Abstract
The combination of high hydrostatic pressure (HHP) and natural antimicrobials can present an interesting efficiency in the decontamination process of cured meat. However, several factors, such as application method and antimicrobial type, must be better understood to improve the process and its total employment. Therefore, this study aimed to evaluate the combined effect (synergism or antagonism) of HHP and natural antimicrobials to inactivate pathogenic and spoilage bacteria in cured meat. After a systematic search of research articles in the databases (Web of Science, Scopus, PubMed, and Science Direct), 20 articles were eligible and resulted in 123 studies for meta-analysis. The effect on Listeria sp., Salmonella serovars, E. coli O157:H7, and total viable counts was investigated considering different application methods (spread onto the surface, incorporated into the product, and active packaging) and antimicrobial types (plant, bacterial and animal origins). Active packs showed the best synergy with HHP, exhibiting a mean effect of 0.78 (CI95: 0.25–1.32) log cfu/g. Antimicrobials of microbial origin (bacteriocins) were more effective in combination with HHP. In addition, Listeria sp. was the most sensitive bacterium considering all investigated combined methods. Hence, the use of natural antimicrobials has the potential to improve the decontamination process when HHP is applied.
1. Introduction
Cured meats are generally delivered to the consumer in a ready-to-eat (RTE) form. Commonly, there is no heat application or other preparation step to kill microorganisms before consumption. Furthermore, handling, slicing, and packaging can lead to cross-contamination [1]. In cured meats, there are several barriers, such as starter cultures, low activity of water, salt and others [2,3,4,5]. However, even though they have another barrier against microbial contamination provided by the curing agents (nitrite and nitrate), those are more effective in inactivating specific pathogenic bacteria and decreasing counts of spoilage microorganisms [6]. In this sense, it can be observed that cured meat products are still a challenge regarding food safety [7].
In recent years, many new conservation technologies have been gaining space in the food industry. High hydrostatic pressure (HHP) is an emerging non-thermal preservation method based on a pressure chamber containing a compression fluid, generally water or alcohol solution, wherein high pressures are applied to inactivate bacteria [8,9]. Usually, food is packed before HHP processing, and thus it can be helpful to treat cross-contamination that happens after the food is processed, such as slicing and packaging. HHP was developed as an alternative to thermal processing, and its mechanism attacks and injures cell walls denaturing important proteins to the microorganism’s metabolism and causing several problems to permeability, transport systems, and cell functionality, which lead to microorganism’s inactivation [10]. HHP efficacy depends on several factors concerning treatment conditions, such as pressure level, time and temperature, and intrinsic properties of the treated meat product, such as initial microbial load and water activity [9,11]. Aside from showing promising results for pathogenic and spoilage bacteria in different types of foods [12,13,14,15], it may be achieved with no adverse effects on original quality when adequate HHP conditions are applied [16]. Since the pressure is applied in isostatic form, it is uniformly distributed throughout the product, which does not deform it and guarantees that the whole food is processed [17]. Furthermore, HHP can be recognized as a waste-free environmentally friendly technology, which means it does not leave any residues that need treatment [18], and its operating costs are similar to conventional thermal processes [10].
Another preservation method becoming more prominent in food production is using antimicrobials of natural origin. This is due to the growing trend in consumers choosing minimally processed foods with a more natural appeal over synthetic antimicrobials [19]. Generally, they are natural defenses created to protect against microorganisms [20], and their efficacy depends on the target microorganism, including its initial microbial load, extrinsic factors (e.g., temperature and atmosphere composition), and food intrinsic properties since each one has specific conditions of water activity, pH and other characteristics [21]. The application form of the antimicrobials, most commonly spreading onto the surface, incorporated in the product or active packaging, is another determinant factor affecting bacterial inactivation on an immediate level.
The mechanism of action of natural antimicrobials is highly variable according to their origin (most commonly plant, animal, or microbial) [22,23]. Microbial-based antimicrobials can be bacteriocins (peptides) or other substances, such as potassium lactate, that bacteria produce to inhibit the growth of other microorganisms [24,25]. Animal-based antimicrobials are mostly enzymes and polysaccharides, but can be any other substance of antimicrobial activity extracted from animals or their secretions. In plant-based antimicrobials, they can assume many forms, such as essential oils, pure substances extracted from plants (e.g., thymol), and mixing substances extracted from plants with some possessing antimicrobial activity [26,27].
Combining different preservation technologies is a good alternative to achieve a final inactivation that can maintain food safety throughout storage. Combining two or more conservation methods creates a hurdle effect and represents an additional barrier for microbial growth; consequently, preserving food for extended periods [28,29]. Overall, combinations of different conservation techniques, such as HHP + ultrasound [30], ultrasound + UV-Vis [31], and ultrasound + antimicrobials [32], are showing promising results in eliminating microorganisms. Likewise, the combination of high hydrostatic pressure and natural antimicrobials has been studied in recent years, and it has been showing successful findings in eliminating microorganisms. However, the complete understanding of the combined effect remains to be elucidated. Thus, this study aimed to collect and analyze data from different articles published to determine the type of antimicrobial and its optimal application to maximize the inactivation of pathogenic and spoilage microorganisms induced by HHP in cured meat products. It is crucial to enable the industrial application of these combined technologies. For bacterial inactivation, we believe that combining high hydrostatic pressure and natural antimicrobials is more advantageous than these individual methods. However, several factors can affect the effectiveness of the combination.
2. Materials and Methods
2.1. Data Search
The present study systematically collected and evaluated all available data in four different databases regarding the role of high hydrostatic pressure in combination with natural antimicrobials on microbial inactivation in cured meat. A publication period has not been previously defined. The papers found in this study were published between 2007 and 2021. This systematic search followed the four-phase flow diagram from Preferred Reporting Items for Systematic Review and Meta-Analyses statement (PRISMA) guidelines [33] to identify, screen, and evaluate the adequacy of the available literature on the theme approached in the study. A predefined search protocol with the computational tool State of the Art through Systematic Review (StArt) was used to assist the selection and extraction of studies [34].
2.2. Focus Questions
The focus questions agree on the population, intervention, comparison, and outcome method (PICO), where we have: (P) microorganisms that contaminate cured meat, (I) combination of high hydrostatic pressure and natural antimicrobials, (C) what type (alone or combined) of treatment was more effective in inactivating targeted microorganisms, and (O) what type (synergistic or antagonistic) of effect natural antimicrobials combined with HHP have in cured meat according to the biological and methodological elements. Beyond the last outcome-based question, this study included the following research questions about cured meat: What type of natural preservative presents the best antimicrobial activity when combined with HHP? What microorganism is affected the most by the combination of treatments? What type of antimicrobial application is most effective in inactivating targeted microorganisms?
2.3. Screening, Eligibility Criteria, and Inclusion
This systematic search of the literature used the exact search string in four different electronic databases: Scopus, Web of Science, ScienceDirect, and PubMed. The screening process took place in May 2022, and potential sources of bias assessment included the choice of keywords for the search string, inclusion/exclusion criteria, and possible missing data (Figure 1).
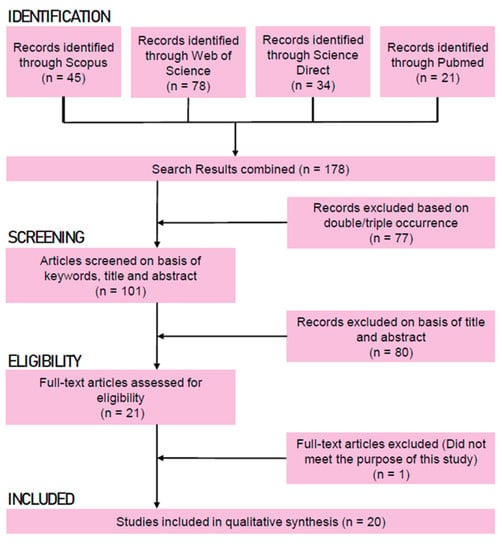
Figure 1.
Prisma flow diagram displaying results of the systematic search. Any paper that could not be eliminated through the process of screening went through the full-text reading.
Aside from summarizing the questions approached in the study, keywords and their synonyms were considered for building search strings to maximize the number of papers retrieved during the search. The strategy developed by Carvalho and Conte-Junior [35] focused on three main points: (i) identification of keywords considering the research questions; (ii) synonyms based on relevant studies about high hydrostatic pressure, natural antimicrobials, and synergy between treatments; and (iii) use of “AND” and “OR” Booleans operators. The search was based on predefined keywords taken from the inclusion criteria (“cured”, “high hydrostatic pressure”, “natural antimicrobials’’ and “combination”) and its synonyms. The search conducted on 6 May 2022, was restricted to original research articles published in English, and no period was established since this is the first meta-analysis about this subject. After the definition of the search strings, they were used in each database in the advanced search as below:
- Search component 1 (SC1): cured OR ham
- Search component 2 (SC2): “high hydrostatic pressure” OR “high pressure processing” OR “high pressure”
- Search component 3 (SC3): natural OR antimicrobial OR antioxidant OR extract OR bacteriocin
- Search component 4 (SC4): combined OR combination
Boolean operator “AND” was used to combine SC1, SC2, SC3, and SC4. The studies identified in each database were uploaded through StArt software, which automatically excluded the replicated papers. After that, a manual exclusion was carried out, and all the remaining articles were screened according to the eligibility criteria (Table 1) by reading the title, abstracts, and keywords.

Table 1.
Inclusion and exclusion criteria.
The search focused on studies that analyze the individual and combined effects of high-pressure processing and natural antimicrobials on bacterial inactivation in cured meat. Screening only considered original research articles while discarding reviews and similar papers with the assistance of the StArt tool [34]. The software sorted out and assessed studies based primarily on title, abstract, and keywords. Other inclusion and exclusion criteria are summarized in Table 1. Subsequently, articles within the eligibility criteria were fully read, and those that met the inclusion criteria were included in this study (n = 20, Figure 1 and Table 2). This study did not consider papers that did not compare the combined treatments with those applied alone in the safety of the products.

Table 2.
Description of the information contained in the articles chosen and used in the data analysis.
2.4. Data Extraction
In an excel sheet, the following treatments were divided into categories: (i) Antimicrobial, (ii) HHP, and (iii) HHP combined with antimicrobial. For each one, microbial counts and standard deviations before and after treatments were extracted and deducted from their control counterparts, aiming to calculate the log reduction of each treatment. Each paper investigated different factors, such as different microbial genera or different application methods. This way, each specific condition of one treatment accounted for an individual study with one effect value. Thus, one paper can be divided into two or more studies (results).
All articles analyzed the effect of the treatments on microbial reduction after application time between 0 and 24 h. The standard deviation for the inactivation was found with the square root of the sum of the squared deviations.
The following classifications for each study were further established to allow data meta-analysis: (i) antimicrobial origin (plant, bacterial or animal), (ii) treatment type (antimicrobial, HHP or combination), and (iii) application method of the antimicrobial (spread onto the surface, incorporated into the product or active packaging) (Table 2). From log reduction values (LRV) and their respective standard deviations, the effect type (synergistic or antagonistic) was calculated by the difference between the log reduction from combined treatments and the sum of the log reductions of both treatments alone. Studies of the most investigated microorganisms in the papers were used, these were Listeria sp., Salmonella serovars, E. coli and total viable count.
2.5. Statistical Analysis
LRV data were used to explore the combined effect of high hydrostatic pressure and natural antimicrobials. The studies were subdivided by (i) microorganism analyzed, (ii) origin of the antimicrobial, and (iii) type of antimicrobial application. The results were calculated using the random effect with a confidence interval of 95%. The overall effect was calculated with the Z test using a significance level of 0.05. The heterogeneity test was performed by I2 test. All data were analyzed using Software Review Manager 5.4. Data were presented in forest plots of each category with the effect values.
2.6. Risk of Bias Assessment
Some possible sources of bias can be cited, such as the inclusion/exclusion criteria, the chosen database, selected language, absence of data in the articles, missing primary data, and the number of papers selected for this study. Thus, it is possible that there is a low number and diversity of studies on a reported topic, such as low diversity of serovars and types of meat product. In addition, the studies were approached separately for some results with high heterogeneity or a low number of studies.
3. Results and Discussion
3.1. Characteristics of Data
The 20 papers selected resulted in 123 studies. Of these, 48 were referred to Listeria, 22 Salmonella, 14 E. coli O157:H7, and 22 total viable counts (TVC). Other microorganisms’ data were not presented because there were insufficient studies to assemble the forest plots and statistical analysis.
3.2. Listeria
Bacterial-based antimicrobials presented a synergistic effect when combined with HHP treatment (Figure 2). This could be related to the fact that bacteriocins have more efficiency against Gram-positive bacteria [51], such as Listeria, since most of the antimicrobials used in this section are bacteriocins. Moreover, this is a result of the mechanism of inactivation of these substances, which, in most cases, destroys the bacterial membrane [28]. A possible explanation of the synergistic effect in the combination is the effect of one method inactivating injured cells by the other method, caused by the sum of different inactivation mechanisms. Another factor may be that the HHP collaborates by pushing the antimicrobial into the cell and/or diffusing the compound in the food.
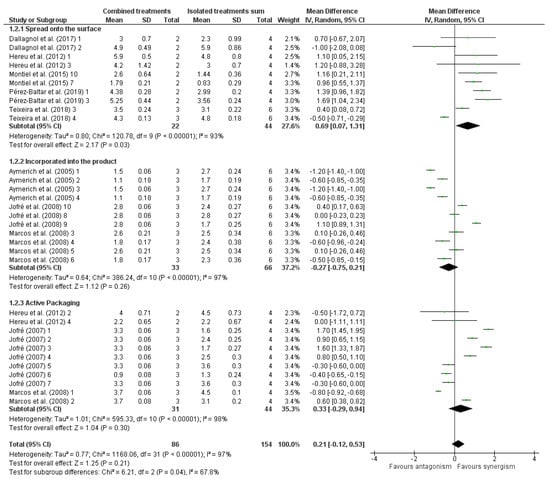
Figure 2.
Forest plot of studies that investigated the individual and combined effects of high hydrostatic pressure and bacterial-based antimicrobials for inactivation of Listeria sp. in cured meat [13,14,15,38,40,41,42,43,44,46].
When antimicrobial was spread onto the product’s surface, antimicrobials of bacterial origin combined with HPP had a synergistic effect on Listeria inactivation. On the other hand, when the antimicrobial was incorporated into the product, there was no synergistic effect (Figure 2). This could indicate more superficial contamination, which may be explained by most analyzed studies inoculating Listeria on the surface of the food. In addition, we suggest that the product’s internal environmental conditions may cause protective effects. The antimicrobial applied in the form of active packaging showed a synergistic effect when combined with HPP (Figure 2). AP system covers the food on a superficial level; thus, this finding may also be explained due to the superficial inoculation of the microorganism. Thus, this method may be promising in overcoming cross-contamination.
Overall, studies evaluating bacterial-based antimicrobials spread onto the surface of the product or added to active packaging in combination with HHP favored synergism, demonstrating a mean effect of 0.70 (CI95: 0.07–1.34) log cfu/g and 0.78 (CI95: 0.25–1.32) log cfu/g, respectively (Figure 2). When observing the AP system in cases of immediate inactivation, such as in the studies analyzed, only the microbicidal effect is relevant since the microbiostatic effect is observed throughout storage time. Considering these similar results, it can be suggested that the AP system possesses the same mechanism as spreading the antimicrobial on the surface.
If analyzing with all forms of application, the combination of antimicrobials of bacterial origin with HHP treatment favored synergism with an overall mean effect of 0.49 (CI95: 0.14–0.83) log cfu/g (Figure 2). With this, it can be concluded that these combined treatments effectively eliminated approximately 0.49 log CFU/g more than the sum of the isolated ones.
For plant-based antimicrobials spread onto the surface of the meat, Pérez-Baltar et al. [13] 2 and Teixeira et al. [14] 2 presented antagonism between treatments (Figure 3). In Pérez-Baltar et al. [13] 2, thymol alone inactivated Listeria similarly to combined treatments, which explains the negative effect value. Additionally, HHP treatment on its own was less effective than the antimicrobial. This result can be related to the fact that Gram-positive bacteria are less susceptible to HHP than Gram-negative ones [9,28]. Nevertheless, when looking at Teixeira et al. [14] 2, it can be observed that the HHP alone reduced Listeria more than the combined treatments (HHP + rosemary extract). It may be attributed to the difference in antimicrobial effectiveness between an isolated compound (thymol) and mixing compounds (rosemary extract). In other words, antimicrobial compounds are present in a smaller concentration in extracts and essential oils than isolated antimicrobial compounds. Similarly, in the study of Pavli et al. [49] 1, neither synergy nor antagonism could be observed for antimicrobials added to active packaging combined with HHP (Figure 3). This is justified by the fact that the oregano applied alone did not affect Listeria, and it combined with HHP similarly reduced Listeria counts. Aside from this, it did not present any synergism with the HHP treatment either, since combined treatments showed the same inactivation as high pressure alone. Furthermore, it is possible to suggest that previous exposure of Listeria to antimicrobial compounds, such as dual lactate salts, may cause a peizo-protective effect on HHP [52]. It is noteworthy that HHP causes alterations in molecular responses, in which Listeria monocytogenes increased the production of cold shock proteins (CSPs) after this treatment [53].
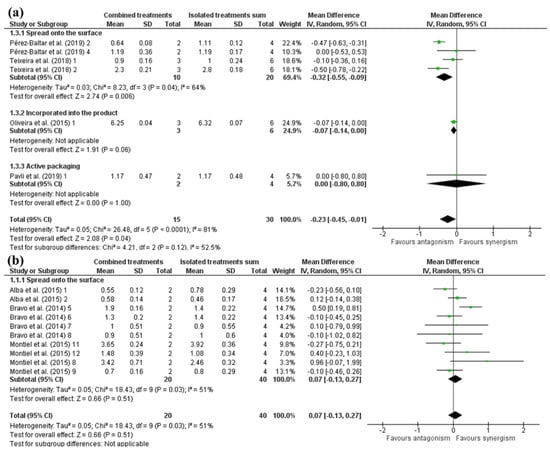
Figure 3.
Forest plot of studies that investigated the individual and combined effects of high hydrostatic pressure and (a) plant-based antimicrobials [13,14,48,49] and (b) animal-based antimicrobials for inactivation of Listeria sp. in cured meat [14,28,46].
Moreover, Pérez-Baltar et al. [13] 4 and Teixeira et al. [14] 1 did not present synergistic nor antagonistic effects, with mean values indicating an additive and a slight antagonistic effect, respectively. With this, the overall mean effect was −0.33 (CI95: −0.55; −10) log cfu/g, showing a prominent antagonistic effect for plant-based antimicrobials in combination with high-pressure treatment (Figure 3). Antimicrobials of plant origin need higher concentrations to achieve significant microbial inactivation than bacterial-based ones [9,28]. Moreover, adding them in large amounts is impossible because they tend to negatively alter the food flavor [9,54]. It makes these antimicrobial substances insufficient to inactivate microorganisms alone and in combination with HHP treatment when higher microbial counts are present.
In other forms of application, Oliveira et al. [48] 1 incorporated the antimicrobial on the product and observed a slight antagonism between treatments (mean effect = −0.07; Figure 3). This is because high-pressure levels (600 MPa) are enough to eliminate high Listeria inoculum levels compared to other studies with lower pressure levels, which could not eliminate Listeria at high load.
Antimicrobials of animal origin spread onto the surface and combined with HHP did not present a synergistic effect (Figure 3). This was caused by the high variability of results between the studies performed. Furthermore, antimicrobials of animal origin are enzymes, which can be denatured and lose their activity when submitted to a high-pressure level [55,56]. Studies that used lactoferrin demonstrated no or little inactivation when the antimicrobial was used alone, with antagonism in most cases for combined treatments. However, when looking at studies that used lactoperoxidase and higher HHP doses, it was noticeable a tendency to antagonism between treatments. In comparison, combined lactoperoxidase with lower HHP doses tended to synergize more.
3.3. Salmonella
Overall, no synergistic effect was observed when analyzing bacterial-based antimicrobials combined with HHP (Figure 4). As aforementioned, most of the substances used in these studies were bacteriocins, which are not as effective for reducing Gram-negative bacteria as Gram-positive ones.
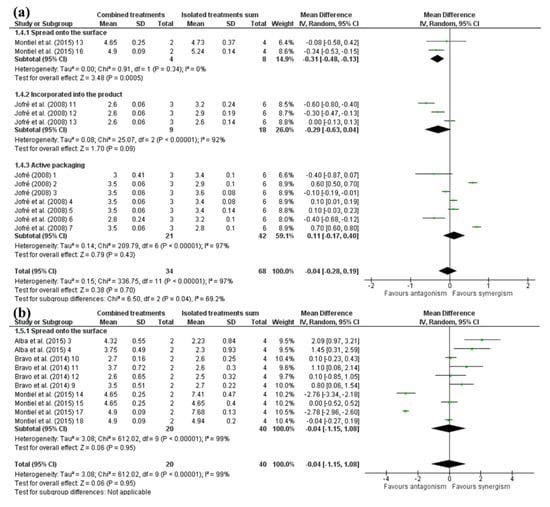
Figure 4.
Forest plot of studies that investigated the individual and combined effects of high hydrostatic pressure and (a) bacterial-based antimicrobials [24,42,46] or (b) animal-based antimicrobials for inactivation of Salmonella serovars in cured meat [12,39,46].
When antimicrobials of bacterial origin were spread onto the surface or incorporated into the product, their combination with HHP was antagonistic (Figure 4). This means that, in most cases, both HHP alone and its combination with these application forms of antimicrobials led to similar inactivation, indicating that the HHP alone is more recommended to inactivate Salmonella.
Otherwise, a tendency synergism was observed for HHP combined with antimicrobials added to active packaging (Figure 4), which may be attributed to the ineffectiveness of the antimicrobials alone in active packaging when looking at the immediate inactivation. In addition, it was observed that the antimicrobials of bacterial origin (enterocin, sakacin or nisin) did not inactivate Salmonella and still allowed for its growth when the effect was analyzed the next day after treatment applications. This is also in agreement that bacteriocins do not work as well in Gram-negative bacteria as in Gram-positive ones [28]. Moreover, active packaging effects are more apparent during product shelf life [57].
3.4. E. coli O157:H7
A possible synergism was observed for the antimicrobials of bacterial origin spread onto the surface with 0.68 (CI95: 0.06–1.31) log cfu/g (Figure 5). Despite this, an interesting difference was observed in isolated results depending on the antimicrobial compound used. Studies that used nisin, Alba et al. [36] 1 and 3, obtained a synergistic effect between HHP and the antimicrobial, while studies that utilized pediocin, Alba et al. [36] 2 and 4, showed a pronounced antagonism where E. coli O157:H7 inactivation was worse in combined treatments than HHP alone. The remaining studies used reuterin and also obtained an apparent antagonism, wherein HHP alone eliminated all the E. coli inoculum.
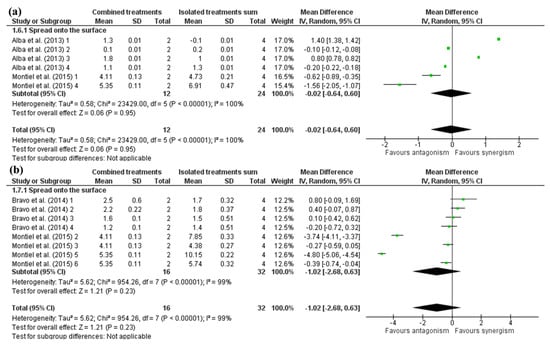
Figure 5.
Forest plot of studies that investigated the individual and combined effects of high hydrostatic pressure and (a) bacterial-based antimicrobials [36,46] or (b) animal-based antimicrobials for inactivation of E. coli O157:H7 in cured meat [39,46].
The same can be noted in some results from animal-based antimicrobials under surface application, such as Montiel et al. [46] 2,3,5 and 6 (lactoferrin and lactoperoxidase). Overall, no synergistic or antagonistic effect was observed (Figure 5), likely due to the low resistance of Gram-negative bacteria to HHP. HHP inactivates a large part of the microbial population, so the comparison with combined treatments has less difference. In addition, efficacy may be enzyme-dependent. Studies evaluating animal-based antimicrobials that used lactoperoxidase achieved more prominent synergism with HHP treatment than lactoferrin, which, in some cases, produced a visible antagonistic effect.
Differences in HHP inactivation between studies could also be due to different food matrices. The Alba et al. [36] studies used dry-cured ham, while the remaining ones evaluated cooked ham. Likewise, Bravo et al. [39] 1, 2, 3 and 4 used cured beef carpaccio, and total elimination of all inoculated E. coli load was not observed, while Montiel et al. [46] 2, 3, 5 and 6 used cooked ham and all E. coli inoculum was inactivated. From these findings, it can be suggested that E. coli O157:H7 are more susceptible to HHP in cooked ham than in other matrices. Syed et al. [58] reviewed that lower water activity in foods causes a protective effect against the effects of HHP. In this way, cooked hams have higher water activity and have more bacterial inactivation efficiency.
3.5. Total Viable Count
Bacterial-based antimicrobials combined with HHP were indicative of a synergistic effect (Figure 6) in an overall view. Under surface application, bacterial-based antimicrobials combined with HHP indicated synergism, while incorporating the substance into the product resulted in a possible antagonism between treatments indicated by Vercammen et al. [50] 4 (Figure 6). This finding agrees with the statement of Dallagnol et al. [15] that a larger part of the contamination in ham comes from cross-contamination during processes such as slicing or packaging, leading to more superficial contamination.
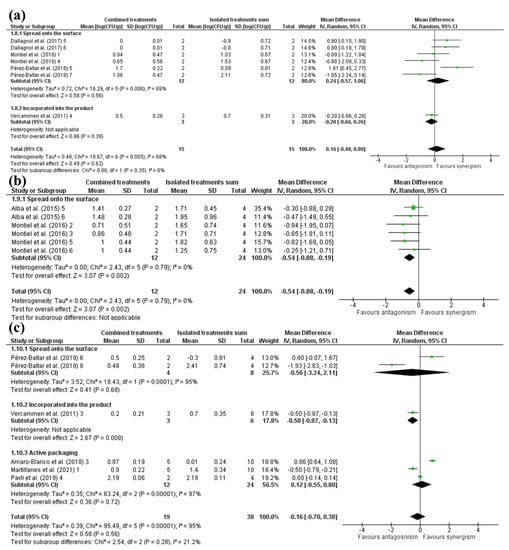
Figure 6.
Forest plot of studies that investigated the individual and combined effects of high hydrostatic pressure and (a) bacterial-based antimicrobials [13,15,47,50], (b) animal-based antimicrobials [12,47] or (c) plant-based antimicrobials for inactivation of total viable count (TVC) in cured meat [13,37,45,49,50].
Antimicrobials of animal origin spread onto the surface in combination with HHP showed an antagonism between treatments (Figure 6) (lactoferrin and lactoperoxidase), which may be explained by the ability of HHP to inactive enzymes, a compound used as an antimicrobial in these studies [55,56]. In the same way, plant-based antimicrobials combined with HHP resulted in a slight antagonistic effect (Figure 6). A possible explanation is that the first treatment can alter bacterial metabolism and favor survival at high pressure applied later, as occurred with Listeria monocytogenes [59].
When spread onto the surface or incorporated into the product, plant-based substances (caprylic acid) combined with HHP presented an antagonism effect (Figure 6). The synergism observed in the study of Pérez-Baltar et al. [13], in which there was bacterial growth after the application of combined treatments, was perceived as negative inactivation when calculating effect, resulting in positive values. On the other hand, plant-based antimicrobials added to active packaging had a synergistic effect with HHP (Figure 6). These findings reaffirm previous findings that active packaging increases the effectiveness of the combination of HHP and antimicrobials.
4. Conclusions
In this study, some knowledge was obtained about the combined application of HHP and natural antimicrobial compounds in a general scenario. This treatment can potentially inactivate pathogenic microorganisms in cured meat synergistically. Bacterial-based antimicrobials were the most promising for combination with high pressure, due to the efficiency of bacteriocins on Gram-positive bacteria, such as Listeria. Consequently, considering the bacteria studied, Listeria sp. is the most sensitive to the combined methods. Regarding the application method, active packaging is more efficient in inactivating pathogens in cured meat when combined with HHP. However, specific factors should be further investigated, such as molecular responses (stress and virulence), mechanisms of combined inactivation and effect on different strains.
Author Contributions
Conceptualization, L.C.d.M.M., D.K.A.D.R. and C.A.C.-J.; study execution, methodology, data curation and statistical analysis, L.C.d.M.M. and D.K.A.D.R.; writing—original draft preparation, L.C.d.M.M. and D.K.A.D.R.; writing—review and editing, D.K.A.D.R., M.L.G.M. and C.A.C.-J.; supervision and project administration, D.K.A.D.R. and C.A.C.-J.; funding acquisition, C.A.C.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) [grant numbers E-26/200.891/2021 and E-26/200.235/2022]; and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number 313119/2020-1 and 300968/2021-3].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings will be available in the database used. However, the data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stratakos, A.C.; Linton, M.; Patterson, M.F.; Koidis, A. Effect of High-Pressure Processing on the Shelf Life, Safety and Organoleptic Characteristics of Lasagne Ready Meals during Storage at Refrigeration and Abuse Temperature. Innov. Food Sci. Emerg. Technol. 2015, 30, 1–7. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Furtado, M.R.; Mutz, Y.S.; Rodrigues, B.L.; Bernardo, Y.A.A.; Baltar, J.D.; Bernardes, P.C.; Estevez, M.; Conte-Junior, C.A. A Chemometric Approach to Establish Underlying Connections between Lipid and Protein Oxidation and Instrumental Color and Texture Characteristics in Brazilian Dry-Cured Loin. Foods 2020, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Cobos, Á.; Díaz, O. Chemical Composition of Meat and Meat Products. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 471–510. ISBN 978-3-642-36605-5. [Google Scholar]
- Zhou, C.; Xia, Q.; Du, L.; He, J.; Sun, Y.; Dang, Y.; Geng, F.; Pan, D.; Cao, J.; Zhou, G. Recent Developments in Off-Odor Formation Mechanism and the Potential Regulation by Starter Cultures in Dry-Cured Ham. Crit. Rev. Food Sci. Nutr. 2022, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mutz, Y.S.; Kaic Alves Rosario, D.; Alves de Aguiar Bernardo, Y.; Paulo Vieira, C.; Vilela Pinto Moreira, R.; Bernardes, P.C.; Conte-Junior, C.A. Unravelling the Relation between Natural Microbiota and Biogenic Amines in Brazilian Dry-Cured Loin: A Chemometric Approach. Int. J. Food Sci. Technol. 2022, 57, 1621–1629. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, Safety, and Regulatory Considerations in the Use of Nitrite and Nitrate from Natural Origin in Meat Products—Invited Review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef] [PubMed]
- Mutz, Y.S.; Rosario, D.K.A.; Paschoalin, V.M.F.; Conte-Junior, C.A. Salmonella enterica: A Hidden Risk for Dry-Cured Meat Consumption? Crit. Rev. Food Sci. Nutr. 2020, 60, 976–990. [Google Scholar] [CrossRef]
- Possas, A.; Pérez-Rodríguez, F.; Valero, A.; García-Gimeno, R.M. Modelling the Inactivation of Listeria monocytogenes by High Hydrostatic Pressure Processing in Foods: A Review. Trends Food Sci. Technol. 2017, 70, 45–55. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Rodrigues, B.L.; Bernardes, P.C.; Conte-Junior, C.A. Principles and Applications of Non-Thermal Technologies and Alternative Chemical Compounds in Meat and Fish. Crit. Rev. Food Sci. Nutr. 2021, 61, 1163–1183. [Google Scholar] [CrossRef]
- Campus, M. High Pressure Processing of Meat, Meat Products and Seafood. Food Eng. Rev. 2010, 2, 256–273. [Google Scholar] [CrossRef]
- Adamcová, M.; van Andel, V.; Strohalm, J.; Houška, M.; Ševčík, R. Effect of High-Pressure Processing and Natural Antimicrobials on the Shelf-Life of Cooked Ham. Czech J. Food Sci. 2019, 37, 57–61. [Google Scholar] [CrossRef] [Green Version]
- de Alba, M.; Bravo, D.; Medina, M. Inactivation of Listeria monocytogenes and Salmonella enteritidis in Dry-Cured Ham by Combined Treatments of High Pressure and the Lactoperoxidase System or Lactoferrin. Innov. Food Sci. Emerg. Technol. 2015, 31, 54–59. [Google Scholar] [CrossRef]
- Pérez-Baltar, A.; Serrano, A.; Bravo, D.; Montiel, R.; Medina, M. Combined Effect of High Pressure Processing with Enterocins or Thymol on the Inactivation of Listeria monocytogenes and the Characteristics of Sliced Dry-Cured Ham. Food Bioprocess. Technol. 2019, 12, 288–297. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Repková, L.; Gänzle, M.G.; McMullen, L.M. Effect of Pressure, Reconstituted RTE Meat Microbiota, and Antimicrobials on Survival and Post-Pressure Growth of Listeria monocytogenes on Ham. Front. Microbiol. 2018, 9, 1979. [Google Scholar] [CrossRef]
- Dallagnol, A.M.; Barrio, Y.; Cap, M.; Szerman, N.; Castellano, P.; Vaudagna, S.R.; Vignolo, G. Listeria Inactivation by the Combination of High Hydrostatic Pressure and Lactocin AL705 on Cured-Cooked Pork Loin Slices. Food Bioprocess. Technol. 2017, 10, 1824–1833. [Google Scholar] [CrossRef]
- Yamamoto, K. Food Processing by High Hydrostatic Pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Yuste, J.; Capellas, M.; Pla, R.; Fung, D.Y.; Mor-Mur, M. High Pressure Processing for Food Safety and Preservation: A Review. J. Rapid Methods Autom. Microbiol. 2001, 9, 1–10. [Google Scholar] [CrossRef]
- Knorr, D. Advantages, Opportunities and Challenges of High Hydrostatic Pressure Application to Food Systems. In Progress in Biotechnology; Hayashi, R., Balny, C., Eds.; High Pressure Bioscience and Biotechnology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 13, pp. 279–287. [Google Scholar]
- Grant, A.; Parveen, S. All Natural and Clean-Label Preservatives and Antimicrobial Agents Used during Poultry Processing and Packaging. J. Food Prot. 2017, 80, 540–544. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’ Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [Green Version]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Control of Pathogenic and Spoilage Microorganisms in Fresh-Cut Fruits and Fruit Juices by Traditional and Alternative Natural Antimicrobials. Compr. Rev. Food Sci. Food Saf. 2009, 8, 157–180. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of Natural Antimicrobial Agents: A Safe Preservation Approach. Active Antimicrobial Food Packaging; IntechOpen: London, UK, 2019; Chapter 2; p. 18. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Garriga, M. Assessment of the Effectiveness of Antimicrobial Packaging Combined with High Pressure to Control Salmonella Sp. in Cooked Ham. Food Control 2008, 19, 634–638. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-based Phenolic Molecules as Natural Preservatives in Comminuted Meats: A Review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.D.; do Rosário, D.K.A.; Weitz, D.A.; Conte-Junior, C.A. Essential Oil Nanoemulsions: Properties, Development, and Application in Meat and Meat Products. Trends Food Sci. Technol. 2022, 121, 1–13. [Google Scholar] [CrossRef]
- Oliveira, T.L.C.; Ramos, A.L.S.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Natural Antimicrobials as Additional Hurdles to Preservation of Foods by High Pressure Processing. Trends Food Sci. Technol. 2015, 45, 60–85. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.-H. Hurdle Technology: A Novel Approach for Enhanced Food Quality and Safety—A Review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of Ultrasound in Combination with Other Technologies in Food Processing: A Review. Ultrason. Sonochemistry 2021, 73, 105506. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; James Feng, L.X.; Bang, W.S.; Yuk, H.-G. Inactivation of Listeria monocytogenes and Natural Microbiota on Raw Salmon Fillets Using Acidic Electrolyzed Water, Ultraviolet Light or/and Ultrasounds. Food Control 2017, 74, 54–60. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Bernardo, Y.A.A.; Mutz, Y.S.; Tiwari, B.; Rajkovic, A.; Bernardes, P.C.; Conte-Junior, C.A. Modelling Inactivation of Staphylococcus Spp. on Sliced Brazilian Dry-Cured Loin with Thermosonication and Peracetic Acid Combined Treatment. Int. J. Food Microbiol. 2019, 309, 108328. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, S.; Camargo, P.F.; Hernandes, E.M.; Thommazo, A.D.; Belgamo, A.; Zamboni, A.; Silva, C. Managing Literature Reviews Information through Visualization. In Proceedings of the Proceedings of the 14th Inter-national Conference on Enterprise Information Systems, Online Streaming, 25–27 April 2022; SciTePress—Science and and Technology Publications; Springer: Berlin/Heidelberg, Germany, 2012; pp. 36–45. [Google Scholar]
- Carvalho, A.P.A.; Conte Junior, C.A. Green Strategies for Active Food Packagings: A Systematic Review on Active Properties of Graphene-Based Nanomaterials and Biodegradable Polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- de Alba, M.; Bravo, D.; Medina, M. Inactivation of Escherichia coli O157:H7 in Dry-Cured Ham by High-Pressure Treatments Combined with Biopreservatives. Food Control 2013, 31, 508–513. [Google Scholar] [CrossRef]
- Amaro-Blanco, G.; Delgado-Adámez, J.; Martín, M.J.; Ramírez, R. Active Packaging Using an Olive Leaf Extract and High Pressure Processing for the Preservation of Sliced Dry-Cured Shoulders from Iberian Pigs. Innov. Food Sci. Emerg. Technol. 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Aymerich, T.; Jofré, A.; Garriga, M.; Hugas, M. Inhibition of Listeria monocytogenes and Salmonella by Natural Antimicrobials and High Hydrostatic Pressure in Sliced Cooked Ham. J. Food Prot. 2005, 68, 173–177. [Google Scholar] [CrossRef]
- Bravo, D.; de Alba, M.; Medina, M. Combined Treatments of High-Pressure with the Lactoperoxidase System or Lactoferrin on the Inactivation of Listeria monocytogenes, Salmonella enteritidis and Escherichia coli O157:H7 in Beef Carpaccio. Food Microbiol. 2014, 41, 27–32. [Google Scholar] [CrossRef]
- Hereu, A.; Bover-Cid, S.; Garriga, M.; Aymerich, T. High Hydrostatic Pressure and Biopreservation of Dry-Cured Ham to Meet the Food Safety Objectives for Listeria monocytogenes. Int. J. Food Microbiol. 2012, 154, 107–112. [Google Scholar] [CrossRef]
- Jofré, A.; Garriga, M.; Aymerich, T. Inhibition of Listeria monocytogenes in Cooked Ham through Active Packaging with Natural Antimicrobials and High-Pressure Processing. J. Food Prot. 2007, 70, 2498–2502. [Google Scholar] [CrossRef]
- Jofré, A.; Garriga, M.; Aymerich, T. Inhibition of Salmonella Sp. Listeria monocytogenes and Staphylococcus aureus in Cooked Ham by Combining Antimicrobials, High Hydrostatic Pressure and Refrigeration. Meat Sci. 2008, 78, 53–59. [Google Scholar] [CrossRef]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. High-Pressure Processing and Antimicrobial Biodegradable Packaging to Control Listeria monocytogenes during Storage of Cooked Ham. Food Microbiol. 2008, 25, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Marcos, B.; Jofré, A.; Aymerich, T.; Monfort, J.M.; Garriga, M. Combined Effect of Natural Antimicrobials and High Pressure Processing to Prevent Listeria monocytogenes Growth after a Cold Chain Break during Storage of Cooked Ham. Food Control 2008, 19, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Martillanes, S.; Rocha-Pimienta, J.; Ramírez, R.; García-Parra, J.; Delgado-Adámez, J. Effect of an Active Packaging with Rice Bran Extract and High-Pressure Processing on the Preservation of Sliced Dry-Cured Ham from Iberian Pigs. LWT 2021, 151, 112128. [Google Scholar] [CrossRef]
- Montiel, R.; Martín-Cabrejas, I.; Medina, M. Reuterin, Lactoperoxidase, Lactoferrin and High Hydrostatic Pressure on the Inactivation of Food-Borne Pathogens in Cooked Ham. Food Control 2015, 51, 122–128. [Google Scholar] [CrossRef]
- Montiel, R.; Martín-Cabrejas, I.; Peirotén, Á.; Medina, M. Reuterin, Lactoperoxidase, Lactoferrin and High Hydrostatic Pressure Treatments on the Characteristics of Cooked Ham. Innov. Food Sci. Emerg. Technol. 2016, 35, 111–118. [Google Scholar] [CrossRef]
- de Oliveira, T.L.C.; Junior, B.R.d.C.L.; Ramos, A.L.S.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Phenolic Carvacrol as a Natural Additive to Improve the Preservative Effects of High Pressure Processing of Low-Sodium Sliced Vacuum-Packed Turkey Breast Ham. LWT Food Sci. Technol. 2015, 64, 1297–1308. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.-J.; Tassou, C.; Chorianopoulos, N. Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing. Materials 2019, 12, 3726. [Google Scholar] [CrossRef] [Green Version]
- Vercammen, A.; Vanoirbeek, K.G.A.; Lurquin, I.; Steen, L.; Goemaere, O.; Szczepaniak, S.; Paelinck, H.; Hendrickx, M.E.G.; Michiels, C.W. Shelf-Life Extension of Cooked Ham Model Product by High Hydrostatic Pressure and Natural Preservatives. Innov. Food Sci. Emerg. Technol. 2011, 12, 407–415. [Google Scholar] [CrossRef]
- Modugno, C.; Loupiac, C.; Bernard, A.; Jossier, A.; Neiers, F.; Perrier-Cornet, J.-M.; Simonin, H. Effect of High Pressure on the Antimicrobial Activity and Secondary Structure of the Bacteriocin Nisin. Innov. Food Sci. Emerg. Technol. 2018, 47, 9–15. [Google Scholar] [CrossRef]
- Serra-Castelló, C.; Jofré, A.; Belletti, N.; Garriga, M.; Bover-Cid, S. Modelling the Piezo-Protection Effect Exerted by Lactate on the High Pressure Resistance of Listeria monocytogenes in Cooked Ham. Food Res. Int. 2021, 140, 110003. [Google Scholar] [CrossRef]
- Wemekamp-Kamphuis, H.H.; Karatzas, A.K.; Wouters, J.A.; Abee, T. Enhanced Levels of Cold Shock Proteins in Listeria monocytogenes LO28 upon Exposure to Low Temperature and High Hydrostatic Pressure. Appl. Environ. Microbiol. 2002, 68, 456–463. [Google Scholar] [CrossRef] [Green Version]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical Composition, Extraction Sources and Action Mechanisms of Essential Oils: Natural Preservative and Limitations of Use in Meat Products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and Applications Beyond Microbial Inactivation. Food Eng Rev 2021, 13, 490–508. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H.N. High-Pressure Inactivation of Enzymes: A Review on Its Recent Applications on Fruit Purees and Juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial Food Packaging: Potential and Pitfalls. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, Q.-A.; Buffa, M.; Guamis, B.; Saldo, J. Factors Affecting Bacterial Inactivation during High Hydrostatic Pressure Processing of Foods: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Serra-Castelló, C.; Ferrocino, I.; Jofré, A.; Cocolin, L.; Bover-Cid, S.; Rantsiou, K. Unravelling the Molecular Mechanisms Underlying the Protective Effect of Lactate on the High-Pressure Resistance of Listeria monocytogenes. Biomolecules 2021, 11, 677. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).