Modified Nano-Fe2O3-Paraffin Wax for Efficient Photovoltaic/Thermal System in Severe Weather Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Materials
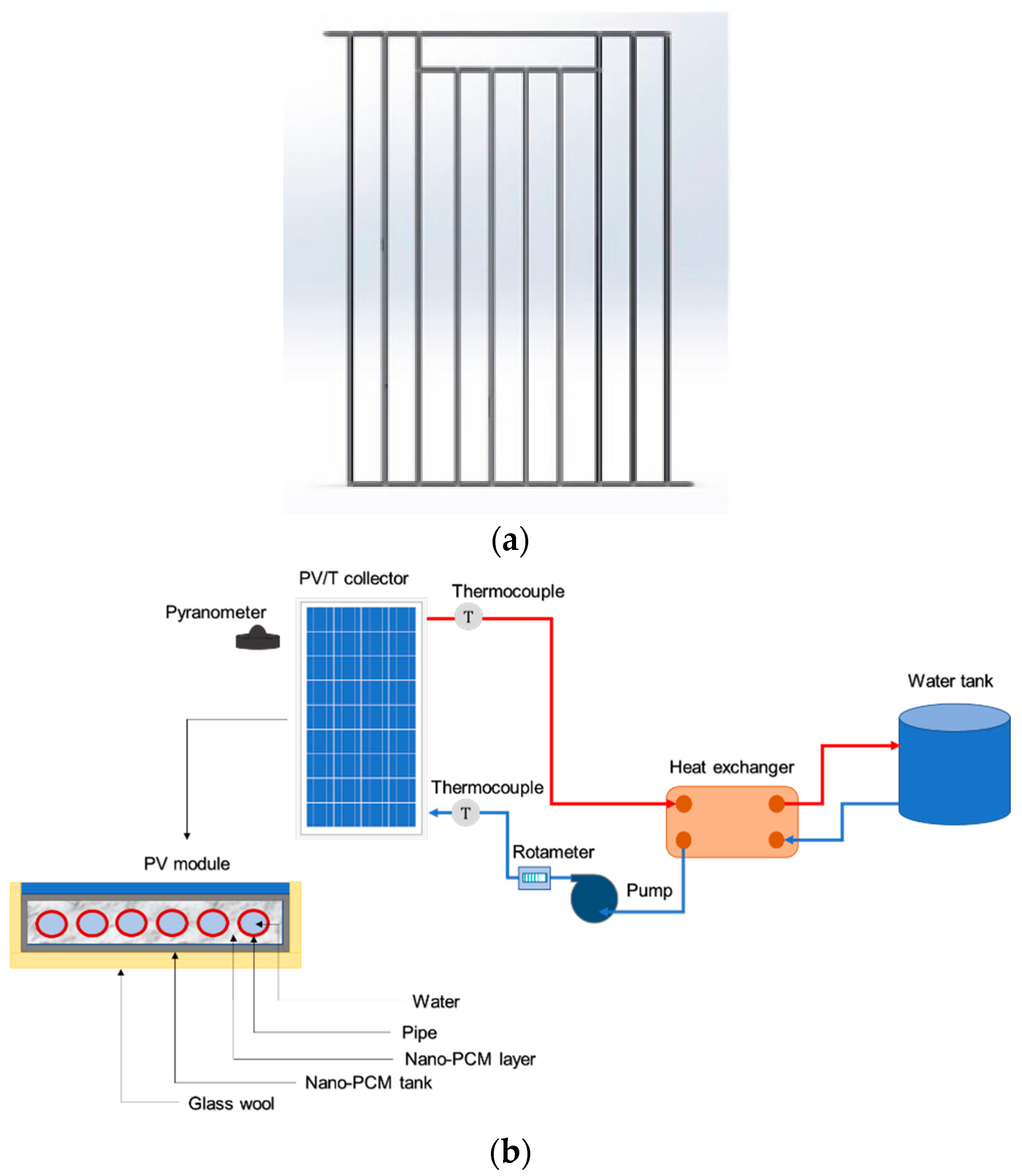
2.3. PVT System Descriptions
2.4. Instrumentations
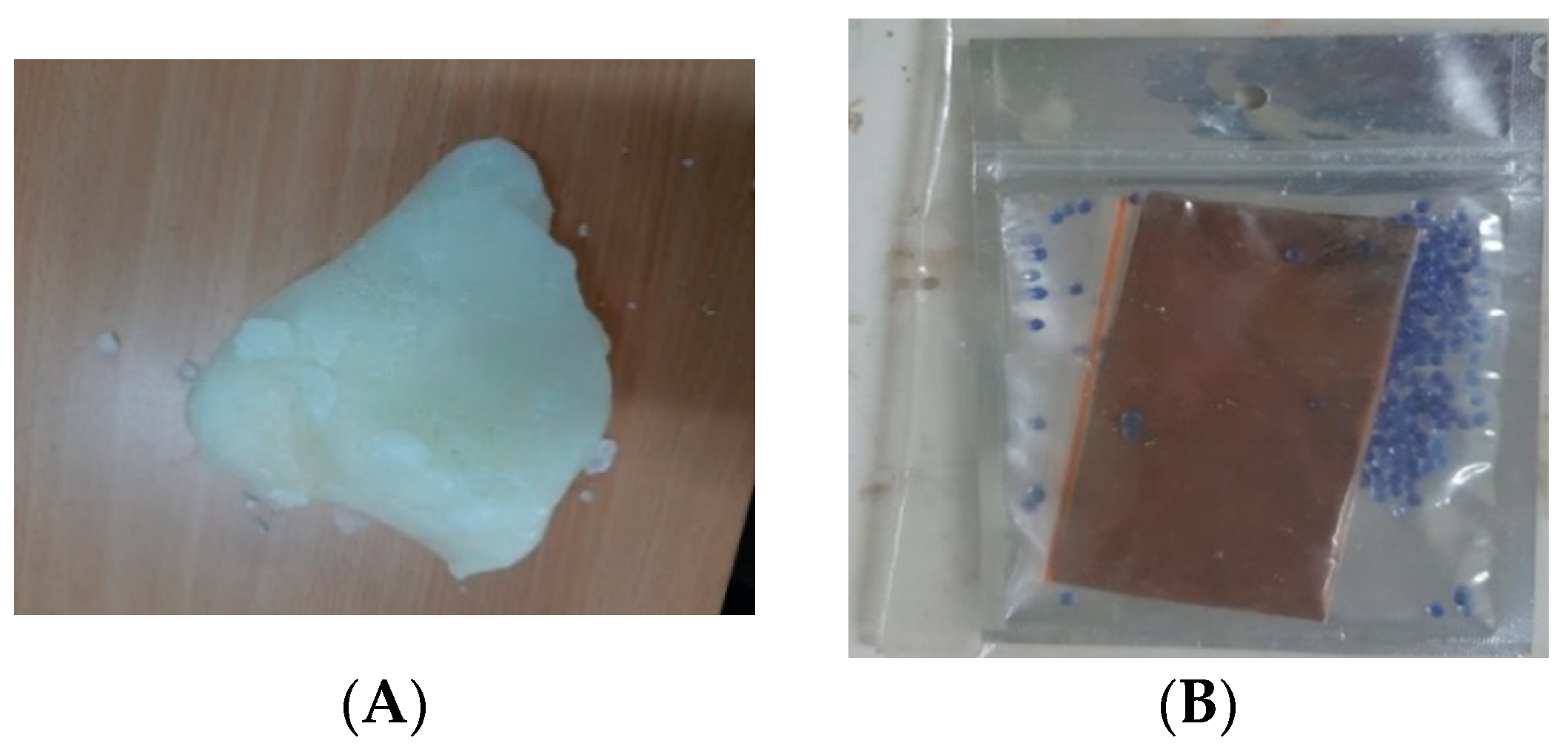
2.5. Preparation of Nano-Paraffin
3. Results and Discussion
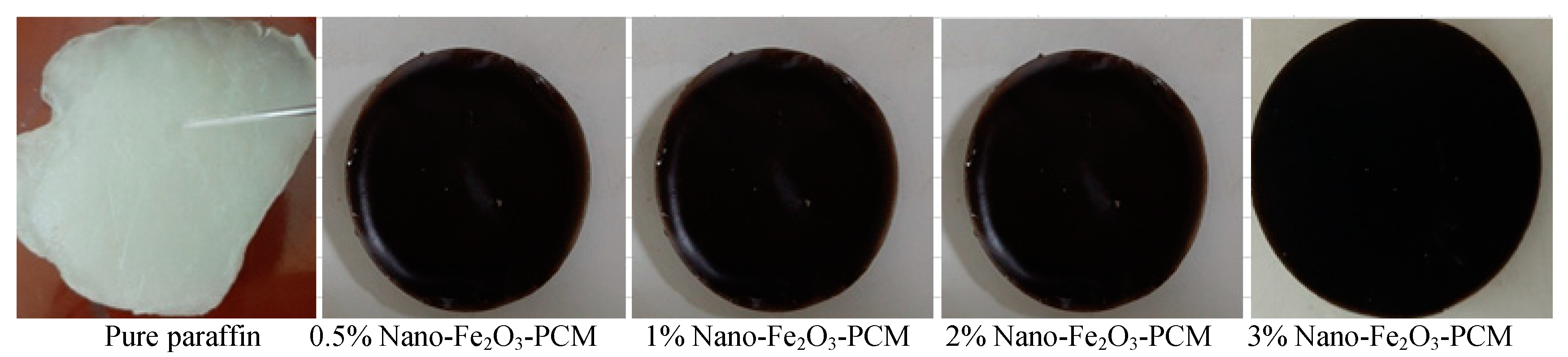
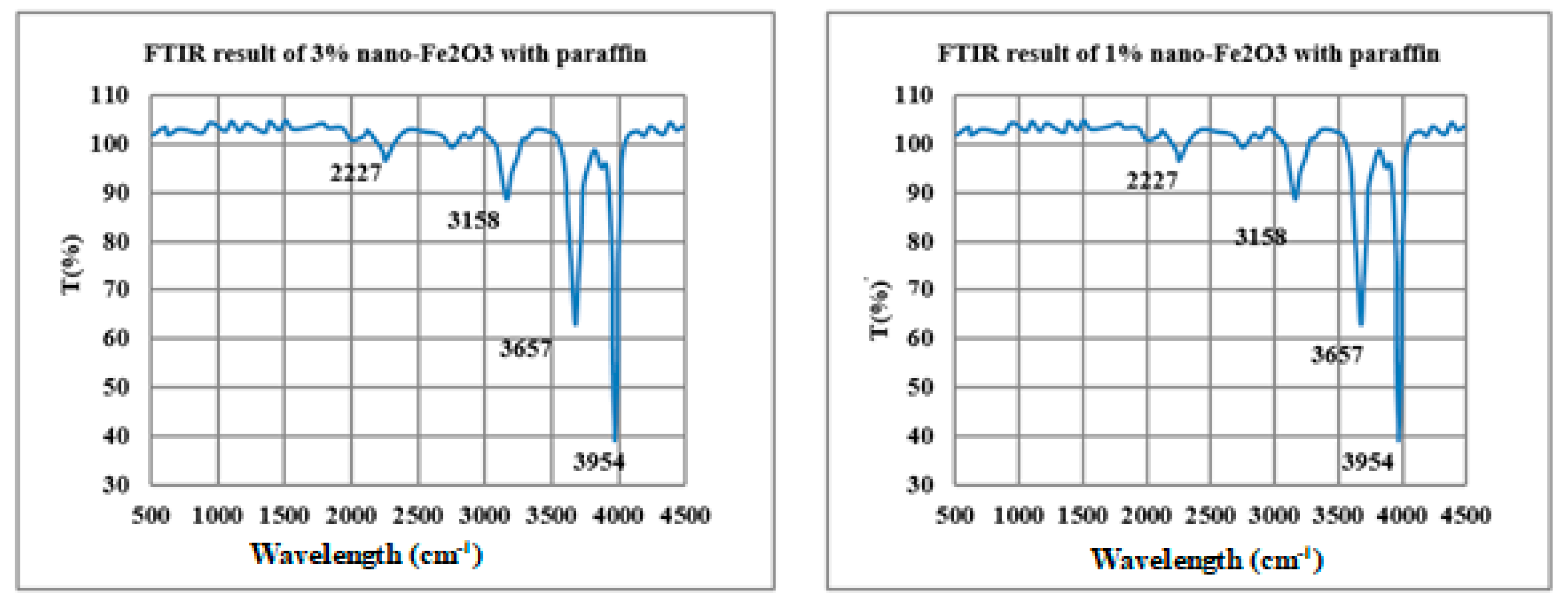
3.1. Optical Properties
3.2. Thermophysical Properties
3.3. Density
3.4. Viscosity
3.5. Thermal Conductivity
3.6. Products’ Stability
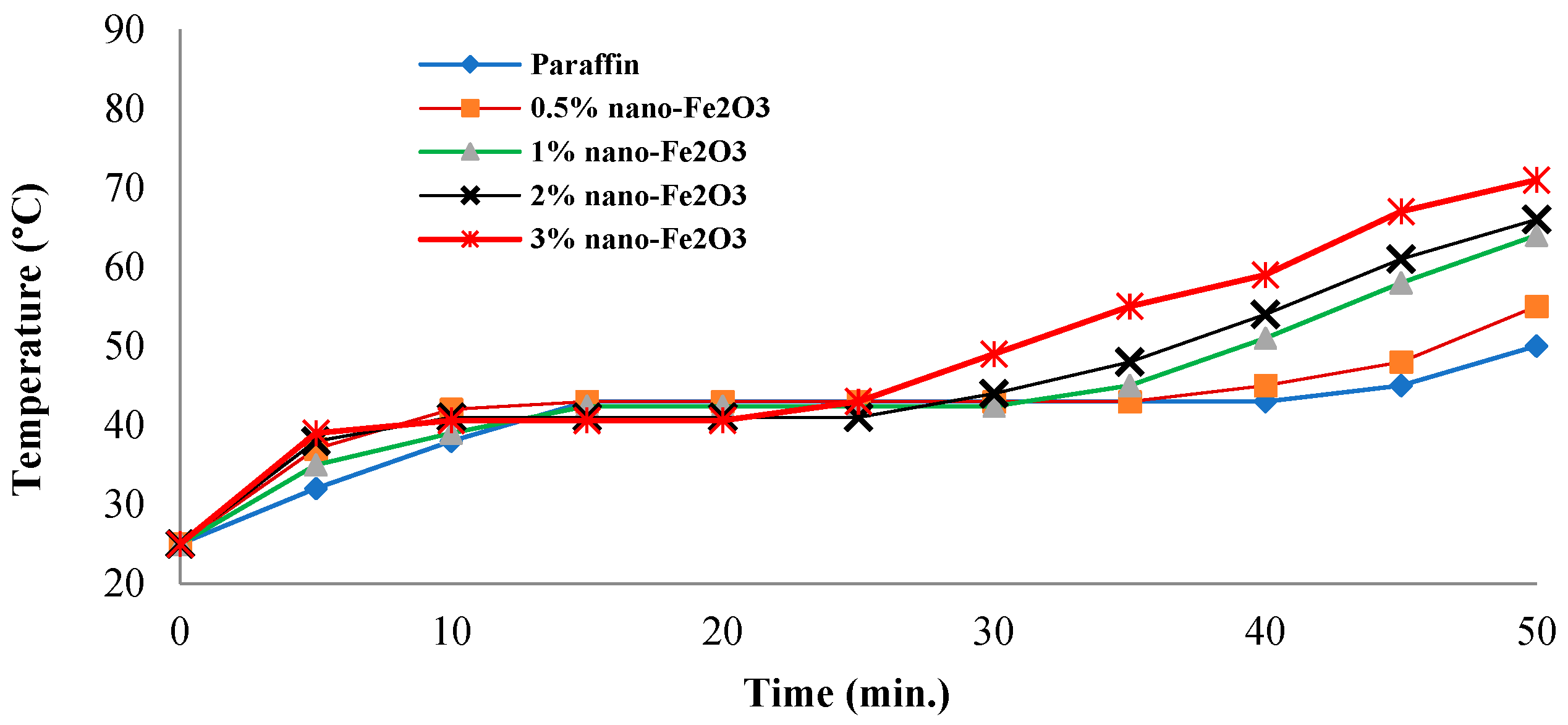
3.7. Charging Period
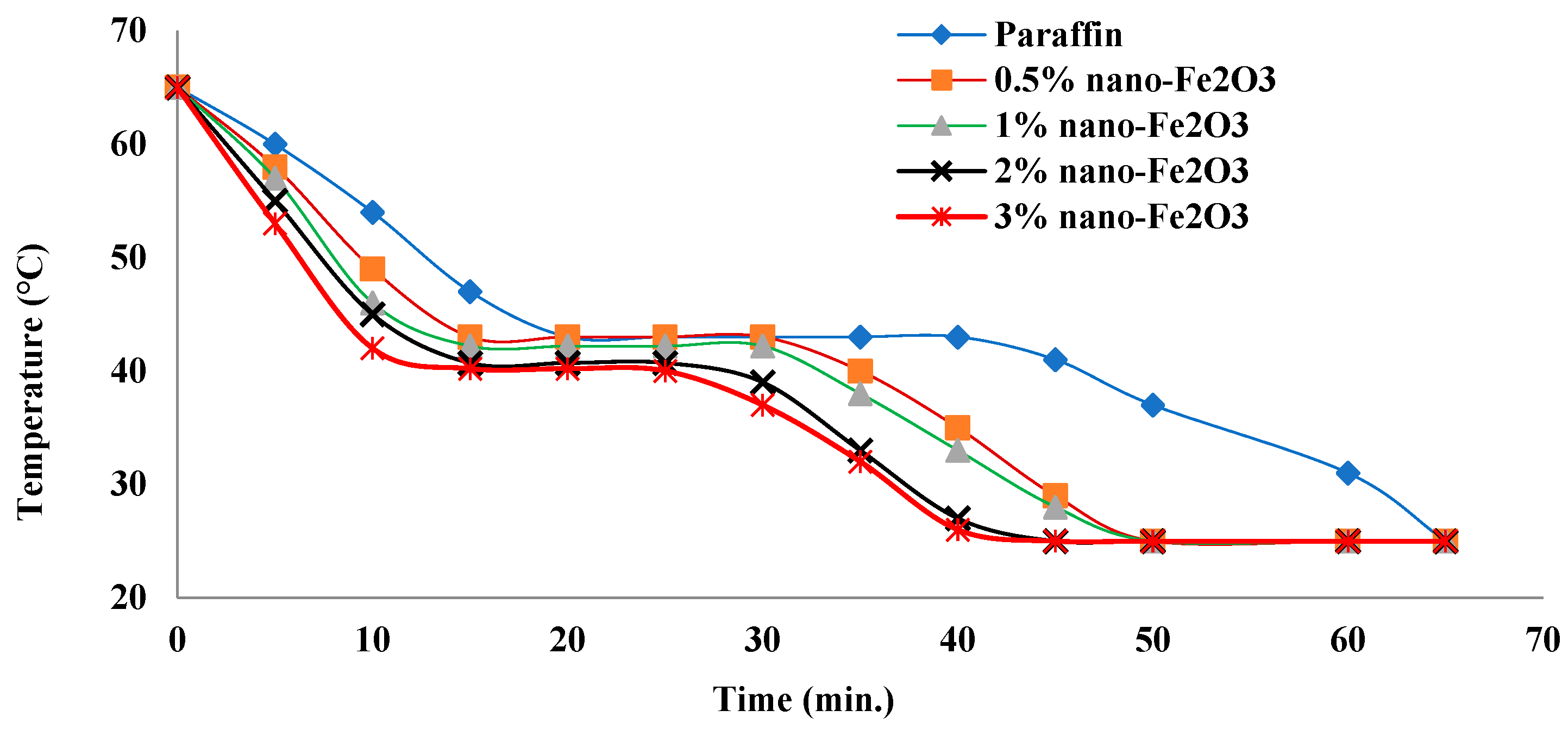
3.8. Discharging Period
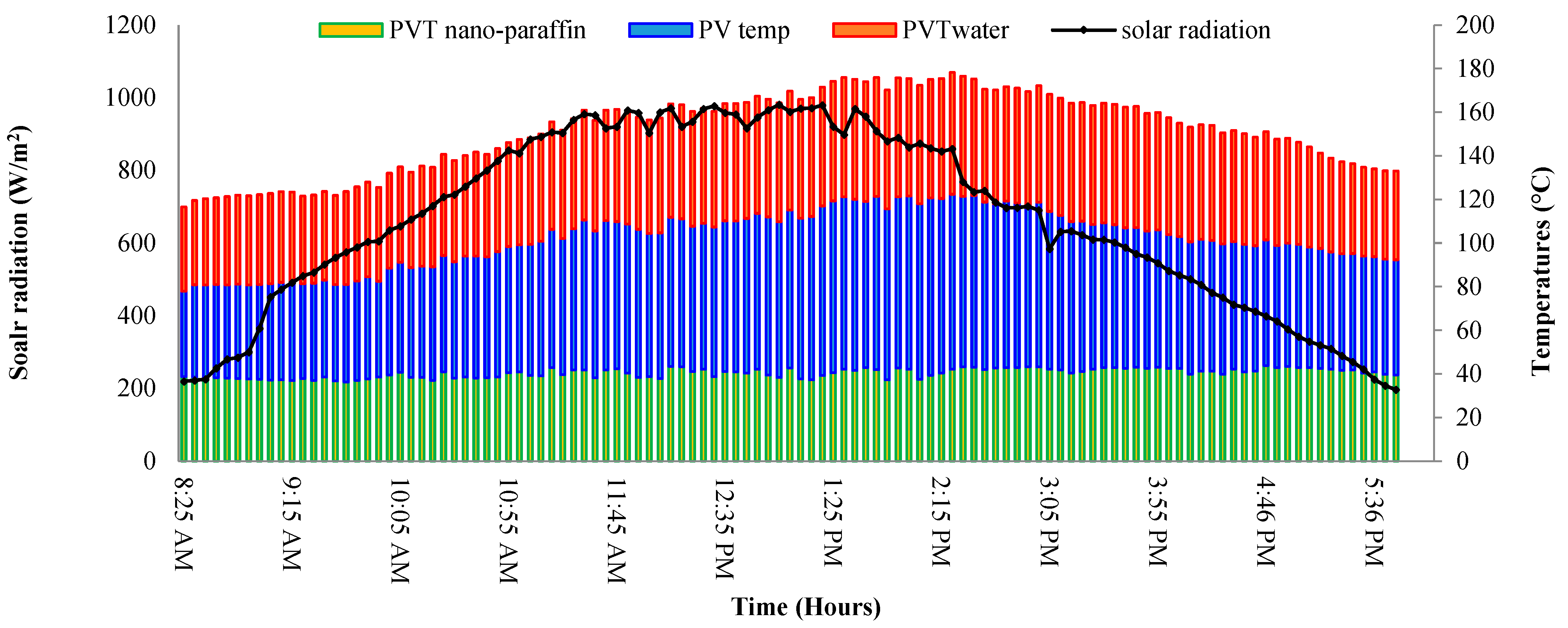
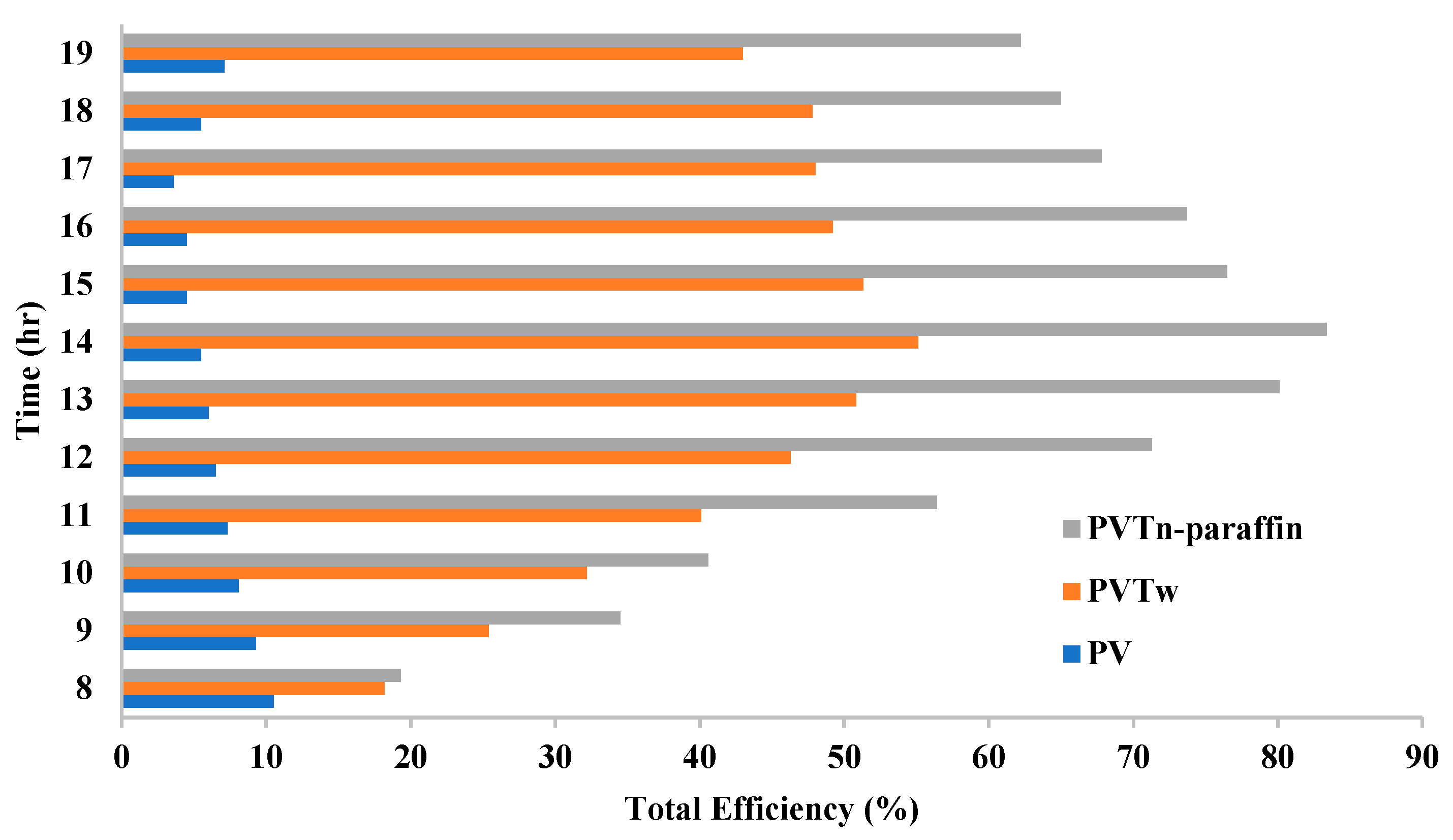
3.9. PVT System Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.M.; Mylsamy, K.; Saravanakumar, P.T.; Anandkumar, R.; Pranav, A. Experimental study on thermal properties of nano-TiO2 embedded paraffin (NEP) for thermal energy storage applications. Mater. Today Proc. 2020, 22, 2153–2159. [Google Scholar] [CrossRef]
- Bornapour, M.; Hemmati, R.; Pourbehzadi, M.; Dastranj, A.; Niknam, T. Probabilistic optimal coordinated planning of molten carbonate fuel cell-CHP and renewable energy sources in microgrids considering hydrogen storage with point estimate method. Energy Convers. Manag. 2020, 206, 112495. [Google Scholar] [CrossRef]
- Al-Maamary, H.M.; Kazem, H.A.; Chaichan, M.T. Changing the energy profile of the GCC states: A review. Int. J. Appl. Eng. Res. (IJAER) 2016, 11, 1980–1988. [Google Scholar]
- Fana, S.; LibLin, Z.; Hea, Y.G. Customer directrix load-based large-scale demand response for integrating renewable energy sources. Electr. Power Syst. Res. 2002, 181, 106175. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Eladla, A.; ElDesoukyb, A. Optimal economic dispatch for multi heat-electric energy source power system. Int. J. Electr. Power Energy Syst. 2019, 110, 21–35. [Google Scholar]
- Eladl, A.A.; El-Afifi, M.I.; Saeed, M.A.; El-Saadawi, M.M. Optimal operation of energy hubs integrated with renewable energy sources and storage devices considering CO2 emissions. Int. J. Electr. Power Energy Syst. 2002, 117, 105719. [Google Scholar] [CrossRef]
- Samykano, M. Role of phase change materials in thermal energy storage: Potential, recent progress and technical challenges. Sustain. Energy Technol. Assess. 2022, 52, 102234. [Google Scholar] [CrossRef]
- Stalin, P.M.J.; Prasad, K.S.; Kumar, K.P.; Hemadri, G.; Rajesh, M.; Kumar, K.P. Performance improvement of solar PV through the thermal management using a nano-PCM. Mater. Today Proc. 2022, 50, 1553–1558. [Google Scholar] [CrossRef]
- Xiong, C.; Sun, J.; Yang, H.; Jiang, H. Real reason for high ideality factor in organic solar cells: Energy disorder. Sol. Energy 2019, 178, 193–200. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Su, H.; Shi, Y.; Liu, Q.; Chen, X.; He, D. Hybrid energy system based on solar cell and self-healing/self-cleaning triboelectric nanogenerator. Nano Energy 2020, 79, 105394. [Google Scholar] [CrossRef]
- Ambreen, T.; Niyas, H.; Kanti, P.; Ali, H.M.; Park, C.W. Experimental investigation on the performance of RT-44HC-nickel foam-based heat sinks for thermal management of electronic gadgets. Int. J. Heat Mass Transf. 2022, 188, 122591. [Google Scholar]
- Fetliński, B.; Malinowski, M. Potential energy yield increase of a solar spectra down-converter equipped photovoltaic device in real operational conditions. Sol. Energy 2018, 165, 148–158. [Google Scholar] [CrossRef]
- Carmona, M.; Bastos, A.P.; García, J.D. Experimental evaluation of a hybrid photovoltaic and thermal solar energy collector with integrated phase change material (PVT-PCM) in comparison with a traditional photovoltaic (PV) module. Renew. Energy 2021, 172, 680–696. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.-N.; Zhou, F. Thermophysical properties and applications of nano-enhanced PCMs: An update review. Energy Convers. Manag. 2020, 214, 112876. [Google Scholar] [CrossRef]
- Abdelrazik, A.; Al-Sulaiman, F.; Saidur, R. Numerical investigation of the effects of the nano-enhanced phase change materials on the thermal and electrical performance of hybrid PV/thermal systems. Energy Convers. Manag. 2020, 205, 112449. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Alwaeli, A.H.A.; Sopian, K.; Kazem, H.A.; Chaichan, M.T. Photovoltaic/Thermal (PV/T) systems: Status and future prospects. Renew. Sustain. Energy Rev. 2017, 77, 109–130. [Google Scholar] [CrossRef]
- Elfasakhany, A. Performance assessment and productivity of a simple-type solar still integrated with nanocomposite energy storage system. Appl. Energy 2016, 183, 399–407. [Google Scholar] [CrossRef]
- Rufuss, D.D.W.; Iniyan, S.; Suganthi, L.; Pa, D. Nanoparticles Enhanced Phase Change Material (NPCM) as Heat Storage in Solar Still Application for Productivity Enhancement. Energy Procedia 2017, 141, 45–49. [Google Scholar] [CrossRef]
- Shareef, A.S.; Rashid, F.L.; Alwan, H.F. Water solar distiller productivity enhancement using solar collector and phase change material (PCM). IOP Conf. Ser. Mater. Sci. Eng. 2020, 671, 012150. [Google Scholar] [CrossRef]
- Behura, A.; Gupta, H.K. Use of nanoparticle-embedded phase change material in solar still for productivity enhancement. Mater. Today Proc. 2021, 45, 3904–3907. [Google Scholar] [CrossRef]
- Kumar, P.M.; Sudarvizhi, D.; Prakash, K.; Anupradeepa, A.; Raj, S.B.; Shanmathi, S.; Sumithra, K.; Surya, S. Investigating a single slope solar still with a nano-phase change material. Mater. Today Proc. 2021, 45, 7922–7925. [Google Scholar] [CrossRef]
- Abdelgaied, M.; Attia, M.E.H.; Kabeel, A.E.; Zayed, M.E. Improving the thermo-economic performance of hemispherical solar distiller using copper oxide nanofluids and phase change materials: Experimental and theoretical investigation. Sol. Energy Mater. Sol. Cells 2022, 238, 111596. [Google Scholar] [CrossRef]
- Saleh, B.; Essa, F.A.; Aly, A.; Alsehli, M.; Panchal, H.; Afzal, A.; Shanmugan, S. Investigating the performance of dish solar distiller with phase change material mixed with Al2O3 nanoparticles under different water depths. Environ. Sci. Pollut. Res. 2022, 29, 28115–28126. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Al-Kayiem, H.H. Evaluation of copper nanoparticles—Paraffin wax compositions for solar thermal energy storage. Sol. Energy 2016, 132, 267–278. [Google Scholar] [CrossRef]
- Kumar, P.M.; Mylsamy, K. Experimental investigation of solar water heater integrated with a nanocomposite phase change material. J. Therm. Anal. 2018, 136, 121–132. [Google Scholar] [CrossRef]
- Sajawal, M.; Rehman, T.U.; Ali, H.M.; Sajjad, U.; Raza, A.; Bhatti, M.S. Experimental thermal performance analysis of finned tube-phase change material based double pass solar air heater. Case Stud. Therm. Eng. 2019, 15, 100543. [Google Scholar] [CrossRef]
- Raj, C.R.; Suresh, S.; Vasudevan, S.; Chandrasekar, M.; Singh, V.K.; Bhavsar, R. Thermal performance of nano-enriched form-stable PCM implanted in a pin finned wall-less heat sink for thermal management application. Energy Convers. Manag. 2020, 226, 113466. [Google Scholar]
- Mahmoud, B.K.; Ibrahim, S.I.; Abass, K.I.; Ali, A.J.; Chaichan, M.T. Flat solar air heater collector with phase change materials for domestic purposes in Iraqi climate. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 022099. [Google Scholar] [CrossRef]
- Shama, A.; Kabeel, A.E.; Moharram, B.M.; Abosheiasha, H.F. Improvement of the thermal properties of paraffin wax using high conductive nanomaterial to appropriate the solar thermal applications. Appl. Nanosci. 2021, 11, 2033–2042. [Google Scholar] [CrossRef]
- Abbas, M.S. Enhancement of the paraffin wax performance in the solar system collector by utilizing alumina (Al2O3) nanoparticles. AIP Conf. Proc. 2021, 2404, 020007. [Google Scholar]
- Gunjo, D.G.; Yadav, V.K.; Sinha, D.K.; Elkotb, M.A.; Ahmed, G.M.S.; Hossain, N.; Abdelmohimen, M.A. Improvement in thermal storage effectiveness of paraffin with addition of aluminum oxide nanoparticles. Materials 2022, 15, 4427. [Google Scholar] [CrossRef] [PubMed]
- Mazman, M.; Cabeza, L.F.; Mehling, H.; Nogues, M.; Evliya, H.; Paksoy, H. Utilization of phase change materials in solar domestic hot water systems. Renew. Energy 2009, 34, 1639–1643. [Google Scholar] [CrossRef]
- Al-Hinti, I.; Al-Ghandoor, A.; Maaly, A.; Abu Naqeera, I.; Al-Khateeb, Z.; Al-Sheikh, O. Experimental investigation on the use of water-phase change material storage in conventional solar water heating systems. Energy Convers. Manag. 2010, 51, 1735–1740. [Google Scholar] [CrossRef]
- Bouadila, S.; Fteïti, M.; Oueslati, M.M.; Guizani, A.; Farhat, A. Enhancement of latent heat storage in a rectangular cavity: Solar water heater case study. Energy Convers. Manag. 2014, 78, 904–912. [Google Scholar] [CrossRef]
- Al-Kayiem, H.H.; Lin, S.C. Performance evaluation of a solar water heater integrated with a PCM nanocomposite TES at various inclinations. Sol. Energy 2014, 109, 82–92. [Google Scholar] [CrossRef]
- Naghavi, M.; Ong, K.; Badruddin, I.; Mehrali, M.; Metselaar, H.S.C. Thermal performance of a compact design heat pipe solar collector with latent heat storage in charging/discharging modes. Energy 2017, 127, 101–115. [Google Scholar] [CrossRef]
- Assari, M.R.; Nasiri, R.; Alipoor, A. Experimental study of charge of paraffin wax along with nanoparticles in an eccentric double tube heat exchanger for storing energy in a solar water heater. Amirkabir J. Mech. Eng. 2017, 50, 1403–1410. [Google Scholar]
- Mandal, S.K.; Kumar, S.; Singh, P.K.; Mishra, S.K.; Bishwakarma, H.; Choudhry, N.P.; Nayak, R.K.; Das, A.K. Performance investigation of CuO-paraffin wax nanocomposite in solar water heater during night. Thermochim. Acta 2019, 671, 36–42. [Google Scholar] [CrossRef]
- Harun, D.; Maulana, I.; Akhyar, M. Increase the heating capacity of solar water heaters through two conditions of placing paraffin in copper tubes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012077. [Google Scholar] [CrossRef]
- Mandal, S.K.; Kumar, S.; Singh, P.K.; Mishra, S.K.; Singh, D. Performance investigation of nanocomposite based solar water heater. Energy 2020, 198, 117295. [Google Scholar] [CrossRef]
- Saravanakumar, P.; Arunkumar, S.; Mansingh, B.B.; Kumar, P.M.; Subbiah, R.; Eswarlal, V.K. Investigating the effect of thermal cycling on thermal characteristics of the nano-silica based phase changing material (PCM). Mater. Today Proc. 2021, 50, 1502–1507. [Google Scholar] [CrossRef]
- Ali, M.A.; Fayaz; Viegas, R.F.; Kumar, M.B.S.; Kannapiran, R.K.; Feroskhan, M. Enhancement of heat transfer in paraffin wax PCM using nano graphene composite for industrial helmets. J. Energy Storage 2019, 26, 100982. [Google Scholar] [CrossRef]
- Manoj Kumar, P.; Mylsamy, K.; Saravanakumar, P.T. Experimental investigations on thermal properties of nano-SiO2/paraffin phase change material (PCM) for solar thermal energy storage applications. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2420–2433. [Google Scholar] [CrossRef]
- Abdelrazik, A.S.; Saidur, R.; Al-Sulaiman, F.A. Thermal regulation and performance assessment of a hybrid photovoltaic/thermal system using different combinations of nano-enhanced phase change materials. Sol. Energy Mater. Sol. Cells 2020, 215, 110645. [Google Scholar] [CrossRef]
- Kazemian, A.; Khatibi, M.; Maadi, S.R.; Ma, T. Performance optimization of a nanofluid-based photovoltaic thermal system integrated with nano-enhanced phase change material. Appl. Energy 2021, 295, 116859. [Google Scholar] [CrossRef]
- Emara, K.; Aliwa, H.; Abdellatif, O.E.; Abd El-hameed, H.M. Experimental investigation for a hybrid aluminum oxide nanofluid-phase change material photovoltaic thermal system based on outdoor test conditions. J. Energy Storage 2022, 50, 104261. [Google Scholar] [CrossRef]
- Rostami, Z.; Heidari, N.; Rahimi, M.; Azimi, N. Enhancing the thermal performance of a photovoltaic panel using nano-graphite/paraffin composite as phase change material. J. Therm. Anal. 2021, 147, 3947–3964. [Google Scholar] [CrossRef]
- Dattu, V.S.C.; Roniki, V.R.; Prasad, P.R.; Tupati, P.R.; Devi, N.G.; Chavakula, R. Influence of Nano-Fe2O3 concentration on thermal characteristics of the water based Nano-fluid. Mater. Today Proc. 2022, 62, 2392–2395. [Google Scholar] [CrossRef]
- Hassan, W.H.; Nile, B.K. Climate change and predicting future temperature in Iraq using CanESM2 and HadCM3 modeling. Model. Earth Syst. Environ. 2020, 7, 737–748. [Google Scholar] [CrossRef]
- Al-Ghezi, M.K.; Mahmoud, B.K.; Alnasser, T.M.; Chaichan, M.T. A Comparative Study of Regression Models and Meteorological Parameters to Estimate the Global Solar Radiation on a Horizontal Surface for Baghdad City, Iraq. Int. J. Renew. Energy Dev. 2021, 11, 71–81. [Google Scholar] [CrossRef]
- Alnasser, T.M.; Mahdy, A.M.; Abass, K.I.; Chaichan, M.T.; Kazem, H.A. Impact of dust ingredient on photovoltaic performance: An experimental study. Sol. Energy 2019, 195, 651–659. [Google Scholar] [CrossRef]
- Holman, J.P. Experimental Methods for Engineers, 8th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Pasupathi, M.K.; Alagar, K.; Mm, M.; Aritra, G. Characterization of Hybrid-nano/Paraffin Organic Phase Change Material for Thermal Energy Storage Applications in Solar Thermal Systems. Energies 2020, 13, 5079. [Google Scholar] [CrossRef]
- Ibrahim, S.I.; Ali, A.H.; Hafidh, S.A.; Chaichan, M.T.; Kazem, H.A.; Ali, J.M.; Isahak, W.N.R.; Alamiery, A. Stability and thermal conductivity of different nano-composite material prepared for thermal energy storage applications. S. Afr. J. Chem. Eng. 2021, 39, 72–89. [Google Scholar] [CrossRef]
- Nourani, M.; Hamdami, N.; Keramat, J.; Moheb, A.; Shahedi, M. Thermal behavior of paraffin-nano-Al2O3 stabilized by sodium stearoyl lactylate as a stable phase change material with high thermal conductivity. Renew. Energy 2016, 88, 474–482. [Google Scholar] [CrossRef]
- Agrawal, R.; Singh, K.D.P.; Sharma, R.K. Experimental investigations on the phase change and thermal properties of nano enhanced binary eutectic phase change material of palmitic acid-stearic acid/CuO nanoparticles for thermal energy storage. Int. J. Energy Res. 2021, 46, 6562–6576. [Google Scholar] [CrossRef]
- Chen, Z.; Shahsavar, A.; Al-Rashed, A.A.; Afrand, M. The impact of sonication and stirring durations on the thermal conductivity of alumina-liquid paraffin nanofluid: An experimental assessment. Powder Technol. 2019, 360, 1134–1142. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, C.; Hu, S.; Yu, X. The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behavior of phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 1208–1212. [Google Scholar] [CrossRef]
- Fan, L.W.; Fang, X.; Wang, X.; Zeng, Y.; Xiao, Y.Q.; Yu, Z.T.; Xu, X.; Hu, Y.C.; Cen, K.F. Effects of various carbon nanofillers on the thermal conductivity and energy storage properties of paraffin-based nanocomposite phase change materials. Appl. Energy 2013, 110, 163–172. [Google Scholar] [CrossRef]
- Xu, B.; Chen, C.; Zhou, J.; Ni, Z.; Ma, X. Preparation of novel microencapsulated phase change material with Cu-Cu2O/CNTs as the shell and their dispersed slurry for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2019, 200, 109980. [Google Scholar] [CrossRef]
- Maleki, M.; Ahmadi, P.T.; Mohammadi, H.; Karimian, H.; Ahmadi, R.; Emrooz, H.B.M. Photo-thermal conversion structure by infiltration of paraffin in three dimensionally interconnected porous polystyrene-carbon nanotubes (PS-CNT) polyHIPE foam. Sol. Energy Mater. Sol. Cells 2018, 191, 266–274. [Google Scholar] [CrossRef]
- Vivekananthan, M.; Amirtham, V.A. Characterisation and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019, 676, 94–103. [Google Scholar] [CrossRef]
- Venkitaraj, K.P.; Suresh, S. Effects of Al2O3, CuO and TiO2 nanoparticles son thermal, phase transition and crystallization properties of solid-solid phase change material. Mech. Mater. 2019, 128, 64–88. [Google Scholar] [CrossRef]
- García-Betanzos, C.I.; Hernández-Sánchez, H.; Bernal-Couoh, T.F.; Quintanar-Guerrero, D.; de la Luz Zambrano-Zaragoza, M. Physicochemical, total phenols and pectin methylesterase changes on quality maintenance on guava fruit (Psidium guajava L.) coated with candeuba wax solid lipid nanoparticles-xanthan gum. Food Res. Int. 2017, 101, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Karimipour, A. Experimental investigation of the effects of temperature and mass fraction on the dynamic viscosity of CuO-paraffin nanofluid. Appl. Therm. Eng. 2018, 128, 189–197. [Google Scholar] [CrossRef]
- Karimipour, A.; Ghasemi, S.; Darvanjooghi, M.H.K.; Abdollahi, A. A new correlation for estimating the thermal conductivity and dynamic viscosity of CuO/liquid paraffin nanofluid using neural network method. Int. Commun. Heat Mass Transf. 2018, 92, 90–99. [Google Scholar] [CrossRef]
- Lebdioua, K.; Aimable, A.; Cerbelaud, M.; Videcoq, A.; Peyratout, C. Influence of different surfactants on Pickering emulsions stabilized by submicronic silica particles. J. Colloid Interface Sci. 2018, 520, 127–133. [Google Scholar] [CrossRef]
- Shahsavar, A.; Khanmohammadi, S.; Karimipour, A.; Goodarzi, M. A novel comprehensive experimental study concerned synthesizes and prepare liquid paraffin-Fe3O4 mixture to develop models for both thermal conductivity & viscosity: A new approach of GMDH type of neural network. Int. J. Heat Mass Transf. 2018, 131, 432–441. [Google Scholar]
- Sedeh, R.N.; Abdollahi, A.; Karimipour, A. Experimental investigation toward obtaining nanoparticles’ surficial interaction with basefluid components based on measuring thermal conductivity of nanofluids. Int. Commun. Heat Mass Transf. 2019, 103, 72–82. [Google Scholar] [CrossRef]
- Shahsavar, A.; Khanmohammadi, S.; Toghraie, D.; Salihepour, H. Experimental investigation and develop ANNs by introducing the suitable architectures and training algorithms supported by sensitivity analysis: Measure thermal conductivity and viscosity for liquid paraffin based nanofluid containing Al2O3 nanoparticles. J. Mol. Liq. 2018, 276, 850–860. [Google Scholar] [CrossRef]
- Venugopal, J.; Prathap, L.; Rao, C.M.P.; Alagesan, J.; Manjunatha, L.H.; Gangodkar, D.; Mohanavel, V.; Sathyamurthy, R. Synthesis and characterization of paraffin wax with carbon nanotubes and magnesium oxide. Energy Rep. 2022, 8, 313–320. [Google Scholar] [CrossRef]
- Hadiya, J.P.; Shukla, A.K.N. Experimental thermal behavior response of paraffin wax as storage unit. J. Therm. Anal. 2016, 124, 1511–1518. [Google Scholar] [CrossRef]
- Akgün, M.; Aydın, O.; Kaygusuz, K. Experimental study on melting/solidification characteristics of a paraffin as PCM. Energy Convers. Manag. 2007, 48, 669–678. [Google Scholar] [CrossRef]
- Al Hattali, B.A.; Husin, N.A. An evaluation study of public schools in the sultanate of Oman: Financial and environmental criteria. In Proceedings of the 2nd International Conference on Government and Public Affairs 2021 (ICOGPA2021), Sintok, Kedah, Malaysia, 6–7 April 2021. [Google Scholar]
- Mohammed, A.; Pignatta, G.; Topriska, E.; Santamouris, M. Canopy Urban Heat Island and Its Association with Climate Conditions in Dubai, UAE. Climate 2020, 8, 81. [Google Scholar] [CrossRef]
- Faraj, T.K.; Tarawneh, Q.Y.; Oroud, I.M. The applicability of the tourism climate index in a hot arid environment: Saudi Arabia as a case study. Int. J. Environ. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kazem, H.A.; Alnaser, N.W.; Gholami, A.; Al-Waeli, A.H.; Alnaser, W.E. Assessment cooling of photovoltaic modules using underground water. Arab. Gulf J. Sci. Res. (AGJSR) 2021, 39, 151–169. [Google Scholar] [CrossRef]
- Khanjari, Y.; Pourfayaz, F.; Kasaeian, A.B. Numerical investigation on using of nanofluid in a water-cooled photovoltaic thermal system. Energy Convers. Manag. 2016, 122, 263–278. [Google Scholar] [CrossRef]
- Sardarabadi, M.; Passandideh-Fard, M.; Maghrebi, M.-J.; Ghazikhani, M. Experimental study of using both ZnO/water nanofluid and phase change material (PCM) in photovoltaic thermal systems. Sol. Energy Mater. Sol. Cells 2017, 161, 62–69. [Google Scholar] [CrossRef]
| Ref. No. | Country | Year | PCM Used | Nanoparticles Added | Main Findings |
|---|---|---|---|---|---|
| Solar distillers | |||||
| [18] | Saudi Arabia | 2016 | Paraffin wax | Nano-Cu | Nano-copper has been added to paraffin to enhance its low thermal conductivity and distiller performance. The addition of nano-Cu to paraffin enhanced the thermal storage process and increased the yield of distilled water by about 125% compared to the usual distiller. This addition caused an increase in the operating time of the distiller after sunset by 6 h. |
| [19] | India | 2017 | Paraffin wax | Nano-CuO | The addition of nano-CuO to paraffin increased the yield of the distillate by about 35%. The daily productivity of the distiller increased when it was made with the composite material by about 34.7% compared to adding pure paraffin to the distiller. |
| [20] | Iraq | 2020 | Polyvinyl pyrrolidone (PVP K-30), polyethylene glycol (PEG 6000) and carboxymethyl cellulose sodium salt (CMC) | - | The daily distilled yield was increased by 30–50% of the distilled water compared to the distiller without PCM. The working time of the distiller with PCM increased and the distillation continued even after sunset. The quantity of distilled water increased by 120%, while the efficiency of the solar still with PCM increased by about 40%. |
| [21] | India | 2021 | Paraffin wax | Nano-CuO | More distillation was achieved using nano-PCM. The highest distillation yield was when 0.3% by weight was added to paraffin, the yield of the distillate improved by 62.74% compared to the conventional solar still. |
| [22] | India | 2021 | Paraffin wax | Nano-Si | Experiments were carried out in hot and humid atmospheres in Coimbatore, India, during the month of April 2020. The addition of paraffin and nano-Si composite material improved the yield of the distiller by 67.07%. While when using pure paraffin, the productivity improvement was only 51.22%. |
| [23] | Egypt | 2022 | Paraffin wax | Nano-CuO | The use of PCM and CuO nanofluid improved the performance of the modified hemispherical distiller by 80.20%. It also reduced the cost of distilled water by up to 90%. |
| [24] | Saudi Arabia | 2022 | Paraffin wax | Nano-Al2O3 | The productivity of the composite material (Nano-Al2O3-paraffin) distiller improved by about 95% compared to the usual distiller. The thermal efficiency of the solar still for this case also increased to 62.4% compared to 30% for the conventional still. |
| Solar air heaters | |||||
| [25] | Malaysia | 2016 | Paraffin wax | Nano-Cu | When nano-Cu was added by weight (0.5%, 1.0%, 1.5%, and 2.0%), the thermal conductivity of the composite materials increased by 14.0%, 23.9%, 42.5%, and 46.3%, respectively. The efficiency of the distiller was improved by adding the composite material by 1.7%. |
| [26] | India | 2019 | Paraffin wax | Nano-SiO2 | The energy efficiency of the solar still containing paraffin and nano-SiO2 improved by 74.79%, while, when adding pure paraffin, it was 69.62% compared to the case of conventional distiller with an efficiency of 58.74%. The thermal conductivity of paraffin was increased by 22.78% by mixing it with nano-SiO2. |
| [27] | Pakistan | 2019 | RT44HC and RT18HC | - | The study was conducted using high melting point RT44HC paraffin and RT18HC low melting point paraffin filled with circular and semicircular finned tubes. The work of the air heater for the previous two cases lasted about two and three hours, respectively, compared to the non-paraffin heater after sunset. These systems achieved total thermal efficiencies of 68.4%, 71.9%, and 53.2% respectively. |
| [28] | India | 2020 | PCM | MWCNTGNT | The authors designed and manufactured an efficient chemically and thermally stable polyethylene glycol 6000 (FS-PCM) heat sink with an addition of 1 wt.% (MWCNTs/graphene nano-sheets) to it. The results showed a significant improvement in thermal conductivity of 61.73% and 84.48%, respectively. The energy storage properties were improved by using the FS-PCM participation, and the heat sink temperature was reduced by 9.77%. |
| [29] | Iraq | 2021 | Paraffin wax | - | The thermal efficiency of the solar heater containing paraffin increased to 16.3% while, in the case without paraffin, it was 12.4%. The time during which the heater can heat the air increased after sunset. |
| [30] | Egypt | 2021 | Paraffin wax | Nano-CuO | It was found from practical experiments that the best concentration of Nano-CuO added to paraffin is 1%, in order to achieve the best improvement in the phase change (melting) period of about 22.22% compared to paraffin. This addition also reduced the solidification time of the composite material to a high level of 66.66% compared to the hardening time of pure paraffin. |
| [31] | China | 2021 | Paraffin wax | Nano-Al2O3 | The addition of nanoparticles to paraffin caused an improvement in the thermal conductivity, which escalates by increasing the added mass fraction, reaching an improvement of up to 15% when adding nano-Al2O3 with a mass fraction of 5%. The dynamic viscosity of paraffin increased by increasing the mass fraction added to it. The experiments proved that the best mass fraction added that gives acceptable results is 1%. |
| [32] | Ethiopia | 2022 | Paraffin wax | Nano-Al2O3 | The melting rate of paraffin was improved by adding nano-Al2O3 by 10%, bringing this improvement to 225%. As for the solidification rate, it was improved by 180%. Paraffin’s ability to store latent and sensible heat has also been relatively reduced. |
| Domestic solar water heaters | |||||
| [33] | Spain | 2009 | 80% Paraffin + 20% stearic acid | Graphene | The use of PCM with graphene caused the water temperature to increase by 3–4 °C within 10–15 min. It also increased the thermal storage efficiency of the heater by 74%. |
| [34] | Jordan | 2010 | Paraffin wax | - | The storage of paraffin in aluminum tubes inside the water heater caused an increase in water temperature by 13–14 °C. The water heater maintained a temperature higher than the surroundings, about 30 °C, as a result of thermal storage. |
| [35] | Tunisia | 2014 | Paraffin wax | - | Two cavities were added to the design of the domestic water heater to store paraffin, which was used to store energy as latent heat. The geyser water stayed hot for 5 h after sunset. Energy efficiency increased up to 35%. |
| [36] | Malaysia | 2014 | Paraffin wax | Nano-Cu | The addition of nano-composite (paraffin and nano-Cu) increased the water retention temperature to 10.8 °C. The solar water heater efficiency has also been increased up to 52.0%. |
| [37] | Malaysia | 2017 | Paraffin wax | - | The different weather conditions of the study area and the rates of flow and withdrawal of hot water showed that the thermal efficiency of the system is about 38–42% on sunny days, while on cloudy days it becomes around 34–36%. |
| [38] | Iran | 2017 | Paraffin wax | Nano-CuO | The addition of nanoparticles to paraffin causes it to reach its melting point faster than in the case of pure paraffin. The temperatures of the upper part of the composite material rise when working with a normal load. |
| [39] | India | 2019 | Paraffin wax | Nano-CuO | The addition of nanoparticles to paraffin caused an increase in its thermal conductivity. The high thermal conductivity of nano-CuO caused most of the heat to be transferred to the water with low absorption from paraffin. The maximum temperature obtained is 57.7 °C. The use of the composite material increased the operating time of the heater during the night. |
| [40] | Indonesia | 2019 | Paraffin wax | - | The water was heated to a temperature of 56 °C when the intensity of the solar radiation was 1289 W/m2. The water temperature is affected by the position of the paraffin-filled copper tubes. |
| [41] | India | 2020 | Paraffin wax | Nano-CuO | The addition of Nano-CuO–paraffin to the water heater caused the water temperature to rise to 61.8 °C when the heater was working in a continuous flow, while when the water was stored for 30 min, the water temperature increased to 80.6 °C. The highest heat transfer rate was 5.32 kW in the case of adding a composite material. |
| [42] | India | 2022 | Paraffin wax | Nano-Si | The addition of nano-Si to paraffin hindered its dissolution at high temperatures. The volume fraction of nanoparticles added up to 1% affects the thermal properties as well as the thermal stability of paraffin. |
| PVT systems | |||||
| [43] | India | 2019 | Paraffin wax | Nano-graphene | When nano-graphene was added to paraffin with a mass fraction of 3%, the thermal conductivity of the product increased by 146% compared to that of pure paraffin. |
| [44] | India | 2019 | Paraffin wax | Nano-SiO2 | When 2.0% of nano-SiO2 was added to paraffin, its thermal conductivity increased by 33.34% compared to pure paraffin. The latent heat of paraffin decreased with the increasing mass fraction of nano-SiO2. The improvement of the properties of the thermoplastic composite material is evident, especially when nanoparticles are added with a low mass fraction. |
| [45] | Saudi Arabia | 2020 | Paraffin wax RT35 Paraffin wax RT25Eutectic of capric-palmitic acidCalcium chloride hexahydrate CaCl2·6H2O | Nano-CuMWCNTGNTNano-Diamond (Di) | The use of a PCM layer between the collector and the PV plate causes the temperature of the PV panel to increase by 7–27%, while if this layer is not added, the panel’s temperature increased by 41%. Calcium chloride hexahydrate CaCl2.6H2O and paraffin RT35 proved to have the best efficiency when nanoparticles were added to them. Additionally, MWCNT was the most effective nanoparticle tested by increasing the cooling of the PV module. |
| [46] | China | 2021 | Paraffin wax | Nano-Al2O3 | The addition of nanoparticles to PCM caused an increase in electrical energy and thermal energy to 136.93 and 377.87 W/m2, respectively, while the energy level decreased to 2.91 W/m2. It was possible to reduce the generated entropy by changing the phase of the PCM at a thickness of 2.75 cm, and by adding nano-Al2O3 with a mass fraction of 8.05% to the paraffin and 8% to the nanofluid. |
| [47] | Egypt | 2022 | paraffin wax RT35 | Nano-Al2O3 | The studied cooling system works by adding paraffin to nano-Al2O3 liquid water with a mass fraction of 0.4%. Circulating this nanofluid and PCM at a rate of 1.6 L/min caused a decrease in the temperature of the PV module by about 12.11 °C compared to a conventional PV. This fluid also increased the electricity generation of the PV panel by a range of 25.33% to 37.81% for a full day of operation compared to the standalone PV panel. The efficiency of the cooled PVT system with this nanofluid and PCM was 82.7%. |
| [48] | Iran | 2022 | Paraffin | Nano-graphene | The rate of heat transfer increased with an increase in the water flow rate through a PVT system that is cooled using a nano-graphite/paraffin composite material. By adding nano-graphite to paraffin with a mass fraction of 1%, the temperature of the PV module in the system was reduced from 63 °C to 37 °C. The electrical efficiency of the upgraded PVT system increased to 21.2%. |
| PCM Type | Paraffin |
|---|---|
| Appearance | White |
| Melting point | 315 K |
| Solidification point | 314 K |
| Density of liquid state | 815 kg/m3 |
| Density of solid state | 910 kg/m3 |
| Latent heat of fusion | 189 kJ/kg |
| Thermal conductivity | 0.21 W/m K |
| Liquid state specific heat | 2.13 kJ/kg |
| Solid state specific heat | 2.22 kJ/kg |
| Material | Nano-Fe2O3 |
|---|---|
| Manufacturer | Sky spring nanomaterial, Inc. |
| Material appearance | Red brown |
| Purity | 99.5% |
| pH degree | 6 |
| Particles’ size | 30–50 nm |
| Density | 5.242 g/cm³ |
| Humidity rate | ≤0.056% |
| Molar mass | 159.69 (g/mole) |
| Melting point | 11,838 K |
| Thermal conductivity | 4.66 (W/m K) |
| The Measure | Symbol | Value |
|---|---|---|
| Length | L | 1.145 m |
| Width | b | 0.505 m |
| Perimeter | P | 3.3 m |
| Area | A | 0.578225 m2 |
| Container thickness | x | 0.0275 m |
| Glass wool thermal conductivity | kgw | 0.042 W/m2 K |
| Measured Parameter | Instrument | Uncertainty |
|---|---|---|
| Mass fraction | Sensitive balance (type EJ6I0-E) | ±0.56 |
| Stability | Zetasizer (ZSN) | ±0.62 |
| TC | HOT DESK TPS 500 | ±0.77 |
| Heat Capacity | HOT DESK TPS 500 | ±0.83 |
| Density | Density tester (type DII-300 L) | ±0.49 |
| Viscosity | Brookfield DV-II+ Pro Viscometer | ±1.05 |
| Temperature | Thermocouple sensor (type pt100) | ±1.13 |
| Coolants’ Flow rates | HC (US Hunter) | ±0.67 |
| Temp. (°C) | Paraffin | Nano-PCM (0.5%) | Nano-PCM (1%) | Nano-PCM (2%) | Nano-PCM (3%) |
|---|---|---|---|---|---|
| Density (kg/m3) | |||||
| 25 | 910 | 936 | 962 | 1014 | 1067 |
| 35 | 860 | 886 | 912 | 964 | 1016 |
| 45 | 815 | 841 | 867 | 919 | 971 |
| 55 | 780 | 806 | 832 | 884 | 936 |
| 65 | 723 | 749 | 775 | 827 | 879 |
| Viscosity (m Pa·s) | |||||
| 25 | 0.093 | 0.095 | 0.096 | 0.097 | 0.098 |
| 35 | 0.062 | 0.065 | 0.066 | 0.068 | 0.069 |
| 45 | 0.053 | 0.055 | 0.056 | 0.057 | 0.058 |
| 55 | 0.49 | 0.053 | 0.055 | 0.058 | 0.059 |
| 65 | 0.042 | 0.044 | 0.046 | 0.047 | 0.049 |
| Thermal conductivity (W/m K) | |||||
| 25 | 0.21 | 0.26 | 0.33 | 0.36 | 0.375 |
| 35 | 0.22 | 0.274 | 0.353 | 0.388 | 0.405 |
| 45 | 0.24 | 0.29 | 0.377 | 0.42 | 0.437 |
| 55 | 0.255 | 0.3 | 0.4 | 0.45 | 0.472 |
| 65 | 0.27 | 0.316 | 0.43 | 0.48 | 0.51 |
| Stability | |||||
| 25 | - | - | - | - | - |
| 35 | - | - | - | - | - |
| 45 | - | 93 | 87 | 66 | 58 |
| 55 | - | 89 | 84 | 59 | 52 |
| 65 | - | 86 | 79 | 53 | 46 |
| Ref. No. | Year | Added PCM | Nanoparticles Added | Added Mass Fraction | Thermal Conductivity Enhancement Rate |
|---|---|---|---|---|---|
| [56] | 2011 | Paraffin Soy wax | CNF (carbon nano-fiber) CNT | 1% 1% | 21.7 5.8 |
| [58] | 2013 | Paraffin | Short-MWCNT Long-MWCNT CNFs CNPs | 5% | 29.62 14.81 11.11 159.25 |
| [57] | 2019 | Paraffin | Cu-Cu2O-CNT | 5% | 58% |
| [59] | 2019 | Paraffin | Polystyrene-Carbon nanotubes (PS-CNT) PolyHIPE foam | - | 62% |
| [60] | 2019 | Erythritol PCM | Graphene | 1% | 53% |
| [61] | 2019 | Pentaerythritol (PE) | Nano-Al2O3 Nano-CuO Nano-TiO2 | 0.1% | 20.9% 6.98% 14.05% |
| [1] | 2019 | Paraffin | Nano-Al2O3 Nano-ZnO2 Nano-SiC | 1% | 3.3% 1.8% 4.2% |
| Current study | Paraffin | Nano-Fe2O3 | 1% | 57.14 | |
| Ref. No. | Year | Added PCM | Nanoparticles Added | Added Mass Fraction | Zeta Potential (Stability) |
|---|---|---|---|---|---|
| [60] | 2017 | Candeuba wax | Xanthan gum | 0.2% | 22–30 |
| [61] | 2018 | Paraffin | Nano-CuO | 1.5% | 40 |
| [62] | 2018 | Paraffin | Nano-CuO | 2.5% | 40 |
| [63] | 2018 | Paraffin | Silica submicronic | 1% | 60 |
| [64] | 2019 | Paraffin | Nano-Fe3O4 | 3% | 50 |
| [65] | 2019 | Paraffin | Nano-TiO2 Nano-CuO | 0.5% | 44.5 45.5 |
| [66] | 2019 | Paraffin | Nano-Al2O3 | 5% | 60 |
| [67] | 2022 | Paraffin | Nano-TiO Nano-MgO | 1% | 90 65 |
| [68] | 2022 | Paraffin | CNT CNT/MgO | 3% | 86 11.1 |
| Current study | Paraffin | Nano-Fe2O3 | 1% | 87 | |
| Ref. No. | Year | Added PCM | Nanoparticles Added | Added Mass Fraction | Total Efficiency (%) |
|---|---|---|---|---|---|
| [75] | 2016 | Paraffin | Nano-Ag Nano-Al2O3 | 0.09 | 92 86.5 |
| [76] | 2017 | Paraffin | Nano-ZnO | 0.2 | 46 |
| [77] | 2018 | Paraffin | Nano-SiC | 0.5 | 84 |
| [78] | 2019 | Paraffin | Nano-Al2O3 | 1 | 68 |
| [79] | 2020 | Paraffin | MWCNT Nano-MgO | 6 | 61 60.6 |
| [80] | 2022 | Paraffin | Nano-Al2O3 Nano-Cu Nano-Ag | 4% | 88.64 88.71 86.63 |
| Current study | Paraffin | Nano-Fe2O3 | 1% | 83.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaichan, M.T.; Mahdi, M.T.; Kazem, H.A.; Al-Waeli, A.H.A.; Fayad, M.A.; Al-Amiery, A.A.; Isahak, W.N.R.W.; Kadhum, A.A.H.; Takriff, M.S. Modified Nano-Fe2O3-Paraffin Wax for Efficient Photovoltaic/Thermal System in Severe Weather Conditions. Sustainability 2022, 14, 12015. https://doi.org/10.3390/su141912015
Chaichan MT, Mahdi MT, Kazem HA, Al-Waeli AHA, Fayad MA, Al-Amiery AA, Isahak WNRW, Kadhum AAH, Takriff MS. Modified Nano-Fe2O3-Paraffin Wax for Efficient Photovoltaic/Thermal System in Severe Weather Conditions. Sustainability. 2022; 14(19):12015. https://doi.org/10.3390/su141912015
Chicago/Turabian StyleChaichan, Miqdam T., Maytham T. Mahdi, Hussein A. Kazem, Ali H. A. Al-Waeli, Mohammed A. Fayad, Ahmed A. Al-Amiery, Wan Nor Roslam Wan Isahak, Abdul Amir H. Kadhum, and Mohd S. Takriff. 2022. "Modified Nano-Fe2O3-Paraffin Wax for Efficient Photovoltaic/Thermal System in Severe Weather Conditions" Sustainability 14, no. 19: 12015. https://doi.org/10.3390/su141912015
APA StyleChaichan, M. T., Mahdi, M. T., Kazem, H. A., Al-Waeli, A. H. A., Fayad, M. A., Al-Amiery, A. A., Isahak, W. N. R. W., Kadhum, A. A. H., & Takriff, M. S. (2022). Modified Nano-Fe2O3-Paraffin Wax for Efficient Photovoltaic/Thermal System in Severe Weather Conditions. Sustainability, 14(19), 12015. https://doi.org/10.3390/su141912015











