 2018, 1227, 507–515. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Stašytė, K.; Duchovskis, P.; Samuolienė, G. The Response of Baby Leaf Lettuce to Selenium Biofortification under Different Lighting Conditions. Biol. Life Sci. Forum. 2021, 3, 10. [Google Scholar]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of Different Ratios of Blue and Red Led Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Jaiwal, P.K.; Chhillar, A.K.; Chaudhary, D.; Jaiwal, R. (Eds.) Nutritional Quality Improvement in Plants; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Santos, J.; Herrero, M.; Mendiola, J.A.; Oliva-Teles, M.T.; Ibáñez, E.; Delerue-Matos, C.; Oliveira, M. Fresh-Cut Aromatic Herbs: Nutritional Quality Stability during Shelf-Life. LWT-Food Sci. Technol. 2014, 59, 101–107. [Google Scholar] [CrossRef]
- Tilman, D. Global Environmental Impacts of Agricultural Expansion: The Need for Sustainable and Efficient Practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Briat, J.; Fobis-Loisy, I.; Grignon, N.; Lobréaux, S.; Pascal, N.; Savino, G.; Thoiron, S.; von Wirén, N.; van Wuytswinkel, O. Cellular and Molecular Aspects of Iron Metabolism in Plants. Biol. Cell 1995, 84, 69–81. [Google Scholar] [CrossRef]
- Bryson, G.M.; Mills, H.A.; Sasseville, D.N.; Jones Jr, J.B.; Barker, A.V. Plant Analysis Handbook III: A Guide to Sampling, Preparation, Analysis, and Interpretation for Agronomic and Horticultural Crops; Micro-Macro Publishing Inc.: Athens, Clarke, 2014. [Google Scholar]
- Schmidt, W.; Thomine, S.; Buckhout, T.J.; Astolfi, S.; Vigani, G. Editorial: Iron Nutrition and Interactions in Plants. Front. Plant Sci. 2020, 10, 1670. [Google Scholar] [CrossRef]
- Briat, J.-F.; Curie, C.; Gaymard, F. Iron Utilization and Metabolism in Plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef]
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular Health Benefits of Specific Vegetable Types: A Narrative Review. Nutrients 2018, 10, 595. [Google Scholar] [CrossRef]
- Briat, J.F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn Nutrition Signals in Arabidopsis Thaliana: Potential Involvement of phosphate starvation response 1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef]
- Pego, J.V.; Kortstee, A.J.; Huijser, C.; Smeekens, S.C.M. Photosynthesis, Sugars and the Regulation of Gene Expression. J. Exp. Bot. 2000, 51, 407–416. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Araújo, G.C.L.; Gonzalez, M.H.; Ferreira, A.G.; Nogueira, A.R.A.; Nóbrega, J.A. Effect of Acid Concentration on Closed-Vessel Microwave-Assisted Digestion of Plant Materials. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 2121–2132. [Google Scholar] [CrossRef]
- Barbosa, J.T.P.; Santos, C.M.M.; Peralva, V.N.; Flores, E.M.M.; Korn, M.; Nóbrega, J.A.; Korn, M.G.A. Microwave-Assisted Diluted Acid Digestion for Trace Elements Analysis of Edible Soybean Products. Food Chem. 2015, 175, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sun, Z.; Chen, C.; Zhang, L.; Zhu, S. Simultaneous Separation and Determination of Fructose, Sorbitol, Glucose and Sucrose in Fruits by HPLC-ELSD. Food Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef]
- Brons, C.; Olieman, C. Study of the High-Performance Liquid Chromatographic Separation of Reducing Sugars, Applied to the Determination of Lactose in Milk. J. Chromatogr. A 1983, 259, 79–86. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Sutulienė, R.; Ragelienė, L.; Samuolienė, G.; Brazaitytė, A.; Urbutis, M.; Miliauskienė, J. The Response of Antioxidant System of Drought-Stressed Green Pea (Pisum sativum L.) Affected by Watering and Foliar Spray with Silica Nanoparticles. Horticulturae 2022, 8, 35. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of Red and Blue Light on Leaf Anatomy, CO2assimilation and the Photosynthetic Electron Transport Capacity of Sweet Pepper (Capsicum annuum L.) Seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal Red:Blue Ratio in Led Lighting for Nutraceutical Indoor Horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Dougher, T.A.O.; Bugbee, B. Long-term Blue Light Effects on the Histology of Lettuce and Soybean Leaves and Stems. J. Am. Soc. Hortic. Sci. 2004, 129, 467–472. [Google Scholar] [CrossRef]
- Bugbee, B. Economics of LED Lighting. In Light Emitting Diodes for Agriculture: Smart Lighting; Springer: Singapore, 2017; pp. 81–99. ISBN 9789811058073. [Google Scholar]
- Zhang, Y.; Ji, J.; Song, S.; Su, W.; Liu, H. Growth, Nutritional Quality and Health-Promoting Compounds in Chinese Kale Grown under Different Ratios of Red:Blue LED Lights. Agronomy 2020, 10, 1248. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Grave, V.; Hao, X. Different Ratios of Red and Blue LED Light Effects on Coriander Productivity and Antioxidant Properties. Acta Hortic. 2016, 1134, 223–229. [Google Scholar] [CrossRef]
- Buckhout, T.J.; Kobayashi, T.; Gao, F.; Robe, K.; Gaymard, F.; Izquierdo, E.; Dubos, C. The Transcriptional Control of Iron Homeostasis in Plants: A Tale of BHLH Transcription Factors? Front. Plant Sci. 2019, 10, 6. [Google Scholar] [CrossRef]
- Connolly, E.L.; Guerinot, M.L. Iron Stress in Plants. Genome Biol. 2002, 3, 1024. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Peña-Olmos, J.E.; Casierra-Posada, F.; Olmos-Cubides, M.A. The Effect of High Iron Doses (Fe2+) on the Growth of Broccoli Plants (Brassica oleracea var. Italica). Agron. Colomb. 2014, 32, 22–28. [Google Scholar] [CrossRef]
- Nenova, V. Effect of Iron Supply on Growth and Photosystem II Efficiency of Pea Plants. Gen. Appl. Plant Physiol. 2006, 32, 81–90. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Varotto, C.; Maiwald, D.; Pesaresi, P.; Jahns, P.; Salamini, F.; Leister, D. The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. Plant J. 2002, 31, 589–599. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Viršilė, A.; Samuolienė, G.; Sakalauskienė, S.; Novičkovas, A.; Miliauskienė, J.; Duchovskis, P. Effect of Blue Light Percentage on Mineral Elements Content in Brassica Microgreens. Acta Hortic. 2020, 1271, 119–126. [Google Scholar] [CrossRef]
- Cavalcante, V.S.; Prado, R.d.M.; Vasconcelos, R.d.L.; Campos, C.N.S.; Cavalcante, V.S.; Prado, R.d.M.; Vasconcelos, R.d.L.; Campos, C.N.S. Iron Concentrations in Sugar Cane (Saccharum officinarum L.) Cultivated in Nutrient Solution. Agrociencia 2016, 50, 867–875. [Google Scholar]
- Hindt, M.N.; Guerinot, M. lou Getting a Sense for Signals: Regulation of the Plant Iron Deficiency Response. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; El-Nakhel, C.; de Micco, V.; Giordano, M.; Pannico, A.; de Pascale, S.; Graziani, G.; Ritieni, A.; Soteriou, G.A.; Kyriacou, M.C.; et al. Morpho-Metric and Specialized Metabolites Modulation of Parsley Microgreens through Selective LED Wavebands. Agronomy 2022, 12, 1502. [Google Scholar] [CrossRef]
- Dorais, M.; Bergstrand, K.-J.; Orsini, F.; Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in Shoot Tissue Pigments, Glucosinolates, and Mineral Elements in Sprouting Broccoli after Exposure to Short-duration Blue Light from Light Emitting Diodes. J. Amer. Soc. Hort. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef]
- Choi, M.-K.; Chang, M.-S.; Eom, S.-H.; Min, K.-S.; Kang, M.-H. Physicochemical Composition Of Buckwheat Microgreens Grown Under Different Light Conditions. J. Korean Soc. Food Sci. Nutr. 2015, 44, 709–715. [Google Scholar] [CrossRef]
- Shin, Y.-S.; Lee, M.-J.; Lee, E.-S.; Ahn, J.; Do, H.-W.; Choi, D.-W.; Jeong, J.-D.; Lee, J.-E.; Kim, M.-K.; Park, J.-U.; et al. Effect of Light Emitting Diodes Treatment on Growth and Mineral Contents of Lettuce (Lactuca sativa L. ‘Chung Chi Ma’). Korean J. Org. Agric. 2013, 21, 659–668. [Google Scholar] [CrossRef]
- Fiorilli, V.; Ferrol, N.; Tang, M.; Xie, X.; Hu, W.; Fan, X.; Chen, H.; Fiorilli, V.; Ferrol, N.; Tang, M.; et al. Interactions Between Phosphorus, Zinc, and Iron Homeostasis in Nonmycorrhizal and Mycorrhizal Plants. Front. Plant Sci. 2019, 10, 1172. [Google Scholar] [CrossRef]
- van den Ende, W.; Ruan, Y.-L.; Weckwerth, W. Multifunctional Fructans and Raffinose Family Oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef]
- van den Ende, W. Sugars Take a Central Position in Plant Growth, Development and, Stress Responses. A Focus on Apical Dominance. Front. Plant Sci. 2014, 5, 313. [Google Scholar] [CrossRef]
- Kelly, N.; Vaštakaitė-Kairienė, V.; Runkle, E.S. Indoor Lighting Effects on Plant Nutritional Compounds. In Plant Factory Basics, Applications and Advances; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–349. [Google Scholar]
- Zargar, S.M.; Kurata, R.; Inaba, S.; Oikawa, A.; Fukui, R.; Ogata, Y.; Agrawal, G.K.; Rakwal, R.; Fukao, Y. Quantitative Proteomics of Arabidopsis Shoot Microsomal Proteins Reveals a Cross-Talk between Excess Zinc and Iron Deficiency. Proteomics 2015, 15, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Quan Ye, Y.; Fan, S.K.; Jin, C.W.; Zheng, S.J. Increased Sucrose Accumulation Regulates Iron-Deficiency Responses by Promoting Auxin Signaling in Arabidopsis Plants. Plant Physiol. 2016, 170, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Agrawal, G.K.; Rakwal, R.; Fukao, Y. Quantitative Proteomics Reveals Role of Sugar in Decreasing Photosynthetic Activity Due to Fe Deficiency. Front. Plant Sci. 2015, 6, 592. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.C.; Nogueira, R.J.M.C.; de Araújo, F.P.; de Melo, N.F.; de Azevedo Neto, A.D. Physiological Responses to Salt Stress in Young Umbu Plants. Environ. Exp. Bot. 2008, 63, 147–157. [Google Scholar] [CrossRef]
- Khokhar, S.; Owusu Apenten, R.K. Iron Binding Characteristics of Phenolic Compounds: Some Tentative Structure–Activity Relations. Food Chem. 2003, 81, 133–140. [Google Scholar] [CrossRef]
- Sun, C.; Wu, T.; Zhai, L.; Li, D.; Zhang, X.; Xu, X.; Ma, H.; Wang, Y.; Han, Z. Reactive Oxygen Species Function to Mediate the Fe Deficiency Response in an Fe-Efficient Apple Genotype: An Early Response Mechanism for Enhancing Reactive Oxygen Production. Front. Plant Sci. 2016, 7, 1726. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Leaf Shape, Growth, and Antioxidant Phenolic Compounds of Two Lettuce Cultivars Grown under Various Combinations of Blue and Red Light-emitting Diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue Light-emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of Light Quality on Main Health-Promoting Compounds and Antioxidant Capacity of Chinese Kale Sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
| R:B Light Ratio | Fe (mg L−1) | PPFD (% of Total PPFD) | |
|---|---|---|---|
| R | B | ||
| 9:1 | 2 | 225 (90%) | 25 (10%) |
| 5 | |||
| 15 | |||
| 3:1 | 2 | 188 (75%) | 62 (25%) |
| 5 | |||
| 15 | |||
| 1:3 | 2 | 62 (25%) | 188 (75%) |
| 5 | |||
| 15 | |||
| Source of Variance | Biometric Indices | Mineral Nutrients (mg g⁻1 DM) | ||||
|---|---|---|---|---|---|---|
| SPAD Index | Leaf No. | Cu | K | Mg | ||
| ‘Corvair F1’ | ||||||
| R:B light ratio | ||||||
| 9:1 | 44.01 a | 9.11 a | 0.012 a | 36.03 a | 8.38 b | |
| 3:1 | 45.39 a | 9.02 a | 0.013 a | 35.68 a | 9.06 a | |
| 1:3 | 44.69 a | 8.73 a | 0.013 a | 35.32 a | 9.17 a | |
| Fe dose, mg L−1 | ||||||
| 2 | 42.08 b | 9.72 a | 0.013 a | 36.82 a | 8.56 a | |
| 5 | 46.79 a | 8.95 b | 0.014 a | 34.35 b | 9.04 a | |
| 15 | 45.22 ab | 8.19 c | 0.011 b | 35.86 a | 9.01 a | |
| R:B light ratio × Fe dose | ||||||
| 9:1 | 2 | 9.83 ab | 42.43 ab | 0.012 abcd | 38.26 a | 8.00 b |
| 5 | 9.33 abc | 44.97 ab | 0.013 ab | 33.72 c | 8.84 ab | |
| 15 | 8.17 cd | 44.64 ab | 0.011 d | 36.12 abc | 8.29 ab | |
| 3:1 | 2 | 10.00 a | 42.70 ab | 0.013 abc | 36.80 ab | 8.93 ab |
| 5 | 8.42 bcd | 46.12 ab | 0.013 abc | 34.09 c | 8.85 ab | |
| 15 | 8.63 abcd | 47.34 ab | 0.011 bcd | 36.16 abc | 9.40 a | |
| 1:3 | 2 | 9.33 abc | 41.13 b | 0.013 abc | 35.42 bc | 8.75 ab |
| 5 | 9.10 abcd | 49.30 a | 0.014 a | 35.25 bc | 9.42 a | |
| 15 | 7.77 d | 43.66 ab | 0.011 cd | 35.31 bc | 9.33 ab | |
| R:B light ratio | NS | NS | NS | NS | ** | |
| Fe dose | ** | ** | ** | ** | NS | |
| R:B light ratio × Fe dose | * | ** | ** | ** | NS | |
| ‘Space F1’ | ||||||
| R:B light ratio | ||||||
| 9:1 (control) | 35.31 a | 10.70 a | 0.011 a | 37.86 a | 9.48 b | |
| 3:1 | 37.10 a | 10.10 ab | 0.012 a | 36.69 a | 10.01 a | |
| 1:3 | 37.37 a | 9.70 b | 0.012 a | 36.63 a | 10.20 a | |
| Fe dose, mg L−1 | ||||||
| 2 (control) | 32.73 b | 10.57 a | 0.016 a | 37.08 a | 9.90 a | |
| 5 | 38.64 a | 10.10 a | 0.011 b | 36.58 a | 9.96 a | |
| 15 | 38.57 a | 9.79 a | 0.008 b | 37.51 a | 9.83 a | |
| R:B light ratio × Fe dose | ||||||
| 9:1 | 2 (control) | 11.32 a | 32.30 c | 0.016 a | 37.72 a | 9.95 ab |
| 5 | 10.40 ab | 36.43 abc | 0.010 ab | 37.22 a | 9.34 cd | |
| 15 | 10.32 ab | 37.31 abc | 0.008 b | 38.64 a | 9.15 d | |
| 3:1 | 2 | 10.00 ab | 32.95 bc | 0.016 a | 36.59 a | 9.92 abc |
| 5 | 10.20 ab | 40.61 a | 0.011 ab | 35.86 a | 10.02 ab | |
| 15 | 10.12 ab | 37.68 ab | 0.008 b | 37.61 a | 10.08 ab | |
| 1:3 | 2 | 10.40 ab | 32.92 bc | 0.015 ab | 36.94 a | 9.83 bc |
| 5 | 9.70 ab | 38.87 a | 0.012 ab | 36.67 a | 10.51 a | |
| 15 | 9.00 b | 40.31 a | 0.008 b | 36.27 a | 10.24 ab | |
| R:B light ratio | NS | * | NS | NS | ** | |
| Fe dose | ** | NS | ** | NS | NS | |
| R:B light ratio × Fe dose | ** | * | ** | NS | ** | |
| Source of Variance | Soluble Sugars (mg g−1 DM) | |||||
|---|---|---|---|---|---|---|
| Fructose | Glucose | Sucrose | Maltose | Raffinose | ||
| ‘Corvair F1’ | ||||||
| R:B light ratio | ||||||
| 9:1 (control) | 2.19 a | 1.54 b | 3.70 a | 2.46 a | 17.45 a | |
| 3:1 | 2.42 a | 1.70 ab | 2.98 a | 2.99 a | 18.56 a | |
| 1:3 | 2.10 a | 2.14 a | 3.26 a | 2.74 a | 14.79 a | |
| Fe dose, mg L−1 | ||||||
| 2 (control) | 1.44 c | 1.34 b | 2.39 b | 3.00 a | 17.49 a | |
| 5 | 2.08 b | 2.01 a | 3.34 ab | 2.15 a | 15.23 a | |
| 15 | 3.18 a | 2.03 a | 4.21 a | 3.04 a | 18.08 a | |
| R:B light ratio × Fe dose | ||||||
| 9:1 | 2 (control) | 1.37 d | 1.40 bc | 3.35 abc | 2.91 a | 18.04 ab |
| 5 | 1.61 d | 1.58 abc | 2.34 bc | 2.08 a | 16.74 ab | |
| 15 | 3.60 a | 1.65 abc | 5.41 a | 2.38 a | 17.56 ab | |
| 3:1 | 2 | 1.30 d | 1.14 c | 1.60 c | 3.97 a | 22.40 b |
| 5 | 2.40 b | 1.90 abc | 3.35 abc | 2.14 a | 19.31 b | |
| 15 | 3.56 a | 2.05 abc | 3.98 ab | 2.87 a | 23.97 a | |
| 1:3 | 2 | 1.67 cd | 1.49 abc | 2.22 bc | 2.13 a | 12.02 ab |
| 5 | 2.24 bc | 2.54 a | 4.32 ab | 2.22 a | 19.64 ab | |
| 15 | 2.38 b | 2.38 ab | 3.23 abc | 3.86 a | 12.72 ab | |
| R:B light ratio | NS | * | NS | NS | NS | |
| Fe dose | ** | ** | ** | NS | NS | |
| R:B light ratio × Fe dose | ** | ** | ** | NS | * | |
| ‘Space F1’ | ||||||
| R:B light ratio | ||||||
| 9:1 (control) | 2.19 a | 3.51 a | 3.43 a | 3.27 a | 14.52 a | |
| 3:1 | 1.96 a | 3.05 a | 3.56 a | 2.51 a | 13.51 a | |
| 1:3 | 2.11 a | 3.53 a | 3.93 a | 2.58 a | 14.54 a | |
| Fe dose, mg L−1 | ||||||
| 2 (control) | 2.27 a | 4.30 a | 2.99 b | 3.29 a | 13.68 a | |
| 5 | 1.95 a | 2.81 b | 3.60 ab | 2.58 a | 13.65 a | |
| 15 | 2.03 a | 2.98 b | 4.33 a | 2.49 a | 15.24 a | |
| R:B light ratio × Fe dose | ||||||
| 9:1 | 2 (control) | 2.09 abc | 4.69 a | 2.77 b | 4.64 a | 13.67 a |
| 5 | 2.16 abc | 2.88 b | 2.99 ab | 2.60 a | 13.54 a | |
| 15 | 2.33 ab | 2.96 b | 4.55 a | 2.56 a | 16.34 a | |
| 3:1 | 2 | 2.07 abc | 3.57 ab | 2.67 b | 2.35 a | 12.29 a |
| 5 | 2.14 abc | 3.05 b | 3.78 ab | 2.64 a | 15.26 a | |
| 15 | 1.66 bc | 2.53 b | 4.23 ab | 2.55 a | 12.99 a | |
| 1:3 | 2 | 2.65 a | 4.64 a | 3.53 ab | 2.87 a | 15.08 a |
| 5 | 1.55 c | 2.50 b | 4.04 ab | 2.51 a | 12.16 a | |
| 15 | 2.11 abc | 3.45 ab | 4.21 ab | 2.37 a | 16.39 a | |
| R:B light ratio | NS | NS | NS | NS | NS | |
| Fe dose | NS | ** | ** | NS | NS | |
| R:B light ratio × Fe dose | ** | ** | ** | NS | NS | |
| Source of Variance | TPC (mg g−1 DM) | ABTS, TEAC (mM g−1 DM) | FRAP, TEAC (µmol g−1 DM) | DPPH (µmol g−1 DM) | |
|---|---|---|---|---|---|
| ‘Corvair F1’ | |||||
| R:B light ratio | |||||
| 9:1 (control) | 5.14 a | 26.25 a | 1.80 c | 3.32 a | |
| 3:1 | 4.94 a | 30.27 b | 3.18 b | 4.20 a | |
| 1:3 | 4.88 a | 32.64 b | 4.22 a | 5.84 b | |
| Fe dose, mg L−1 | |||||
| 2 (control) | 4.47 b | 29.21 a | 3.48 a | 5.46 a | |
| 5 | 5.26 a | 28.21 a | 2.66 a | 3.47 b | |
| 15 | 5.23 a | 31.73 a | 3.06 a | 4.43 ab | |
| R:B light ratio × Fe dose | |||||
| 9:1 | 2 (control) | 4.85 abc | 24.76 bc | 2.82 abc | 5.49 ab |
| 5 | 5.36 bc | 23.58 c | 1.01 d | 1.52 c | |
| 15 | 5.21 bc | 30.42 abc | 1.57 cd | 2.95 bc | |
| 3:1 | 2 | 4.80 abc | 30.02 abc | 3.79 ab | 4.34 abc |
| 5 | 5.31 bc | 29.19 abc | 2.56 bcd | 4.10 abc | |
| 15 | 4.69 ab | 31.59 abc | 2.56 bcd | 4.18 abc | |
| 1:3 | 2 | 3.74 a | 32.84 ab | 3.83 ab | 6.54 a |
| 5 | 5.03 bc | 31.87 ab | 4.43 a | 4.80 abc | |
| 15 | 5.87 c | 33.20 a | 4.43 a | 6.18 ab | |
| R:B light ratio | NS | ** | ** | ** | |
| Fe dose | ** | NS | NS | * | |
| R:B light × Fe | * | * | ** | ** | |
| ‘Space F1’ | |||||
| R:B light ratio | |||||
| 9:1 (control) | 4.53 ab | 29.66 a | 3.81 a | 3.89 a | |
| 3:1 | 5.27 a | 31.59 a | 3.99 a | 5.75 b | |
| 1:3 | 4.06 b | 33.12 a | 3.70 a | 6.22 b | |
| Fe dose, mg L−1 | |||||
| 2 (control) | 4.87 a | 30.88 a | 3.80 a | 4.71 a | |
| 5 | 4.63 a | 33.49 a | 3.89 a | 5.39 a | |
| 15 | 4.35 a | 30.02 a | 3.82 a | 5.77 a | |
| R:B light ratio | |||||
| 9:1 | 2 (control) | 4.89 abc | 31.24 ab | 3.69 a | 3.45 b |
| 5 | 3.60 c | 30.18 ab | 4.66 a | 4.40 b | |
| 15 | 5.09 abc | 27.57 b | 3.08 a | 3.83 b | |
| 3:1 | 2 | 5.77 ab | 31.92 ab | 4.28 a | 6.64 ab |
| 5 | 6.14 a | 32.76 ab | 3.50 a | 5.49 ab | |
| 15 | 3.89 c | 30.10 ab | 4.19 a | 5.13 ab | |
| 1:3 | 2 | 3.96 c | 29.46 ab | 3.42 a | 4.02 b |
| 5 | 4.14 bc | 37.53 a | 3.50 a | 6.29 ab | |
| 15 | 4.07 c | 32.38 ab | 4.17 a | 8.35 a | |
| R:B light ratio | * | NS | NS | ** | |
| Fe dose | NS | NS | NS | NS | |
| R:B light ratio × Fe | * | NS | NS | ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaštakaitė-Kairienė, V.; Brazaitytė, A.; Miliauskienė, J.; Runkle, E.S. Red to Blue Light Ratio and Iron Nutrition Influence Growth, Metabolic Response, and Mineral Nutrients of Spinach Grown Indoors. Sustainability 2022, 14, 12564. https://doi.org/10.3390/su141912564
Vaštakaitė-Kairienė V, Brazaitytė A, Miliauskienė J, Runkle ES. Red to Blue Light Ratio and Iron Nutrition Influence Growth, Metabolic Response, and Mineral Nutrients of Spinach Grown Indoors. Sustainability. 2022; 14(19):12564. https://doi.org/10.3390/su141912564
Chicago/Turabian StyleVaštakaitė-Kairienė, Viktorija, Aušra Brazaitytė, Jurga Miliauskienė, and Erik S. Runkle. 2022. "Red to Blue Light Ratio and Iron Nutrition Influence Growth, Metabolic Response, and Mineral Nutrients of Spinach Grown Indoors" Sustainability 14, no. 19: 12564. https://doi.org/10.3390/su141912564
APA StyleVaštakaitė-Kairienė, V., Brazaitytė, A., Miliauskienė, J., & Runkle, E. S. (2022). Red to Blue Light Ratio and Iron Nutrition Influence Growth, Metabolic Response, and Mineral Nutrients of Spinach Grown Indoors. Sustainability, 14(19), 12564. https://doi.org/10.3390/su141912564









