Sustainable Production of Biodiesel from Novel and Non-Edible Ailanthus altissima (Mill.) Seed Oil from Green and Recyclable Potassium Hydroxide Activated Ailanthus Cake and Cadmium Sulfide Catalyst
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Heterogeneous Catalyst
2.2.1. Calcined Ailanthus Cake (CAC)
2.2.2. KOH-Activated Ailanthus Cake (KAC)
2.2.3. Cadmium Sulphide Nanoparticles (CdS)
2.3. Biodiesel Synthesis Procedure
3. Results and Discussion
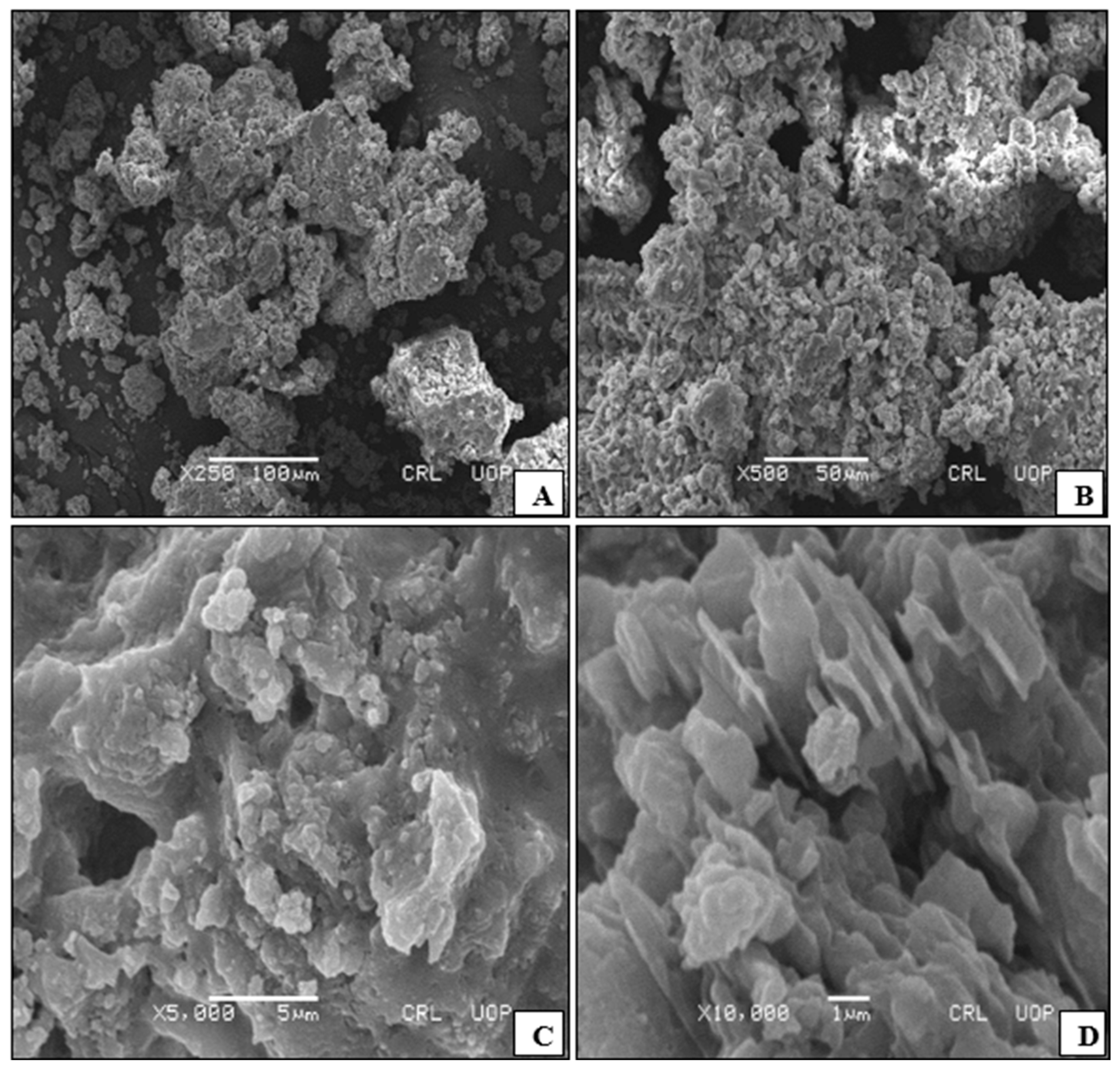
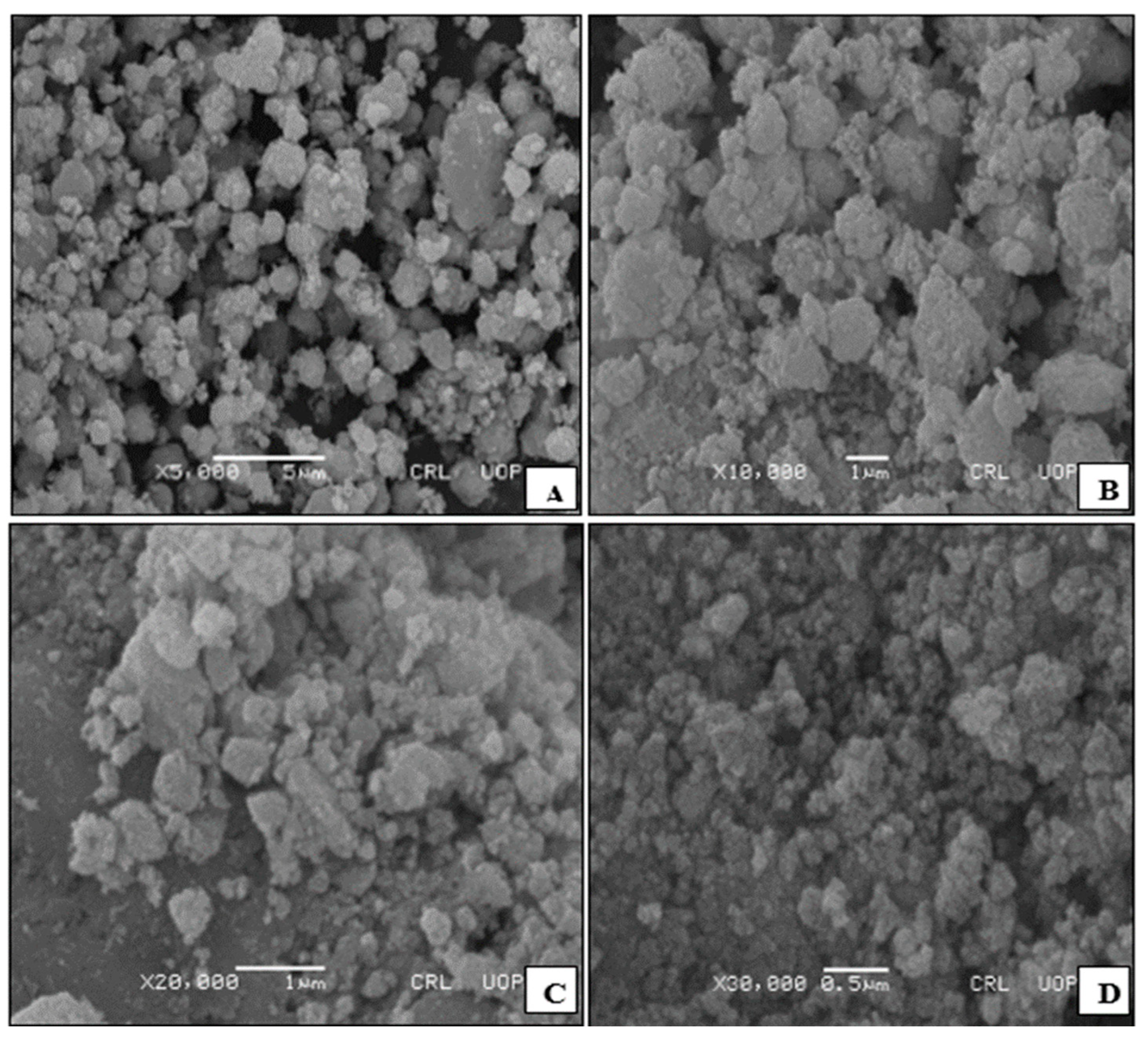
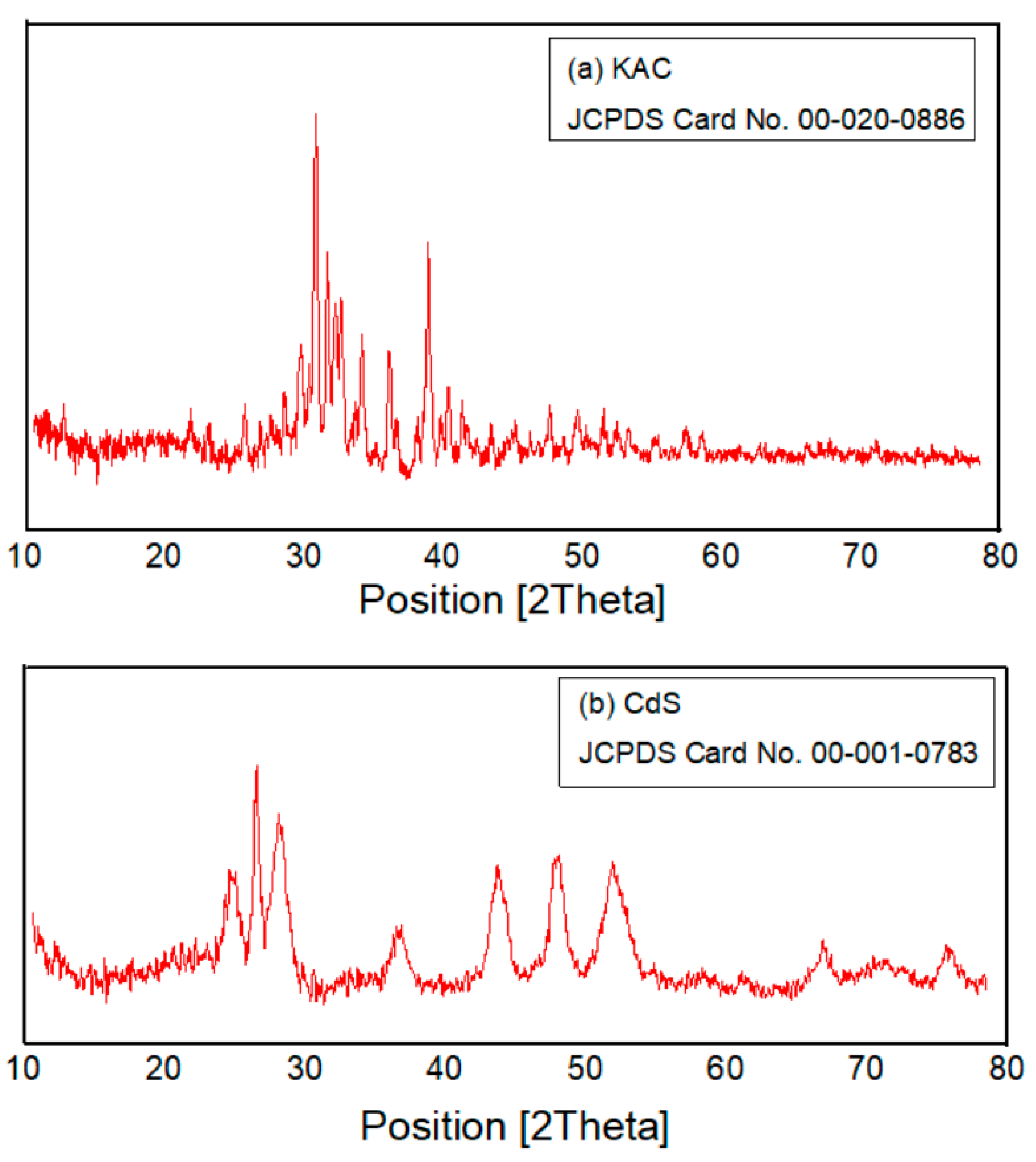
3.1. Characterisation of Catalysts
3.2. Biodiesel Synthesis Using Ailanthus altissima Seed Oil
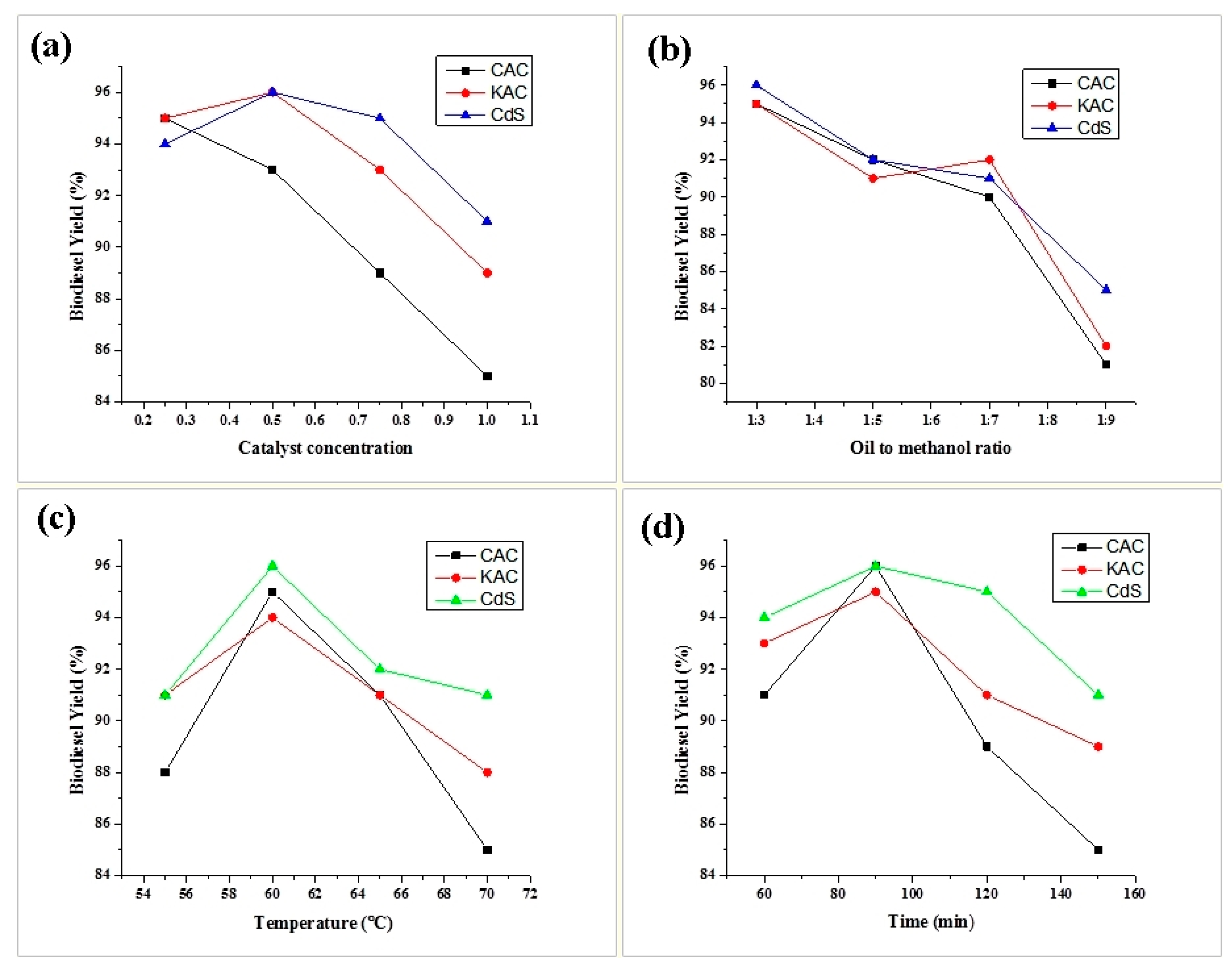
3.2.1. Optimisation of Biodiesel Yield
Effect of Catalyst Concentration on Biodiesel Yield
Effect of Alcohol-to-Oil Ratio on Biodiesel Yield
Effect of Reaction Temperature on Biodiesel Yield
Effect of Reaction Time on Biodiesel Yield
3.3. Fuel Properties of Biodiesel
3.4. Characterisation of Synthesised Biodiesel
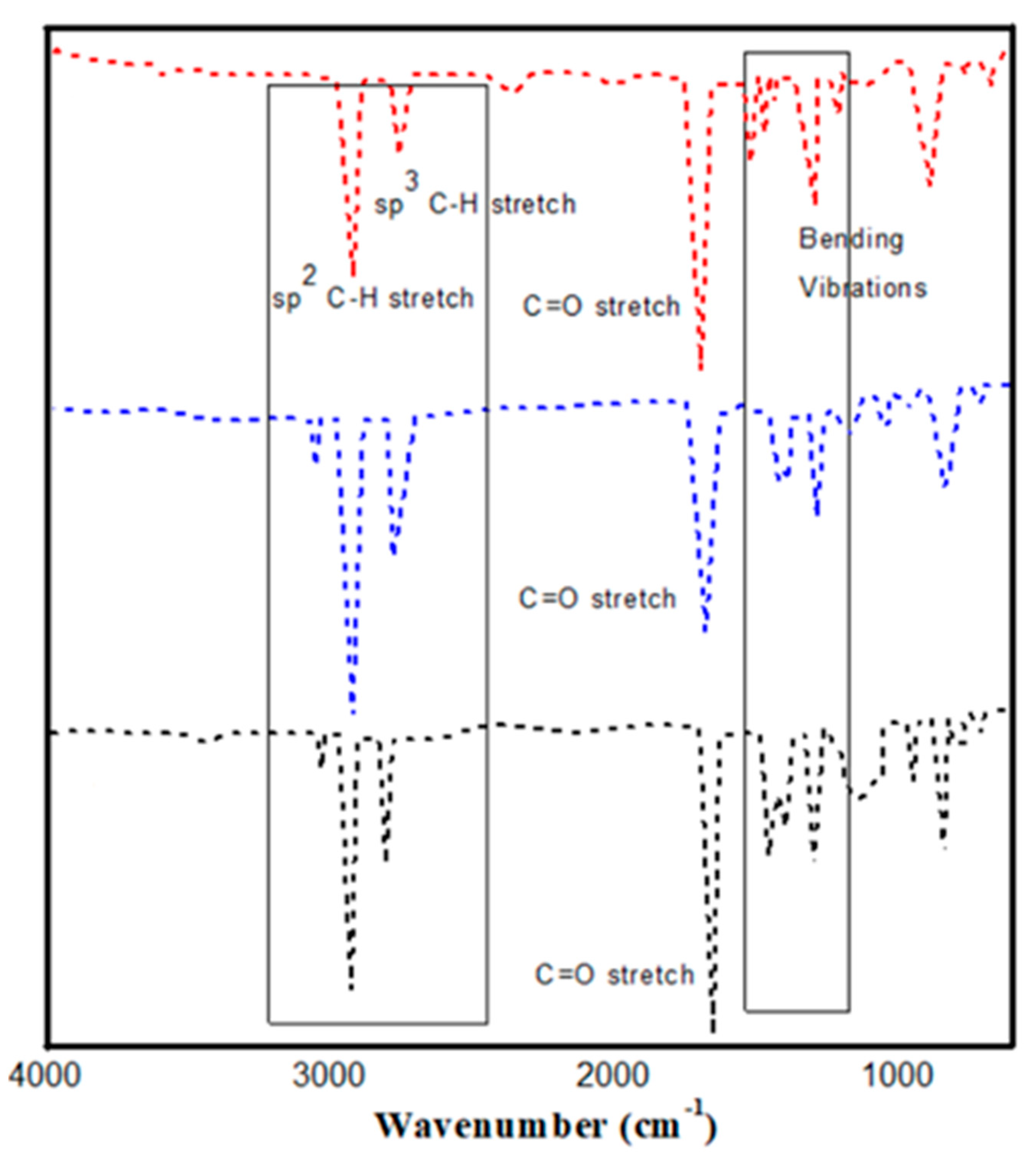
3.4.1. FTIR of Biodiesel
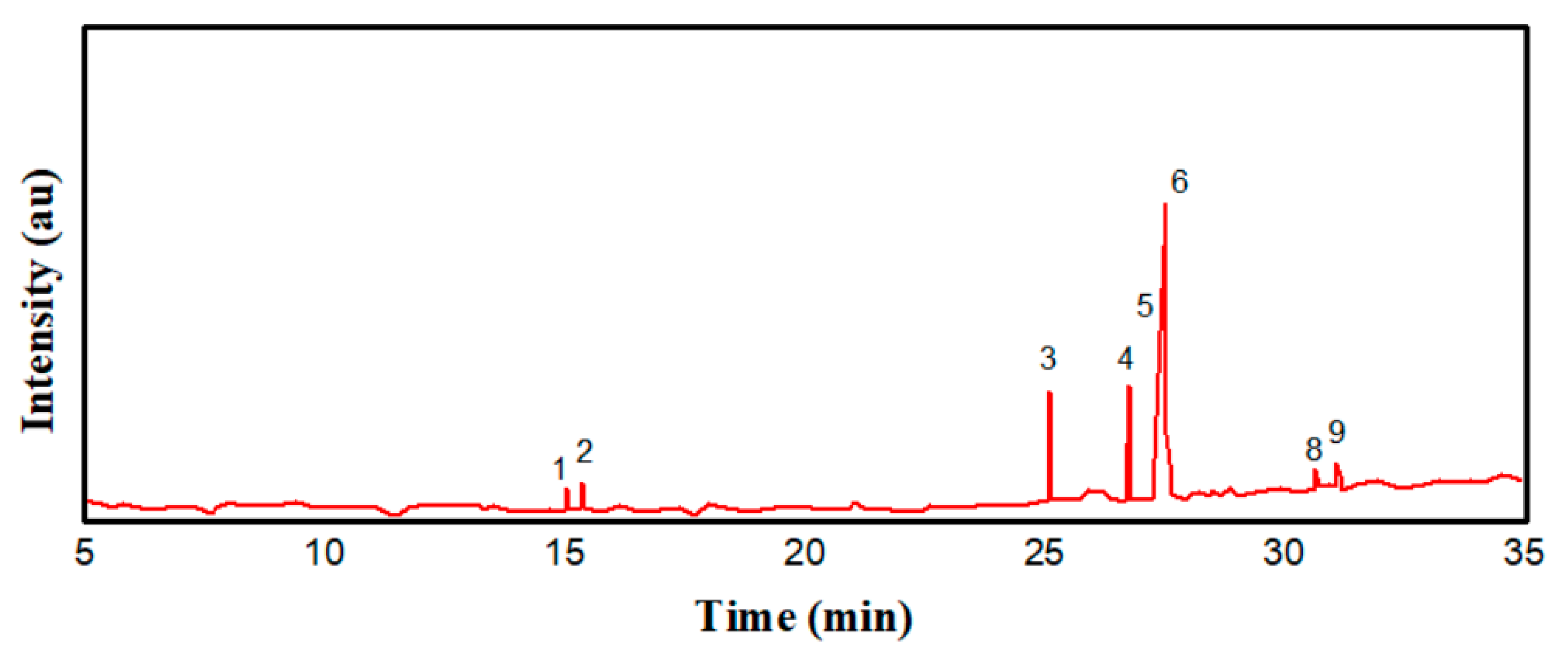
3.4.2. GCMS Analysis of Ailanthus altissima Oil Biodiesel
4. Conclusions
- The biodiesel (AAOBD) synthesis from highly efficient, novel non-edible seed oil of Ailanthus altissima (AAO) via a trans-esterification process is strongly recommended as a suitable source of biofuel on industrial scale due to its low cost, high productivity and eco-friendly nature.
- In the present study, promising yields of biodiesel with KAC and CdS (94.3 and 93.5%, respectively) were achieved.
- The optimum operating conditions for trans-esterification of Ailanthus altissima seed oil (AAO) are 3:1 methanol-to-oil molar ratio, 90 min reaction time, 60 °C temperature and 600 rpm. Therefore, it can be concluded that heterogeneous green catalysts are ideal to use due to their limited preparation time and cost-effective nature.
- This study is also optimistic towards efficient and reusable heterogeneous catalyst along with a sustainable oil source that establishes a way to reduce overall biodiesel production cost.
- Fuel properties of Ailanthus altissima biodiesel, such as specific gravity, flash point, pour point, kinematic viscosity, density, total acid number and sulphur content, were thoroughly investigated and met the requirements of international fuel standards. Low sulphur content of 0.0002 wt.% indicates a high value and pollution-free quality of the synthesised biodiesel.
- However, further investigation is suggested concerning the economic feasibility of AAO methyl ester in the fuel market. Furthermore, in the light of this investigation, kinetic and thermodynamic study on methyl ester formation from Ailanthus altissima seed oil and combustion characteristics, which are very sensitive to fuel quality, will be explored in future research.
Author Contributions
Funding
Conflicts of Interest
References
- Munir, M.; Saeed, M.; Ahmad, M.; Waseem, A.; Sultana, S.; Zafar, M.; Srinivasan, G.R. Optimization of novel Lepidium perfoliatum Linn. Biodiesel using zirconium-modified montmorillonite clay catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 44, 6632–6647. [Google Scholar] [CrossRef]
- Shah, S.H.; Raja, I.A.; Rizwan, M.; Rashid, N.; Mahmood, Q.; Shah, F.A.; Pervez, A. Potential of microalgal biodiesel production and its sustainability perspectives in Pakistan. Renew. Sustain. Energy Rev. 2018, 81, 76–92. [Google Scholar] [CrossRef]
- Chaichan, M.T. Performance and emission characteristics of CIE using hydrogen, biodiesel, and massive EGR. Int. J. Hydrogen Energy 2018, 43, 5415–5435. [Google Scholar] [CrossRef]
- Kim, D.S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. Energy 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Nizami, A.-S.; Sultana, S.; Zafar, M.; Rehan, M.; Srinivasan, G.R.; Ali, A.M.; et al. Biodiesel production from novel non-edible caper (Capparis spinosa L.) seeds oil employing Cu–Ni doped ZrO2 catalyst. Renew. Sustain. Energy Rev. 2021, 138, 110558. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Rehan, M.; Nizami, A.-S.; Zafar, M.; Arshad, M.; Sultana, S. Sustainable production of bioenergy from novel non-edible seed oil (Prunus cerasoides) using bimetallic impregnated montmorillonite clay catalyst. Renew. Sustain. Energy Rev. 2019, 109, 321–332. [Google Scholar] [CrossRef]
- Crespi-Perellino, N.; Guicciardi, A.; Malyszko, G.; Arlandini, E.; Ballabio, M.; Minghetti, A. Occurrence of indole alkaloids in Ailanthus altissima cell cultures. J. Nat. Prod. 1986, 49, 1010–1014. [Google Scholar] [CrossRef]
- Changmai, B.; Vanlalveni, C.; Ingle, A.P.; Bhagat, R.; Rokhum, L. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef]
- Nasreen, S.; Liu, H.; Khan, R.; Zhu, X.C.; Skala, D. Transesterification of soybean oil catalyzed by Sr-doped cinder. Energy Convers. Manag. 2015, 95, 272–280. [Google Scholar] [CrossRef]
- Nasreen, S.; Liu, H.; Skala, D.; Waseem, A.; Wan, L. Preparation of biodiesel from soybean oil using La/Mn oxide catalyst. Fuel Process. Technol. 2015, 131, 290–296. [Google Scholar] [CrossRef]
- Nasreen, S.; Liu, H.; Qureshi, L.A.; Sissou, Z.; Lukic, I.; Skala, D. Cerium-manganese oxide as catalyst for transesterification of soybean oil with subcritical methanol. Fuel Process. Technol. 2016, 148, 76–84. [Google Scholar]
- Shaheen, A.; Sultana, S.; Lu, H.; Ahmad, M.; Asma, M.; Mahmood, T. Assessing the potential of different nano-composite (MgO, Al2O3 -CaO and TiO2) for efficient conversion of Silybum eburneum seed oil to liquid biodiesel. J. Mol. Liq. 2018, 249, 511–521. [Google Scholar] [CrossRef]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Ali, R.M. Smart utilization of jatropha (Jatropha curcas Linnaeus) seeds for biodiesel production: Optimization and mechanism. Ind. Crops Prod. 2018, 111, 407–413. [Google Scholar]
- Goud, B.S.; Suresh, Y.; Annapurna, S.; Singh, A.; Bhikshamaiah, G. Green Synthesis and Characterization of Cadmium Sulphide Nanoparticles. Mater. Today Proc. 2016, 3, 4003–4008. [Google Scholar]
- Rozina Asif, S.; Ahmad, M.; Zafar, M.; Ali, N. Prospects and potential of fatty acid methyl esters of some non-edible seed oils for use as biodiesel in Pakistan. Renew. Sustain. Energy Rev. 2017, 74, 687–702. [Google Scholar]
- Adepoju, T.F. Optimization processes of biodiesel production from pig and neem (Azadirachta indica a. Juss) seeds blend oil using alternative catalysts from waste biomass. Ind. Crops Prod. 2020, 149, 11. [Google Scholar]
- Sadia, H.; Ahmad, M.; Zafar, M.; Sultana, S.; Azam, A.; Khan, M.A. Variables effecting the optimization of non edible wild safflower oil biodiesel using alkali catalyzed transesterification. Int. J. Green Energy 2013, 10, 53–62. [Google Scholar] [CrossRef]
- Westermann, P.; Jørgensen, B.; Lange, L.; Ahring, B.K.; Christensen, C.H. Maximizing renewable hydrogen production from biomass in a bio/catalytic refinery. Int. J. Hydrogen Energy 2007, 32, 4135–4141. [Google Scholar] [CrossRef]
- Yasin, G.; Bhanger, M.I.; Ansari, T.M.; Naqvi, S.M.S.R.; Talpur, F.N. Quality of commercial high speed diesel and its environmental impact. J. Pet. Technol. Altern. Fuels 2012, 3, 29–35. [Google Scholar]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste cooking oil as an alternate feedstock for biodiesel production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, M.; Muraleedharan, C. A comparative study of vegetable oil methyl esters (biodiesels). Energy 2011, 36, 2129–2137. [Google Scholar]
- Anwar, F.; Rashid, U.; Ashraf, M.; Nadeem, M. Okra (Hibiscus esculentus) seed oil for biodiesel production. Appl. Energy 2010, 87, 779–785. [Google Scholar]


| S. No. | Tests | Method | Ailanthus altissima Biodiesel (Current Study) | American (ASTM D-6751) | European EN-14214 | China GB/T 20828-2007 |
|---|---|---|---|---|---|---|
| 1. | Color | Visual | 2 | _ | _ | _ |
| 2. | Flash Point °C (PMCC) | ASTM D-93 | 72.6 | |||
| 3. | Density at 15 °C Kg/L | ASTM D-1298 | 0.800 | _ | 0.86–0.89 g/m3 | _ |
| 4. | Kinematic Viscosity at 40 °C cSt | ASTM D-445 | 4.12 | 1.9–6.0 | 3.4–5.0 | _ |
| 5. | Pour Point °C | ASTM D-97 | −8 | |||
| 6. | Cloud Point °C | ASTM D-2500 | −11.33 | _ | _ | _ |
| 7. | Sulphur wt.% | ASTM D-4294 | 0.0002 | _ | _ | _ |
| 8. | Total Acid No. mg KOH/gm | ASTM D-974 | 0.162 | 0.020 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabeen, M.; Munir, M.; Abbas, M.M.; Ahmad, M.; Waseem, A.; Saeed, M.; Kalam, M.A.; Zafar, M.; Sultana, S.; Mohamed, A.; et al. Sustainable Production of Biodiesel from Novel and Non-Edible Ailanthus altissima (Mill.) Seed Oil from Green and Recyclable Potassium Hydroxide Activated Ailanthus Cake and Cadmium Sulfide Catalyst. Sustainability 2022, 14, 10962. https://doi.org/10.3390/su141710962
Jabeen M, Munir M, Abbas MM, Ahmad M, Waseem A, Saeed M, Kalam MA, Zafar M, Sultana S, Mohamed A, et al. Sustainable Production of Biodiesel from Novel and Non-Edible Ailanthus altissima (Mill.) Seed Oil from Green and Recyclable Potassium Hydroxide Activated Ailanthus Cake and Cadmium Sulfide Catalyst. Sustainability. 2022; 14(17):10962. https://doi.org/10.3390/su141710962
Chicago/Turabian StyleJabeen, Munazza, Mamoona Munir, Muhammad Mujtaba Abbas, Mushtaq Ahmad, Amir Waseem, Muhammad Saeed, Md Abul Kalam, Muhammad Zafar, Shazia Sultana, Abdullah Mohamed, and et al. 2022. "Sustainable Production of Biodiesel from Novel and Non-Edible Ailanthus altissima (Mill.) Seed Oil from Green and Recyclable Potassium Hydroxide Activated Ailanthus Cake and Cadmium Sulfide Catalyst" Sustainability 14, no. 17: 10962. https://doi.org/10.3390/su141710962
APA StyleJabeen, M., Munir, M., Abbas, M. M., Ahmad, M., Waseem, A., Saeed, M., Kalam, M. A., Zafar, M., Sultana, S., Mohamed, A., & Chaudhry, B. (2022). Sustainable Production of Biodiesel from Novel and Non-Edible Ailanthus altissima (Mill.) Seed Oil from Green and Recyclable Potassium Hydroxide Activated Ailanthus Cake and Cadmium Sulfide Catalyst. Sustainability, 14(17), 10962. https://doi.org/10.3390/su141710962









