Spatial Variation of Microbial Community Structure and Its Driving Environmental Factors in Two Forest Types in Permafrost Region of Greater Xing′an Mountains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sample Collection
2.3. Determination of Soil Physical and Chemical Properties
2.4. Soil Microbial Illumina Sequencing
2.5. Data Processing Analysis
3. Results
3.1. Soil Physicochemical Properties
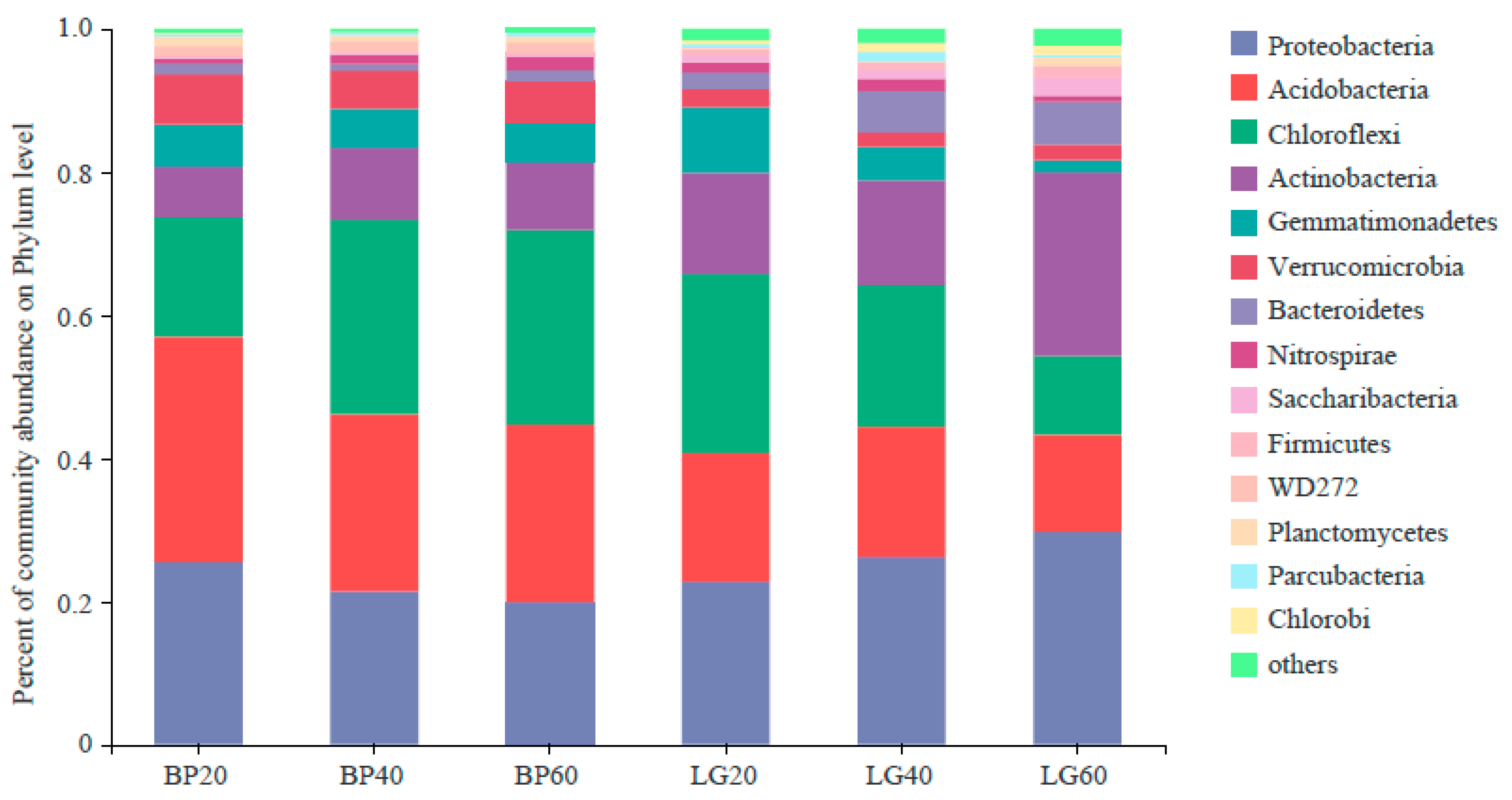
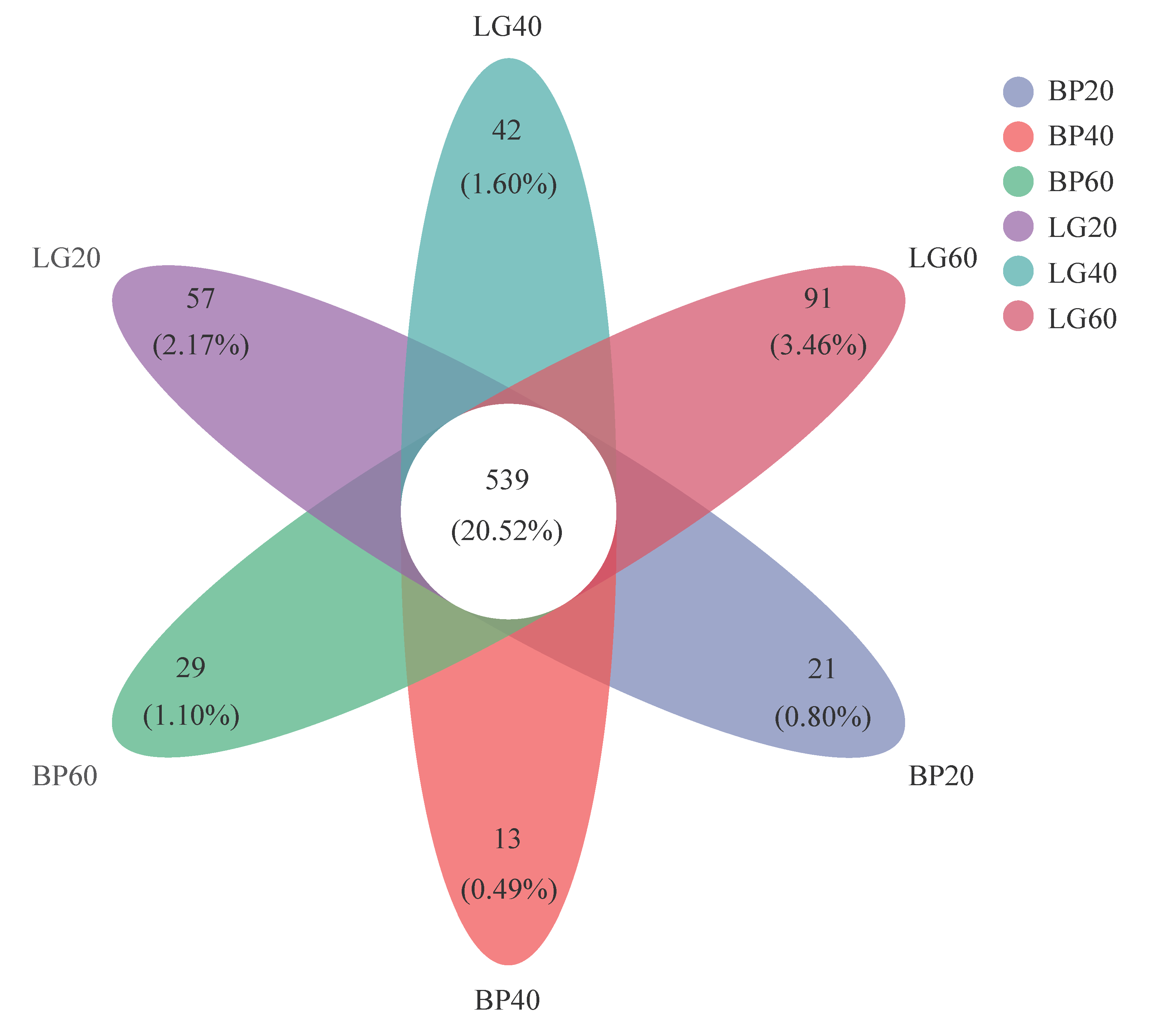
3.2. Composition of Soil Microbial Community
3.3. Richness and Diversity of Soil Microbial Community
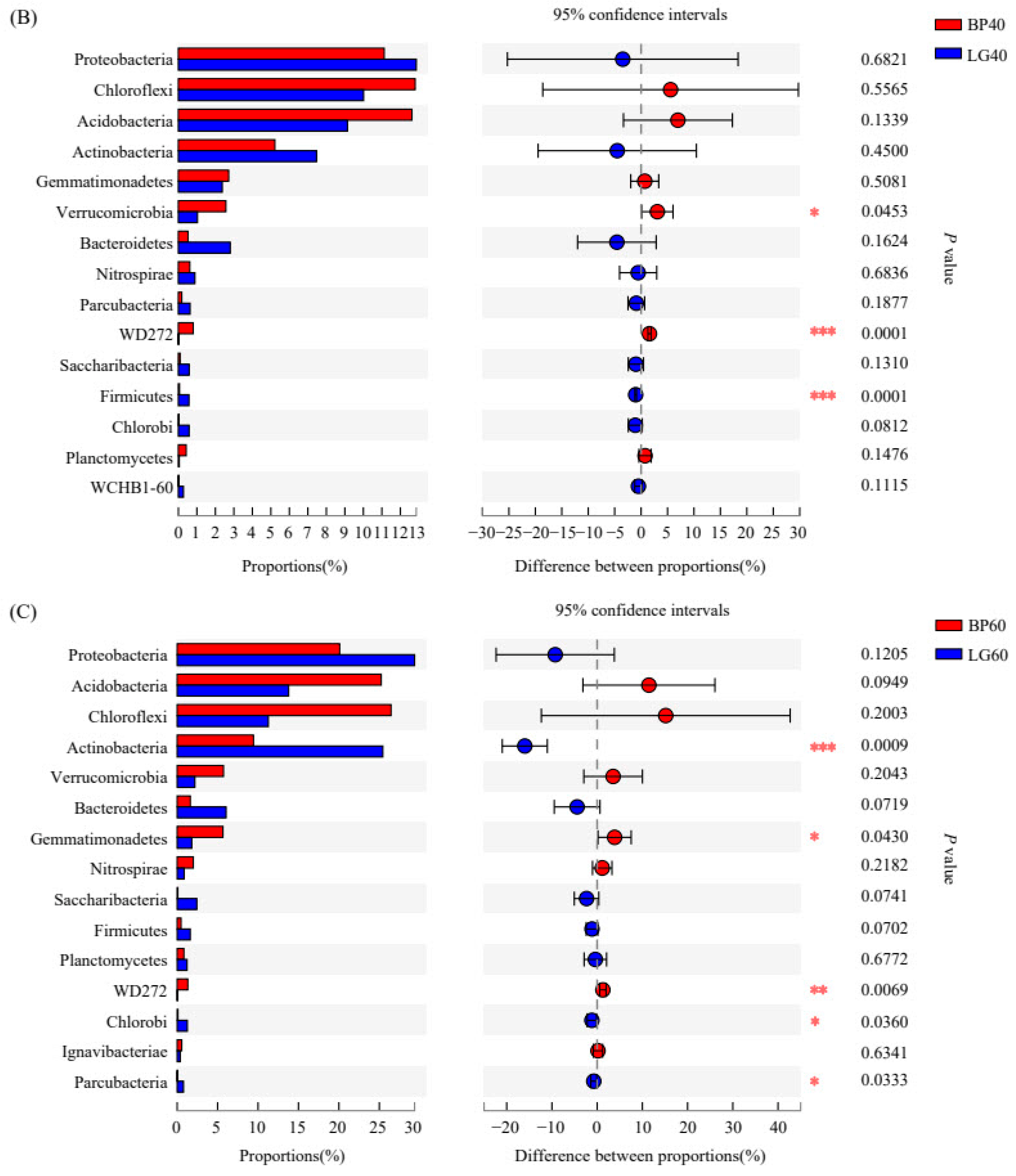
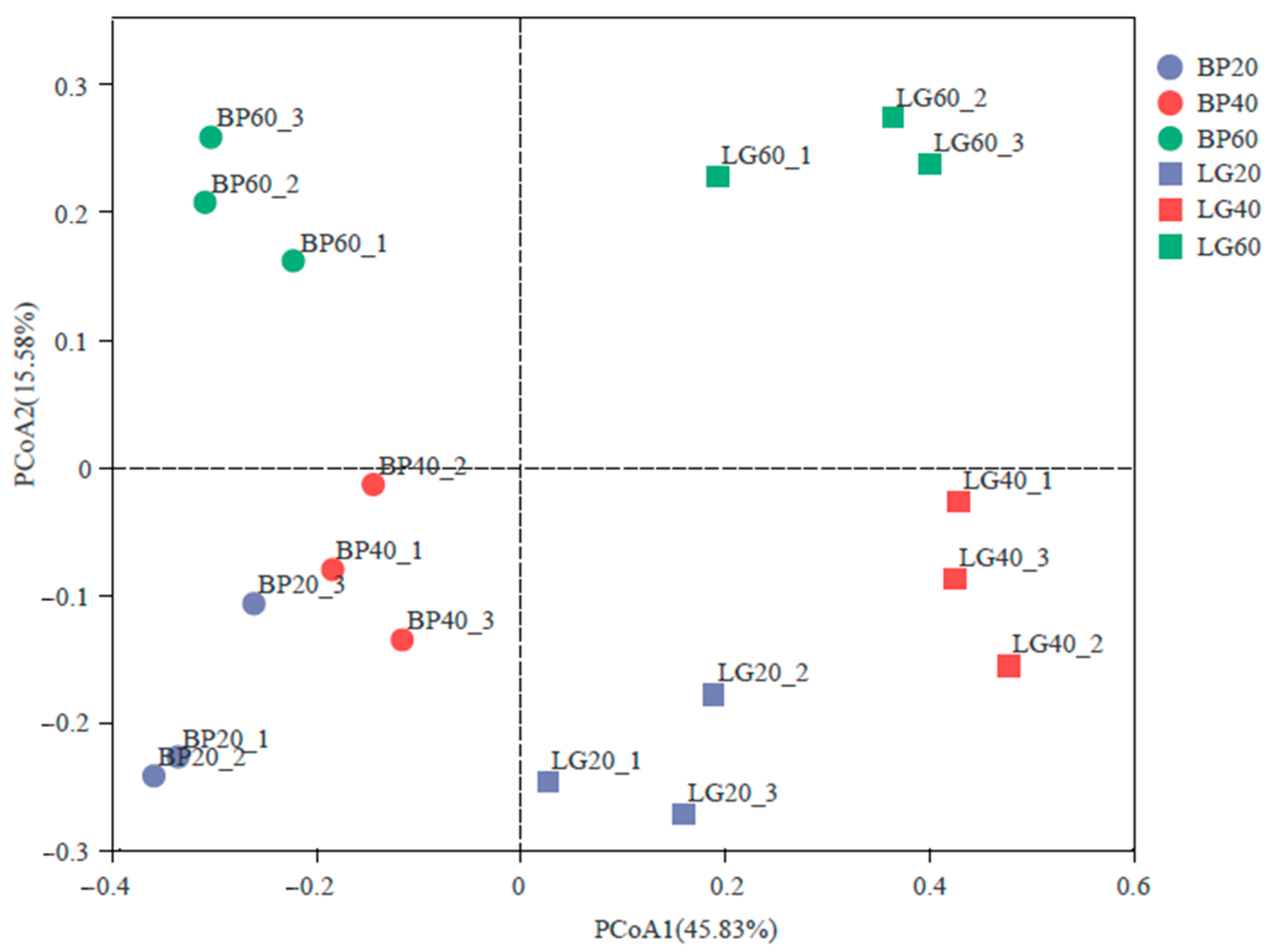
3.4. Structure of Soil Microbial Community
3.5. Relationship between Soil Microbial Community and Physicochemical Properties
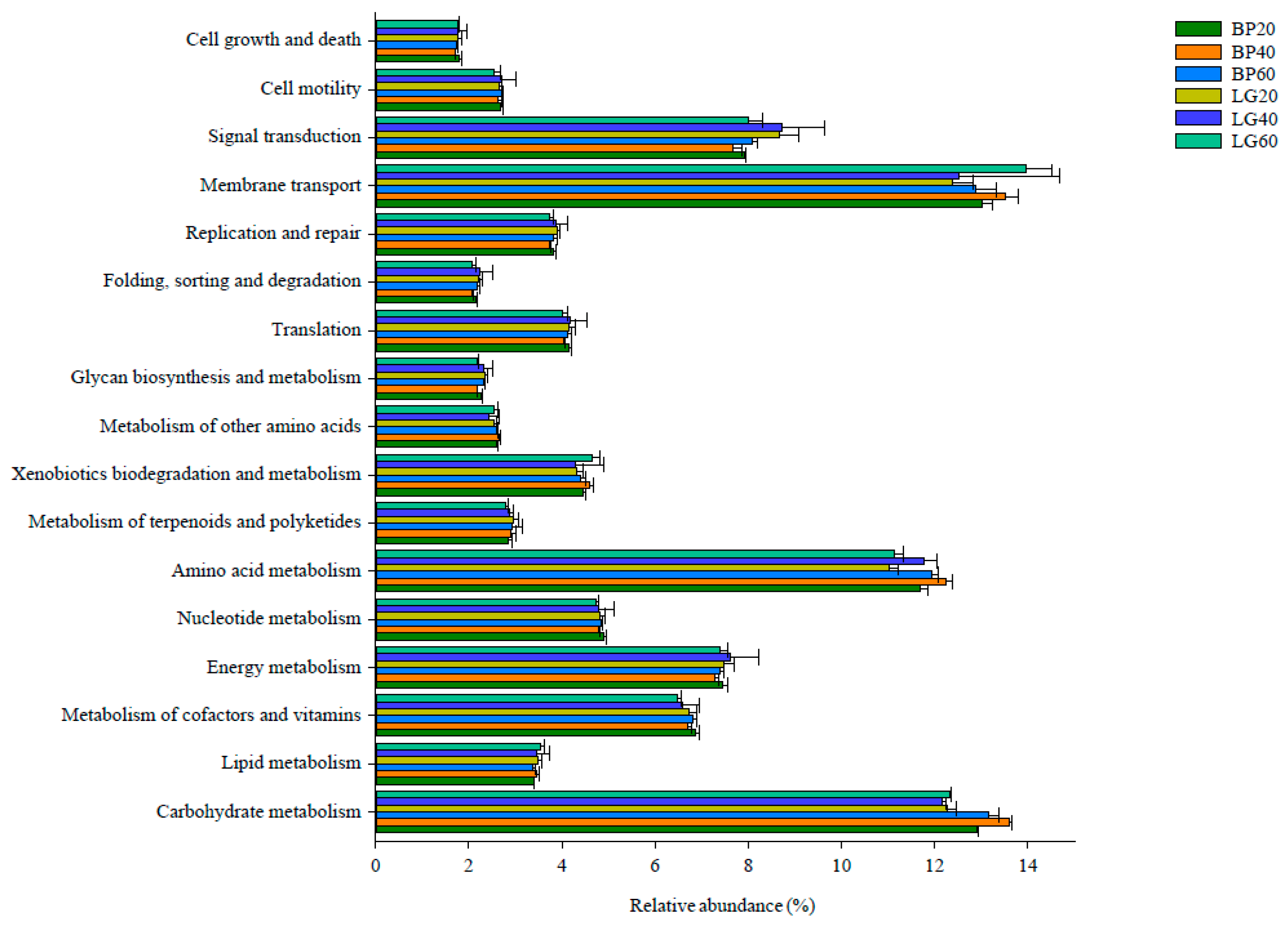
3.6. Predicted Functional Analysis for Microbial Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.M.; Lee, M.J.; Jung, J.Y.; Hwang, C.Y.; Kim, M.; Ro, H.M.; Chun, J.; Lee, Y.K. Vertical distribution of bacterial community is associated with the degree of soil organic matter decomposition in the active layer of moist acidic tundra. J. Microbiol. 2016, 54, 713–723. [Google Scholar] [CrossRef]
- Feng, J.J.; Wang, C.; Lei, J.S.; Yang, Y.F.; Yan, Q.Y.; Zhou, X.S.; Tao, X.Y.; Ning, D.L.; Yuan, M.T.M.; Qin, Y.J.; et al. Warming-induced permafrost thaw exacerbates tundra soil carbon decomposition mediated by microbial community. Microbiome 2020, 8, 3. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; McGuire, A.D.; Schadel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef]
- Yergeau, E.; Hogues, H.; Whyte, L.G.; Greer, C.W. The functional potential of high Arctic permafrost revealed by metagenomic sequencing, qPCR and microarray analyses. ISME J. 2010, 4, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Averill, C.; Hawkes, C.V. Ectomycorrhizal fungi slow soil carbon cycling. Ecol. Lett. 2016, 19, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Ye, J.; Wei, Y.L.; Yan, S.K.; Yuan, H.S. Soil depth-dependent C/N stoichiometry and fungal and bacterial communities along a temperate forest succession gradient. Catena 2021, 207, 105613. [Google Scholar] [CrossRef]
- Bolaji, A.J.; Wan, J.C.; Manchur, C.L.; Lawley, Y.; de Kievit, T.R.; Fernando, W.G.D.; Belmonte, M.F. Microbial community dynamics of soybean (Glycine max) is affected by cropping sequence. Front. Microbiol. 2021, 12, 632280. [Google Scholar] [CrossRef]
- Shahzad, T.; Chenu, C.; Genet, P.; Barot, S.; Perveen, N.; Mougin, C.; Fontaine, S. Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol. Biochem. 2015, 80, 146–155. [Google Scholar] [CrossRef]
- Jorgenson, M.T.; Harden, J.; Kanevskiy, M.; O’Donnell, J.; Wickland, K.; Ewing, S.; Manies, K.; Zhuang, Q.L.; Shur, Y.; Striegl, R.; et al. Reorganization of vegetation, hydrology and soil carbon after permafrost degradation across heterogeneous boreal landscapes. Environ. Res. Lett. 2013, 8, 035017. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Liu, M.H.; Feng, F.J.; Cai, T.J.; Tang, S.J. Soil microbial community response differently to the frequency and strength of freeze-thaw events in a Larix gmelinii forest in the Daxing’an Mountains, China. Front. Microbiol. 2020, 11, 1164. [Google Scholar] [CrossRef]
- Yasmeen, S.; Wang, X.C.; Zhao, H.Y.; Zhu, L.J.; Yuan, D.Y.; Li, Z.S.; Zhang, Y.D.; Ahmad, S.; Han, S.J. Contrasting climate-growth relationship between Larix gmelinii and Pinus sylvestris var. mongolica along a latitudinal gradient in Daxing’an Mountains, China. Dendrochronologia 2019, 58, 125645. [Google Scholar] [CrossRef]
- Wei, H.; Man, X.L. Increased litter greatly enhancing soil respiration in Betula platyphylla forests of permafrost region, Northeast China. Forests 2021, 12, 89. [Google Scholar] [CrossRef]
- Fang, X.M.; Yu, D.P.; Zhou, W.M.; Zhou, L.; Dai, L.M. The effects of forest type on soil microbial activity in Changbai Mountain, Northeast China. Ann. Forest Sci. 2016, 73, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.X.; Man, X.L.; Cai, T.J.; Xiao, R.H.; Ge, Z.X. Increasing soil organic carbon and nitrogen stocks along with secondary forest succession in permafrost region of the Daxing’an Mountains, Northeast China. Glob. Ecol. Conserv. 2020, 24, e01258. [Google Scholar] [CrossRef]
- Gao, J.; Wu, Y.Y.; Liu, B.Y.; Zhao, R.K.; Liu, A.Q.; Li, X.; Chen, Q.Z.; Sun, L.W.; Guo, X.P.; Liu, H.J. Vertical distribution patterns of element concentrations in podetia of Cladonia rangiferina from Huzhong Natural Reserve, Heilongjiang, China. Pol. J. Environ. Stud. 2021, 30, 103–110. [Google Scholar] [CrossRef]
- Ping, X.Y.; Chang, Y.; Liu, M.; Hu, Y.M.; Huang, W.T.; Shi, S.X.; Jia, Y.C.; Li, D.K. Carbon emission and redistribution among forest carbon pools, and change in soil nutrient content after different severities of forest fires in Northeast China. Forests 2022, 13, 110. [Google Scholar] [CrossRef]
- Jin, H.J.; Chang, X.L.; Luo, D.L.; He, R.X.; Lu, L.Z.; Yang, S.Z.; Guo, D.X.; Chen, X.M.; Harris, S.A. Evolution of permafrost in Northeast China since the Late Pleistocene. Sci. Cold Arid Reg. 2016, 8, 269–296. [Google Scholar] [CrossRef]
- Li, W.K.; Niu, S.K.; Liu, X.D.; Wang, J.M. Short-term response of the soil bacterial community to differing wildfire severity in Pinus tabulaeformis stands. Sci. Rep. 2019, 9, 1148. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, A.; Kushwaha, P.; Jim, A.; Troch, P.A.; Maier, R. New soil, old plants, and ubiquitous microbes: Evaluating the potential of incipient basaltic soil to support native plant growth and influence belowground soil microbial community composition. Sustainability 2020, 12, 4209. [Google Scholar] [CrossRef]
- Jiang, L.; Song, Y.Y.; Sun, L.; Ma, X.Y.; Wang, X.W.; Song, C.C.; Hou, A.X.; Wang, L.L. Distribution of carbon and nitrogen cycle microbes along permafrost peatland profile in Northeast China. Environ. Prog. Sustain. 2021, 40, 13707. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.J.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D. Community Ecology Package, R Package, Version 2.4-2; R Foundation for Statistical Computing: Vienna, Austria, 2017. Available online: https://cran.r-project.org/web/packages/vegan/index.html(accessed on 28 June 2022).
- Braak, C.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0. 2012. Available online: http://www.canoco5.com (accessed on 28 June 2022).
- Ke, H.; Li, J.H.; Zhang, X.Q.; Hu, J. Bacterial community structure and predicted metabolic function of landfilled municipal solid waste in China. Sustainability 2022, 14, 3144. [Google Scholar] [CrossRef]
- Deng, J.; Gu, Y.F.; Zhang, J.; Xue, K.; Qin, Y.J.; Yuan, M.; Yin, H.; He, Z.L.; Wu, L.Y.; Schuur, E.A.G.; et al. Shifts of tundra bacterial and archaeal communities along a permafrost thaw gradient in Alaska. Mol. Ecol. 2015, 24, 222–234. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Kim, H.M.; Jung, J.Y.; Nam, S.; Ju, H.T.; Kim, M.; Lee, Y.K. Distinct taxonomic and functional profiles of the microbiome associated with different soil horizons of a moist tussock tundra in Alaska. Front. Microbiol. 2019, 10, 1442. [Google Scholar] [CrossRef] [Green Version]
- Yokota, M.; Guan, Y.P.; Fan, Y.; Zhang, X.M.; Yang, W. Vertical and temporal variations of soil bacterial and archaeal communities in wheat-soybean rotation agroecosystem. PeerJ 2022, 10, 12868. [Google Scholar] [CrossRef]
- Jin, X.Y.; Jin, H.J.; Iwahana, G.; Marchenko, S.S.; Luo, D.L.; Li, X.Y.; Liang, S.H. Impacts of climate-induced permafrost degradation on vegetation: A review. Adv. Clim. Chang. Res. 2021, 12, 29–47. [Google Scholar] [CrossRef]
- Aksenov, A.S.; Shirokova, L.S.; Kisil, O.Y.; Kolesova, S.N.; Lim, A.G.; Kuzmina, D. Bacterial number and genetic diversity in a permafrost peatland (Western Siberia): Testing a link with organic matter quality and elementary composition of a peat soil profile. Diversity 2021, 13, 328. [Google Scholar] [CrossRef]
- Dong, X.F.; Liu, C.; Ma, D.L.; Wu, Y.F.; Man, H.R.; Wu, X.W.; Li, M.; Zang, S.Y. Organic carbon mineralization and bacterial community of active layer soils response to short-term warming in the Great Hing’an Mountains of Northeast China. Front. Microbiol. 2021, 12, 802213. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Wang, J.; Yao, J.H.; Li, X.W.; Lin, Y.H.; Du, F.K. Soil bacterial characteristics under four habitats with different vegetation communities on the Qinghai-Tibetan Plateau. Wetlands 2021, 41, 58. [Google Scholar] [CrossRef]
- Qi, D.D.; Feng, F.J.; Fu, Y.M.; Ji, X.M.; Liu, X.F. Effects of soil microbes on forest recovery to climax community through the regulation of nitrogen cycling. Forests 2020, 11, 1027. [Google Scholar] [CrossRef]
- Wang, Z.H.; Bai, Y.; Hou, J.F.; Li, F.; Li, X.Q.; Cao, R.; Deng, Y.Y.; Wang, H.B.; Jiang, Y.R.; Yang, W.Q. The changes in soil microbial communities across a subalpine forest successional series. Forests 2022, 13, 289. [Google Scholar] [CrossRef]
- Shi, Y.; Xiang, X.J.; Shen, C.C.; Chu, H.Y.; Neufeld, J.D.; Walker, V.K.; Grogan, P. Vegetation-associated impacts on Arctic tundra bacterial and microeukaryotic communities. Appl. Environ. Microb. 2015, 81, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.W.; Lu, Z.T.; Yang, K.; Zhu, J.J. Impacts of conversion from secondary forests to larch plantations on the structure and function of microbial communities. Appl. Soil Ecol. 2017, 111, 73–83. [Google Scholar] [CrossRef]
- Ao, L.; Zhao, M.C.; Li, X.; Sun, G.Y. Different urban forest tree species affect the assembly of the soil bacterial and fungal community. Microb. Ecol. 2022, 83, 447–458. [Google Scholar] [CrossRef]
- Mateos-Rivera, A.; Yde, J.C.; Wilson, B.; Finster, K.W.; Reigstad, L.J.; Ovreas, L. The effect of temperature change on the microbial diversity and community structure along the chronosequence of the sub-arctic glacier forefield of Styggedalsbreen (Norway). FEMS Microbiol. Ecol. 2016, 92, fiw038. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.H.; Hu, Y.M.; Chen, B.D.; Zhang, Y.; Thiele, J.; Shi, R.J.; Liu, M.; Bu, R.C. Soil pH and plant diversity shape soil bacterial community structure in the active layer across the latitudinal gradients in continuous permafrost region of Northeastern China. Sci. Rep. 2018, 8, 5619. [Google Scholar] [CrossRef]
- Fu, D.G.; Wu, X.N.; Qiu, Q.T.; Duan, C.Q.; Jones, D.L. Seasonal variations in soil microbial communities under different land restoration types in a subtropical mountains region, Southwest China. Appl. Soil Ecol. 2020, 153, 103634. [Google Scholar] [CrossRef]
- Dong, H.Y.; Shao, S.; Liang, C.F.; Xu, Q.F.; Chen, J.H.; Qin, H. Impacts of litter composition on the structure and functional pathways of soil microbial community during Phyllostachys edulis expansion. Agronomy 2022, 12, 220. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef]
- Fujii, K.; Yamada, T.; Hayakawa, C.; Nakanishi, A.; Funakawa, S. Decoupling of protein depolymerization and ammonification in nitrogen mineralization of acidic forest soils. Appl. Soil Ecol. 2020, 153, 103572. [Google Scholar] [CrossRef]
- Blume-Werry, G.; Milbau, A.; Teuber, L.M.; Johansson, M.; Dorrepaal, E. Dwelling in the deep-strongly increased root growth and rooting depth enhance plant interactions with thawing permafrost soil. New Phytol. 2019, 223, 1328–1339. [Google Scholar] [CrossRef]
- Adamczyk, M.; Ruthi, J.; Frey, B. Root exudates increase soil respiration and alter microbial community structure in alpine permafrost and active layer soils. Environ. Microbiol. 2021, 23, 2152–2168. [Google Scholar] [CrossRef]



| Samples | pH | TOC (g/kg) | TN (g/kg) | TP (g/kg) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | SWC (%) |
|---|---|---|---|---|---|---|---|
| BP20 | 4.99 ± 0.07 b | 57.86 ± 5.45 a | 3.45 ± 0.35 a | 1.39 ± 0.25 a | 8.27 ± 0.49 b | 12.00 ± 2.49 a | 30.87 ± 3.04 a |
| BP40 | 5.17 ± 0.11 a | 31.47 ± 4.37 b | 2.72 ± 0.26 b | 0.73 ± 0.13 b | 11.16 ± 1.27 a | 8.04 ± 0.93 b | 36.21 ± 5.32 a |
| BP60 | 5.04 ± 0.03 b | 16.15 ± 2.61 c | 1.41 ± 0.12 c | 0.47 ± 0.32 b | 12.16 ± 2.18 a | 7.31 ± 1.08 b | 21.44 ± 1.40 b |
| LG20 | 5.67 ± 0.14 ab | 39.27 ± 4.32 a | 3.07 ± 0.43 a | 1.80 ± 0.33 a | 8.43 ± 0.35 b | 5.73 ± 0.35 a | 39.10 ± 4.69 a |
| LG40 | 5.79 ± 0.06 a | 20.01 ± 2.08 b | 2.23 ± 0.39 b | 0.61 ± 0.48 b | 10.99 ± 1.53 a | 3.48 ± 0.73 b | 32.50 ± 6.57 a |
| LG60 | 5.54 ± 0.08 b | 15.20 ± 3.63 b | 1.09 ± 0.17 c | 0.39 ± 0.11 b | 11.31 ± 2.76 a | 3.41 ± 0.52 b | 23.23 ± 2.46 b |
| Samples | Shannon-Wiener | Simpson | ACE | Chao1 |
|---|---|---|---|---|
| BP20 | 5.49 ± 0.08 a | 0.013 ± 0.004 a | 1328.35 ± 37.48 a | 1305.26 ± 38.52 a |
| BP40 | 5.12 ± 0.21 b | 0.015 ± 0.003 a | 1295.32 ± 53.04 a | 1288.06 ± 65.80 a |
| BP60 | 5.15 ± 0.16 b | 0.014 ± 0.002 a | 1343.42 ± 46.35 a | 1344.03 ± 47.65 a |
| LG20 | 5.42 ± 0.07 b | 0.010 ± 0.003 b | 1452.31 ± 50.18 a | 1417.03 ± 49.59 a |
| LG40 | 5.07 ± 0.24 c | 0.016 ± 0.002 a | 1325.23 ± 23.41 b | 1309.08 ± 39.42 b |
| LG60 | 5.78 ± 0.25 a | 0.008 ± 0.004 b | 1413.24 ± 33.86 a | 1390.13 ± 26.58 a |
| Factor | Forest Type | Depth | Forest Type × Depth | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Shannon | 3.84 | 0.074 | 7.72 | 0.007 | 6.82 | 0.010 |
| Simpson | 3.03 | 0.108 | 4.84 | 0.029 | 1.88 | 0.195 |
| ACE | 14.19 | 0.003 | 6.34 | 0.013 | 1.90 | 0.019 |
| Chao1 | 7.50 | 0.018 | 4.05 | 0.045 | 1.54 | 0.025 |
| Phyla | 26.27 | <0.001 | 7.15 | 0.025 | 3.10 | 0.028 |
| Genera | 18.24 | <0.001 | 5.52 | 0.041 | 4.15 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Cui, Y.; Ma, D.; Li, X.; Liu, L. Spatial Variation of Microbial Community Structure and Its Driving Environmental Factors in Two Forest Types in Permafrost Region of Greater Xing′an Mountains. Sustainability 2022, 14, 9284. https://doi.org/10.3390/su14159284
Song D, Cui Y, Ma D, Li X, Liu L. Spatial Variation of Microbial Community Structure and Its Driving Environmental Factors in Two Forest Types in Permafrost Region of Greater Xing′an Mountains. Sustainability. 2022; 14(15):9284. https://doi.org/10.3390/su14159284
Chicago/Turabian StyleSong, Dandan, Yuanquan Cui, Dalong Ma, Xin Li, and Lin Liu. 2022. "Spatial Variation of Microbial Community Structure and Its Driving Environmental Factors in Two Forest Types in Permafrost Region of Greater Xing′an Mountains" Sustainability 14, no. 15: 9284. https://doi.org/10.3390/su14159284
APA StyleSong, D., Cui, Y., Ma, D., Li, X., & Liu, L. (2022). Spatial Variation of Microbial Community Structure and Its Driving Environmental Factors in Two Forest Types in Permafrost Region of Greater Xing′an Mountains. Sustainability, 14(15), 9284. https://doi.org/10.3390/su14159284





