The Environmental Impact of Lowering Dietary Crude Protein in Finishing Pig Diets—The Effect on Ammonia, Odour and Slurry Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals and Measurements
2.2. Chemical Analysis
2.3. Statistical Analysis
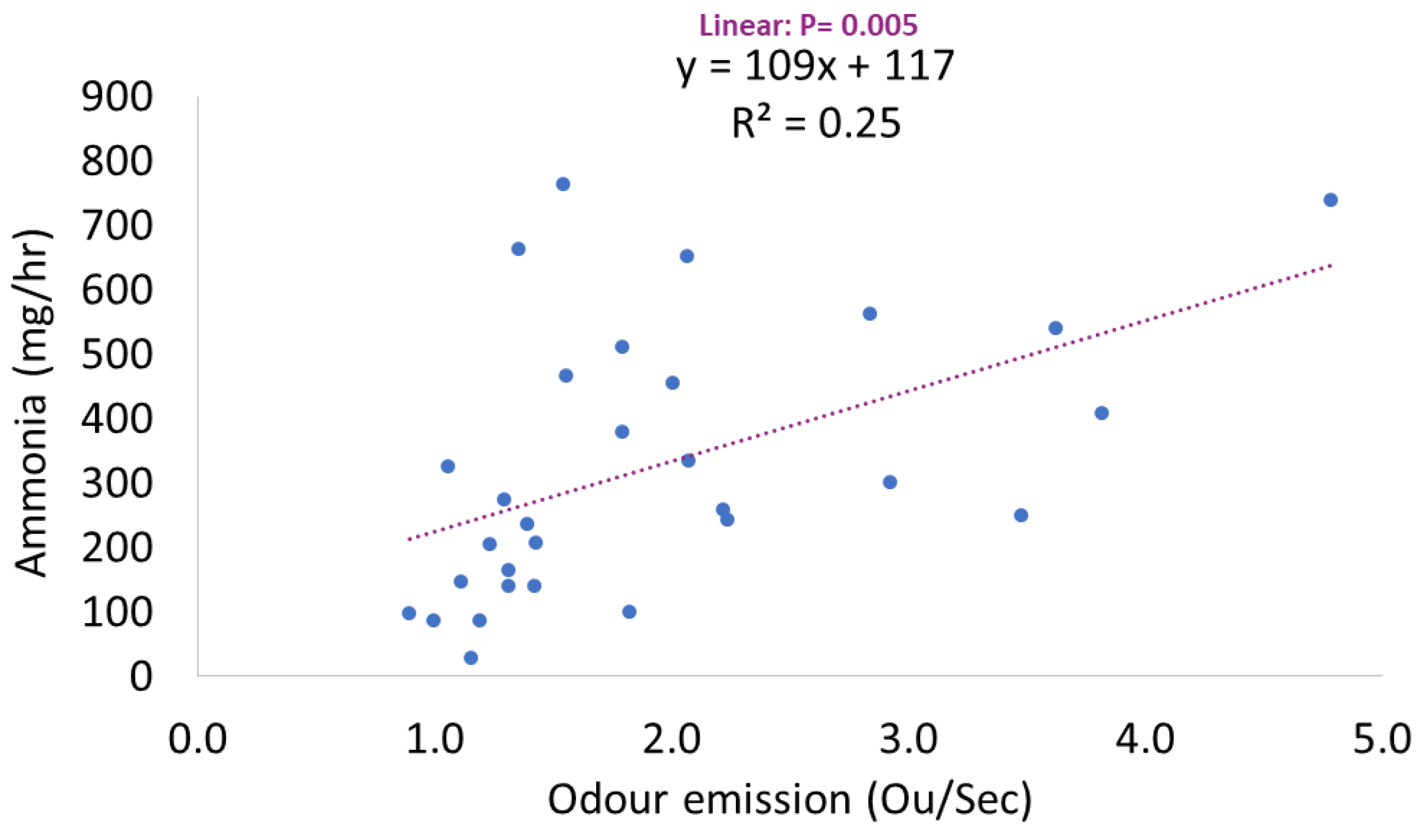
3. Results
3.1. Diet Composition
3.2. Experimental Measurements
4. Discussion
4.1. Diet Analysis
4.2. Pig Performance, Water Usage, Slurry Output and Ammonia and Odour Emissions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Emission Database for Global Atmospheric Research (EDGAR), Release Version 4.3. Available online: https://edgar.jrc.ec.europa.eu/overview.php?v=432_AP (accessed on 21 June 2022).
- Prosser, J.I.; Hink, L.; Gubry-Rangin, C.; Nicol, G.W. Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Change Biol. 2019, 26, 103–118. [Google Scholar] [CrossRef]
- Philippe, F.-X.; Cabaraux, J.-F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Banhazi, T.M.; Seedorf, J.; Rutley, D.L.; Pitchford, W.S. Identification of risk factors for sub-optimal housing conditions in Australian piggeries: Part 2. Airborne pollutants. J. Agric. Saf. Health 2008, 14, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, E.P.M.; Amon, B.; Ammon, C.; Zollitsch, W.; Winiwarter, W. Evaluating the potential of dietary crude protein manipulation in reducing ammonia emissions from cattle and pig manure: A meta-analysis. Nutr. Cycl. Agroecosyst. 2018, 110, 161–175. [Google Scholar] [CrossRef]
- Magowan, E.; Kennedy, T.; Mansoor, F.; Farmer, L.J. Ammonia and Odour Abatement Methods for the NI Pig Industry. DAERA E&I Project 13/04/03. 2015. Available online: http://www.afbini.gov.uk/sites/afbini.gov.uk/files/publications/ammonia_and_odour_creation_and_abatement_2015_0.pdf (accessed on 22 September 2022).
- Morazán, H.; Alvarez-Rodriguez, J.; Seradj, A.R.; Balcells, J.; Babot, D. Trade-offs among growth performance, nutrient digestion and carcass traits when feeding low protein and/or high neutral-detergent fiber diets to growing-finishing pigs. Anim. Feed Sci. Technol. 2015, 207, 168–180. [Google Scholar] [CrossRef]
- Aarnink, A.; Verstegen, M. Nutrition, key factor to reduce environmental load from pig production. Livest. Sci. 2007, 109, 194–203. [Google Scholar] [CrossRef]
- Mroz, Z.; Jongbloed, A.W.; Lenis, N.P.; Vreman, K. Water in pig nutrition: Physiology, allowances and environmental implications. Nutr. Res. Rev. 1995, 8, 137–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- British Society of Animal Science. Nutrient Requirements Standards for Pigs; Whittemore, C.T., Hazzledine, M.J., Close, W.H., Eds.; BSAS: Penicuik, UK, 2003. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- European Commission regulation (EC). No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, 54, 1–130. [Google Scholar]
- Riles, P. Multi-Element Analysis of Trace Metals in Animal Feed Using ICP-OES; Agilent Technologies: Melbourne, Australia, 2018. [Google Scholar]
- Ottosen, M.; Mackenzie, S.G.; Filipe, J.A.; Misiura, M.M.; Kyriazakis, I. Changes in the environmental impacts of pig production systems in Great Britain over the last 18 years. Agric. Syst. 2021, 189, 103063. [Google Scholar] [CrossRef]
- Tallentire, C.; Mackenzie, S.; Kyriazakis, I. Environmental impact trade-offs in diet formulation for broiler production systems in the UK and USA. Agric. Syst. 2017, 154, 145–156. [Google Scholar] [CrossRef]
- Krul, E.S. Calculation of Nitrogen-to-Protein Conversion Factors: A Review with a Focus on Soy Protein. J. Am. Oil Chem. Soc. 2019, 96, 339–364. [Google Scholar] [CrossRef]
- Mariotti, F.; Mirand, P.H. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Boisen, S.; Bech-Andersen, S.; Eggum, B.O. A Critical View on the Conversion Factor 6.25 from Total Nitrogen to Protein Nitrogen to Protein. Acta Agric. Scand. 1987, 37, 299–304. [Google Scholar] [CrossRef]
- Beattie, V.; Ball, M.E.; McCracken, K.; Muns Vila, R.; Grodon, F.; Smyth, S.; Bradford, R.; Magowan, E. Low protein diets in late finishing: Performance and excretion. In Proceedings of the Nutritional Solutions to Environmental Challenges, Hillsborough, UK, 30 October 2020. [Google Scholar]
- Ball, M.E.; Beattie, V.; Smyth, S.; McCracken, K.; McCormack, U.; Muns Vila, R.; Gordon, F.; Bradford, R.; Magowan, E. Dietary crude protein and lysine levels for finishing pigs. Nutritional Solutions to Environmental Challenges. In Proceedings of the Nutritional Solutions to Environmental Challenges, Hillsborough, UK, 30 October 2020. [Google Scholar]
- Kay, R.M. Ammonia emission from pigs buildings and characteristics of slurry produced by pigs offered low crude protein diets. In Proceedings of the International Symposium Ammonia and Odour Control from Animal Production Facilities, Vinkelord, The Netherlands, 6–10 October 1997; pp. 245–252. [Google Scholar]
- Webb, J.; Broomfield, M.; Jones, S.; Donovan, B. Ammonia and odour emissions from UK pig farms and nitrogen leaching from outdoor pig production. A review. Sci. Total Environ. 2014, 470–471, 865–875. [Google Scholar] [CrossRef]
- National Research Council. Nutrients Requitements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Portejoie, S.; Dourmad, J.; Martinez, J.; Lebreton, Y. Effect of lowering dietary crude protein on nitrogen excretion, manure composition and ammonia emission from fattening pigs. Livest. Prod. Sci. 2004, 91, 45–55. [Google Scholar] [CrossRef]
- Pratt, E.; Rose, S.; Keeling, A. Effect of ambient temperature on losses of volatile nitrogen compounds from stored laying hen manure. Bioresour. Technol. 2002, 84, 203–205. [Google Scholar] [CrossRef]
- Nutrient Management Guide RB209. AHDB. 2021. Available online: https://ahdb.org.uk/knowledge-library/rb209-section-2-organic-materials (accessed on 28 June 2022).
- Oster, M.; Reyer, H.; Ball, E.; Fornara, D.; McKillen, J.; Sørensen, K.U.; Poulsen, H.D.; Andersson, K.; Ddiba, D.; Rosemarin, A.; et al. Bridging Gaps in the Agricultural Phosphorus Cycle from an Animal Husbandry Perspective—The Case of Pigs and Poultry. Sustainability 2018, 10, 1825. [Google Scholar] [CrossRef]
- Hove, N.C.; Demeyer, P.; Van der Heyden, C.; Van Weyenberg, S.; Van Langenhove, H. Improving the repeatability of dynamic olfactometry according to EN 13725: A case study for pig odour. Biosyst. Eng. 2017, 161, 70–79. [Google Scholar] [CrossRef]
- Bindelle, J.; Leterme, P.; Budgen, A. Nutritional and environmental consequences of dietary fibre in pig nutrition: A review. Biotechnol. Agron. Soc. Environ. 2008, 12, 69–80. [Google Scholar]
- Sungback, C.; Okhwa, H.; Sungkwon, P. Effect of Dietary Protein Levels on Composition of Odorous Compounds and Bacterial Ecology in Pig Manure. Asian Australas. J. Anim. Sci. 2015, 28, 1362–1370. [Google Scholar]
- Claus, R.; Weiler, U.; Herzog, A. Physiological aspects of androstenone and skatole formation in the boar—A review with experimental data. Meat Sci. 1994, 38, 289–305. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Hayes, E.; Curran, T.; Dodd, V. Odour and ammonia emissions from intensive pig units in Ireland. Bioresour. Technol. 2005, 97, 940–948. [Google Scholar] [CrossRef] [PubMed][Green Version]
| 180 g/kg CP | 150 g/kg CP | 130 g/kg CP | |
|---|---|---|---|
| Ingredient, g/kg | |||
| Barley | 210 | 275 | 250 |
| Wheat | 263 | 344 | 447 |
| Maize | 175 | 155 | 155 |
| Maize DDGS | 30 | - | - |
| Pollard | 75 | 50 | 50 |
| Rapeseed meal | 50 | 50 | 50 |
| Soybean meal | 164 | 80 | - |
| Soya oil | 10 | 10 | 12 |
| DeviGainPG a | - | 10 | 10 |
| l-Lysine HCl | 3.3 | 5.0 | 5.0 |
| dl-Methionine | 0.4 | 1.0 | 0.3 |
| l-Threonine | 0.9 | 1.7 | 1.4 |
| l-Tryptophan | - | 0.3 | 0.2 |
| Limestone | 8.8 | 9.1 | 9.4 |
| Mono dicalcium phosphate | 2.1 | 2.8 | 3.5 |
| Salt | 4.4 | 3.3 | 3.3 |
| Mineral and vitamins b | 3.0 | 3.0 | 3.0 |
| Formulated composition, g/kg | |||
| Crude protein | 182.2 | 150.0 | 120.8 |
| Oil A | 32.4 | 29.6 | 30.6 |
| Crude Fibre | 39.3 | 36.5 | 35.0 |
| Ash | 45.5 | 40.5 | 37.6 |
| Total Lysine | 11.0 | 11.1 | 9.0 |
| Total Methionine | 3.2 | 3.8 | 2.8 |
| Total Methionine and cysteine | 6.6 | 6.75 | 5.4 |
| Total Threonine | 7.4 | 7.5 | 6.0 |
| Total Tryptophan | 2.3 | 2.1 | 1.7 |
| Lysine:CP ratio | 0.06 | 0.07 | 0.07 |
| Calcium | 6.9 | 7.0 | 7.0 |
| Available phosphorus | 3.2 | 3.0 | 3.0 |
| Salt | 6.5 | 6.5 | 6.5 |
| Digestible Energy (MJ/kg) | 14.0 | 14.0 | 14.0 |
| Nutrient, g/kg | 180 g/kg CP | 150 g/kg CP | 130 g/kg CP |
|---|---|---|---|
| Dry matter | 875.4 | 870.0 | 867.8 |
| Crude protein | 183.0 | 148.0 | 132.0 |
| Oil A | 29.0 | 24.6 | 25.3 |
| Crude fibre | 43.3 | 34.8 | 35.7 |
| Ash | 39.2 | 32.6 | 32.7 |
| Gross energy (MJ/kg) | 16.42 | 16.07 | 16.00 |
| Alanine | 8.20 | 5.60 | 5.20 |
| Arginine | 10.90 | 7.20 | 6.80 |
| Aspartic acid | 14.30 | 9.40 | 8.70 |
| Glutamic acid | 35.80 | 29.80 | 28.70 |
| Glycine | 7.60 | 5.60 | 5.30 |
| Histidine | 4.40 | 3.50 | 3.00 |
| Iso-leucine | 7.10 | 4.90 | 4.80 |
| Leucine | 13.30 | 9.70 | 8.70 |
| Lysine | 9.80 | 9.20 | 8.80 |
| Methionine | 2.80 | 2.40 | 2.60 |
| Phenylalanine | 7.30 | 5.50 | 5.50 |
| Proline | 14.30 | 10.90 | 9.90 |
| Serine | 7.90 | 5.90 | 5.50 |
| Threonine | 6.60 | 5.80 | 5.60 |
| Tyrosine | 2.90 | 1.90 | 1.50 |
| Valine | 9.00 | 6.20 | 6.00 |
| Tryptophan | 2.10 | 1.60 | 1.60 |
| Lysine:CP ratio | 0.053 | 0.062 | 0.067 |
| 180 g/kg CP | 150 g/kg CP | 130 g/kg CP | SED | p-Value | p Lin | |
|---|---|---|---|---|---|---|
| Intake (kg/d) | 3.40 | 3.49 | 3.34 | 0.149 | 0.603 | 0.797 |
| ADG (kg/d) | 1.28 | 1.37 | 1.35 | 0.053 | 0.182 | 0.139 |
| FCR (kg/kg) | 2.68 | 2.57 | 2.49 | 0.152 | 0.493 | 0.240 |
| 180 g/kg CP | 150 g/kg CP | 130 g/kg CP | SED | p-Value | p Lin | |
|---|---|---|---|---|---|---|
| Nitrogen intake (g) | 280.3 b | 259.5 b | 200.9 a | 18.89 | <0.001 | <0.001 |
| Ammonia (mg/h) | 434 b | 338 b | 231 a | 44.8 | <0.001 | <0.001 |
| Water usage (L/day) | 6.3 | 5.4 | 4.6 | 0.68 | 0.055 | 0.017 |
| Slurry output (L/day) | 3.3 b | 2.3 a | 2.0 a | 0.34 | 0.001 | <0.00 |
| Slurry DM (%) | 8.5 a | 10.4 ab | 12.7 b | 1.47 | 0.017 | 0.005 |
| Odour emission (OuE/Second) | 2.13 | 1.92 | 1.70 | 0.408 | 0.567 | 0.290 |
| Hydrogen Sulphide (ppm) | 2.13 b | 1.38 a | 1.19 a | 0.353 | 0.031 | 0.010 |
| 180 g/kg CP | 150 g/kg CP | 130 g/kg CP | SED | p-Value | p Lin | |
|---|---|---|---|---|---|---|
| Nitrogen (g/kg fresh) | 1.89 a | 2.44 ab | 2.68 b | 0.310 | 0.048 | 0.015 |
| Phosphate (g/kg fresh) | 2.95 | 3.78 | 3.82 | 0.564 | 0.231 | 0.111 |
| Potash (g/kg fresh) | 6.41 | 6.34 | 6.55 | 0.612 | 0.962 | 0.833 |
| MgO (g/kg fresh) | 1.39 | 1.64 | 1.68 | 0.264 | 0.485 | 0.247 |
| Sulphate (g/kg fresh) | 1.97 | 2.79 | 2.85 | 0.489 | 0.122 | 0.053 |
| ZnO (g/kg fresh) | 0.07 a | 0.10 b | 0.12 b | 0.012 | 0.002 | <0.001 |
| Nitrogen (g/kg DMB) | 22.65 | 22.02 | 21.76 | 2.202 | 0.914 | 0.673 |
| Phosphate (g/kg DMB) | 33.70 | 33.98 | 30.91 | 2.769 | 0.468 | 0.344 |
| Potash (g/kg DMB) | 75.78 b | 59.86 a | 53.29 a | 6.171 | 0.003 | <0.001 |
| MgO (g/kg DMB) | 15.84 | 14.38 | 13.12 | 1.159 | 0.076 | 0.025 |
| Sulphate (g/kg DMB) | 22.86 | 23.55 | 21.8 | 3.329 | 0.871 | 0.783 |
| ZnO (g/kg DMB) | 0.82 | 0.86 | 0.94 | 0.065 | 0.185 | 0.079 |
| Nitrogen in slurry output (g/d) | 7.19 b | 5.44 a | 5.24 a | 0.801 | 0.844 | 0.018 |
| Phosphate in slurry output (g/d) | 10.58 | 8.32 | 7.54 | 1.460 | 0.116 | 0.042 |
| Potash in slurry output (g/d) | 23.95 b | 14.19 a | 12.79 a | 1.392 | <0.001 | <0.001 |
| MgO in slurry output (g/d) | 4.85 | 3.55 | 3.26 | 0.656 | 0.054 | 0.020 |
| Sulphate in slurry output (g/d) | 6.67 | 5.85 | 5.53 | 1.018 | 1.486 | 0.252 |
| ZnO in slurry output (g/d) | 0.25 | 0.21 | 0.23 | 0.024 | 0.440 | 0.352 |
| 180 g/kg CP | 150 g/kg CP | 130 g/kg CP | SED | p-Value | p Lin | |
|---|---|---|---|---|---|---|
| Acetic acid (ng/g) | 4.28 | 4.15 | 4.236 | 0.109 | 0.442 | 0.580 |
| 1H-Pyrrole, 3-methyl-(ng/g) | 7.53 | 7.54 | 7.52 | 0.027 | 0.663 | 0.696 |
| Disulfide, dimethyl (ng/g) | 7.55 | 7.56 | 7.56 | 0.011 | 0.287 | 0.199 |
| Toluene (ng/g) | 8.24 | 8.26 | 8.25 | 0.011 | 0.242 | 0.219 |
| 4-Octene, (E)-(ng/g) | 9.27 | 9.25 | 9.27 | 0.011 | 0.218 | 0.919 |
| 2-Heptanone (ng/g) | 12.31 | 12.32 | 12.31 | 0.007 | 0.270 | 0.341 |
| Nonane (ng/g) | 12.53 | 12.51 | 12.54 | 0.034 | 0.614 | 0.829 |
| Butyrolactone (ng/g) | 13.46 | 13.58 | 13.54 | 0.075 | 0.305 | 0.244 |
| Benzaldehyde (ng/g) | 14.61 | 14.59 | 14.59 | 0.020 | 0.572 | 0.327 |
| Dimethyl trisulfide (ng/g) | 14.82 | 14.82 | 14.82 | 0.007 | 0.984 | 0.928 |
| Phenol (ng/g) | 15.20 | 15.19 | 15.18 | 0.012 | 0.249 | 0.099 |
| 2-Octanone (ng/g) | 15.42 | 15.42 | 15.43 | 0.007 | 0.313 | 0.146 |
| 2-Nonanone (ng/g) | 18.23 | 18.23 | 18.26 | 0.018 | 0.219 | 0.144 |
| 2-Decanone (ng/g) | 20.70 b | 20.68 a | 20.68 a | 0.004 | <0.001 | <0.001 |
| Indole (ng/g) | 23.20 | 23.19 | 23.19 | 0.008 | 0.476 | 0.281 |
| Skatole (ng/g) | 25.25 a | 25.25 a | 25.28 b | 0.013 | 0.007 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ball, M.E.E.; Smyth, S.; Beattie, V.E.; McCracken, K.J.; McCormack, U.; Muns, R.; Gordon, F.J.; Bradford, R.; Reid, L.A.; Magowan, E. The Environmental Impact of Lowering Dietary Crude Protein in Finishing Pig Diets—The Effect on Ammonia, Odour and Slurry Production. Sustainability 2022, 14, 12016. https://doi.org/10.3390/su141912016
Ball MEE, Smyth S, Beattie VE, McCracken KJ, McCormack U, Muns R, Gordon FJ, Bradford R, Reid LA, Magowan E. The Environmental Impact of Lowering Dietary Crude Protein in Finishing Pig Diets—The Effect on Ammonia, Odour and Slurry Production. Sustainability. 2022; 14(19):12016. https://doi.org/10.3390/su141912016
Chicago/Turabian StyleBall, M. Elizabeth E., Sam Smyth, Violet E. Beattie, Kelvin J. McCracken, Ursula McCormack, Ramon Muns, Fred J. Gordon, Raymond Bradford, L. Alanna Reid, and Elizabeth Magowan. 2022. "The Environmental Impact of Lowering Dietary Crude Protein in Finishing Pig Diets—The Effect on Ammonia, Odour and Slurry Production" Sustainability 14, no. 19: 12016. https://doi.org/10.3390/su141912016
APA StyleBall, M. E. E., Smyth, S., Beattie, V. E., McCracken, K. J., McCormack, U., Muns, R., Gordon, F. J., Bradford, R., Reid, L. A., & Magowan, E. (2022). The Environmental Impact of Lowering Dietary Crude Protein in Finishing Pig Diets—The Effect on Ammonia, Odour and Slurry Production. Sustainability, 14(19), 12016. https://doi.org/10.3390/su141912016






