Microscopic Damage to Limestone under Acidic Conditions: Phenomena and Mechanisms
Abstract
:1. Introduction
2. Test Section
2.1. Test Sample Preparation
2.2. Test Methods and Apparatus
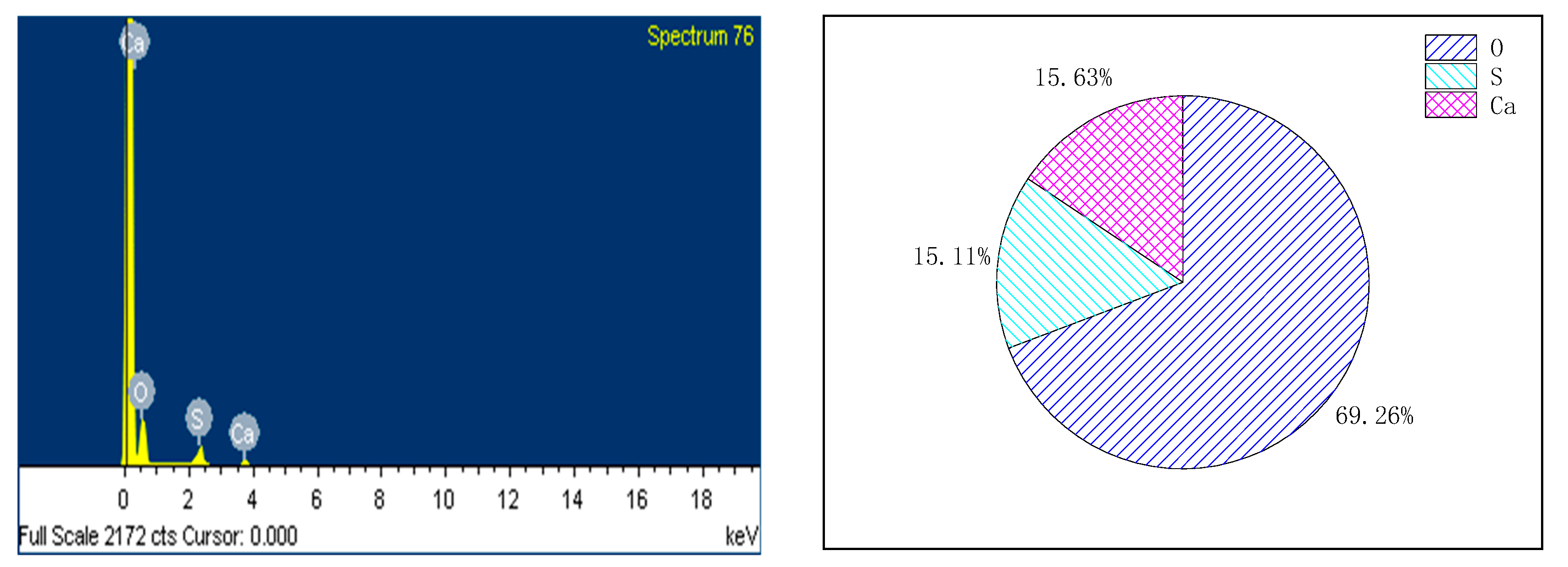
3. Analysis of Morphological Changes in Appearance
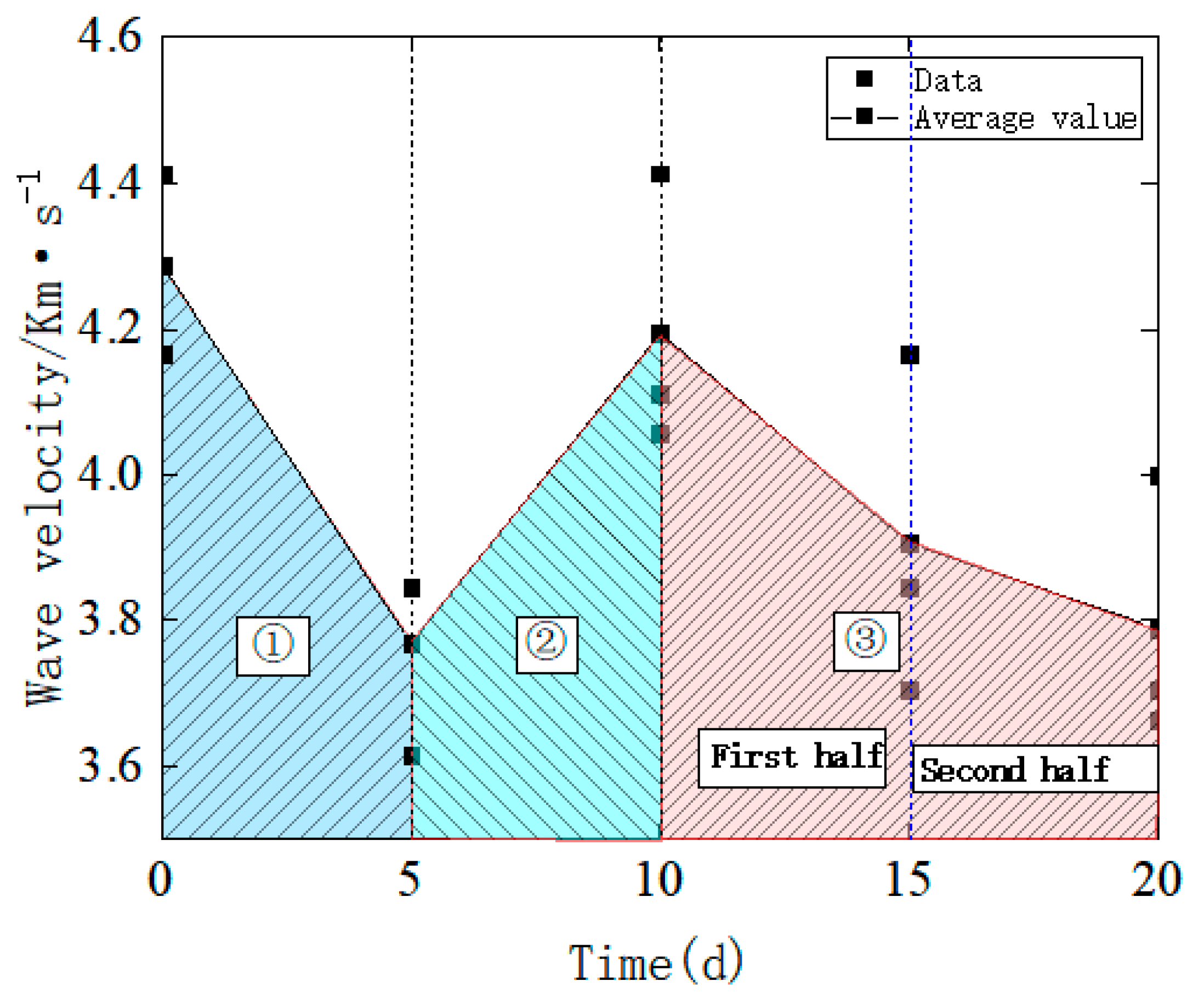
4. Wave Speed Test
4.1. P-Wave Velocity Analysis
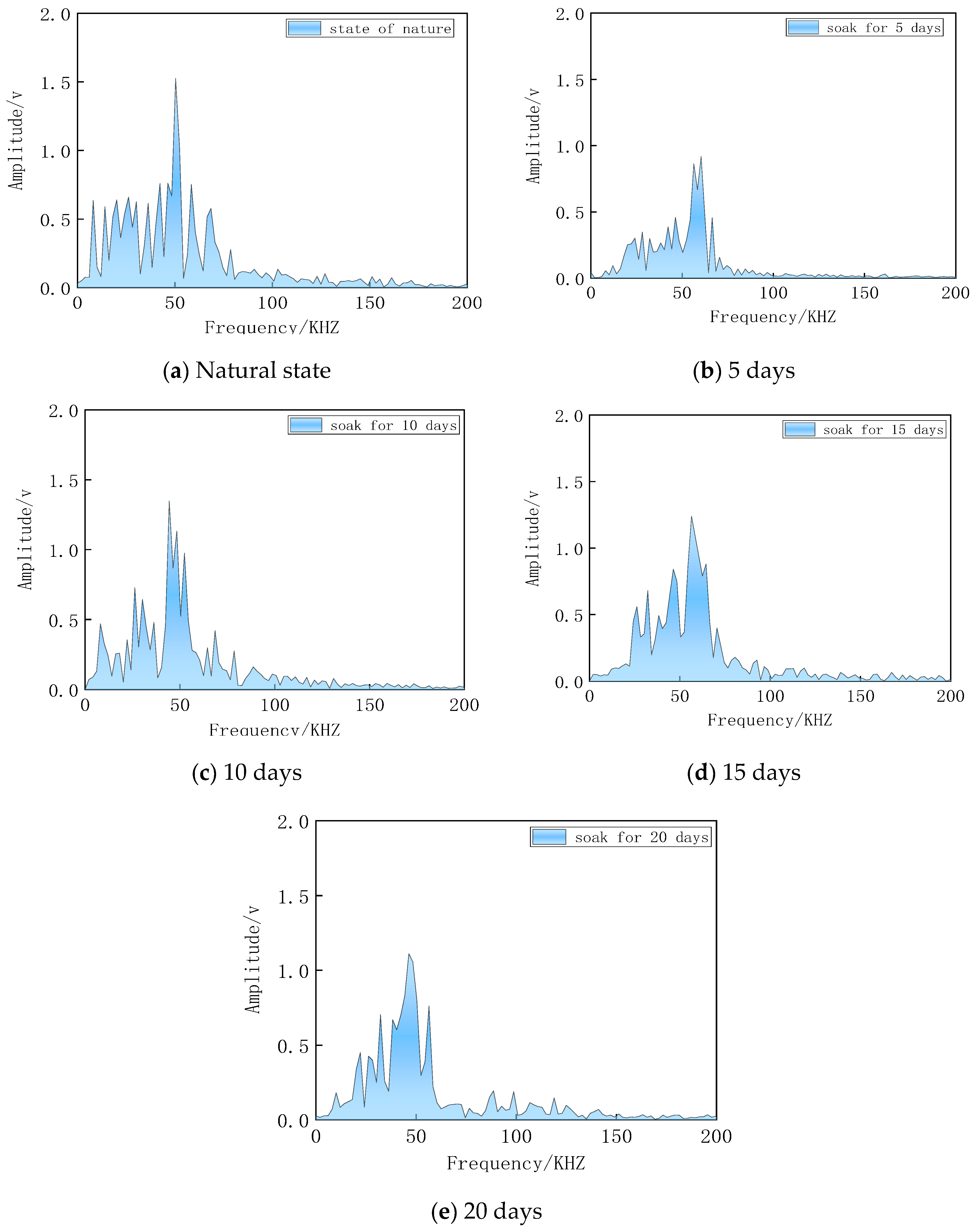
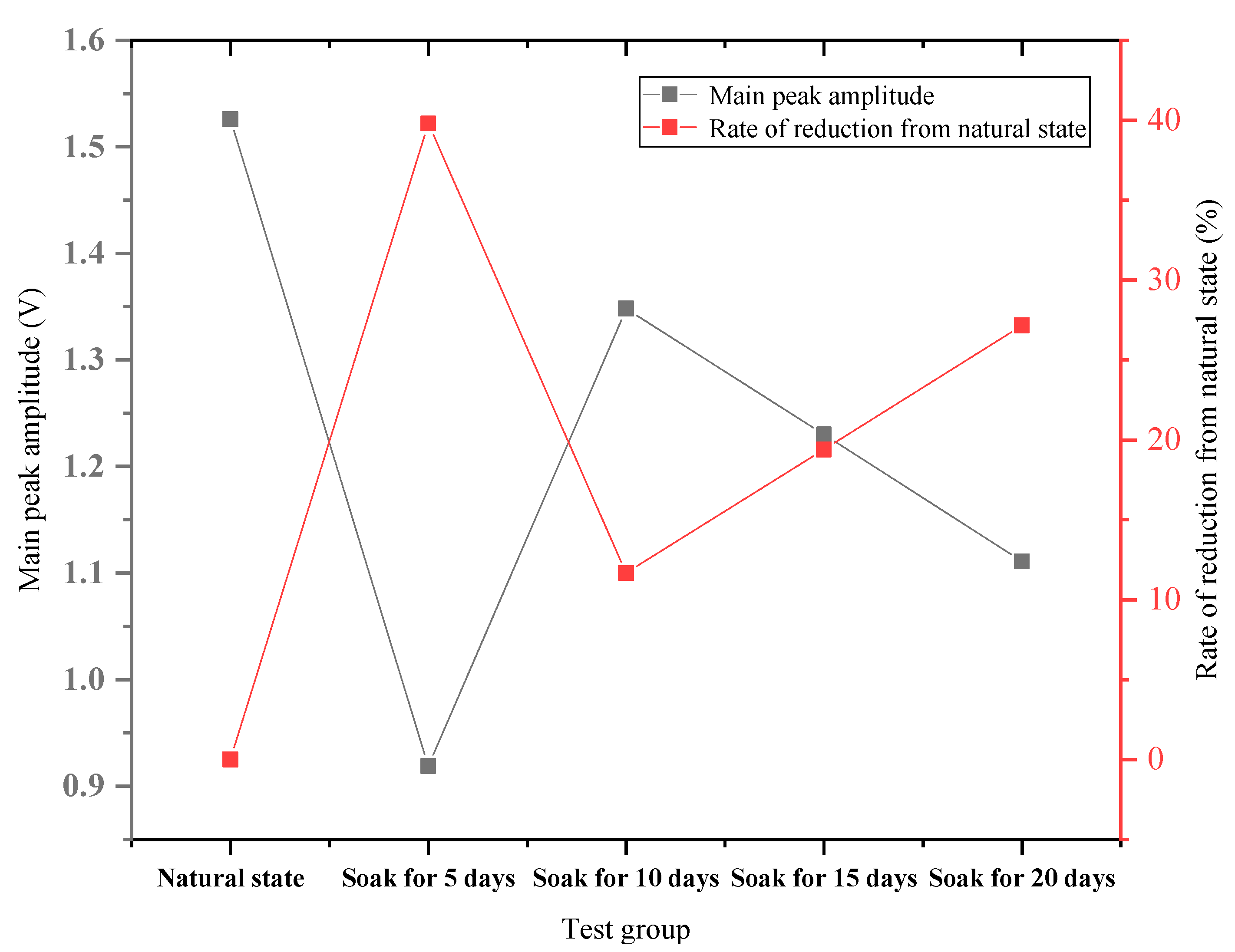
4.2. Acoustic Frequency Domain Signal Analysis
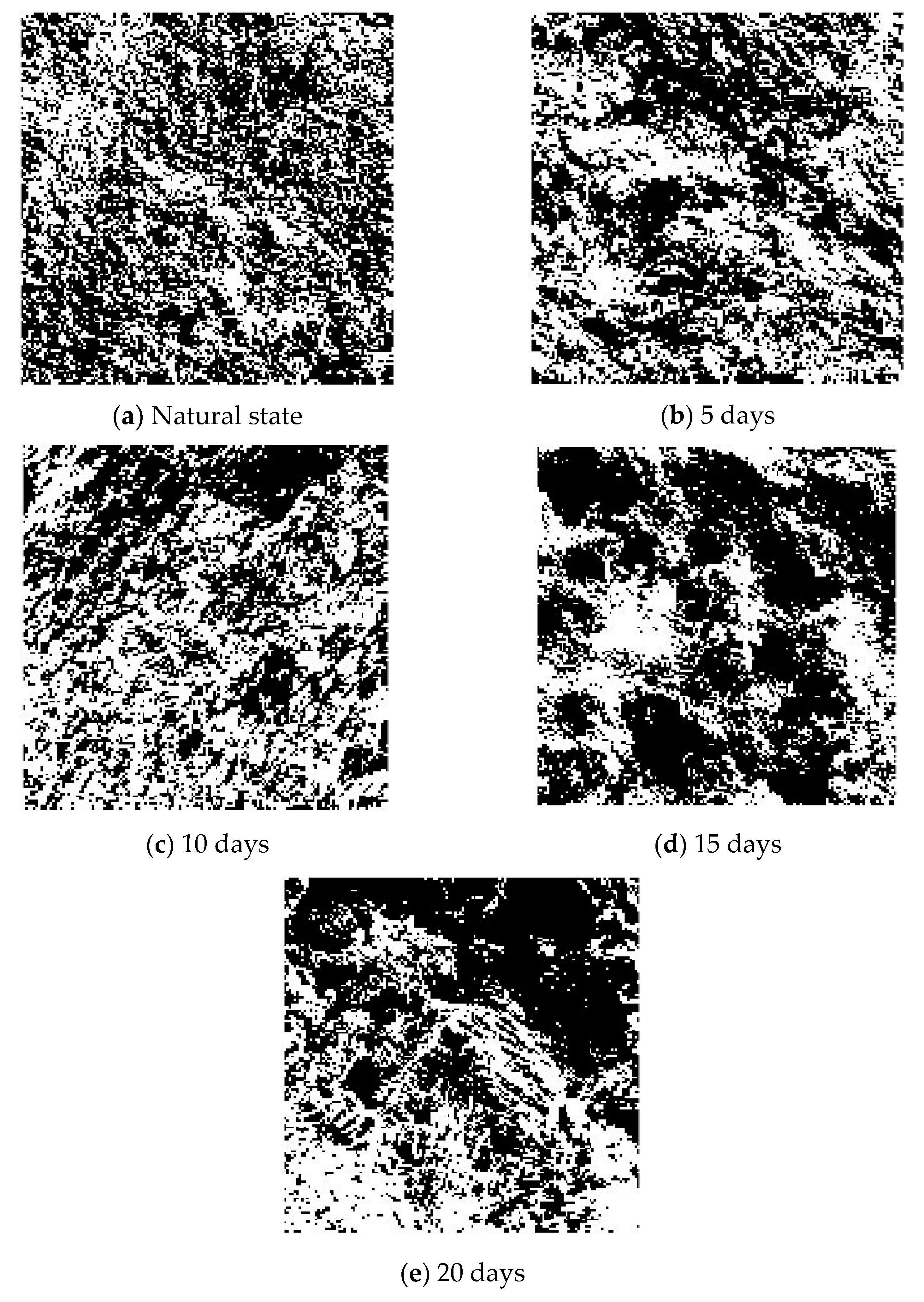
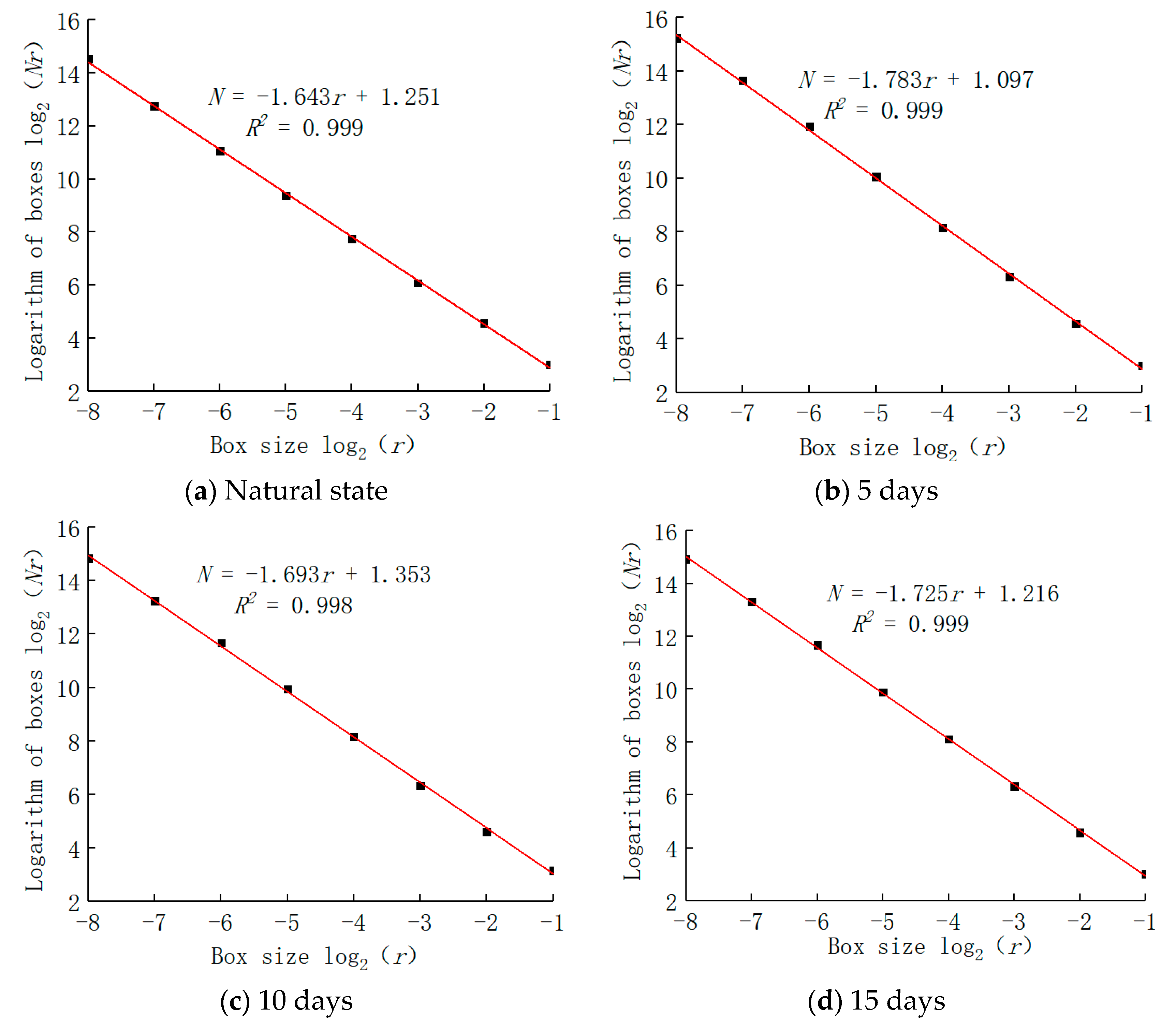
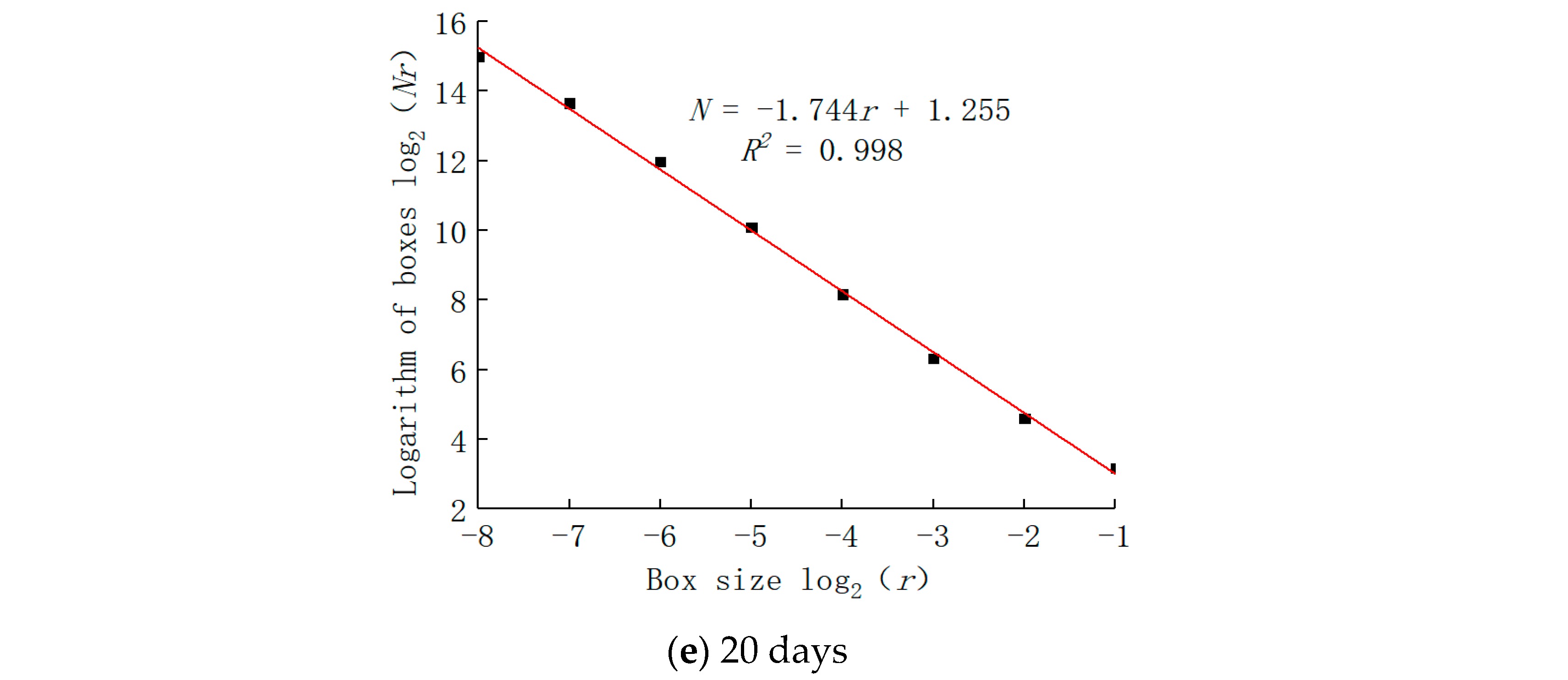
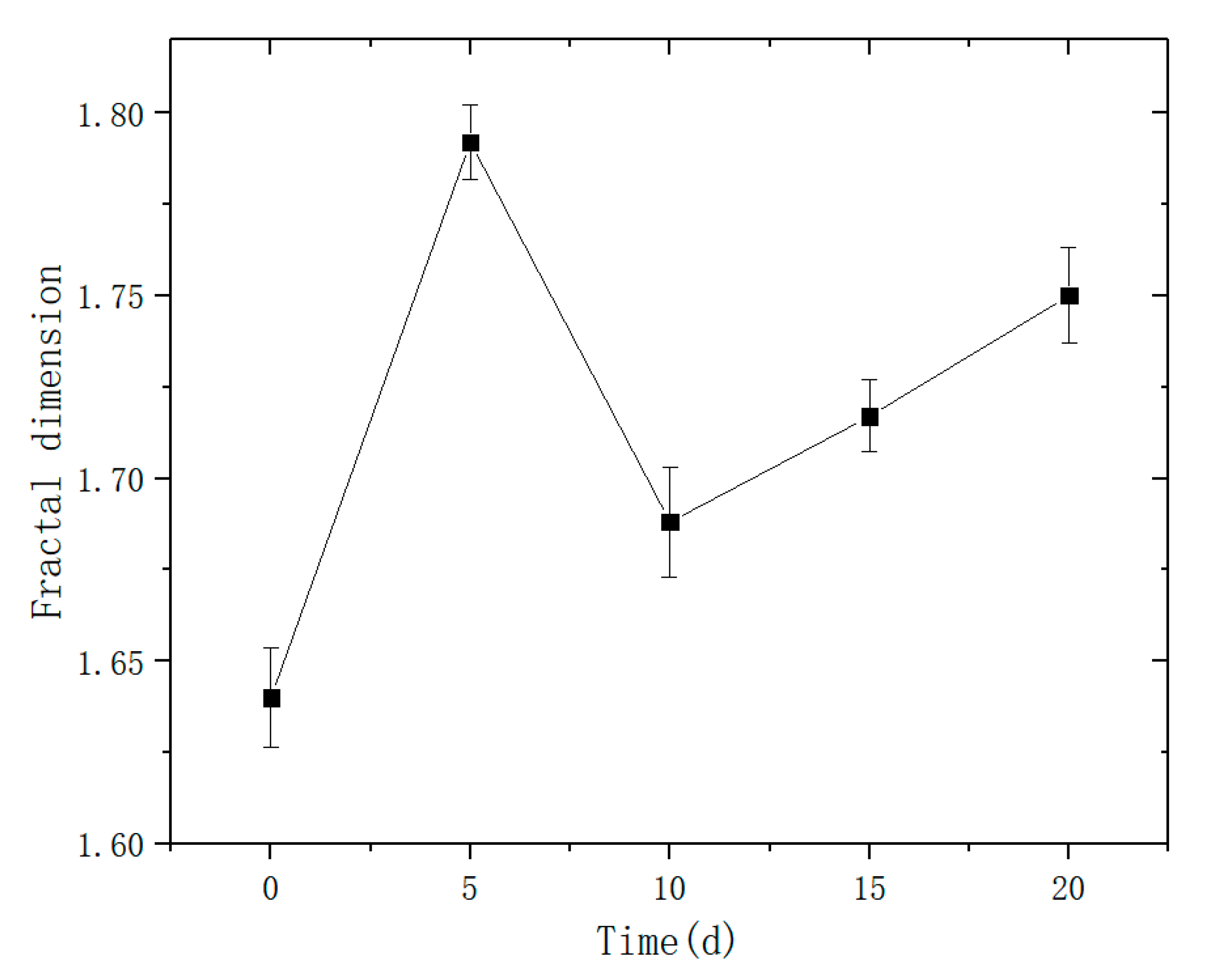
5. Fractal Characteristics
6. Conclusions
- (1)
- The analysis of limestone acidification in the sulfuric acid solution environment shows that the internal damage to rock increases as a whole in the sulfuric acid solution immersion environment; however, this is not a single linear trend, and the damage decreases after a certain period of time. This is because the sulfuric acid solution immersion will produce calcium sulfate crystals in the micro cracks, filling part of the internal crack space, and will reduce the damage degree in this period.
- (2)
- Through the analysis of acoustic time-domain signal and frequency-domain signal, the results show that the rock-wave velocity in the time-domain state is degraded to varying degrees, compared with the natural state; the acoustic signal has an obvious absorption effect on high frequency, and the variation trend of the main peak amplitude with the soaking time is consistent.
- (3)
- The pore distribution of a limestone mesostructure under acidic conditions has fractal characteristics; the fractal dimension D first increases, then decreases, and then increases with the increase in soaking time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.H.; Yang, J.; Zhang, D.F.; Peng, H. Effects of wetting and cyclic wetting–drying on tensile strength of sandstone with a low clay mineral content. Rock Mech. Rock Eng. 2017, 50, 485–491. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Cai, X.; Ma, D.; Chen, L.; Wang, S.; Tan, L. Dynamic tensile properties of sandstone subjected to wetting and drying cycles. Constr. Build. Mater. 2018, 182, 215–232. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Niu, Y.X.; Shang, X.J.; Ye, P.; Zhou, R.; Gao, F. Deterioration of physical and mechanical properties of rocks by cyclic drying and wetting. Geofluids 2021, 2021, 6661107. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Feng, X.; Zhang, H.; Feng, J. Effects of Acid Sulfate and Chloride Ion on the Pore Structure and Mechanical Properties of Sandstone Under Dynamic Loading. Rock Mech. Rock Eng. 2021, 54, 6105–6121. [Google Scholar] [CrossRef]
- Tong, R.; Liu, H.; Liu, J.; Shi, Y.; Xie, L.; Ban, S. Meso-Mechanical Characteristics of Granite with Natural Cracks after Mud Acid Corrosion. Energies 2022, 15, 721. [Google Scholar] [CrossRef]
- Morsy, S.; Hetherington, C.; Sheng, J. Effect of low-concentration HCl on the mineralogy, physical and mechanical properties, and recovery factors of some shales. J. Unconv. Oil Gas Resour. 2015, 9, 94–102. [Google Scholar] [CrossRef]
- Feng, X.T.; Chen, S.L.; Li, S.J. Effects of water chemistry on microcracking and compressive strength of granite. Int. J. Rock Mech. Min. Sci. 2001, 38, 557–568. [Google Scholar] [CrossRef]
- Yu, L.Y.; Zhang, Z.Q.; Wu, J.Y.; Liu, R.; Qin, H.; Fan, P. Experimental study on the dynamic fracture mechanical properties of limestone after chemical corrosion. Theor. Appl. Fract. Mech. 2020, 108, 102620. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Z.L.; Liu, X.R.; Sheng, Y.; Yang, D. Micro-damage evolution and macro-mechanical property degradation of limestone due to chemical effects. Int. J. Rock Mech. Min. Sci. 2018, 110, 257–265. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, K.P.; Gao, R.G.; Li, J.; Zhang, J. Influence of chemical corrosion on pore structure and mechanical properties of sandstone. Geofluids 2019, 2019, 7320536. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, H.; Taheri, A.; Ke, B.; Liu, C.; Yang, X. Degradation of physical and mechanical properties of sandstone subjected to freeze-thaw cycles and chemical erosion. Cold Reg. Sci. Technol. 2018, 155, 37–46. [Google Scholar] [CrossRef]
- Li, H.M.; Li, H.G.; Wang, K.L.; Liu, C. Effect of rock composition microstructure and pore characteristics on its rock mechanics properties. Int. J. Min. Sci. Technol. 2018, 28, 303–308. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.P.; Duan, L.C.; Tan, S. Prediction of rock abrasivity and hardness from mineral composition. Int. J. Rock Mech. Min. Sci. 2021, 140, 104658. [Google Scholar] [CrossRef]
- Zhang, W.Q.; LU, C. Effects of mineral content on limestone properties with exposure to different temperatures. J. Pet. Sci. Eng. 2020, 188, 106941. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Sun, C.; Xiong, Y.; Wu, W.; Zhang, Y.; Li, N. Kinetics study of surface reaction between acid and sandstone based on the rotation disk instrument. Chem. Technol. Fuels Oils 2020, 55, 765–777. [Google Scholar] [CrossRef]
- Marieni, C.; Matter, J.M.; Teagle, D.A.H. Experimental study on mafic rock dissolution rates within CO2-Sea water rock systems. Geochim. Et Cosmochim. Acta 2020, 272, 259–275. [Google Scholar] [CrossRef]
- Ivanishin, I.B.; Nasr-El-din, H.A. Effect of calcium content on the dissolution rate of Dolomites in HCl acid. J. Pet. Sci. Eng. 2021, 202, 108463. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Su, G.S.; Wu, Z.K.; Liu, F.T. Experimental Investigation on Influence of Acidic Dry-Wet Cycles on Karst Limestone Deterioration and Damage. Geofluids 2022, 2022, 8562226. [Google Scholar] [CrossRef]
- Lai, J.; Guo, J.C.; Ma, Y.X.; Zhou, H.Y.; Wang, S.B.; Liu, Y.X. Effect of Acid-Rock Reaction on the Microstructure and Mechanical Property of Tight Limestone. Rock Mech. Rock Eng. 2022, 55, 35–49. [Google Scholar] [CrossRef]
- Ding, W.X.; Wang, H.Y.; Chen, H.J.; Ma, T. Mechanical Damage and Chemical Dissolution Kinetic Features of Limestone under Coupled Mechanical-Hydrological-Chemical Effects. Geofluids 2021, 2021, 1810768. [Google Scholar] [CrossRef]
- Niazi, F.S.; Piñán-Llamas, A.; Fekadu, F. Impact of chemical weathering on microstructures and mechanical properties of karstic limestone. Geotech. Lett. 2021, 11, 281–293. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Z.L.; Eshiet, K.I.-I.; Sheng, Y.; Liu, X.R.; Yang, D. Experimental Investigation of the Permeability and Mechanical Behaviours of Chemically Corroded Limestone Under Different Unloading Conditions. Rock Mech. Rock Eng. 2020, 53, 1587–1603. [Google Scholar] [CrossRef]
- Scrivano, S.; Gaggero, L. An experimental investigation into the salt-weathering susceptibility of building limestones. Rock Mech. Rock Eng. 2020, 53, 5329–5343. [Google Scholar] [CrossRef]
- Yao, H.; Ma, D.; Xiong, J. Study on the Influence of Different Aqueous Solutions on the Mechanical Properties and Microstructure of Limestone. J. Test. Eval. 2020, 49, 3776–3794. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, W.; Yao, X.; Liang, P.; Sun, L.; Liu, X. Study on Spectrum Characteristics and Clustering of Acoustic Emission Signals from Rock Fracture. Circuits Syst. Signal Process. 2020, 39, 1133–1145. [Google Scholar] [CrossRef]
- Wu, S.; Qin, G.; Cao, J. Deformation, Failure, and Acoustic Emission Characteristics under Different Lithological Confining Pressures. Materials 2022, 15, 4257. [Google Scholar] [CrossRef]
- Du, K.; Li, X.; Tao, M.; Wang, S. Experimental study on acoustic emission (AE) characteristics and crack classification during rock fracture in several basic lab tests. Int. J. Rock Mech. Min. Sci. 2020, 133, 104411. [Google Scholar] [CrossRef]
- Zhang, M.M.; Liang, L.X.; Jiang, S.L. Influence of carbonate rocks with different pore structures on the time-frequency characteristics of acoustic waves. Broken Block Oil Gas Field 2016, 23, 825–828. [Google Scholar]
- Ersoy, H.; Atalar, C.; Sünnetci, M.O.; Kolaylı, H.; Karahan, M.; Ersoy, A.F. Assessment of damage on geo-mechanical and micro-structural properties of weak calcareous rocks exposed to fires using thermal treatment coefficient. Eng. Geol. 2021, 284, 106046. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.; Klaver, J.; Dewanckele, J.; Amann-Hildenbrand, A.; Gaus, G.; Littke, R. An Integrated Imaging Study of the Pore Structure of the Cobourg Limestone—A Potential Nuclear Waste Host Rock in Canada. Minerals 2021, 11, 1042. [Google Scholar] [CrossRef]
- Wu, X.; Guo, Q.; Zhu, Y.; Ren, F.; Zhang, J.; Wu, X.; Cai, M. Pore structure and crack characteristics in high-temperature granite under water-cooling. Case Stud. Therm. Eng. 2021, 28, 101646. [Google Scholar] [CrossRef]
- Li, X.; Wei, W.; Wang, L.; Ding, P.; Zhu, L.; Cai, J. A new method for evaluating the pore structure complexity of digital rocks based on the relative value of fractal dimension. Mar. Pet. Geol. 2022, 141, 105694. [Google Scholar] [CrossRef]
- Huang, D.; Chang, X.; Tan, Y.; Fang, K.; Yin, Y. From rock microstructure to macromechanical properties based on fractal dimensions. Adv. Mech. Eng. 2019, 11, 168781401983636. [Google Scholar] [CrossRef]
- Cao, R.H.; Wang, C.S.; Yao, R.B.; Hu, T.; Lei, D.X.; Lin, H.; Zhao, Y.L. Effects of cyclic freeze-thaw treatments on the fracture characteristics of sandstone under different fracture modes: Laboratory testing. Theor. Appl. Fract. Mech. 2020, 109, 102738. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, X.; Luo, H.; Long, L.; Liu, C. Microscopic Damage to Limestone under Acidic Conditions: Phenomena and Mechanisms. Sustainability 2022, 14, 11771. https://doi.org/10.3390/su141811771
Chen X, Liu X, Luo H, Long L, Liu C. Microscopic Damage to Limestone under Acidic Conditions: Phenomena and Mechanisms. Sustainability. 2022; 14(18):11771. https://doi.org/10.3390/su141811771
Chicago/Turabian StyleChen, Xingming, Xiaoping Liu, Haoming Luo, Linjian Long, and Chuanju Liu. 2022. "Microscopic Damage to Limestone under Acidic Conditions: Phenomena and Mechanisms" Sustainability 14, no. 18: 11771. https://doi.org/10.3390/su141811771
APA StyleChen, X., Liu, X., Luo, H., Long, L., & Liu, C. (2022). Microscopic Damage to Limestone under Acidic Conditions: Phenomena and Mechanisms. Sustainability, 14(18), 11771. https://doi.org/10.3390/su141811771





