Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review
Abstract
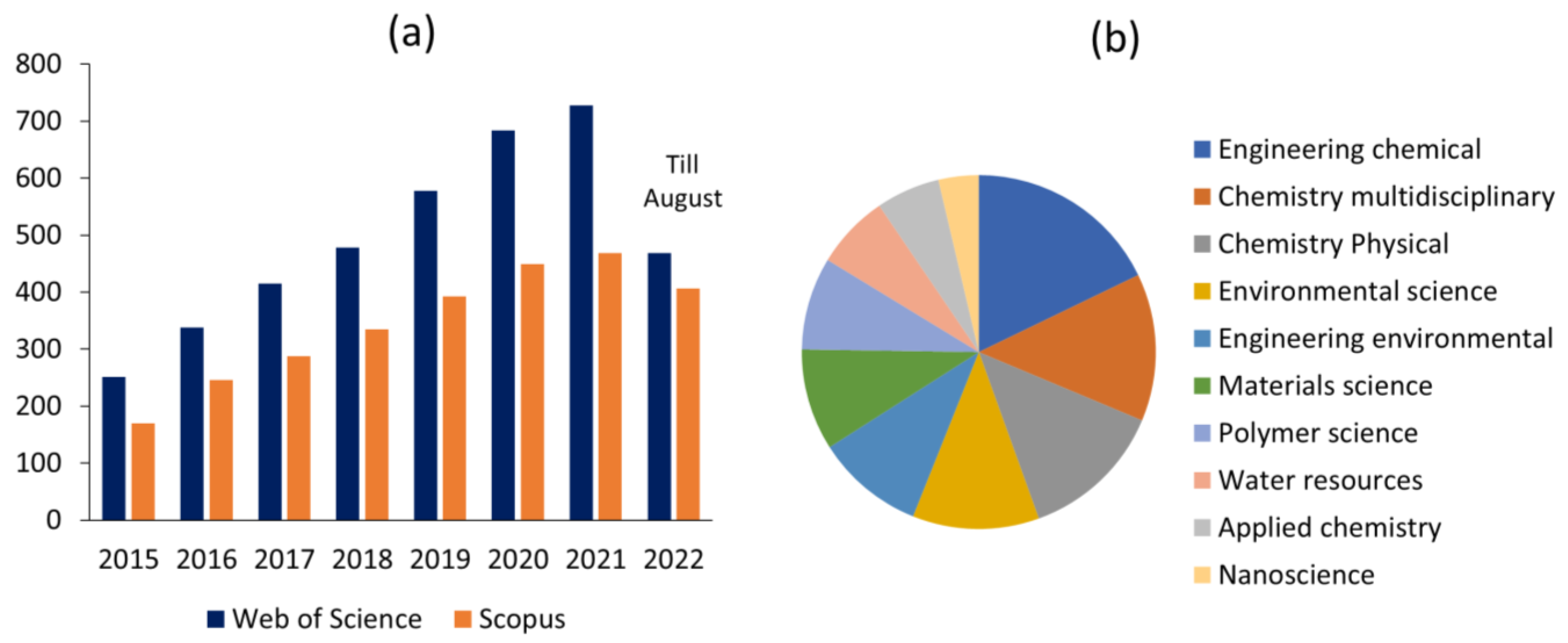
1. Introduction
2. Types of Anionic Dyes
2.1. Reactive Dye
2.2. Direct Dye
2.3. Acid Dye
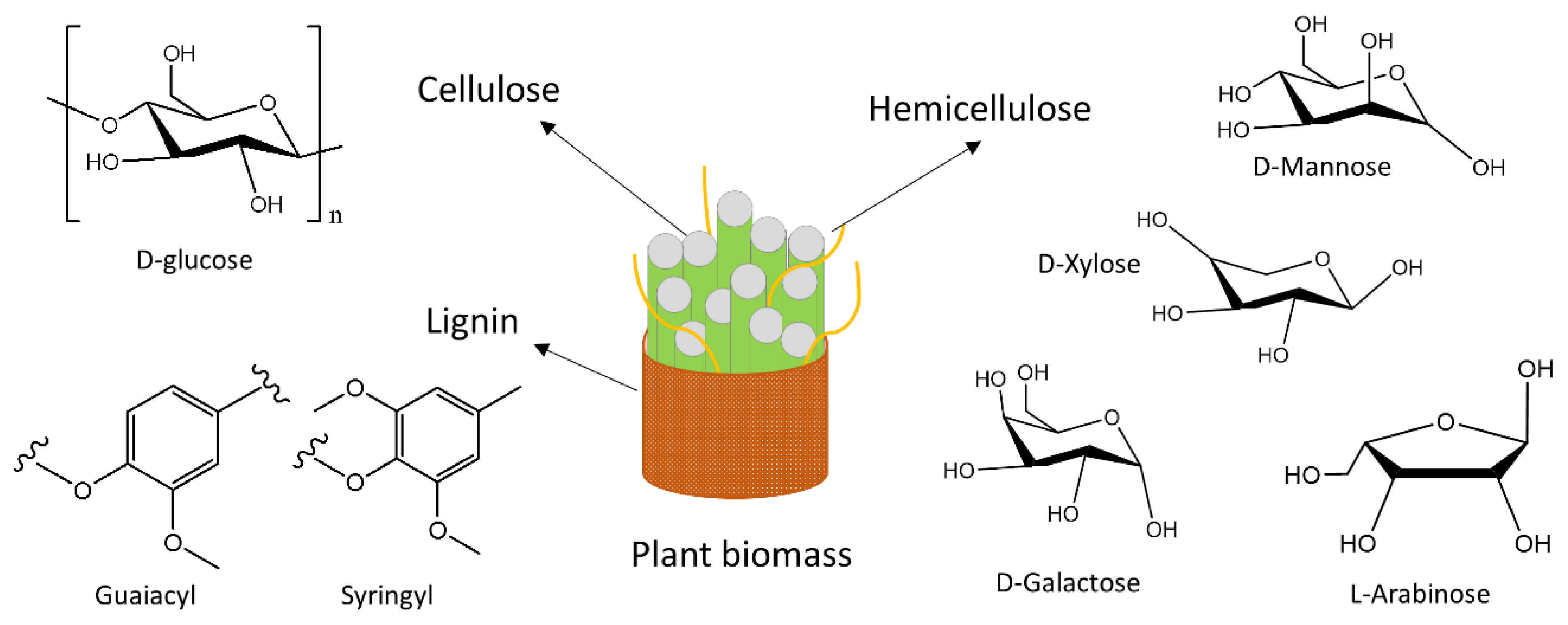
3. Chemical Nature of Plant-Derived Agricultural Wastes
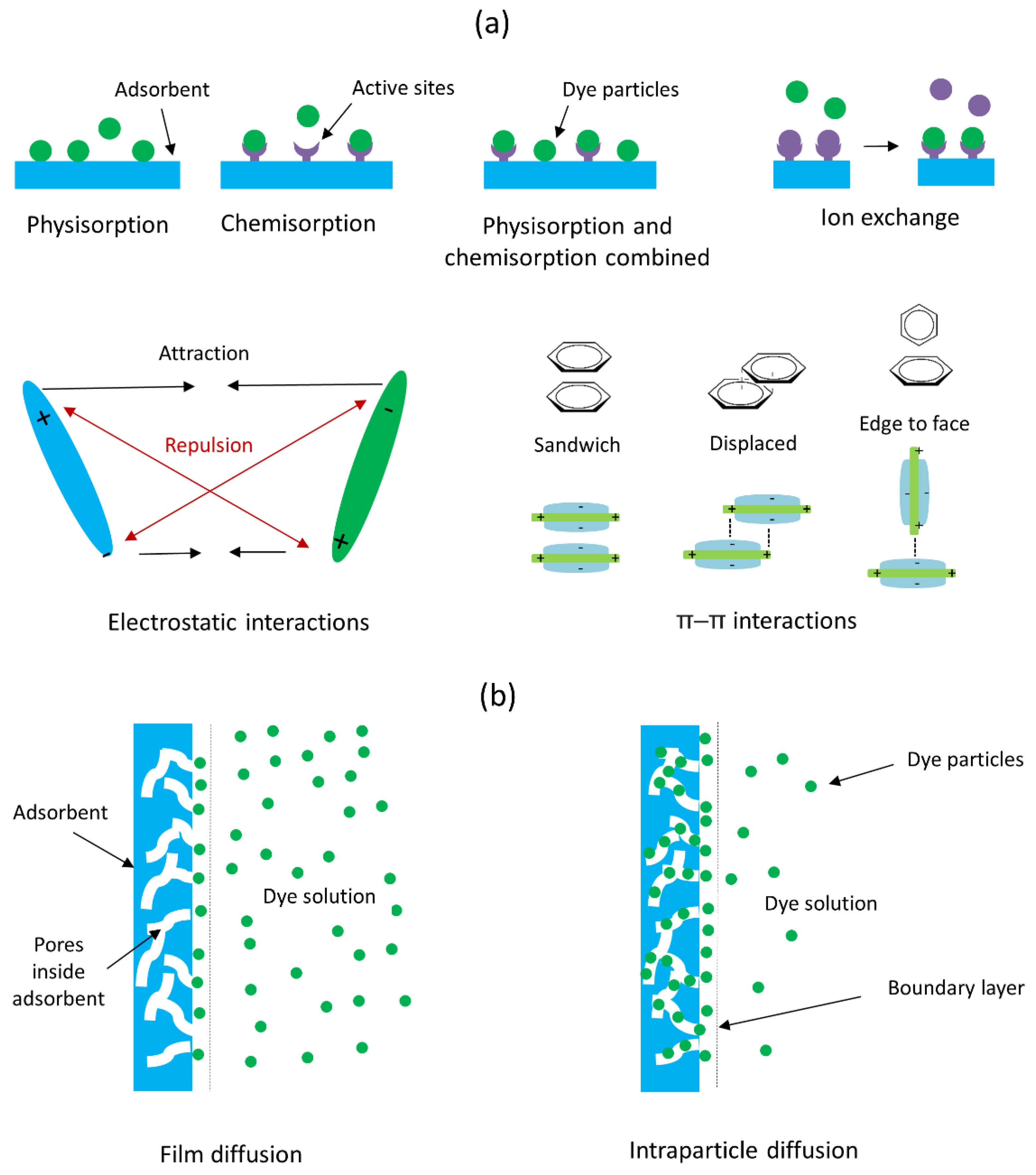
4. Adsorbents for Anionic Dyes
4.1. Mechanical Modifications
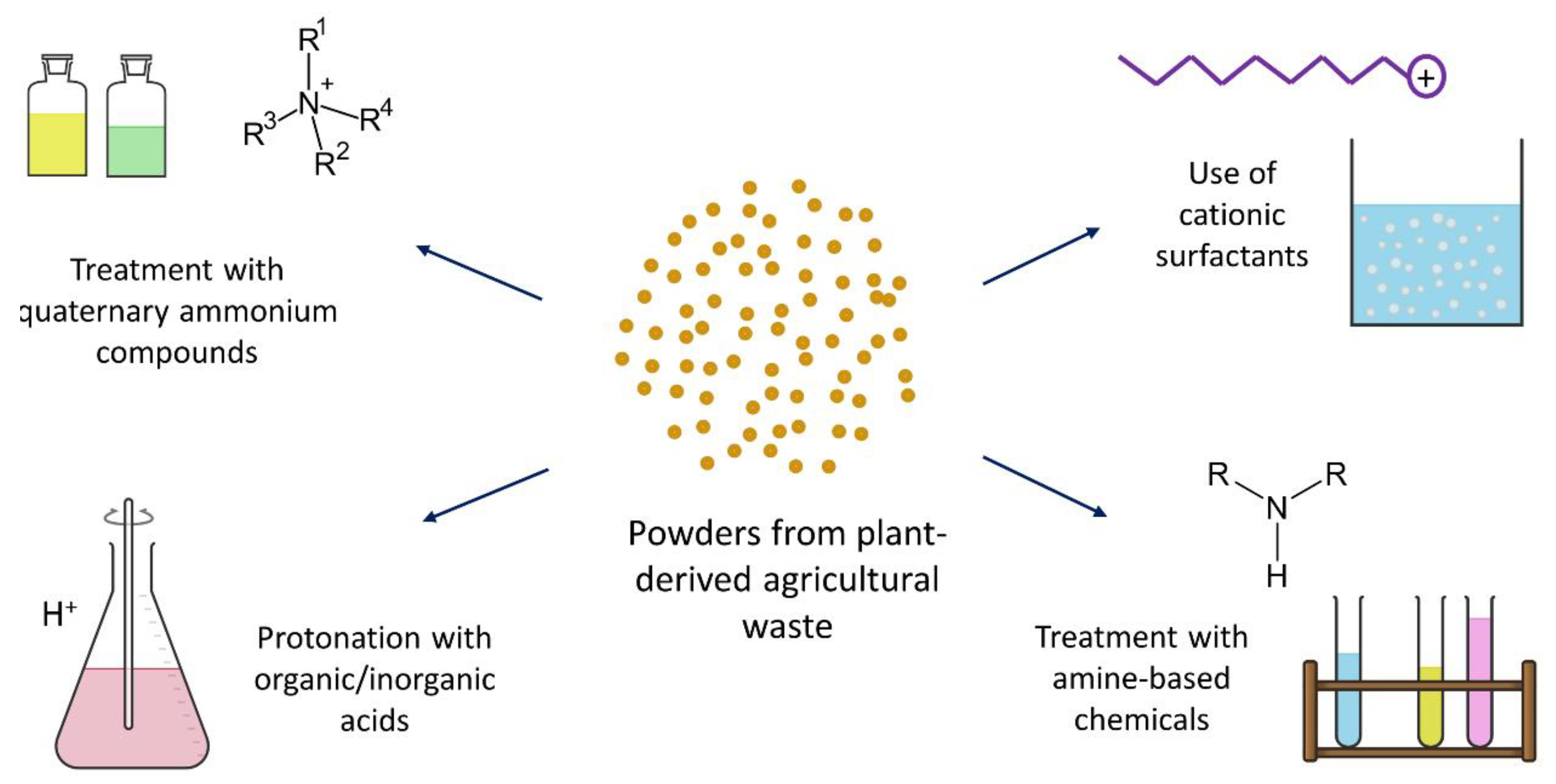
4.2. Chemical Modifications
| Resources | Chemical Modification | Modification Type | Dye | qmax (mg/g) | Reference |
|---|---|---|---|---|---|
| Wheat straw | Grafting with 2-dimethylamino ethyl methacrylate monomer | Amine-based | Orange II | 506 | [58] |
| Wheat straw | Treatment with hexadecylpyridinium bromide | Cationic surfactant | Light Green | 70.01 ± 3.39 | [47] |
| Peanut husk | Modified by hexadecylpyridinium bromide | Cationic surfactant | Light Green | 60.5 | [46] |
| Wheat straw | Treatment with epichlorohydrin, N,N-dimethylformamide, ethylenediamine, and trimethylamine | Amine-based | Acid Red 73 | 714.3 | [12] |
| Wheat straw | Treatment with epichlorohydrin, N,N-dimethylformamide, ethylenediamine, and trimethylamine | Amine-based | Reactive Red 24 | 285.7 | [12] |
| Barley straw | Treatment with hexadecylpyridinium chloride monohydrate | Cationic surfactant | Acid Blue 40 | 51.95 | [49] |
| Barley straw | Treatment with hexadecylpyridinium chloride monohydrate | Cationic surfactant | Reactive Blue 4 | 31.5 | [49] |
| Corn stalks | Treatment by cetylpyridinium bromide | Cationic surfactant | Acid Red | 30.77 | [15] |
| Corn stalks | Treatment by cetylpyridinium bromide | Cationic surfactant | Acid Orange | 31.06 | [15] |
| Banana peel | Reinforcement with nanoparticles and chitosan | Amine-based | Reactive Orange 5 | 125 | [14] |
| Sawdust | Coating with polyaniline | Amine-based | Acid Red G | 212.97 | [45] |
| Sawdust | Treatment with cetyltrimethylammonium bromide | Cationic surfactant | Congo Red | 9.1 | [50] |
| Sawdust | Treatment with concentrate HCl | Acid treatment | Reactive Red 196 | 13.39 | [13] |
| Rice husk | Treatment with hydroxypropyloctadecyldimethylammonium | Quaternary ammonium compounds | Diamine Green B | 207.15 | [52] |
| Rice husk | Treatment with hydroxypropyloctadecyldimethylammonium | Quaternary ammonium compounds | Acid Black 24 | 268.88 | [52] |
| Rice husk | Treatment with hydroxypropyloctadecyldimethylammonium | Quaternary ammonium compounds | Congo Red | 580.09 | [52] |
| Peanut husk | Treatment with alginate and CaCl2 | Metal salt | Drimarine Black CL-B | 40.98 | [55] |
| Peanut husk | Treatment by hexadecylpyridinium bromide in batch mode | Cationic surfactant | Light Green | 146.2 ± 2.4 | [63] |
| Peanut husk | Hydrochloric acid treatment | Acid treatment | Drimarine Black CL-B | 51.02 | [55] |
| Oil palm empty fruit bunches | Silylation | Amine-based | Procion Red | 208.33 | [57] |
| Orange peel | Treatment with dichloroethane, methyl amine, and acetic acid | Amine-based | Congo Red | 163 | [16] |
| Cotton gin trash | Cationized by chitosan | Amine-based | Acid Blue 25 | 151.52 | [7] |
| Aquatic plant | Phosphoric acid treatment and low-temperature activation | Acid treatment | Direct Red 89 | 15.96 | [54] |
| Coffee waste | Treatment with polyethylenimine | Amine-based | Reactive Black 5 | 77.52 | [18] |
| Coffee waste | Treatment with polyethylenimine | Amine-based | Congo Red | 34.36 | [18] |
| Hardwood kraft pulp | Grafting cellulose nanocrystals with ethylenediamine | Amine-based | Acid Red GR | 555.6 | [44] |
| Palm kernel shell | Quaternized by N-(3-chloro-2-hydroxypropyl) trimethylammonium chloride | Quaternary ammonium compounds | Reactive Black 5 | 207.5 | [53] |
| Fermentation waste | Protonated by nitric acid | Acid treatment | Reactive Black 5 | 185.2 | [56] |
| Waste coir pith | Treatment with hexadecyltrimethylammonium solution | Cationic surfactant | Acid Brilliant Blue | 159 | [51] |
| Waste coir pith | Treatment with hexadecyltrimethylammonium solution | Cationic surfactant | Procion Orange | 89 | [51] |
| Wood residue | Aluminum oxide modification | Metal oxide | Reactive Blue 19 | 29.83 | [64] |
| Wood biowaste | Aluminum oxide modification | Metal oxide | Reactive Blue 19 | 441.9 | [59] |
4.3. Thermal Modifications
4.4. The Effect of Process Conditions
5. Adsorption Isotherms
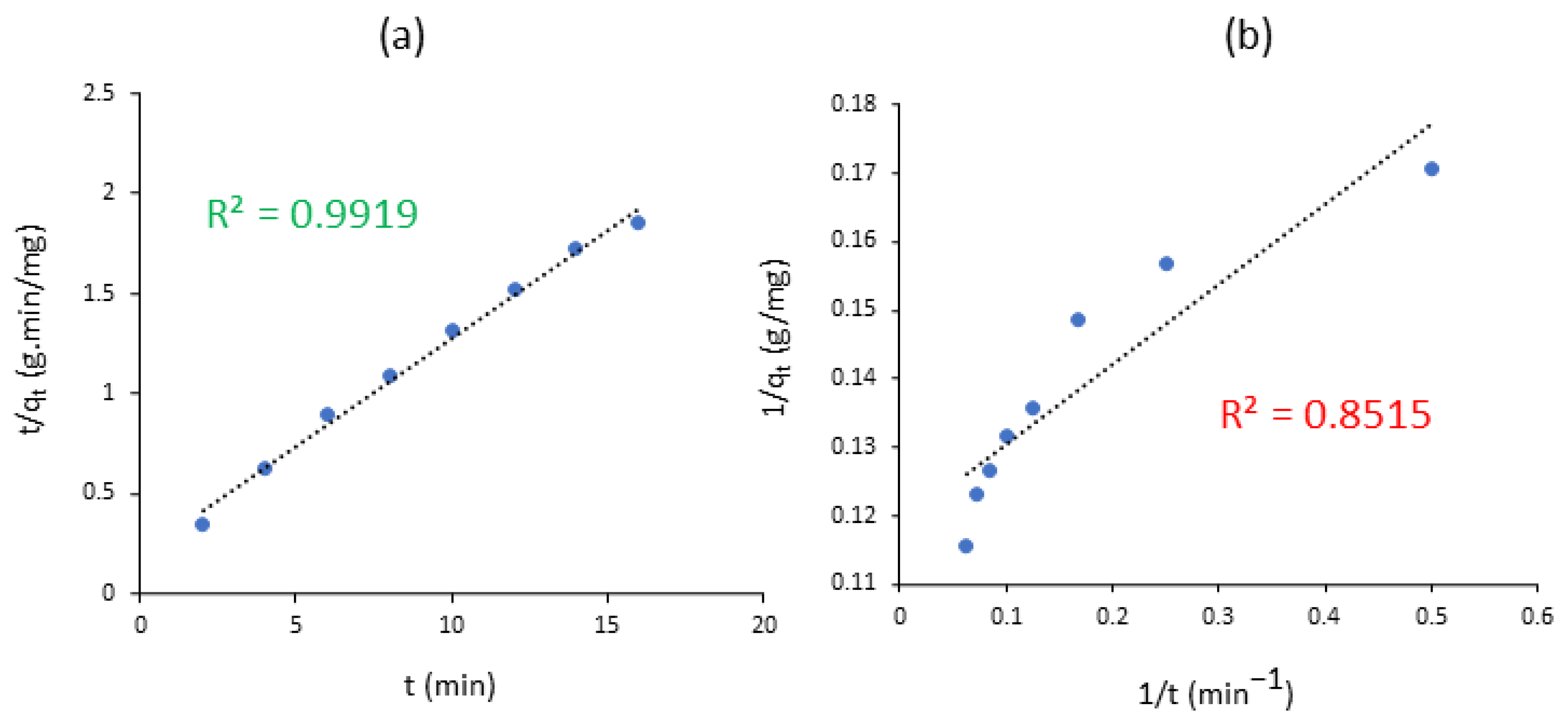
6. Kinetics and Diffusion
7. Thermodynamics of Adsorption
8. Circularity and Sustainability
9. Conclusions and Future Scopes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haque, A.N.M.A.; Remadevi, R.; Wang, X.; Naebe, M. Sorption properties of fabricated film from cotton gin trash. Mater. Today Proc. 2020, 31, S221–S226. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Rojas, O.J.; Wang, X.; Naebe, M. Kinetics and equilibrium adsorption of methylene blue onto cotton gin trash bioadsorbents. Cellulose 2020, 27, 6485–6504. [Google Scholar] [CrossRef]
- Ray, R.C.; Behera, S.S. Solid state fermentation for production of microbial cellulases. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–79. [Google Scholar]
- Haque, A.N.M.A.; Remadevi, R.; Wang, X.; Naebe, M. Adsorption of anionic Acid Blue 25 on chitosan-modified cotton gin trash film. Cellulose 2020, 27, 9437–9456. [Google Scholar] [CrossRef]
- Roa, K.; Oyarce, E.; Boulett, A.; ALSamman, M.; Oyarzún, D.; Pizarro, G.D.C.; Sánchez, J. Lignocellulose-based materials and their application in the removal of dyes from water: A review. Sustain. Mater. Technol. 2021, 29, e00320. [Google Scholar] [CrossRef]
- Mishra, S.; Cheng, L.; Maiti, A. The utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104901. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Rachna, K.; Agarwal, A.; Singh, N. Rice husk and Sodium hydroxide activated Rice husk for removal of Reactive yellow dye from water. Mater. Today Proc. 2019, 12, 573–580. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.-Y.; Yue, Q.-Y.; Zhong, Q.-Q. Preparation and utilization of wheat straw bearing amine groups for the sorption of acid and reactive dyes from aqueous solutions. J. Hazard. Mater. 2010, 182, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Doltabadi, M.; Alidadi, H.; Davoudi, M. Comparative study of cationic and anionic dye removal from aqueous solutions using sawdust-based adsorbent. Environ. Prog. Sustain. Energy 2016, 35, 1078–1090. [Google Scholar] [CrossRef]
- Abdelghaffar, F. Biosorption of anionic dye using nanocomposite derived from chitosan and silver Nanoparticles synthesized via cellulosic banana peel bio-waste. Environ. Technol. Innov. 2021, 24, 101852. [Google Scholar] [CrossRef]
- Soldatkina, L.; Zavrichko, M. Equilibrium, kinetic, and thermodynamic studies of anionic dyes adsorption on corn stalks modified by cetylpyridinium bromide. Colloids Interfaces 2018, 3, 4. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Kim, D.-S. Adsorption of anionic azo dye Congo Red from aqueous solution by Cationic Modified Orange Peel Powder. J. Mol. Liq. 2016, 220, 540–548. [Google Scholar] [CrossRef]
- Tahir, M.A.; Bhatti, H.N.; Iqbal, M. Solar Red and Brittle Blue direct dyes adsorption onto Eucalyptus angophoroides bark: Equilibrium, kinetics and thermodynamic studies. J. Environ. Chem. Eng. 2016, 4, 2431–2439. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef]
- Broadbent, A.D. Basic Principles of Textile Coloration; Society of Dyers and Colorists Bradford: Bradford, UK, 2001; Volume 132. [Google Scholar]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Islam, M.A. The contribution of different vinyl sulphone-reactive dyes to an effluent. J. Taibah Univ. Sci. 2015, 9, 594–600. [Google Scholar] [CrossRef][Green Version]
- Sekar, N. Acid dyes. In Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes; Clark, M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 486–514. [Google Scholar]
- Chakraborty, J. Fundamentals and Practices in Colouration of Textiles; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Haque, A.N.M.A.; Zhang, Y.; Naebe, M. A review on lignocellulose/poly (vinyl alcohol) composites: Cleaner approaches for greener materials. Cellulose 2021, 28, 10741–10764. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Naebe, M. Lemongrass (Cymbopogon): A review on its structure, properties, applications and recent developments. Cellulose 2018, 25, 5455–5477. [Google Scholar] [CrossRef]
- Sanderson, K. Lignocellulose: A chewy problem. Nature 2011, 474, S12–S14. [Google Scholar] [CrossRef] [PubMed]
- Değermenci, G.D.; Değermenci, N.; Ayvaoğlu, V.; Durmaz, E.; Çakır, D.; Akan, E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Clean. Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Aziz, E.K.; Abdelmajid, R.; Rachid, L.M.; Mohammadine, E.H. Adsorptive removal of anionic dye from aqueous solutions using powdered and calcined vegetables wastes as low-cost adsorbent. Arab. J. Basic Appl. Sci. 2018, 25, 93–102. [Google Scholar] [CrossRef]
- Rodríguez, A.; García, J.; Ovejero, G.; Mestanza, M. Adsorption of anionic and cationic dyes on activated carbon from aqueous solutions: Equilibrium and kinetics. J. Hazard. Mater. 2009, 172, 1311–1320. [Google Scholar] [CrossRef]
- Stavrinou, A.; Aggelopoulos, C.; Tsakiroglou, C. Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: Adsorption kinetics and equilibrium isotherms as a tool. J. Environ. Chem. Eng. 2018, 6, 6958–6970. [Google Scholar] [CrossRef]
- Jain, S.N.; Tamboli, S.R.; Sutar, D.S.; Jadhav, S.R.; Marathe, J.V.; Shaikh, A.A.; Prajapati, A.A. Batch and continuous studies for adsorption of anionic dye onto waste tea residue: Kinetic, equilibrium, breakthrough and reusability studies. J. Clean. Prod. 2020, 252, 119778. [Google Scholar] [CrossRef]
- El Messaoudi, N.; Dbik, A.; El Khomri, M.; Sabour, A.; Bentahar, S.; Lacherai, A. Date stones of Phoenix dactylifera and jujube shells of Ziziphus lotus as potential biosorbents for anionic dye removal. Int. J. Phytoremediat. 2017, 19, 1047–1052. [Google Scholar] [CrossRef]
- Namasivayam, C.; Prabha, D.; Kumutha, M. Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour. Technol. 1998, 64, 77–79. [Google Scholar] [CrossRef]
- Reddy, M.S.; Sivaramakrishna, L.; Reddy, A.V. The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J. Hazard. Mater. 2012, 203, 118–127. [Google Scholar] [CrossRef]
- Tunç, Ö.; Tanacı, H.; Aksu, Z. Potential use of cotton plant wastes for the removal of Remazol Black B reactive dye. J. Hazard. Mater. 2009, 163, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Parimelazhagan, V.; Jeppu, G.; Rampal, N. Continuous Fixed-Bed Column Studies on Congo Red Dye Adsorption-Desorption Using Free and Immobilized Nelumbo nucifera Leaf Adsorbent. Polymers 2021, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfi, R.; Karoui, S.; Mougin, K.; Ghorbal, A. Adsorptive removal of cationic and anionic dyes from aqueous solution by utilizing almond shell as bioadsorbent. Euro-Mediterr. J. Environ. Integr. 2017, 2, 20. [Google Scholar] [CrossRef]
- Farah, J.Y.; Elgendy, N. Performance, kinetics and equilibrium in biosorption of anionic dye Acid Red 14 by the waste biomass of Saccharomyces cerevisiae as a low-cost biosorbent. Turk. J. Eng. Environ. Sci. 2013, 37, 146–161. [Google Scholar]
- Alhujaily, A.; Yu, H.; Zhang, X.; Ma, F. Adsorptive removal of anionic dyes from aqueous solutions using spent mushroom waste. Appl. Water Sci. 2020, 10, 183. [Google Scholar] [CrossRef]
- Grabi, H.; Lemlikchi, W.; Derridj, F.; Lemlikchi, S.; Trari, M. Efficient native biosorbent derived from agricultural waste precursor for anionic dye adsorption in synthetic wastewater. Biomass Convers. Biorefinery 2021, 1–18. [Google Scholar] [CrossRef]
- Grabi, H.; Derridj, F.; Lemlikchi, W.; Guénin, E. Studies of the potential of a native natural biosorbent for the elimination of an anionic textile dye Cibacron Blue in aqueous solution. Sci. Rep. 2021, 11, 9705. [Google Scholar] [CrossRef]
- Banerjee, S.; Dastidar, M. Use of jute processing wastes for treatment of wastewater contaminated with dye and other organics. Bioresour. Technol. 2005, 96, 1919–1928. [Google Scholar] [CrossRef]
- Mbarki, F.; Kesraoui, A.; Seffen, M.; Ayrault, P. Kinetic, thermodynamic, and adsorption behavior of cationic and anionic dyes onto corn stigmata: Nonlinear and stochastic analyses. Water Air Soil Pollut. 2018, 229, 95. [Google Scholar] [CrossRef]
- Jin, L.; Li, W.; Xu, Q.; Sun, Q. Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 2015, 22, 2443–2456. [Google Scholar] [CrossRef]
- Lyu, W.; Yu, M.; Feng, J.; Yan, W. Highly crystalline polyaniline nanofibers coating with low-cost biomass for easy separation and high efficient removal of anionic dye ARG from aqueous solution. Appl. Surf. Sci. 2018, 458, 413–424. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, W.; Liu, L.; Zhu, H.; Jiao, Y.; Zhang, S.; Han, R. Effective adsorption of light green anionic dye from solution by CPB modified peanut in column mode. J. Mol. Liq. 2015, 211, 909–914. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, B.; Xiao, W.; Han, R. Adsorption behavior of light green anionic dye using cationic surfactant-modified wheat straw in batch and column mode. Environ. Sci. Pollut. Res. 2013, 20, 5558–5568. [Google Scholar] [CrossRef] [PubMed]
- Oei, B.C.; Ibrahim, S.; Wang, S.; Ang, H.M. Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution. Bioresour. Technol. 2009, 100, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Fatimah, I.; Ang, H.-M.; Wang, S. Adsorption of anionic dyes in aqueous solution using chemically modified barley straw. Water Sci. Technol. 2010, 62, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Seyghali, B.; Mohammad-Khah, A.; Zanjanchi, M.A. Highly efficient adsorption of anionic dyes from aqueous solutions using sawdust modified by cationic surfactant of cetyltrimethylammonium bromide. J. Surfactants Deterg. 2012, 15, 557–565. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sureshkumar, M. Anionic dye adsorption characteristics of surfactant-modified coir pith, a ‘waste’lignocellulosic polymer. J. Appl. Polym. Sci. 2006, 100, 1538–1546. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114. [Google Scholar] [CrossRef]
- Koay, Y.S.; Ahamad, I.S.; Nourouzi, M.M.; Abdullah, L.C.; Choong, T.S.Y. Development of novel low-cost quaternized adsorbent from palm oil agriculture waste for reactive dye removal. BioResources 2014, 9, 66–85. [Google Scholar] [CrossRef]
- Benabbas, K.; Zabat, N.; Hocini, I. Study of the chemical pretreatment of a nonconventional low-cost biosorbent (Callitriche obtusangula) for removing an anionic dye from aqueous solution. Euro-Mediterr. J. Environ. Integr. 2021, 6, 54. [Google Scholar] [CrossRef]
- Noreen, S.; Bhatti, H.N.; Nausheen, S.; Sadaf, S.; Ashfaq, M. Batch and fixed bed adsorption study for the removal of Drimarine Black CL-B dye from aqueous solution using a lignocellulosic waste: A cost affective adsorbent. Ind. Crops Prod. 2013, 50, 568–579. [Google Scholar] [CrossRef]
- Won, S.W.; Kim, H.-J.; Choi, S.-H.; Chung, B.-W.; Kim, K.-J.; Yun, Y.-S. Performance, kinetics and equilibrium in biosorption of anionic dye Reactive Black 5 by the waste biomass of Corynebacterium glutamicum as a low-cost biosorbent. Chem. Eng. J. 2006, 121, 37–43. [Google Scholar] [CrossRef]
- Saputra, O.A.; Nauqinida, M.; Pujiasih, S.; Kusumaningsih, T.; Pramono, E. Improvement of anionic and cationic dyes removal in aqueous solution by Indonesian agro-waste oil palm empty fruit bunches through silylation approach. Groundw. Sustain. Dev. 2021, 13, 100570. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, K.; Gao, M.; Bai, Y.; Chen, L.; Ma, H. Effectively removal of cationic and anionic dyes by pH-sensitive amphoteric adsorbent derived from agricultural waste-wheat straw. J. Taiwan Inst. Chem. Eng. 2017, 76, 65–72. [Google Scholar] [CrossRef]
- Velinov, N.; Radović Vučić, M.; Petrović, M.; Najdanović, S.; Kostić, M.; Mitrović, J.; Bojić, A. The influence of various solvents’ polarity in the synthesis of wood biowaste sorbent: Evaluation of dye sorption. Biomass Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Reza, R.A. Decontamination of cationic and anionic dyes in single and binary mode from aqueous phase by mesoporous pulp waste. Environ. Prog. Sustain. Energy 2015, 34, 724–735. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, I.; Karabacakoğlu, B.; Tümsek, F. Adsorption of textile dye onto activated carbon prepared from industrial waste by ZnCl2 activation. J. Int. Environ. Appl. Sci. 2008, 3, 381–389. [Google Scholar]
- Bhomick, P.C.; Supong, A.; Baruah, M.; Pongener, C.; Sinha, D. Pine Cone biomass as an efficient precursor for the synthesis of activated biocarbon for adsorption of anionic dye from aqueous solution: Isotherm, kinetic, thermodynamic and regeneration studies. Sustain. Chem. Pharm. 2018, 10, 41–49. [Google Scholar] [CrossRef]
- Zhao, B.; Xiao, W.; Shang, Y.; Zhu, H.; Han, R. Adsorption of light green anionic dye using cationic surfactant-modified peanut husk in batch mode. Arab. J. Chem. 2017, 10, S3595–S3602. [Google Scholar] [CrossRef]
- Velinov, N.; Mitrović, J.; Kostić, M.; Radović, M.; Petrović, M.; Bojić, D.; Bojić, A. Wood residue reuse for a synthesis of lignocellulosic biosorbent: Characterization and application for simultaneous removal of copper (II), Reactive Blue 19 and cyprodinil from water. Wood Sci. Technol. 2019, 53, 619–647. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.-B.; Liu, W.-W.; Miao, M.; Cheng, B.-J.; Zhu, S.-W. Novel synthesis of a versatile magnetic adsorbent derived from corncob for dye removal. Bioresour. Technol. 2015, 190, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Markets and Markets, Activated Carbon Market by Type, Application (Liquid Phase (Water Treatment, Foods & Beverages, Pharmaceutical & Medical), Gas Phase (Industrial, Automotive), and Region (APAC, North America, Europe, Middle East, South America)—Global Forecast to 2026. 2022. Available online: https://www.marketsandmarkets.com/Market-Reports/activated-carbon-362.html (accessed on 15 August 2022).
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Büchel, K.H.; Moretto, H.-H.; Werner, D. Industrial Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Belyaeva, O.; Krasnova, T.; Semenova, S. Effect of modification of granulated activated carbons with ozone on their properties. Russ. J. Appl. Chem. 2011, 84, 597–601. [Google Scholar] [CrossRef]
- Delamar, M.; Desarmot, G.; Fagebaume, O.; Hitmi, R.; Pinsonc, J.; Savéant, J.-M. Modification of carbon fiber surfaces by electrochemical reduction of aryl diazonium salts: Application to carbon epoxy composites. Carbon 1997, 35, 801–807. [Google Scholar] [CrossRef]
- García, A.B.; Martínez-Alonso, A.; y Leon, C.A.L.; Tascón, J.M. Modification of the surface properties of an activated carbon by oxygen plasma treatment. Fuel 1998, 77, 613–624. [Google Scholar] [CrossRef]
- Otake, Y.; Jenkins, R.G. Characterization of oxygen-containing surface complexes created on a microporous carbon by air and nitric acid treatment. Carbon 1993, 31, 109–121. [Google Scholar] [CrossRef]
- Silva, A.R.; Freire, C.; De Castro, B.; Freitas, M.; Figueiredo, J. Anchoring of a nickel (II) Schiff base complex onto activated carbon mediated by cyanuric chloride. Microporous Mesoporous Mater. 2001, 46, 211–221. [Google Scholar] [CrossRef]
- Theamwong, N.; Intarabumrung, W.; Sangon, S.; Aintharabunya, S.; Ngernyen, Y.; Hunt, A.J.; Supanchaiyamat, N. Activated carbons from waste Cassia bakeriana seed pods as high-performance adsorbents for toxic anionic dye and ciprofloxacin antibiotic remediation. Bioresour. Technol. 2021, 341, 125832. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Gupta, V.; Sharma, P. Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: Effect of activation conditions. J. Colloid Interface Sci. 2017, 493, 228–240. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Ponnuchamy, M. Performance of activated carbon derived from Cymbopogon winterianus distillation waste for scavenging of aqueous toxic anionic dye Congo red: Comparison with commercial activated carbon. Sep. Sci. Technol. 2020, 55, 1970–1983. [Google Scholar] [CrossRef]
- Alhogbi, B.G.; Altayeb, S.; Bahaidarah, E.; Zawrah, M.F. Removal of anionic and cationic dyes from wastewater using activated carbon from palm tree fiber waste. Processes 2021, 9, 416. [Google Scholar] [CrossRef]
- Extross, A.; Waknis, A.; Tagad, C.; Gedam, V.; Pathak, P. Adsorption of congo red using carbon from leaves and stem of water hyacinth: Equilibrium, kinetics, thermodynamic studies. Int. J. Environ. Sci. Technol. 2022, 1–38. [Google Scholar] [CrossRef]
- Ravenni, G.; Cafaggi, G.; Sárossy, Z.; Nielsen, K.R.; Ahrenfeldt, J.; Henriksen, U. Waste chars from wood gasification and wastewater sludge pyrolysis compared to commercial activated carbon for the removal of cationic and anionic dyes from aqueous solution. Bioresour. Technol. Rep. 2020, 10, 100421. [Google Scholar] [CrossRef]
- Paredes-Laverde, M.; Salamanca, M.; Diaz-Corrales, J.D.; Flórez, E.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the removal of an anionic dye in textile wastewaters by adsorption on ZnCl2 activated carbons from rice and coffee husk wastes: A combined experimental and theoretical study. J. Environ. Chem. Eng. 2021, 9, 105685. [Google Scholar] [CrossRef]
- Güzel, F.; Sayğılı, H.; Sayğılı, G.A.; Koyuncu, F. New low-cost nanoporous carbonaceous adsorbent developed from carob (Ceratonia siliqua) processing industry waste for the adsorption of anionic textile dye: Characterization, equilibrium and kinetic modeling. J. Mol. Liq. 2015, 206, 244–255. [Google Scholar] [CrossRef]
- Malik, P.K. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of Acid Yellow 36. Dye. Pigment. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Zubair, M.; Mu’azu, N.D.; Jarrah, N.; Blaisi, N.I.; Aziz, H.A.; A Al-Harthi, M. Adsorption behavior and mechanism of methylene blue, crystal violet, eriochrome black T, and methyl orange dyes onto biochar-derived date palm fronds waste produced at different pyrolysis conditions. Water Air Soil Pollut. 2020, 231, 240. [Google Scholar] [CrossRef]
- Periyaraman, P.M.; Karan, S.; Ponnusamy, S.K.; Vaidyanathan, V.; Vasanthakumar, S.; Dhanasekaran, A.; Subramanian, S. Adsorption of an anionic dye onto native and chemically modified agricultural waste. Environ. Eng. Manag. J. 2019, 18, 257–270. [Google Scholar]
- Bouhadjra, K.; Lemlikchi, W.; Ferhati, A.; Mignard, S. Enhancing removal efficiency of anionic dye (Cibacron blue) using waste potato peels powder. Sci. Rep. 2021, 11, 2090. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F.; Önal, Y. Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J. Clean. Prod. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Gupta, V. Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J. Colloid Interface Sci. 2009, 340, 16–26. [Google Scholar] [CrossRef]
- Landin-Sandoval, V.; Mendoza-Castillo, D.; Seliem, M.; Mobarak, M.; Villanueva-Mejia, F.; Bonilla-Petriciolet, A.; Navarro-Santos, P.; Reynel-Ávila, H. Physicochemical analysis of multilayer adsorption mechanism of anionic dyes on lignocellulosic biomasses via statistical physics and density functional theory. J. Mol. Liq. 2021, 322, 114511. [Google Scholar] [CrossRef]
- Demirbas, E.; Nas, M. Batch kinetic and equilibrium studies of adsorption of Reactive Blue 21 by fly ash and sepiolite. Desalination 2009, 243, 8–21. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Tanzifi, M.; Yaraki, M.T.; Kiadehi, A.D.; Hosseini, S.H.; Olazar, M.; Bharti, A.K.; Agarwal, S.; Gupta, V.K.; Kazemi, A. Adsorption of Amido Black 10B from aqueous solution using polyaniline/SiO2 nanocomposite: Experimental investigation and artificial neural network modeling. J. Colloid Interface Sci. 2018, 510, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, P.; Palaniyappan, M.; Priyadharshini, M.; Vignesh, A.; Thanjiappan, A.; Sebastina Anne Fernando, P.; Tanvir Ahmed, R.; Srinath, R. Adsorption of basic dye onto raw and surface-modified agricultural waste. Environ. Prog. Sustain. Energy 2014, 33, 87–98. [Google Scholar] [CrossRef]
- Iakovleva, E.; Sillanpää, M.; Maydannik, P.; Liu, J.T.; Allen, S.; Albadarin, A.B.; Mangwandi, C. Manufacturing of novel low-cost adsorbent: Co-granulation of limestone and coffee waste. J. Environ. Manag. 2017, 203, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Bukhari, S.N.A. Study of isothermal, kinetic, and thermodynamic parameters for adsorption of cadmium: An overview of linear and nonlinear approach and error analysis. Bioinorg. Chem. Appl. 2018, 2018, 3463724. [Google Scholar] [CrossRef]
- Dada, A.O.; Latona, D.F.; Ojediran, O.J.; Nath, O.O. Adsorption of Cu (II) onto bamboo supported manganese (BS-Mn) nanocomposite: Effect of operational parameters, kinetic, isotherms, and thermodynamic studies. J. Appl. Sci. Environ. Manag. 2016, 20, 409–422. [Google Scholar] [CrossRef]
- Kezerle, A.; Velic, N.; Hasenay, D.; Kovacevic, D. Lignocellulosic materials as dye adsorbents: Adsorption of methylene blue and Congo red on Brewers’ spent grain. Croat. Chem. Acta 2018, 91, 53–65. [Google Scholar] [CrossRef]
- Farzana, N.; Uddin, M.Z.; Haque, M.M.; Haque, A.N.M.A. Dyeability, kinetics and physico-chemical aspects of Bombyx mori muslin silk fabric with bi-functional reactive dyes. J. Nat. Fibers 2018, 17, 986–1000. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Hussain, M.; Smriti, S.A.; Siddiqa, F.; Farzana, N. Kinetic Study of Curcumin on Modal Fabric. Tekstilec 2018, 61, 27–32. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Hussain, M.; Siddiqa, F.; Haque, M.; Islam, G. Adsorption Kinetics of Curcumin on Cotton Fabric. Tekstilec 2018, 61, 76–81. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- El-Khaiary, M.I.; Malash, G.F.; Ho, Y.-S. On the use of linearized pseudo-second-order kinetic equations for modeling adsorption systems. Desalination 2010, 257, 93–101. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Ho, Y.-S. Isotherms for the sorption of lead onto peat: Comparison of linear and non-linear methods. Pol. J. Environ. Stud. 2006, 15, 81–86. [Google Scholar]
- Perez-Ameneiro, M.; Vecino, X.; Cruz, J.; Moldes, A. Wastewater treatment enhancement by applying a lipopeptide biosurfactant to a lignocellulosic biocomposite. Carbohydr. Polym. 2015, 131, 186–196. [Google Scholar] [CrossRef]
- Islam, G.N.; Ke, G.; Haque, A.N.M.A.; Islam, A. Effect of ultrasound on dyeing of wool fabric with acid dye. Int. J. Ind. Chem. 2017, 8, 425–431. [Google Scholar] [CrossRef][Green Version]
- Roy, A.; Adhikari, B.; Majumder, S. Equilibrium, kinetic, and thermodynamic studies of azo dye adsorption from aqueous solution by chemically modified lignocellulosic jute fiber. Ind. Eng. Chem. Res. 2013, 52, 6502–6512. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Farzana, N.; Smriti, S.; Islam, M. Kinetics and thermodynamics of silk dyeing with turmeric extract. AATCC J. Res. 2018, 5, 8–14. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Badawy, A.A.; Essawy, H.A. Improvement of dyes removal from aqueous solution by Nanosized cobalt ferrite treated with humic acid during coprecipitation. J. Nanostructure Chem. 2019, 9, 281–298. [Google Scholar] [CrossRef]



| Resources | Particle Size (µm) | Dye | qmax (mg/g) | Reference |
|---|---|---|---|---|
| Waste tea residue | 0.3475 | Acid Blue 25 | 127.14 | [31] |
| Date stones and jujube shells | 50–100 | Congo Red | 45.08–59.55 | [32] |
| Waste banana pith | >53 | Direct Red | 5.92 | [33] |
| Waste banana pith | >53 | Acid Brilliant Blue | 4.42 | [33] |
| Jujuba seeds | 53–150 | Congo Red | 55.56 | [34] |
| Cotton plant wastes | 75–500 | Remazol Black B | 35.7–50.9 | [35] |
| Lotus | <100 | Congo Red | 0.783–1.179 | [36] |
| Almond shell | 100–500 | Eriochrome Black T | 123.92 | [37] |
| Waste of corn silk | 250–500 | Reactive Blue 19 | 71.6 | [27] |
| Waste of corn silk | 250–500 | Reactive Red 218 | 63.3 | [27] |
| Banana peel, cucumber peel, and potato peel | 250–500 | Orange G | 20.9–40.5 | [30] |
| Eucalyptus bark | 250–700 | Solar Red BA | 43.5 | [17] |
| Eucalyptus bark | 250–700 | Solar Brittle Blue A | 49 | [17] |
| Saccharomyces cerevisiae (yeast) | 315–400 | Acid Red 14 | 18–23 | [38] |
| Mushroom waste | <400 | Direct Red 5B | 18 | [39] |
| Mushroom waste | <400 | Direct Black 22 | 15.46 | [39] |
| Mushroom waste | <400 | Direct Black 71 | 20.19 | [39] |
| Mushroom waste | <400 | Reactive Black 5 | 14.62 | [39] |
| Ash seed | ≤1000 | Cibacron Blue | 67.114 | [40] |
| Bean peel | ≤1000 | Cibacron Blue | 28.490 | [41] |
| Jute processing waste | 10,000 | Congo Red | 13.18 | [39,42] |
| Corn stigmata | Ground (size not mentioned) | Indigo Carmine | 63.7 | [39,43] |
| Cotton gin trash | Film form | Acid Blue 25 | 35.46 | [7] |
| Resources | Surface Area (m2/g) | Anionic Dye | qmax (mg/g) | Reference |
|---|---|---|---|---|
| Java citronella | Not reported | Congo Red | 4.29 | [77] |
| Palm tree waste | 648.90 | Congo Red | 10.4 | [78] |
| Water hyacinth | Not reported | Congo Red | 14.367 | [79] |
| Wastewater sludge | 98.8 | Amaranth | 19.6 | [80] |
| Coffee husk | 613 ± 14 | Indigo Carmine | 36.63 | [81] |
| Carob waste | 921.07 | Reactive Black 5 | 36.90 | [82] |
| Rick husk | 272 | Acid Yellow 36 | 86.9 | [83] |
| Pinecone | 878.07 | Alizarin Red S | 118.06 | [62] |
| Vegetable waste | Not reported | Eriochrome Black T | 120.50 | [28] |
| Date palm fronds | 431.82 | Methyl Orange | 163.132 | [84] |
| Sawdust | 516.3 | Acid Yellow 36 | 183.8 | [83] |
| Hazelnut bagasse | 1489 | Acid Blue 350 | 450 | [61] |
| Psyllium stalks | Not reported | Coomassie Brilliant Blue | 237.2 | [83,85] |
| Waste potato peels | Not reported | Cibacron Blue | 270.3 | [83,86] |
| Pulp waste | 1022.46 | Methyl Orange | 285.71 | [60,85] |
| Date palm fronds | 431.82 | Eriochrome Black T | 309.59 | [84] |
| Grape waste | 1455 | Acid yellow 36 | 386 | [84,87] |
| Pink shower | 283.4 | Congo Red | 970 | [75] |
| Modification Technique | Dye | Adsorbent | R2 | Reference | |
|---|---|---|---|---|---|
| Langmuir Model | Freundlich Model | ||||
| Mechanical | Congo Red | Jujuba seeds | 0.999 | 0.987–0.989 | [34] |
| Mechanical | Reactive Black 5 | Spent mushroom waste | 0.997 | 0.907 | [39] |
| Mechanical | Cibacron Blue | Ash seed | 0.9357 | 0.98 | [40] |
| Mechanical | Cibacron Blue | Bean peel | 0.9822 | 0.9414 | [41] |
| Mechanical | Direct Red 5B | Spent mushroom waste | 0.998 | 0.918 | [39] |
| Mechanical | Direct Black 22 | Spent mushroom waste | 0.996 | 0.905 | [39] |
| Mechanical | Acid Blue 25 | Waste tea residue | 0.9197–0.9309 | Not reported | [31] |
| Mechanical | Congo Red | Bottom ash and deoiled soya | 0.79–0.99 | 0.85–0.97 | [88] |
| Mechanical | Congo Red | Jute processing Waste | 0.9916 | 0.9912 | [42] |
| Chemical | Congo Red | Sawdust | 0.9418–0.9867 | 0.9040–0.9876 | [50] |
| Chemical | Congo Red | Rice husk | 0.9939–0.9988 | 0.9672–0.9861 | [52] |
| Chemical | Congo Red | Palm tree fiber waste | 0.996 | 0.857 | [78] |
| Chemical | Congo Red | Coffee Waste | 0.99 | 0.67 | [18] |
| Chemical | Diamine Green B | Rice husk | 0.9209–0.9731 | 0.8085–0.8988 | [52] |
| Chemical | Reactive Black 5 | Palm kernel Shell | 0.901 | 0.854 | [53] |
| Chemical | Reactive Black 5 | Coffee Waste | 0.96 | 0.93 | [18] |
| Chemical | Reactive Black 5 | Fermentation Wastes | 0.994–0.999 | Not reported | [56] |
| Chemical | Acid Blue 25 | Cotton gin trash | 0.967–0.996 | 0.875–0.947 | [7] |
| Chemical | Direct Red 89 | Aquatic Plant | 0.995 | 0.999 | [54] |
| Chemical | Acid Black 24 | Rice husk | 0.9887–0.9975 | 0.9715–0.9823 | [52] |
| Thermal | Eriochrome Black T | Date palm Fronds | 0.694–0.953 | 0.711–0.975 | [84] |
| Thermal | Congo Red | Java Citronella | 0.904–0.957 | 0.994–0.999 | [77] |
| Thermal | Eriochrome Black T | Vegetable Waste | 0.853–0.978 | 0.965–0.996 | [28] |
| Adsorbent | Modification Technique | Dye | ΔH° (kJ/mol) | ΔG° (kJ/mol) | ΔS° (J/mol K) | References |
|---|---|---|---|---|---|---|
| Bean peel | Mechanical | Cibacron Blue | −32.36 | −3.97 to −4.89 | −92.21 | [41] |
| Ash seed | Mechanical | Cibacron Blue | −28.95 | −18.01 to −18.37 | −35.36 | [40] |
| Spent mushroom waste | Mechanical | Direct Red 5B | 0.99 | −0.79 to −1.83 | 1.63 | [39] |
| Spent mushroom waste | Mechanical | Direct Black 22 | 0.59 | −0.69 to −1.33 | 1.65 | [39] |
| Spent mushroom waste | Mechanical | Direct Black 71 | 3.79 | −3.32 to −7.47 | 7.02 | [39] |
| Spent mushroom waste | Mechanical | Reactive Black 5 | 0.62 | −0.15 to −0.77 | 0.21 | [39] |
| Vegetable waste | Mechanical | Eriochrome Black T | 44.51 | −2.72 to −5.89 | 158.51 | |
| Jujuba seeds | Mechanical | Congo Red | 12.94 | −3.49 to −6.43 | 57.9 | [34] |
| Sawdust | Chemical | Acid Red G | 28.7 | −1.07 to −3.14 | 103.4 | [45] |
| Orange peel | Chemical | Congo Red | 19 | −1.88 to −3.23 | 70 | [16] |
| Peanut husk | Chemical | Drimarine Black CL-B | −23.74 | −0.22 to −2.9 | 72 | [55] |
| Coffee waste | Chemical | Reactive Black 5 | 8.28 | −9.66 to −11.08 | 59.99 | [18] |
| Coffee waste | Chemical | Congo Red | 35.05 | −3.76 to −8.17 | 130.59 | [18] |
| Rice husk | Chemical | Diamine Green B | 15.06 | −3.46 to −4.39 | 35.11 | [52] |
| Rice husk | Chemical | Acid Black 24 | 16.32 | −2.65 to −3.10 | 43.52 | [52] |
| Rice husk | Chemical | Congo Red | 2.96 | −2.98 to −3.37 | 19.57 | [52] |
| Wheat straw | Chemical | Reactive Red 24 | 4.53 | −29.05 to −32.93 | 135.1 | [12] |
| Waste coir pith | Chemical | Procion Orange | 25.69 | −8.43 to −11.15 | 111.52 | [51] |
| Waste coir pith | Chemical | Acid Brilliant Blue | 70.09 | −5.65 to −11.37 | 245.64 | [51] |
| Water hyacinth | Thermal | Congo Red | −189.01 to −918.94 | −3001.04 to −12,254.1 | 16.39 to 53.97 | [79] |
| Coffee husk | Thermal | Indigo Carmine | 21.72 to 35.83 | −46.71 to −61.05 | 229.9 to 287.6 | [81] |
| Java citronella | Thermal | Congo Red | −0.007 to −0.029 | 7.9 to 30.2 | −6 to −92 | [77] |
| Pinecone | Thermal | Alizarin Red S | 29.13 | −2.75 to −4.39 | 82 | [62] |
| Vegetable waste | Thermal | Eriochrome Black T | −10.08 | −2.76 to 3.43 | −22.32 | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, A.N.M.A.; Sultana, N.; Sayem, A.S.M.; Smriti, S.A. Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review. Sustainability 2022, 14, 11098. https://doi.org/10.3390/su141711098
Haque ANMA, Sultana N, Sayem ASM, Smriti SA. Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review. Sustainability. 2022; 14(17):11098. https://doi.org/10.3390/su141711098
Chicago/Turabian StyleHaque, Abu Naser Md Ahsanul, Nigar Sultana, Abu Sadat Muhammad Sayem, and Shamima Akter Smriti. 2022. "Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review" Sustainability 14, no. 17: 11098. https://doi.org/10.3390/su141711098
APA StyleHaque, A. N. M. A., Sultana, N., Sayem, A. S. M., & Smriti, S. A. (2022). Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review. Sustainability, 14(17), 11098. https://doi.org/10.3390/su141711098








