Utilization of Colored Extracts for the Formulation of Ecological Friendly Plant-Based Green Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagent
2.2. Preparation of Fruit Peels Extracts
2.3. Determination of Total Phenolic Content (TPC)
2.4. Determination of DPPH Radical Scavenging Activity
2.5. Development of O/W Emulsion with Fruit Peel Extracts
2.6. Basic Characterization of Formulations
2.6.1. pH and Stability Testing
2.6.2. Spreadability
2.6.3. Sun Protection Factor
2.6.4. Photostability
2.7. Antioxidant Activity of Cream
2.8. Statistical Analysis
3. Results and Discussion
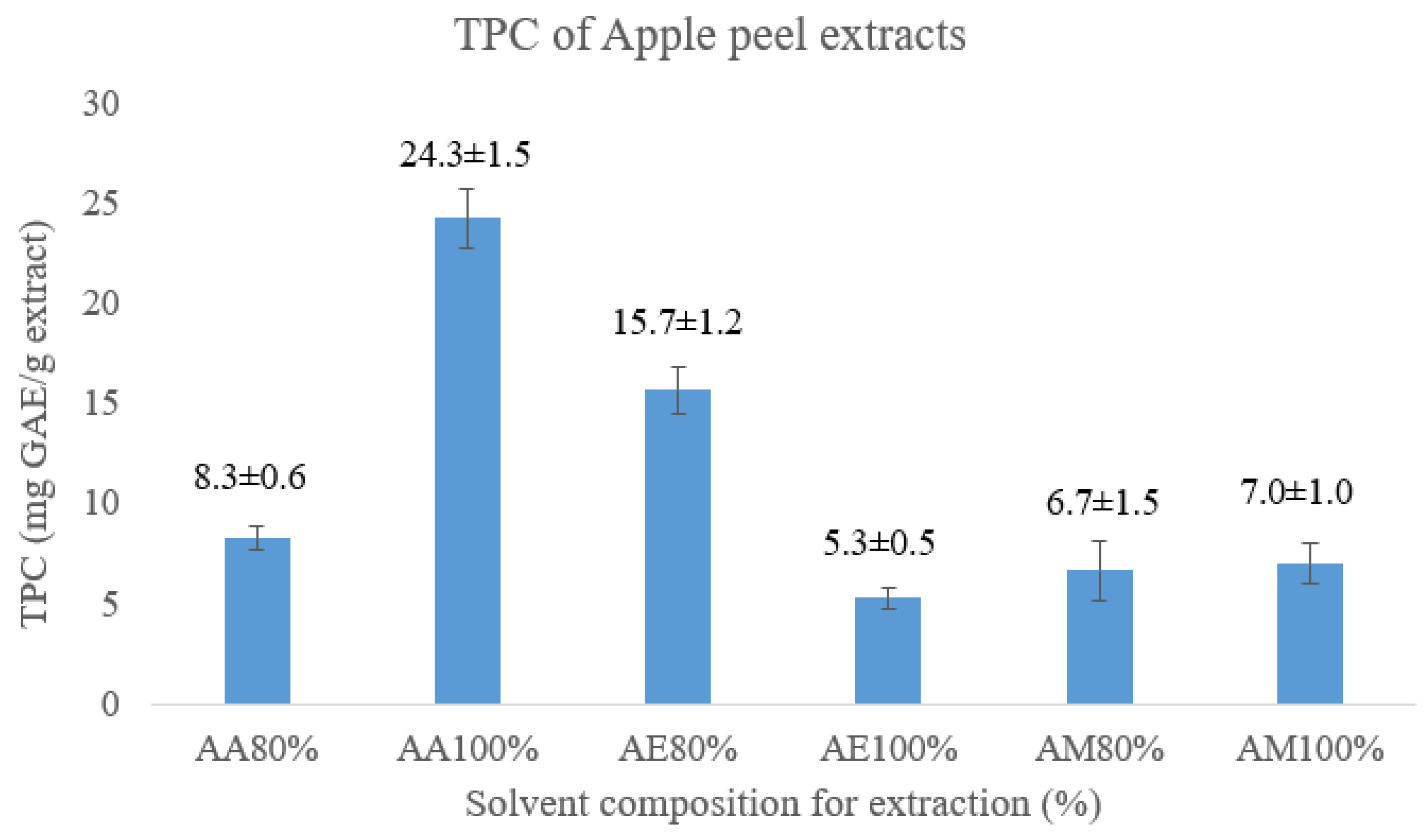
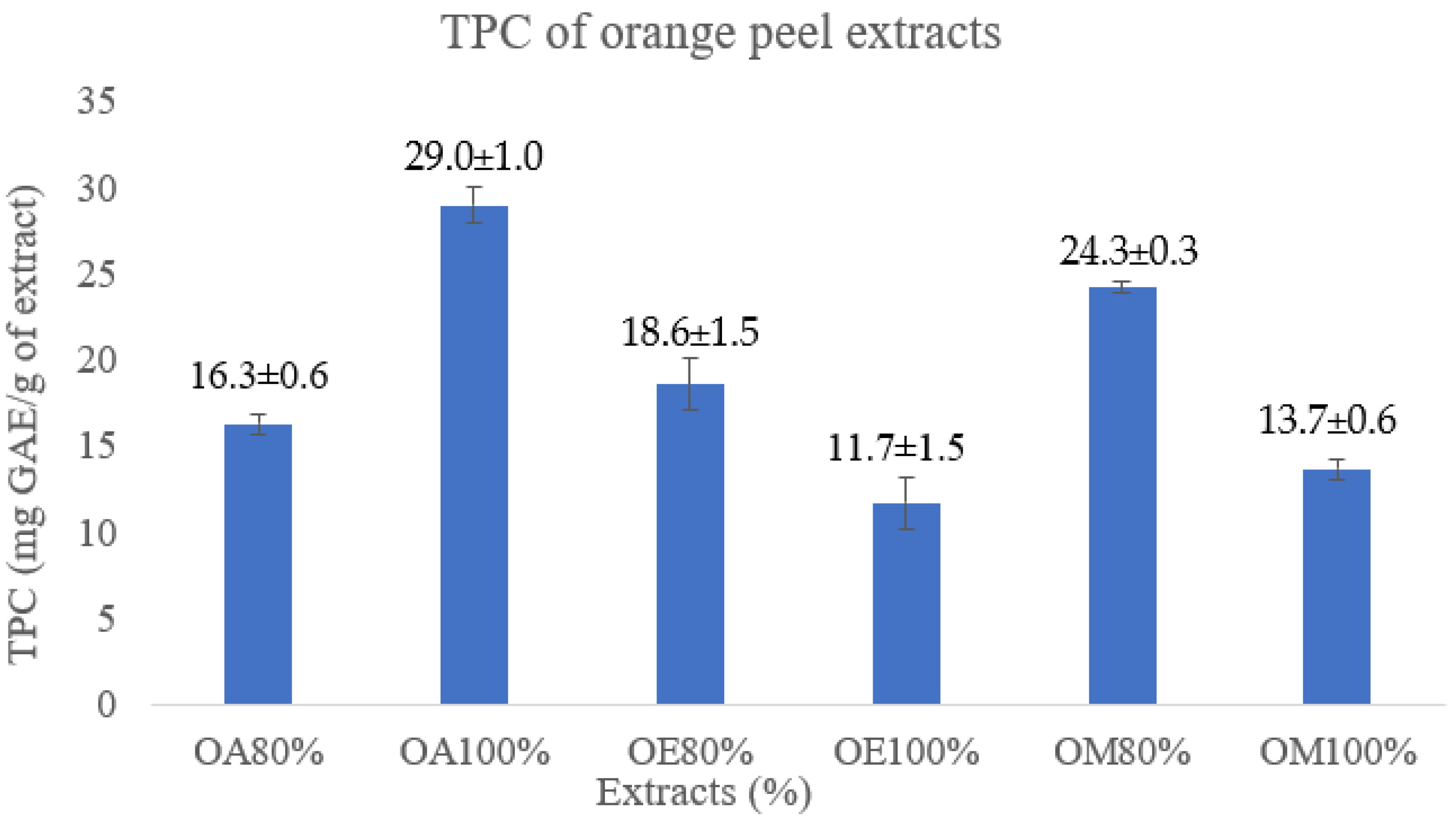
3.1. Extraction and Determination of Total Phenolic Contents (TPC)
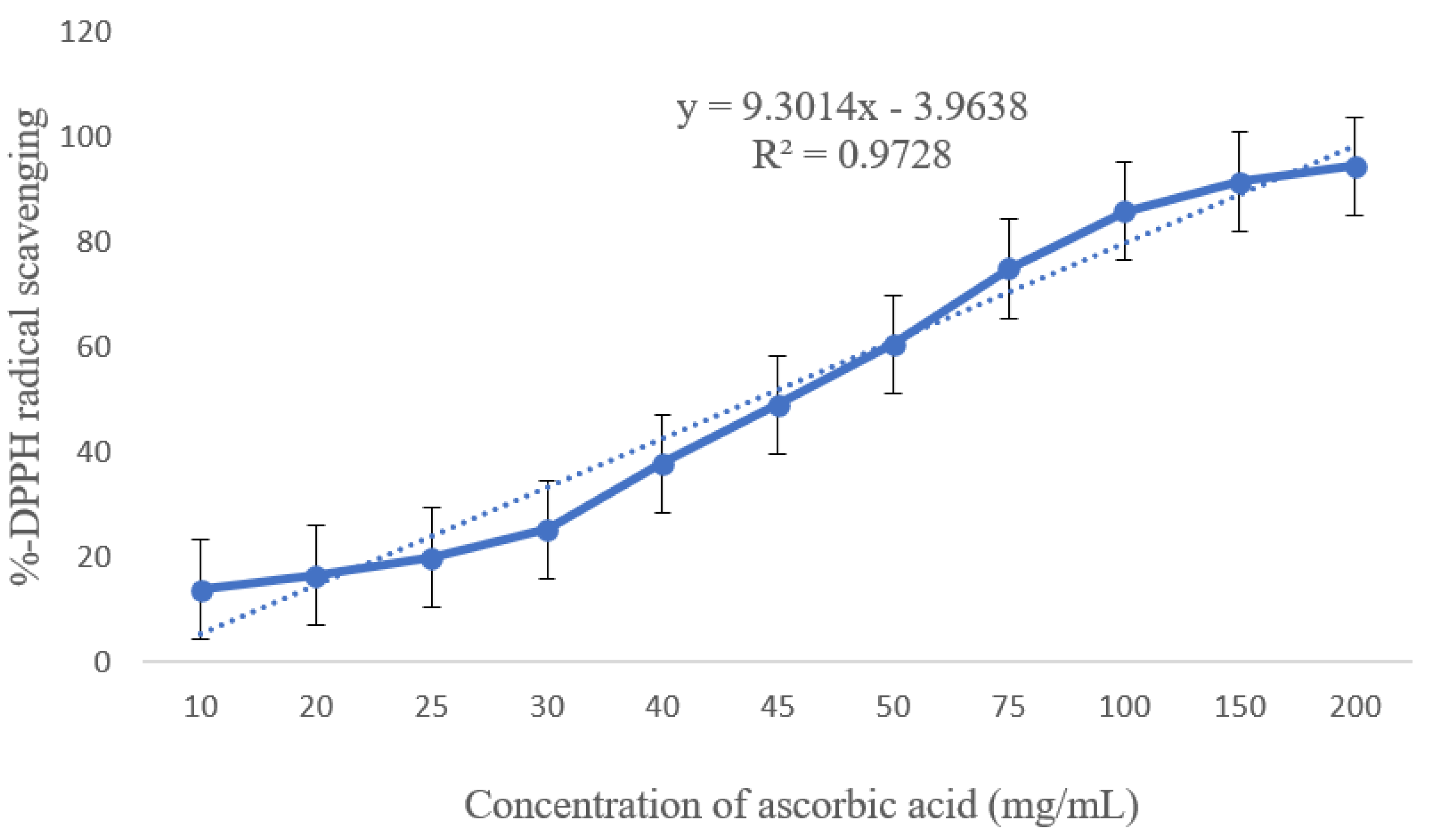
3.2. Antioxidant Activity
3.3. Incorporation of Extracts into Emulsion
3.4. Emulsion Stability Studies
3.5. Characterization of the Emulsions
3.5.1. Spreadability
3.5.2. Sun Protection Factor (SPF)
3.5.3. Photostability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goyal, N.; Jerold, F. Biocosmetics: Technological advances and future outlook. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Akram, W.; Adeel, S.; Amin, N.; Hosseinnezhad, M.; Haq, E.U. Environmental-friendly extraction of Peepal (Ficus Religiosa) bark-based reddish brown tannin natural dye for silk coloration. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hussaan, M.; Iqbal, N.; Adeel, S.; Azeem, M.; Tariq Javed, M.; Raza, A. Microwave-assisted enhancement of milkweed (Calotropis procera L.) leaves as an eco-friendly source of natural colorants for textile. Environ. Sci. Pollut. Res. 2017, 24, 5089–5094. [Google Scholar] [CrossRef] [PubMed]
- Kaderides, K.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A.M. Potential of pomegranate peel extract as a natural additive in foods. Trends Food Sci. Technol. 2021, 115, 380–390. [Google Scholar] [CrossRef]
- Karray, A.; Krayem, N.; Saad, H.B.; Sayari, A. Spirulina platensis, Punica granatum peel, and moringa leaves extracts in cosmetic formulations: An integrated approach of in vitro biological activities and acceptability studies. Environ. Sci. Pollut. Res. 2021, 28, 8802–8811. [Google Scholar] [CrossRef]
- Choi, D.-Y.; Lee, Y.-J.; Hong, J.T.; Lee, H.-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.J.S.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Aseervatham, G.S.B.; Sivasudha, T.; Jeyadevi, R.; Arul Ananth, D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans—An overview. Environ. Sci. Pollut. Res. 2013, 20, 4356–4369. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, J.-Y.; Lee, H.J.; Park, K.Y.; Ma, Y.-K.; Lee, S.-H.; Cho, J.A.; Kim, W.-S.; Park, K.-H.; Moon, J.-H. Isolation and identification of phenolic compounds from an Asian pear (Pyrus pyrifolia Nakai) fruit peel. Food Sci. Biotechnol. 2011, 20, 1539–1545. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Kazłowska, K.; Hsu, T.; Hou, C.-C.; Yang, W.-C.; Tsai, G.-J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Nagendra Prasad, K.; Yang, B.; Yang, S.; Chen, Y.; Zhao, M.; Ashraf, M.; Jiang, Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem. 2009, 116, 1–7. [Google Scholar] [CrossRef]
- Simic, M.G.; Jovanovic, S.V. Inactivation of Oxygen Radicals by Dietary Phenolic Compounds in Anticarcinogenesis. In Food Phytochemicals for Cancer Prevention II; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; Volume 547, pp. 20–32. [Google Scholar]
- González-Montelongo, R.; Lobo, G.; Gonzalez, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Correia, P.; Araújo, P.; Ribeiro, C.; Oliveira, H.; Pereira, A.R.; Mateus, N.; de Freitas, V.; Brás, N.F.; Gameiro, P.; Coelho, P.; et al. Anthocyanin-Related Pigments: Natural Allies for Skin Health Maintenance and Protection. Antioxidants 2021, 10, 1038. [Google Scholar] [CrossRef]
- Hubner, A.; Sobreira, F.; Vetore Neto, A.; Pinto, C.A.S.d.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharm. Biol. 2010, 48, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Bandonienė, D.; Murkovic, M.; Pfannhauser, W.; Venskutonis, P.; Gruzdienė, D. Detection and activity evaluation of radical scavenging compounds by using DPPH free radical and on-line HPLC-DPPH methods. Eur. Food Res. Technol. 2002, 214, 143–147. [Google Scholar] [CrossRef]
- Sharma, T.; Tyagi, V.; Bansal, M. Determination of sun protection factor of vegetable and fruit extracts using UV–Visible spectroscopy: A green approach. Sustain. Chem. Pharm. 2020, 18, 100347. [Google Scholar] [CrossRef]
- Jarzycka, A.; Lewińska, A.; Gancarz, R.; Wilk, K.A. Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J. Photochem. Photobiol. B 2013, 128, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M.C. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatogra A 1998, 823, 331–337. [Google Scholar] [CrossRef]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef]
- Tongkaew, P.; Tohraman, A.; Bungaramphai, R.; Mitrpant, C.; Aydin, E. Kluai Hin (Musa sapientum Linn.) peel as a source of functional polyphenols identified by HPLC-ESI-QTOF-MS and its potential antidiabetic function. Sci. Rep. 2022, 12, 4145. [Google Scholar] [CrossRef]
- Abozed, S.S.; El-kalyoubi, M.; Abdelrashid, A.; Salama, M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann. Agric. Sci. 2014, 59, 63–67. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Phenolic acids contribution to antioxidant activities and comparative assessment of phenolic content in mango pulp and peel. S. Afr. J. Bot. 2018, 116, 158–163. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Abla, M.J.; Banga, A.K. Quantification of skin penetration of antioxidants of varying lipophilicity. Int. J. Cosmet. Sci. 2013, 35, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Blaak, J.; Staib, P. The Relation of pH and Skin Cleansing. Curr. Probl. Dermatol. 2018, 54, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Afsharnezhad, M.; Shahangian, S.; Panahi, E.; Sariri, R. Evaluation of the antioxidant activity of extracts from some fruit peels. Casp. J. Environ. Sci. 2017, 15, 213–222. [Google Scholar]
- Nesseem, D. Formulation of sunscreens with enhancement sun protection factor response based on solid lipid nanoparticles. Int. J. Cosmet. Sci. 2011, 33, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Oh, B.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. The relation between the amount of sunscreen applied and the sun protection factor in Asian skin. J. Am. Acad. Dermatol. 2010, 62, 218–222. [Google Scholar] [CrossRef]
- Berkey, C.; Oguchi, N.; Miyazawa, K.; Dauskardt, R. Role of sunscreen formulation and photostability to protect the biomechanical barrier function of skin. Biochem. Biophys. Rep. 2019, 19, 100657. [Google Scholar] [CrossRef] [PubMed]




| Ingredients | Apple | Banana | Orange | Control Base |
|---|---|---|---|---|
| Water | Q.S. | Q.S. | Q.S. | Q.S. |
| EDTA | 0.05% | 0.05% | 0.05% | 0.05% |
| Glycerine | 2.5% | 2.5% | 2.5% | 2.5% |
| Polawax | 5% | 5% | 5% | 5% |
| Shea butter | 1% | 1% | 1% | 1% |
| Stearic acid | 0.3% | 0.3% | 0.3% | 0.3% |
| Petrolatum | 1% | 1% | 1% | 1% |
| Extract | 4% | 4% | 4% | 0% |
| Almond Oil | 1% | 1% | 1% | 1% |
| Perfume | 1% | 1% | 1% | 1% |
| Parabens | 0.2% | 0.2% | 0.2% | 0.2% |
| Sample | Extract | Color | SPF | Photostability | Antioxidant Activity |
|---|---|---|---|---|---|
| CRB | None | White | 4.0 ± 0.2 | - | None |
| ACR | Apple | Cream | 20.0 ± 0.4 | 95% | 88.0% |
| BCR | Banana | Gold brown | 19.0 ± 0.2 | 98% | 87.2% |
| OCR | Orange | Orange | 18.3 ± 0.1 | 97% | 80.0% |
| Sample | pH (0 Time) | pH (30 Days) | pH (After Centrifuge) |
|---|---|---|---|
| CRB | 5.07 ± 0.1 | 5.03 ± 0.2 | 5.06 ± 0.1 |
| ACR | 5.45 ± 0.3 | 5.41 ± 0.1 | 5.45 ± 0.1 |
| BCR | 5.53 ± 0.1 | 5.52 ± 0.2 | 5.53 ± 0.3 |
| OCR | 4.89 ± 0.2 | 4.85 ± 0.4 | 4.87 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeel, S.; Habiba, M.; Kiran, S.; Iqbal, S.; Abrar, S.; Hassan, C.M. Utilization of Colored Extracts for the Formulation of Ecological Friendly Plant-Based Green Products. Sustainability 2022, 14, 11758. https://doi.org/10.3390/su141811758
Adeel S, Habiba M, Kiran S, Iqbal S, Abrar S, Hassan CM. Utilization of Colored Extracts for the Formulation of Ecological Friendly Plant-Based Green Products. Sustainability. 2022; 14(18):11758. https://doi.org/10.3390/su141811758
Chicago/Turabian StyleAdeel, Shahid, Maryam Habiba, Shumaila Kiran, Sarosh Iqbal, Shazia Abrar, and Ch Moazzam Hassan. 2022. "Utilization of Colored Extracts for the Formulation of Ecological Friendly Plant-Based Green Products" Sustainability 14, no. 18: 11758. https://doi.org/10.3390/su141811758
APA StyleAdeel, S., Habiba, M., Kiran, S., Iqbal, S., Abrar, S., & Hassan, C. M. (2022). Utilization of Colored Extracts for the Formulation of Ecological Friendly Plant-Based Green Products. Sustainability, 14(18), 11758. https://doi.org/10.3390/su141811758






