Effect of Salinity and Temperature on the Seed Germination and Seedling Growth of Desert Forage Grass Lasiurus scindicus Henr.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Lasiurus scindicus Seeds
2.2. Gemination Experiment
2.3. Measurement of Growth Parameters
2.4. Chlorophyll Estimation
2.5. Estimation of Proline Content
2.6. Data Analysis
3. Results
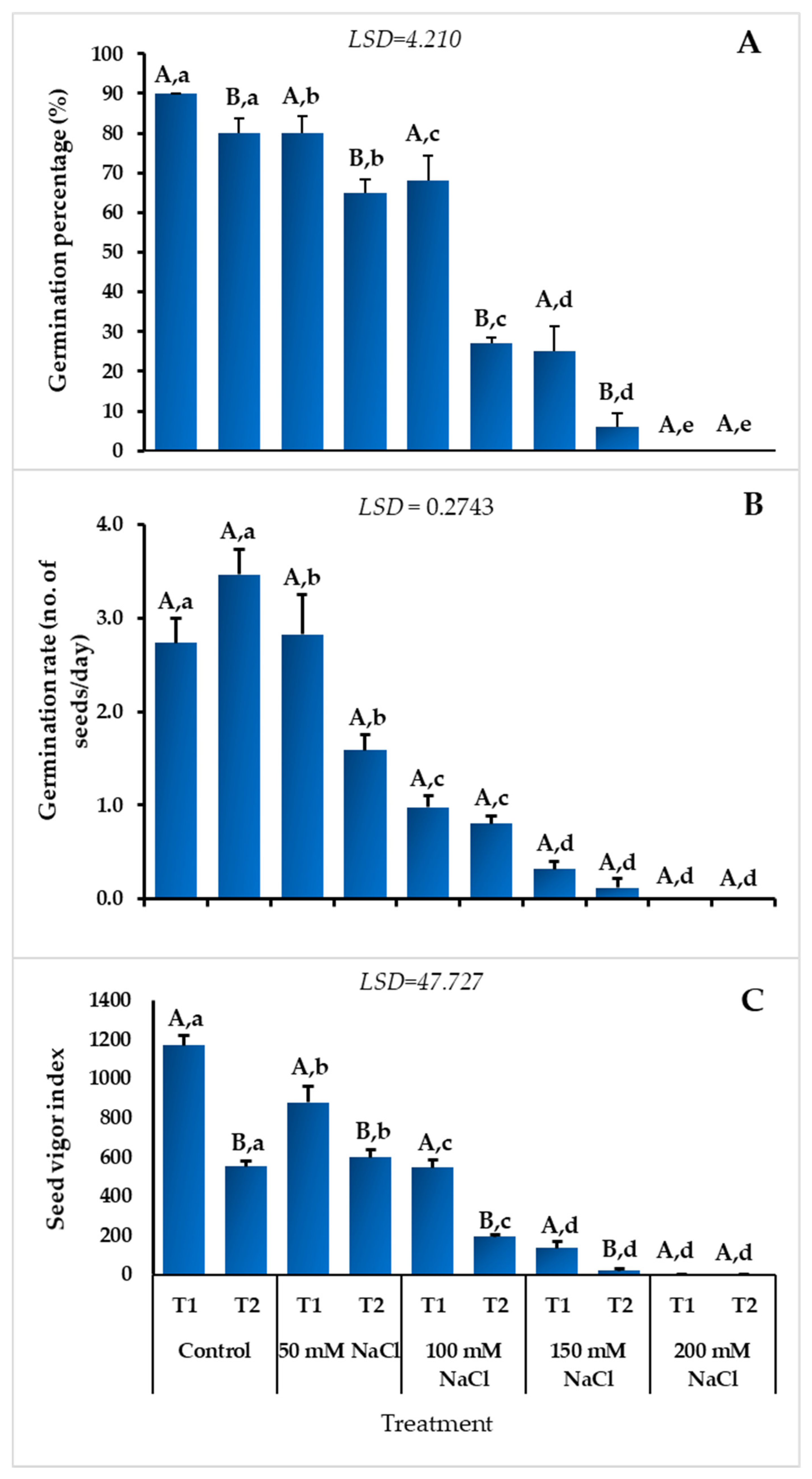
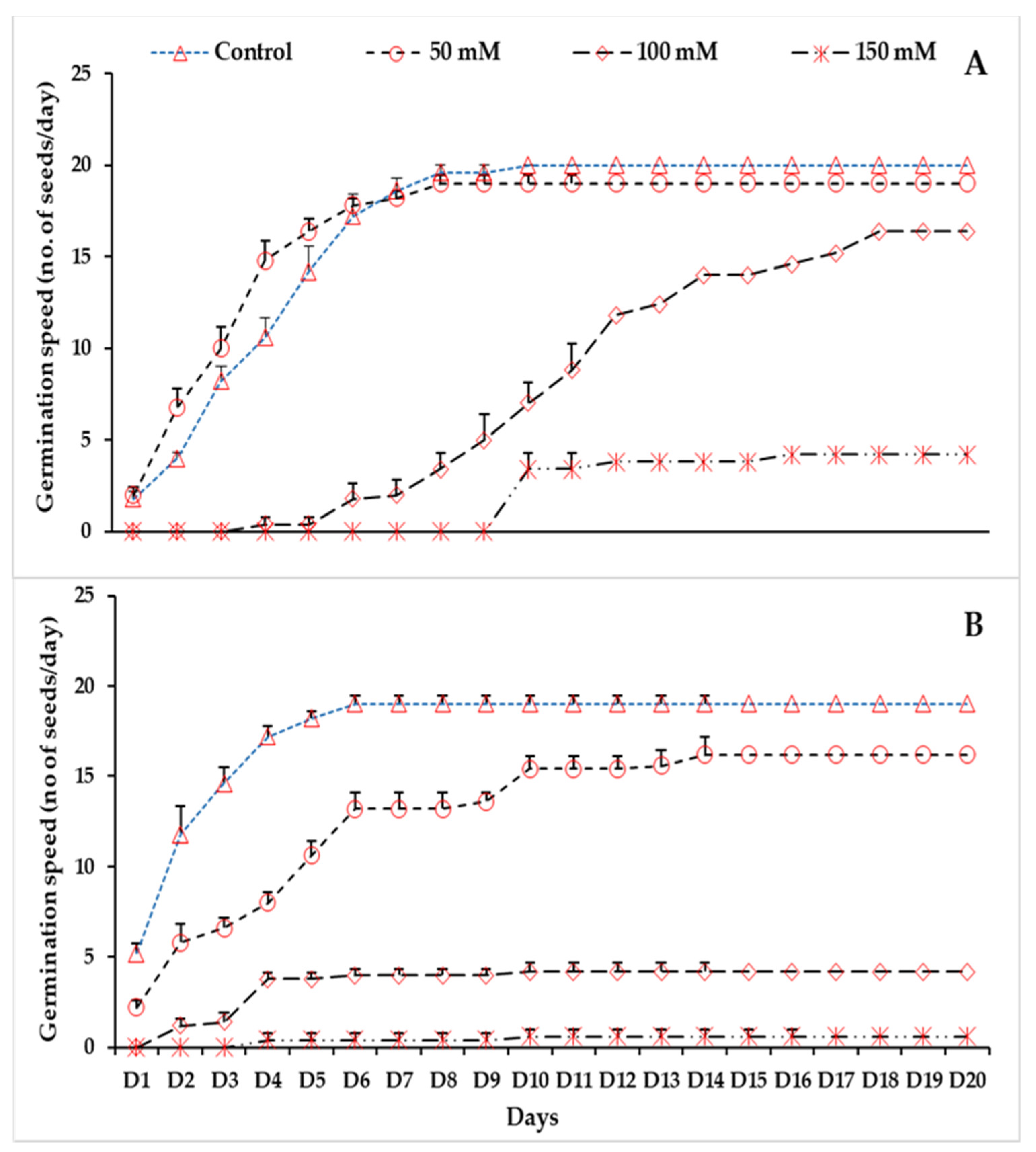
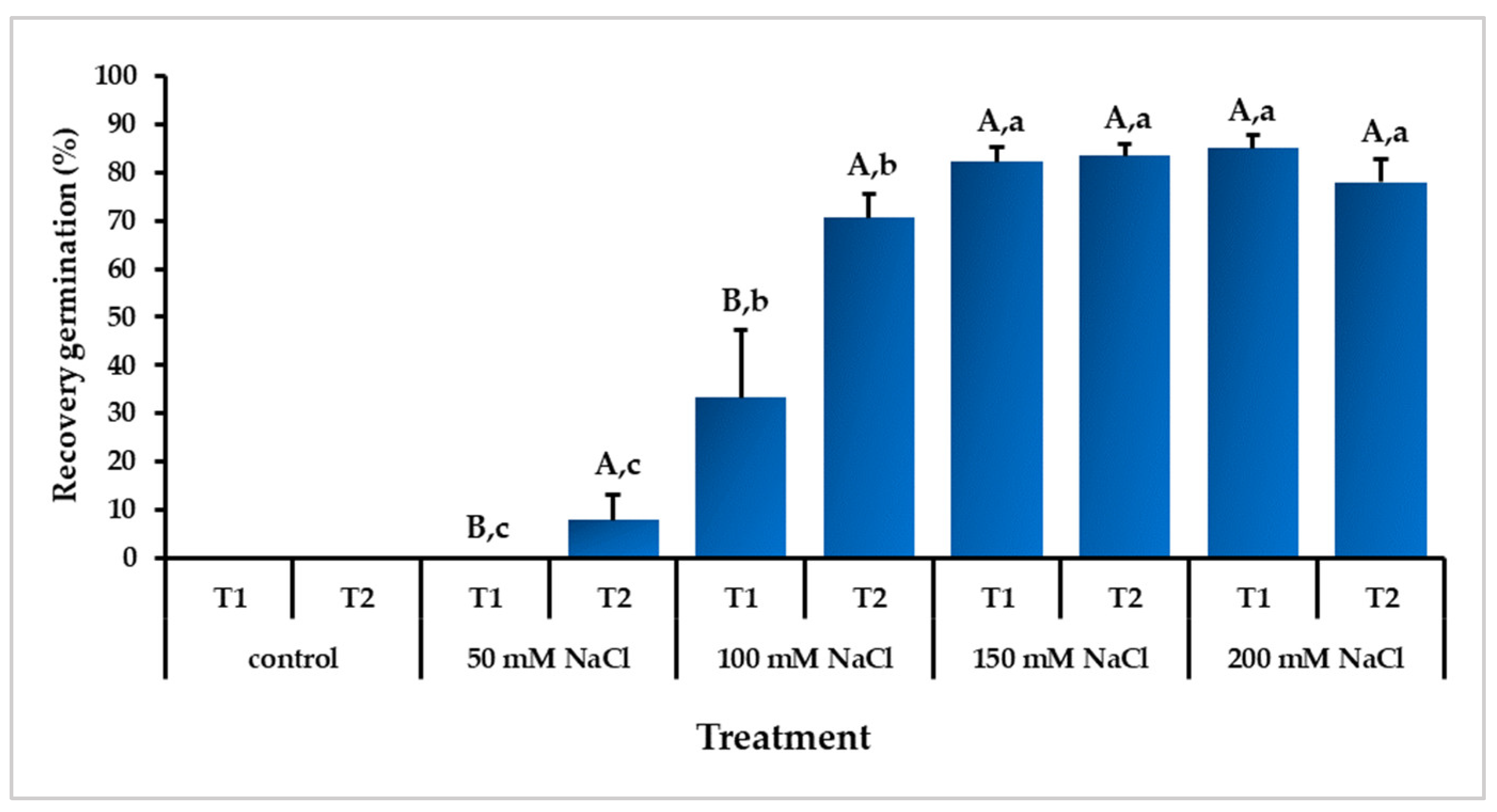
3.1. Effect of Temperature and Salinity on Germination Indices
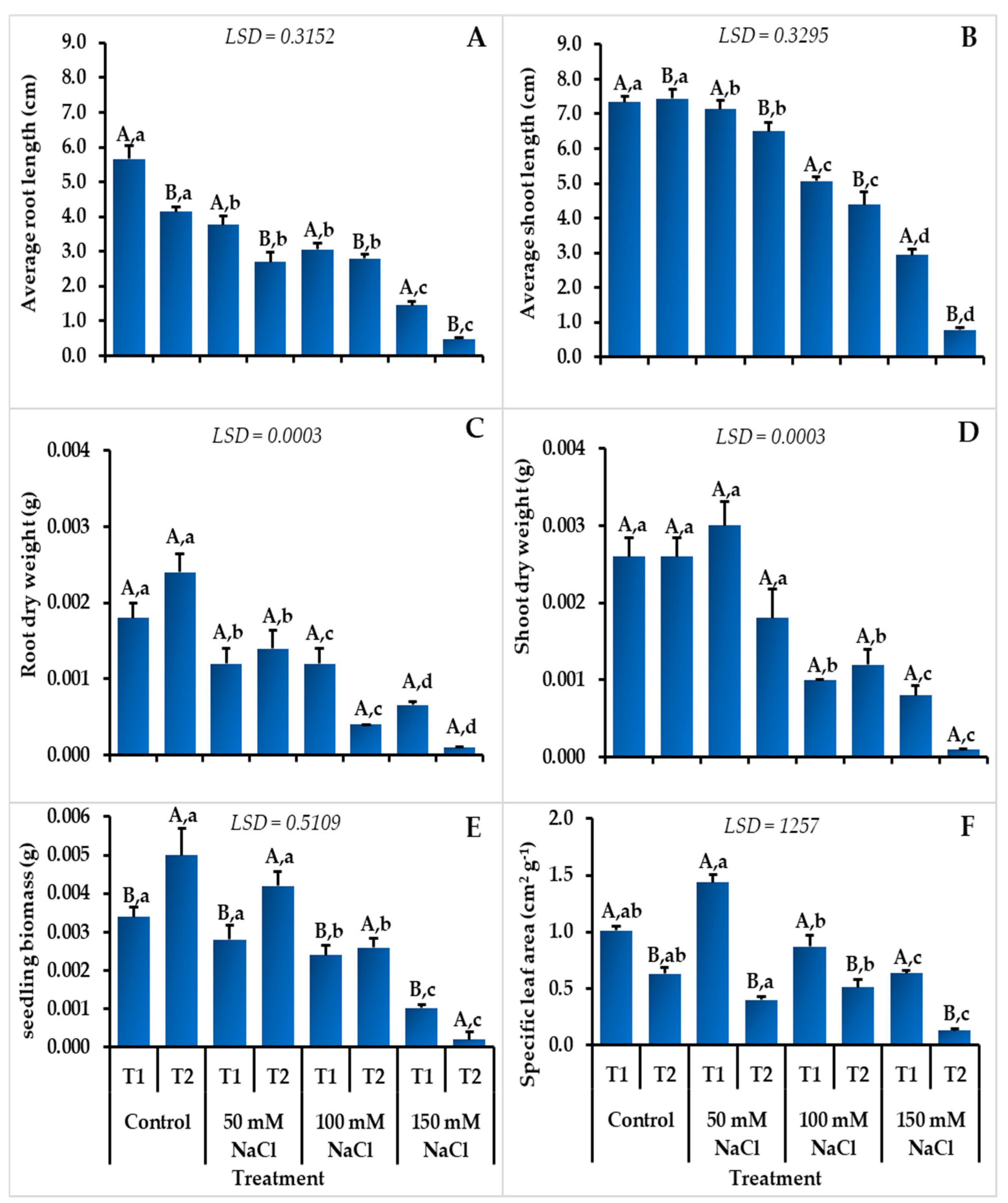
3.2. Seedling Growth
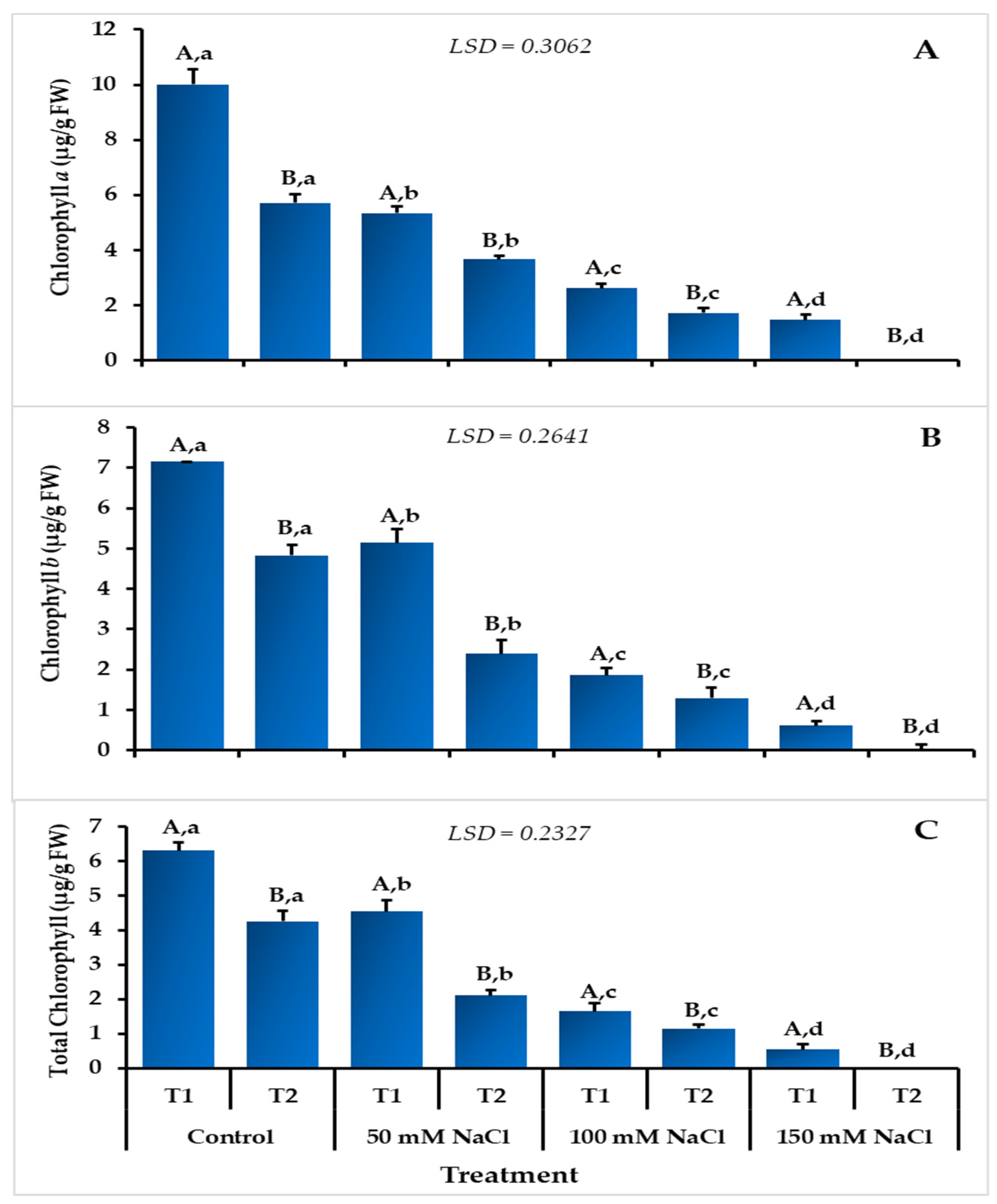
3.3. Effect of Salinity and Temperature on Chlorophyll Content of L. scindicus Seedlings
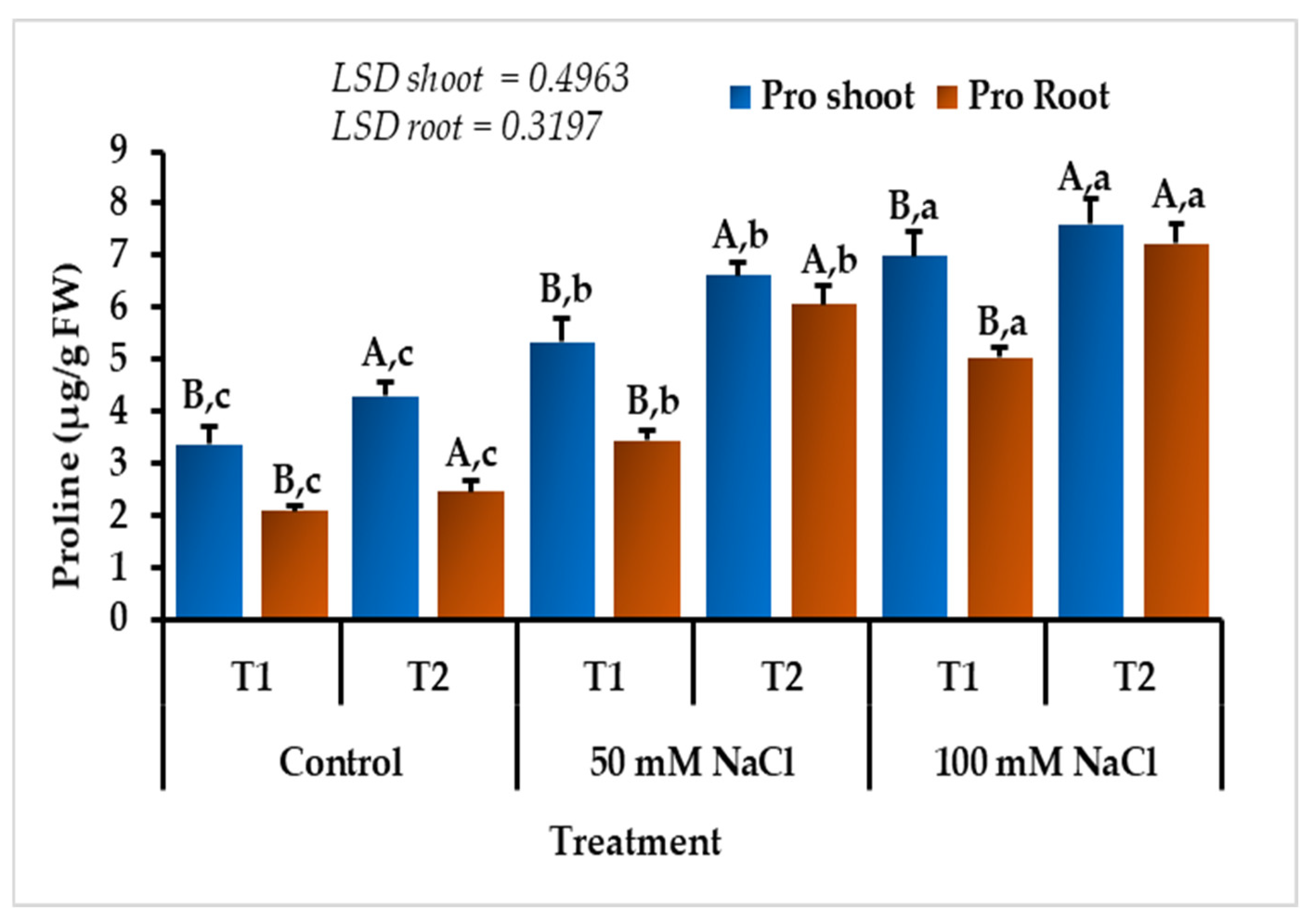
3.4. Effect of Salinity and Temperature on the Shoot and Root Proline Content of L. scindicus Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poudel, R.; Finnie, S.; Rose, D.J. Effects of wheat kernel germination time and drying temperature on compositional and end-use properties of the resulting whole wheat flour. J. Cereal Sci. 2019, 86, 33–40. [Google Scholar] [CrossRef]
- Nimbalkar, M.S.; Pawar, N.V.; Pai, S.R.; Dixit, G.B. Synchronized variations in levels of essential amino acids during germination in grain Amaranth. Braz. J. Bot. 2020, 43, 481–491. [Google Scholar] [CrossRef]
- Weber, D. Adaptive mechanisms of halophytes in desert regions. In Salinity and Water Stress; Springer: Berlin/Heidelberg, Germany, 2009; pp. 179–185. [Google Scholar]
- Farooq, S.; Onen, H.; Tad, S.; Ozaslan, C.; Mahmoud, S.F.; Brestic, M.; Zivcak, M.; Skalicky, M.; El-Shehawi, A.M. The influence of environmental factors on seed germination of Polygonum perfoliatum L.: Implications for management. Agronomy 2021, 11, 1123. [Google Scholar] [CrossRef]
- Fenner, M. Seeds: The Ecology of Regeneration in Plant Communities; CAB International: Wallingford, UK, 2000. [Google Scholar]
- Gairola, S.; Shabana, H.A.; Al Ketbi, A.; Mahmoud, T. Seed germination behavior of halophytes distributed in arid arabian deserts: A review of current research. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–17. [Google Scholar]
- Etesami, H.; Noori, F. Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–22. [Google Scholar]
- Malik, J.A.; AlQarawi, A.A.; Dar, B.A.; Hashem, A.; Alshahrani, T.S.; AlZain, M.N.; Habib, M.M.; Javed, M.M.; Abd-Allah, E.F. Arbuscular mycorrhizal fungi isolated from highly saline “sabkha habitat” soil alleviated the NaCl-induced stress and improved Lasiurus scindicus Henr. growth. Agriculture 2022, 12, 337. [Google Scholar] [CrossRef]
- Zhang, T.T.; Qi, J.G.; Gao, Y.; Ouyang, Z.T.; Zeng, S.L.; Zhao, B. Detecting soil salinity with MODIS time series VI data. Ecol. Indic. 2015, 52, 480–489. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.G.; Wang, S.; Shi, W.; Liu, R.; Feng, G.; Song, J. Salinity affects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol. Biochem. 2015, 95, 41–48. [Google Scholar] [CrossRef]
- Qu, X.X.; Huang, Z.Y.; Baskin, J.M.; Baskin, C.C. Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte shrub Halocnemum strobilaceum. Ann. Bot. 2008, 101, 293–299. [Google Scholar] [CrossRef] [Green Version]
- El-Keblawy, A.; Gairola, S.; Bhatt, A. Maternal salinity environment affects salt tolerance during germination in Anabasis setifera: A facultative desert halophyte. J. Arid. Land 2016, 8, 254–263. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; De Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Dai, X.; Huo, Z.; Wang, H. Simulation for response of crop yield to soil moisture and salinity with artificial neural network. Field Crops Res. 2011, 121, 441–449. [Google Scholar] [CrossRef]
- Din, B.U.; Sarfraz, S.; Xia, Y.; Kamran, M.A.; Javed, M.T.; Sultan, T.; Munis, M.F.H.; Chaudhary, H.J. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 2019, 183, 109466. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Fayaz, F.; Zahedi, M. Beneficial effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) nutritional status and tolerance indices under soil salinity stress. J. Plant Nutr. 2021, 45, 185–201. [Google Scholar] [CrossRef]
- Sofy, M.; Mohamed, H.; Dawood, M.; Abu-Elsaoud, A.; Soliman, M. Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch. Agron. Soil Sci. 2021, 1–22. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Lin, X.; Sun, C. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Prerna, D.I.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Vasantharaja, R.; Chaturvedi, S.; Shkolnik, D. Influence of nanoscale micro-nutrient α-Fe2O3 on seed germination, seedling growth, translocation, physiological effects and yield of rice (Oryza sativa) and maize (Zea mays). Plant Physiol. Biochem. 2021, 162, 564–580. [Google Scholar] [CrossRef]
- Koger, C.H.; Reddy, K.N.; Poston, D.H. Factors affecting seed germination, seedling emergence, and survival of texasweed (Caperonia palustris). Weed Sci. 2004, 52, 989–995. [Google Scholar] [CrossRef]
- Bidgoly, R.O.; Balouchi, H.; Soltani, E.; Moradi, A. Effect of temperature and water potential on Carthamus tinctorius L. seed germination: Quantification of the cardinal temperatures and modeling using hydrothermal time. Ind. Crops Prod. 2018, 113, 121–127. [Google Scholar] [CrossRef]
- Khaeim, H.; Kende, Z.; Jolánkai, M.; Kovács, G.P.; Gyuricza, C.; Tarnawa, Á. Impact of temperature and water on seed germination and seedling growth of maize (Zea mays L.). Agronomy 2022, 12, 397. [Google Scholar] [CrossRef]
- Blackport, R.; Screen, J.A. Insignificant effect of Arctic amplification on the amplitude of midlatitude atmospheric waves. Sci. Adv. 2020, 6, eaay2880. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Qaiser, M. Halophytes of Pakistan: Characteristics, distribution and potential economic usages. In Sabkha Ecosystems; Springer: Berlin/Heidelberg, Germany, 2006; pp. 129–153. [Google Scholar]
- Bai, H.; Xiao, D.; Wang, B.; Liu, D.L.; Feng, P.; Tang, J. Multi-model ensemble of CMIP6 projections for future extreme climate stress on wheat in the North China Plain. Int. J. Climatol. 2021, 41, E171–E186. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Change Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Mondoni, A.; Rossi, G.; Orsenigo, S.; Probert, R.J. Climate warming could shift the timing of seed germination in alpine plants. Ann. Bot. 2012, 110, 155–164. [Google Scholar] [CrossRef]
- Utset, A.; Borroto, M. A modeling-GIS approach for assessing irrigation effects on soil salinisation under global warming conditions. Agric. Water Manag. 2001, 50, 53–63. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Chen, Y.; Yang, C.; Shi, D. Effects of salt and alkali stresses on growth and ion balance in rice (Oryza sativa L.). Plant Soil Environ. 2011, 57, 286–294. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Ansari, F.; Hassan, N.; Al-Shamsi, N. Salinity, temperature and light affect germination of Salsola imbricata. Seed Sci. Technol. 2007, 35, 272–281. [Google Scholar] [CrossRef]
- Gorai, M.; Neffati, M. Germination responses of Reaumuria vermiculata to salinity and temperature. Ann. Appl. Biol. 2007, 151, 53–59. [Google Scholar] [CrossRef]
- Khan, M.A.; Gulzar, S. Light, salinity, and temperature effects on the seed germination of perennial grasses. Am. J. Bot. 2003, 90, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shi, W.; Liu, R.; Xu, Y.; Sui, N.; Zhou, J.; Feng, G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; De Boeck, H.J.; Hou, F. Effects of temperature and salinity on seed germination of three common grass species. Front. Plant Sci. 2021, 12, 731433. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, D.; Zhang, H.; Tian, Y.; Japhet, W.; Wang, P. Germination responses of Medicago ruthenica seeds to salinity, alkalinity, and temperature. J. Arid. Environ. 2009, 73, 135–138. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Ansari, F.; Al-Shamsi, N. Effects of temperature and light on salinity tolerance during germination in two desert glycophytic grasses, Lasiurus scindicus and Panicum turgidum. Grass Forage Sci. 2011, 66, 173–182. [Google Scholar] [CrossRef]
- Sanadya, S.K.; Shekhawat, S.S.; Sahoo, S. Sewan grass: A potential forage grass in arid environments. In Grasses and Grassland-New Perspectives; IntechOpen Limited: London, UK, 2021. [Google Scholar]
- Assaeed, A.M.; Al-Doss, A.A. Seedling competition of Lasiurus scindicus and Rhazya stricta in response to water stress. J. Arid. Environ. 2001, 49, 315–320. [Google Scholar] [CrossRef]
- Chaudhary, S.A.; Al-Jowaid, A.A.A. Vegetation of the Kingdom of Saudi Arabia; National Agricultural and Water Research Center, Ministry of Agriculture and Water: Riyadh, Saudi Arabia, 1999. [Google Scholar]
- Cunningham, P.L. Plants included in the diet of Arabian sand gazelle (Reem) from Saudi Arabia. J. King Saud Univ.-Sci. 2013, 25, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.S. Sustainable use of marginal lands to improve food security in the United Arab Emirates. J. Exp. Biol. Agric. Sci. 2017, 5, S41–S49. [Google Scholar] [CrossRef]
- Chauhan, S.S. Desertification control and management of land degradation in the Thar desert of India. Environmentalist 2003, 23, 219–227. [Google Scholar] [CrossRef]
- Naz, N.; Rafique, T.; Hameed, M.; Ashraf, M.; Batool, R.; Fatima, S. Morpho-anatomical and physiological attributes for salt tolerance in sewan grass (Lasiurus scindicus Henr.) from Cholistan Desert, Pakistan. Acta Physiol. Plant. 2014, 36, 2959–2974. [Google Scholar] [CrossRef]
- El-Sheikh, M.A.; Thomas, J.; Alatar, A.A.; Hegazy, A.K.; Abbady, G.A.; Alfarhan, A.H.; Okla, M.I. Vegetation of Thumamah Nature Park: A managed arid land site in Saudi Arabia. Rend. Lincei 2013, 24, 349–367. [Google Scholar] [CrossRef]
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.-M.; Lutts, S. Osmotic and ionic effects of NaCl on germination, early seedling growth, and ion content of Atriplex halimus (Chenopodiaceae). Can. J. Bot. 2002, 80, 297–304. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1980; Volume 2, pp. 137–139. [Google Scholar]
- Almodares, A.; Hadi, M.; Dosti, B. Effects of salt stress on germination percentage and seedling growth in sweet sorghum cultivars. J. Biol. Sci. 2007, 7, 1492–1495. [Google Scholar] [CrossRef] [Green Version]
- El-Keblawy, A. Salinity effects on seed germination of the common desert range grass, Panicum turgidum. Seed Sci. Technol. 2004, 32, 873–878. [Google Scholar] [CrossRef]
- Shiade, S.R.G.; Boelt, B. Seed germination and seedling growth parameters in nine tall fescue varieties under salinity stress. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 485–494. [Google Scholar] [CrossRef]
- AL-Shoaibi, A.A. Combined effects of salinity and temperature on germination, growth and gas exchange in two cultivars of Sorghum bicolor. J. Taibah Univ. Sci. 2020, 14, 812–822. [Google Scholar] [CrossRef]
- Cherifi, K.; Boufous, E.H.; Boubaker, H.; Msanda, F. Comparative Salt Tolerance Study of Some Acacia Species at Seed Germination Stage. arXiv 2016, arXiv:1610.06033. [Google Scholar]
- Ben Zetta, H.; Amrani, S.; Nacer, A.J.A.E.; Research, E. Effects of pre-germination treatments, salt and water stresses on germination of Acacia ehrenbergiana Hayne and Acacia seyal Del.(Mimosoideae): Two Algerian native species. Appl. Ecol. Environ. Res. 2017, 15, 355–368. [Google Scholar] [CrossRef]
- Zhumabekova, Z.; Xu, X.; Wang, Y.; Song, C.; Kurmangozhinov, A.; Sarsekova, D. Effects of sodium chloride and sodium sulfate on Haloxylon ammodendron seed germination. Sustainability 2020, 12, 4927. [Google Scholar] [CrossRef]
- Chiu, R.S.; Nahal, H.; Provart, N.J.; Gazzarrini, S. The role of the Arabidopsis FUSCA3 transcription factor during inhibition of seed germination at high temperature. BMC Plant Biol. 2012, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High Temperature-Induced Abscisic Acid Biosynthesis and Its Role in the Inhibition of Gibberellin Action in Arabidopsis Seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, K.; Eskandari, H. Effects of salt stress on germination and early seedling growth of rice (Oryza sativa) cultivars in Iran. Afr. J. Biotechnol. 2011, 10, 17789–17792. [Google Scholar] [CrossRef]
- Guo, C.; Shen, Y.; Shi, F. Effect of temperature, light, and storage time on the seed germination of Pinus bungeana Zucc. ex Endl.: The role of seed-covering layers and abscisic acid changes. Forests 2020, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Mwando, E.; Han, Y.; Angessa, T.T.; Zhou, G.; Hill, C.B.; Zhang, X.Q.; Li, C. Genome-wide association study of salinity tolerance during germination in barley (Hordeum vulgare L.). Front. Plant Sci. 2020, 11, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uçarlı, C. Effects of salinity on seed germination and early seedling stage. In Abiotic Stress in Plants; IntechOpen: London, UK, 2020; p. 211. [Google Scholar]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Nisar, F.; Gul, B.; Khan, M.A.; Hameed, A. Germination and recovery responses of heteromorphic seeds of two co-occurring Arthrocnemum species to salinity, temperature and light. S. Afr. J. Bot. 2019, 121, 143–151. [Google Scholar] [CrossRef]
- Bhatt, A.; Santo, A. Germination and recovery of heteromorphic seeds of Atriplex canescens (Amaranthaceae) under increasing salinity. Plant Ecol. 2016, 217, 1069–1079. [Google Scholar] [CrossRef]
- Arias, C.; Serrat, X.; Moysset, L.; Perissé, P.; Nogués, S. Morpho-physiological responses of alamo switchgrass during germination and early seedling stage under salinity or water stress conditions. BioEnergy Res. 2018, 11, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Asaadi, A.M. Investigation of salinity stress on seed germination of Trigonella foenum-graecum. Res. J. Biol. Sci. 2009, 4, 1152–1155. [Google Scholar]
- Krishnamurthy, L.; Serraj, R.; Hash, C.T.; Dakheel, A.J.; Reddy, B.V.J.E. Screening sorghum genotypes for salinity tolerant biomass production. Euphytica 2007, 156, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Ahamed, K.U.; Hakeem, K.R.; Ozturk, M.; Fujita, M. Plant responses and tolerance to high temperature stress: Role of exogenous phytoprotectants. In Crop Production and Global Environmental Issues; Springer: Berlin/Heidelberg, Germany, 2015; pp. 385–435. [Google Scholar]
- Borsai, O.; Hassan, M.A.; Negrușier, C.; Raigón, M.D.; Boscaiu, M.; Sestraș, R.E.; Vicente, O. Responses to salt stress in Portulaca: Insight into its tolerance mechanisms. Plants 2020, 9, 1660. [Google Scholar] [CrossRef]
- Boutraa, T.; Akhkha, A.; Al-Shoaibi, A.K. Evaluation of growth and gas exchange rates of two local saudi wheat cultivars grown under heat stress conditions. Pak. J. Bot. 2015, 47, 27–34. [Google Scholar]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Oh, S.; Arora, R.; Kim, D. Proline accumulation in response to high temperature in winter-acclimated shoots of Prunus persica: A response associated with growth resumption or heat stress? Can. J. Plant Sci. 2016, 96, 630–638. [Google Scholar] [CrossRef] [Green Version]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef]
- Signorelli, S. The fermentation analogy: A point of view for understanding the intriguing role of proline accumulation in stressed plants. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [Green Version]
- Ashrafijou, M.; Noori, S.S.; Darbandi, A.I.; Saghafi, S.J.P. Effect of salinity and radiation on proline accumulation in seeds of canola (Brassica napus L.). Plant Soil Environ. 2010, 56, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Cvikrová, M.; Gemperlová, L.; Martincová, O.; Vanková, R. Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol. Biochem. 2013, 73, 7–15. [Google Scholar] [CrossRef]
| Species | Country | Site | Location | Seed Color | Seed Weight |
|---|---|---|---|---|---|
| Lasiurus scindicus | Saudi Arabia | Escarpment habitats of Thumamah National Park, Riyadh | 25°08′13.7″ N 46°36′00.9″ E | Dark Brown | 4.02 ± 0.1494 g/1000 seeds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, J.A.; AlQarawi, A.A.; AlZain, M.N.; Dar, B.A.; Habib, M.M.; Ibrahim, S.N.S. Effect of Salinity and Temperature on the Seed Germination and Seedling Growth of Desert Forage Grass Lasiurus scindicus Henr. Sustainability 2022, 14, 8387. https://doi.org/10.3390/su14148387
Malik JA, AlQarawi AA, AlZain MN, Dar BA, Habib MM, Ibrahim SNS. Effect of Salinity and Temperature on the Seed Germination and Seedling Growth of Desert Forage Grass Lasiurus scindicus Henr. Sustainability. 2022; 14(14):8387. https://doi.org/10.3390/su14148387
Chicago/Turabian StyleMalik, Jahangir A., AbdulAziz A. AlQarawi, Mashail N. AlZain, Basharat A. Dar, Muhammad M. Habib, and Salah Nasser S. Ibrahim. 2022. "Effect of Salinity and Temperature on the Seed Germination and Seedling Growth of Desert Forage Grass Lasiurus scindicus Henr." Sustainability 14, no. 14: 8387. https://doi.org/10.3390/su14148387
APA StyleMalik, J. A., AlQarawi, A. A., AlZain, M. N., Dar, B. A., Habib, M. M., & Ibrahim, S. N. S. (2022). Effect of Salinity and Temperature on the Seed Germination and Seedling Growth of Desert Forage Grass Lasiurus scindicus Henr. Sustainability, 14(14), 8387. https://doi.org/10.3390/su14148387









