Plant Nutrition for Human Health: A Pictorial Review on Plant Bioactive Compounds for Sustainable Agriculture
Abstract
1. Introduction
2. Methodology of the Review
3. Plant Nutrition and Sustainable Agriculture
- By 2030, ending hunger;
- By 2030, ending all malnutrition forms;
- By 2030, doubling agricultural productivity;
- By 2030, ensuring sustainable food production systems [27].
4. Plant Nutrients Uptake and Their Physiological Functions
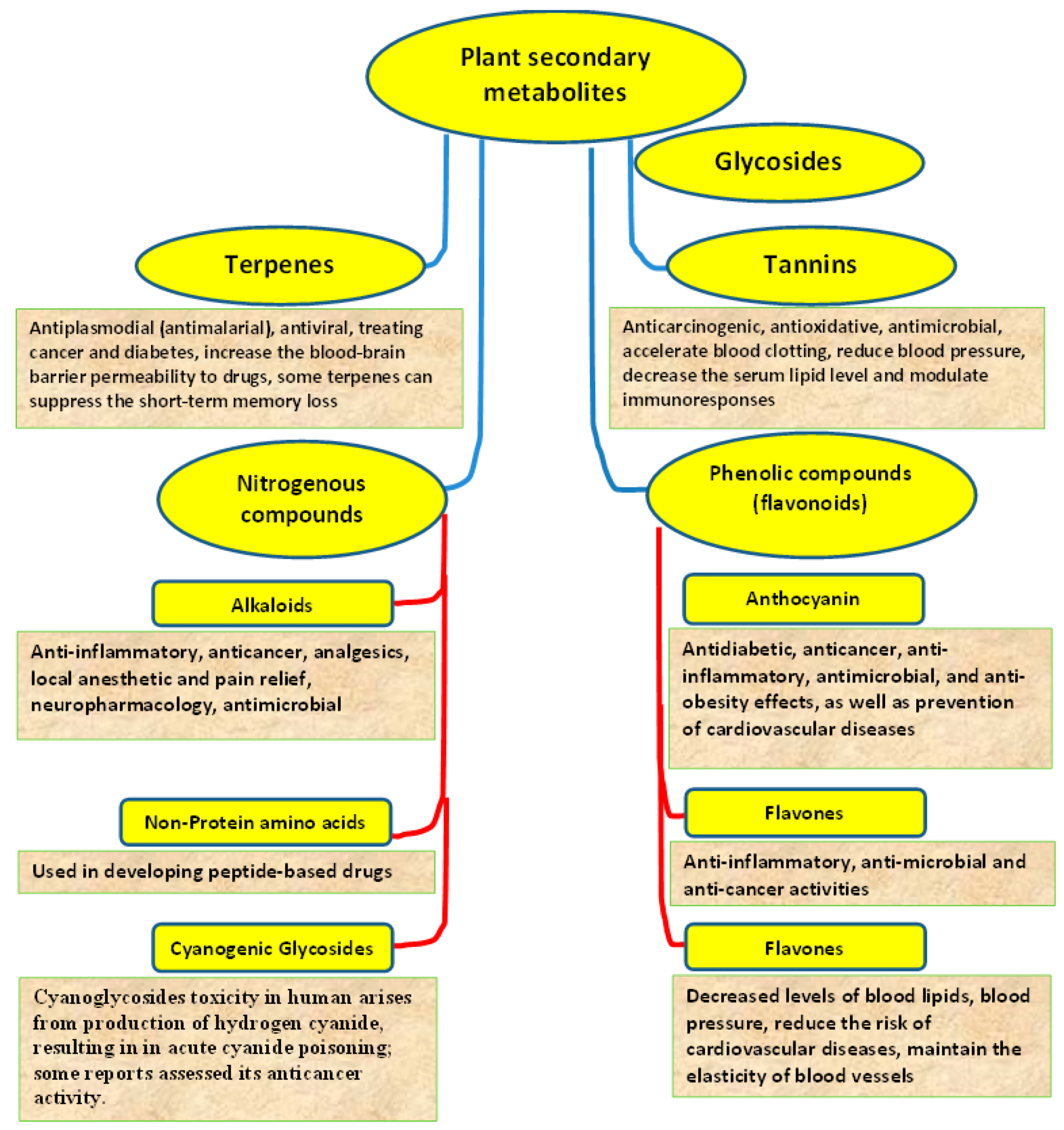
5. Medicinal Plants and Their Bioactive Compounds
| Common Name(s) | Scientific Name | Plant Family | Commonly Used Plant Part(s) | Common Uses | Refs. |
|---|---|---|---|---|---|
| Adzuki bean | Vigna angularis (Willd.) Ohwi and H. Ohash | Fabaceae | Seeds | Seeds for cooking with rice | [92] |
| Andean lupin, pearl lupin | Lupinus mutabilis Sweet | Fabaceae | Seeds | Seeds for culinary use | [93] |
| African yam bean | Sphenostylis stenocarpa Hochst ex. A. Rich. Harms | Fabaceae | Seeds, tubers | Seeds for culinary use | [94] |
| Bambara nut or groundnut | Vigna stenocarpa L. Verdc | Fabaceae | Seeds | Seeds for foods and beverages | [95] |
| Deer-eye beans, donkey-eye bean | Mucuna spp. | Fabaceae | Seeds | Seeds for culinary use | [96] |
| Jack beans, sword bean | Canavalia spp. | Fabaceae | Pods and seeds | Pods as vegetable; seeds for culinary use | [97] |
| Ground bean, Hausa groundnut | Macrotyloma geocarpum (Harms.) Maréchal and Baudet | Fabaceae | Seeds like peanuts | Seeds for culinary use | [98] |
| Hyacinth bean, lablab bean | Lablab purpureus L. | Fabaceae | Leaves, seeds | Seeds used culinarily | [99] |
| Horse gram | Macrotyloma uniflorum (Lam.) Verdc. | Fabaceae | Seeds | Seeds for culinary use | [100] |
| Peavines, vetchlings | Lathyrus spp. | Fabaceae | Pods, seeds | Seeds for culinary use | [101] |
| Moth bean | Vigna aconitifolia (Jacq.) Marechal | Fabaceae | Pods, seeds | Seeds for culinary use | [102] |
| Stinky bean | Parkia speciosa Hassk. | Fabaceae | Pods, seeds | Pods and seeds for culinary use | [103] |
| Rattlepods | Crotalaria spp. | Fabaceae | Leaves, flowers, pods, seeds | Leafy vegetable; seeds for culinary use | [104] |
| Rice bean | Vigna umbellata (Thunb.) Ohwi and H. Ohashi | Fabaceae | Seeds | Seeds for culinary use | [105] |
| White lead tree, subabul | Leucaena leucocephala (Lam.) de Wit | Fabaceae | Pods | Young pods used as vegetable | [106] |
| Winged bean | Psophocarpus tetragonolobus (L.) D.C. | Fabaceae | Leaves, seeds, bean pods, roots | Entire winged been plant is edible | [91] |
| Amaranth | Amaranthus spp. | Amaranthaceae | Leaves, seeds | Leafy vegetable, oil, pigments | [107] |
| Black nightshade | Solanum nigrum L. | Solanaceae | Leaves, fruits (berries) | Leaves and berries as food prepared by cooking | [108] |
| Common purslane | Portulaca oleracea L. | Portulacaceae | Leaves, stem | Leafy vegetable | [109] |
| Curcuma | Curcuma spp. | Zingiberaceae | Rhizomes, roots, leaves | Roots edible, rhizomes culinary | [110] |
| Bitter melon, spiny gourd | Momordica spp. | Cucurbitaceae | Fruits | Fruits as vegetable | [91] |
| Indian poke | Phytolacca acinose Roxb. | Phytolaccaceae | Leaves | Leafy vegetable | [91] |
| Mallow leaves | Corchorus spp. | Malvaceae | Leaves | Leafy vegetable | [91] |
| Parsnip | Pastinaca sativa L. | Apiaceae | Roots | Root vegetable like carrot | [91] |
| Prickly pear | Opuntia spp. | Cactaceae | Leaves or cladodes | Leaves cooked as a vegetable | [96] |
| Squash, pumpkin | Cucurbita spp. | Cucurbitaceae | Leaves, fruits, seeds | Fruits for culinary | [111] |
| Sea kale | Crambe spp. | Brassicaceae | Leaves | Leafy vegetable | [91] |
| Tassel hyacinth | Leopoldia comosa (L.) Parl. | Asparagaceae | Bulbs | Bulbs as vegetable | [112] |
| Tomatillo | Physalis philadelphica Lam. | Solanaceae | Fruits | Green fruits as vegetable | [91] |
| Yellow cresses | Rorippa indica (L.) Hiern | Brassicaceae | Tender shoots, leaves | Leafy vegetable | [91] |
| Water spinach | Ipomoea aquatica Forssk. | Convolvulaceae | Leaves | Leafy vegetable | [113] |
| Water leaf, Ceylon spinach | Talinum triangulare (Jacq.) Willd. | Talinaceae | Leaves | Leafy vegetable | [114] |
| Plant Species | Therapeutic Agent | Target Disease | Ref. |
|---|---|---|---|
| Artemisia annua L. | Artemisinin | Malignant treatment | [116] |
| Artemisia obtusiloba Ledeb. | Arglabin | Cancer chemotherapy | [81] |
| Amorpha fruticose L. | Monoterpene and sesquiterpene | Antibacterial, insecticidal, and cytotoxic effects | [85] |
| Calophyllum lanigerum Miq. | Calanolide A | Type-1 HIV | [85] |
| Capsicum annum L. | Capsaicin | Postherpetic neuralgia treatment | [81] |
| Cannabis sativa L. | Dronabinol; Cannabidol | Chronic neuropathic pain | [81] |
| Caragana sinica (Buc’hoz) Rehder | Collagen and aggrecan | Preventing degradation of cartilages | [117] |
| Carthamus tinctorius L. | Serotonin/N-feruloyl serotonin | Preventing degradation of cartilages | [118] |
| Cephalotaxus harringtonia (Knight ex J.Forbes) K.Koch | Homo-harringtonine | Oncology treatment | [81] |
| Colchicum spp. | Colchicine | Gout disease | [81] |
| Conium macularum L. | Coniine | Poisonous, neurotoxin | [119] |
| Cinchona succirubra Pav. ex Klotzsch | Quinine | Antimalarial | [120] |
| Dalbergia sissoo Roxb. ex DC. | Flavonoids (Tectorigenin) | Anti-degradation of cartilage proteins | [121] |
| Euphorbia peplus L. | Ingenol mebutate | Actinic keratosis treatment | [81] |
| Galanthus woronowii Losinsk. | Galantamine | Alzheimer’s disease | [122] |
| Galega officinalis L. | Metformin | Anti-diabetic | [85] |
| Larrea tridentata (DC.) Coville | Masoprocol | Cancer chemotherapy | [81] |
| Nigella sativa L. | Thymoquinone | Osteoarthritis treatment | [115] |
| Oroxylum indicum (L.) Kurz | Oroxylin A | Anti-degrading markers of cartilages | [123] |
| Papaver somniferum L. | Morphine | Acute pulmonary disease and breath shortness | [124] |
| Papaver somniferum L. | Codeine | Analgesic and anti-diarrheal properties | [125] |
| Papaver bracteatum Lindl. | Thebaine (paramorphine) | Analgesic | [126] |
| Phyla nodiflora (L.) Greene | Nepetin | Osteoarthritis treatment | [127] |
| Rhus succedanea L. | Rhoifolin | Preventing degradation of cartilages | [128] |
| Pueraria lobata (Willd.) Ohwi | Puerarin | Against cartilage degradation | [129] |
| Sarcotheca griffithii (Planch.) Hallier f. | Crude extract | Cough | [130] |
| Solanum spp. | Nicotine | Anti-inflammatory and stimulant | [131] |
| Solanum tuberosum L. | Solanine | Anticarcinogenic | [132] |
| Solanum lycopersicum L. | Tomatine | Anticancer and immune effects | [133] |
| Talinum triangulare (Jacq.) Willd. | Acrylamide and phaeophytin | Cuts, wound, scabies, and peptic ulcer | [134] |
| Taxus brevifolia Nutt. | Paclitaxel | Antimitotic agent for various cancers | [81] |
6. Plant Nutrition Management for Human Health
- How can the world increase crop productivity to double its current amount, especially under the global nutrient imbalance?
- How can the world guarantee this production to double or triple, particularly in developing countries such as African nations under unbalanced inputs of human nutrition?
- What is the role of precision or smart farming in accelerating the adoption by farmers of more solutions for precise nutrient management?
- What are the sustainable solutions for decreasing the losses of nutrients, such that their wastes along the whole agri-food chain are halved?
- To what extent can the nutrient cycles in the farming of crops and livestock be made closed?
- What are the key measures to improve and sustain soil health?
- What is the main role of mineral nutrition of different crops and its changes in a changing climate?
- To what extent can applied fertilizers reduce greenhouse gas emissions?
- What is the main role of cropping systems in producing high crop quality and more nutritious foods?
- To what extent can we monitor nutrients for implementation of 4R nutrient stewardship?
6.1. Plant Nutrition under Climate Change
6.2. Plant Nutrition under Pollution
| Studied Plant | Abiotic Stress Details | Plant Bioactive Compound and Its Response | Ref. |
|---|---|---|---|
| Robinia pseudoacacia L. | Applied Cd at 0.45 and 4.5 mg Cd kg−1 | Elevated CO2 (up to 750 ppm) may promote synthesis of total flavonoids under Cd stress | [194] |
| Potato (Solanum tuberosum L.) | Drought stress by discontinued irrigation for 6 weeks after 88 days planting | No significant effect of drought on phenolic compounds, anthocyanins, or antioxidant activity | [195] |
| Feverfew (Tanacetum parthenium (L.) Sch. Bip.) | Drought stress (irrigation intervals 4, 8, and 12 days as control, moderate, extreme) | Under drought stress, the yield of essential oils decreased by 30%, and phenols and nano-silicon increased plant drought tolerance | [196] |
| Cistus clusii Dunal | Drought stress (days of drought 15, 30, 50) | Increase phenolics (flavonols, epigallocatechin gallate) | [197] |
| Crataegus laevigata (Poir.) DC. | Drought (water deficit for 10 days vs. watering daily) | Increase phenolics (chlorogenic acid, (-)-epicatechin) | [198] |
| Glycine max (L.) Merr. | Drought stress (non-irrigated field) | Increased alkaloids (trigonelline) | [199] |
| Hypericum brasiliense Choisy | Drought stress (water stress (waterlogging and drought)) | Increased terpenoids (betulinic acid) | [200] |
| Pepper (Capsicum annuum L.) | In vitro, supplemented MS with 200 µM CdCl2 | ZnO-NPs induced mitigation of Cd-toxicity by increased activity of enzymatic antioxidants | [201] |
| Giant Juncao (Pennisetum giganteum Ten. ex Steud.) | Salt stress (250, 500 mM NaCl) | Salicylic acid mitigated the adverse impacts of salt stress, which decreased flavonoids by enhancing the content of chlorogenic acid | [202] |
| Brassica nigra L. | Salt stress (100 and 150 mM NaCl) | Salinity enhanced forming of many bioactives e.g., phytosterols, and tocopherols | [203] |
| Faba bean: Vicia faba L. | Salt stress at 150 mM NaCl | Salinity induced accumulation of flavonoids, phenols, and tannins, in response to ZnO-NPs | [204] |
| Calotropis procera (Aiton) | Salt stress in 3 experiments up to 320 mM NaCl using Petri dishes and hydropriming | Seed priming with thiourea and ascorbic acid increased tolerance to salinity up to 120 mM by increasing phenolic acids (gallic, caffeic, p–coumaric, p–benzoic, and sinapic acid) | [205] |
| Wheat (Triticum aestivum L.) | Salt stress (150 mM) | Priming with 0.12% Cu-chitosan-NP induced an increase in β-carotenoids, total carotenoids | [206] |
| Catharanthus roseus (L.) G. Don | Cold stress (4 °C) in growth chamber | Decreased alkaloids (vindoline) | [207] |
| Glycine max (L.) Merr. | Cold stress (10 °C) | Increased phenolics (genistein, daidzein) | [208] |
| Solanum Lycopersicon L. | Cold stress (6 and 3 °C) | Increased terpenoids (δ-elemene, α-humulene, and β-caryophyllene) | [209] |
| Withania somnifera (L.) Dunal | Cold stress (4 °C) under controlled environment | Increased terpenoids (withanolide A; withferin A) | [210] |
| Zea mays L. | Cold stress (10 °C) | Increased phenolics (pelargonidin) | [211] |
6.3. Plant Nutrition under Stressful Soil
7. General Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stratton, A.E.; Kuhl, L.; Blesh, J. Ecological and Nutritional Functions of Agroecosystems as Indicators of Smallholder Resilience. Front. Sustain. Food Syst. 2020, 4, 543914. [Google Scholar] [CrossRef]
- Goncharova, N.A.; Merzlyakova, N.V. Food Shortages and Hunger as a Global Problem. Food Sci. Technol. 2022, 42, e70621. [Google Scholar] [CrossRef]
- Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The Molecular–Physiological Functions of Mineral Macronutrients and Their Consequences for Deficiency Symptoms in Plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- Roberts, D.P.; Mattoo, A.K. Sustainable Crop Production Systems and Human Nutrition. Front. Sustain. Food Syst. 2019, 3, 72. [Google Scholar] [CrossRef]
- Cakmak, I. Plant Nutrition Research: Priorities to Meet Human Needs for Food in Sustainable Ways. Plant Soil 2002, 247, 3–24. [Google Scholar] [CrossRef]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant Nutrition under Climate Change and Soil Carbon Sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Moses, T.; Goossens, A. Plants for Human Health: Greening Biotechnology and Synthetic Biology. J. Exp. Bot. 2017, 68, 4009–4011. [Google Scholar] [CrossRef]
- Bruulsema, T.; Cakmak, I.; Dobermann, A.; Gerard, B.; Majumdar, K.; McLaughlin, M.; Reidsma, P.; Vanlauwe, B.; Wollenberg, E.K.; Zhang, F.; et al. Scientific Panel on Responsible Plant Nutrition. A New Paradigm for Plant Nutrition. Issue Brief 01. Available online: https://www.apni.net/downloads/sprpnIss01.pdf (accessed on 4 April 2022).
- Kumar, V.; Srivastava, A.; Suprasanna, P. Plant Nutrition and Food Security in the Era of Climate Change; Academic Press: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and Biological Properties of Satureja Cuneifolia Ten. and Thymus Spinulosus Ten.: Two Wild Officinal Species of Conservation Concern in Apulia (Italy). A Preliminary Survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and Antimicrobial Properties of Essential Oils from Four Wild Taxa of Lamiaceae Family Growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWRs) Threatened and Endemic to Italy: Urgent Actions for Protection and Use. Biology 2022, 11, 193. [Google Scholar] [CrossRef]
- Larson-Meyer, E.D.; Ruscigno, M. Plant-Based Sports Nutrition: Expert Fueling Strategies for Training, Recovery, and Performance, 1st ed.; Human Kinetics: Champaign, IL, USA, 2020. [Google Scholar]
- Masoodi, M.H.; Rehman, M.U. Edible Plants in Health and Diseases: Volume 1: Cultural, Practical and Economic Value; Springer Nature: Singapore, 2022; ISBN 9789811648793. [Google Scholar]
- Masoodi, M.H.; Rehman, M.U. Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties; Springer: Singapore, 2022; ISBN 9789811649585. [Google Scholar]
- Savage, E. Healing Through Nutrition: The Essential Guide to 50 Plant-Based Nutritional Sources; ROCKRIDGE Press: Emeryville, CA, USA, 2020; ISBN 978-1-64152-813-9. [Google Scholar]
- Summer, E. Plant-based high-protein diet: The Athletes Nutrition Guide with Easy Recipes to Burn Fat. In How to Use Vegetable-Based Protein and Boost Energy for Muscle Growth and Athletic Performance Improvement; Independently Published: Chicago, IL, USA, 2020; ISBN 9798628313237. [Google Scholar]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.-Y.; Lu, Y.; Jiao, J.; Fu, J.-X.; Xu, X.-J.; Yao, L.; Fu, Y.-J. Application of UV-B Radiation for Enhancing the Accumulation of Bioactive Phenolic Compounds in Pigeon Pea [Cajanus Cajan (L.) Millsp.] Hairy Root Cultures. J. Photochem. Photobiol. B 2022, 228, 112406. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaini, H.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana Peels as a Bioactive Ingredient and Its Potential Application in the Food Industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Paulo, F.; Tavares, L.; Santos, L. Extraction and Encapsulation of Bioactive Compounds from Olive Mill Pomace: Influence of Loading Content on the Physicochemical and Structural Properties of Microparticles. J. Food Meas. Charact. 2022. [Google Scholar] [CrossRef]
- Patil, S.; Vedashree, M.; Murthy, P.S. Valorization of Coffee Leaves as a Potential Agri-Food Resource: Bio-Active Compounds, Applications and Future Prospective. Planta 2022, 255, 67. [Google Scholar] [CrossRef]
- Fageria, N.K. Soil Fertility and Plant Nutrition Research under Controlled Conditions: Basic Principles and Methodology. J. Plant Nutr. 2005, 28, 1975–1999. [Google Scholar] [CrossRef]
- Fageria, N.K. Soil Fertility and Plant Nutrition Research under Field Conditions: Basic Principles and Methodology. J. Plant Nutr. 2007, 30, 203–223. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Alshaal, T.A.; Shehata, S.A.; Domokos-Szabolcsy, É.; Elhawat, N.; Prokisch, J.; Fári, M.; Marton, L. Plant Nutrition: From Liquid Medium to Micro-Farm. In Sustainable Agriculture Reviews 14; Ozier-Lafontaine, H., Lesueur-Jannoyer, M., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2014; Volume 14, pp. 449–508. ISBN 978-3-319-06015-6. [Google Scholar]
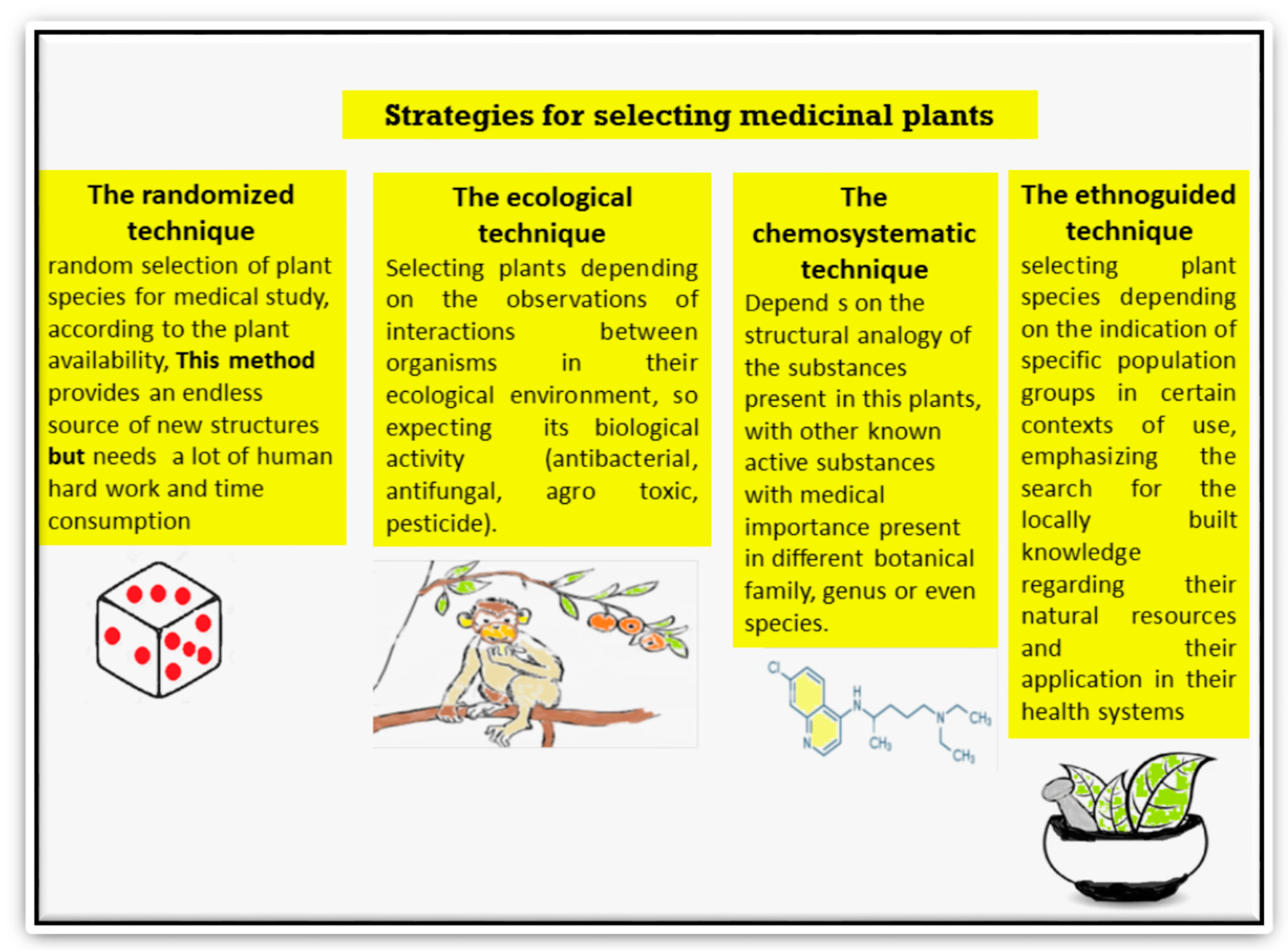
- Barbosa, W.L.R.; Nascimento, M.S.; do Pinto, L.N.; Maia, F.L.C.; Sousa, A.J.A.; Júnior, J.O.C.S.; Monteiro, M.M.; de Oliveira, D.R. Selecting Medicinal Plants for Development of Phytomedicine and Use in Primary Health Care; InTech Open: Rijeka, Croatia, 2012; ISBN 978-953-307-805-2. [Google Scholar]
- United Nations Sustainable Development Goals. Sustainable Development Knowledge Platform. Available online: https://sustainabledevelopment.un.org/sdgs (accessed on 20 April 2022).
- Dordas, C. Role of Nutrients in Controlling Plant Diseases in Sustainable Agriculture: A Review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 443–460. ISBN 978-90-481-2665-1. [Google Scholar]
- Isaac, W.-A.; Felix, N.; Ganpat, W.G.; Saravanakumar, D.; Churaman, J. Sustainable Climate-Smart Agricultural Solutions to Improve Food and Nutrition Security in Trinidad and Tobago. In Development, Political, and Economic Difficulties in the Caribbean; Bissessar, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 167–195. ISBN 978-3-030-02993-7. [Google Scholar]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for Agricultural and Environmental Sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Agrawal, S.; Kumar, V.; Kumar, S.; Shahi, S.K. Plant Development and Crop Protection Using Phytonanotechnology: A New Window for Sustainable Agriculture. Chemosphere 2022, 299, 134465. [Google Scholar] [CrossRef]
- Cappelli, S.L.; Domeignoz-Horta, L.A.; Loaiza, V.; Laine, A.-L. Plant Biodiversity Promotes Sustainable Agriculture Directly and via Belowground Effects. Trends Plant Sci. 2022, 27, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Ditta, A.; Arshad, M. Applications and Perspectives of Using Nanomaterials for Sustainable Plant Nutrition. Nanotechnol. Rev. 2016, 5, 209–229. [Google Scholar] [CrossRef]
- Perea Vélez, Y.S.; Carrillo-González, R.; González-Chávez, M. Interaction of Metal Nanoparticles–Plants–Microorganisms in Agriculture and Soil Remediation. J. Nanopart. Res. 2021, 23, 206. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Koopaee, H.K.; Rezaei, V.; Esmailizadeh, A. DNA Biosensors Techniques and Their Applications in Food Safety, Environmental Protection and Biomedical Research: A Mini-Review. J. Cell Dev. Biol. 2020, 3, 28–35. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Bangar, M.A.; Baldrich, E.; Muñoz, F.J.; Mulchandani, A. Conducting Polymer Nanowire-Based Chemiresistive Biosensor for the Detection of Bacterial Spores. Biosens. Bioelectron. 2010, 25, 2309–2312. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, L.; Wan, Y.; Gu, Z. Pathogenic Detection and Phenotype Using Magnetic Nanoparticle-Urease Nanosensor. Sens. Actuators B Chem. 2018, 259, 428–432. [Google Scholar] [CrossRef]
- Podar, D.; Maathuis, F.J.M. Primary Nutrient Sensors in Plants. iScience 2022, 25, 104029. [Google Scholar] [CrossRef]
- Marles, R.J. Mineral Nutrient Composition of Vegetables, Fruits and Grains: The Context of Reports of Apparent Historical Declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Li, J.; Huang, K.; Yang, X.; Chen, J.; Liu, X.; Cao, J.; Chen, S.; Shen, C.; et al. Fruit and Vegetable Consumption, Cardiovascular Disease, and All-Cause Mortality in China. Sci. China Life Sci. 2022, 65, 119–128. [Google Scholar] [CrossRef]
- Bharali, B.; Chetia, H.; Kalita, J.J.; Mosahari, P.V.; Chhillar, A.K.; Bora, U. Engineered Nanomaterials in Plants: Sensors, Carriers, and Bio-Imaging. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 87, pp. 133–157. ISBN 978-0-12-821320-9. [Google Scholar]
- Karthika, K.S.; Rashmi, I.; Parvathi, M.S. Biological Functions, Uptake and Transport of Essential Nutrients in Relation to Plant Growth. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 1–49. ISBN 978-981-10-9043-1. [Google Scholar]
- White, P.J.; Brown, P.H. Plant Nutrition for Sustainable Development and Global Health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A. Mineral Nutrition of Plants under Soil Water Deficit Condition: A Review. In Soil Water Deficit and Physiological Issues in Plants; Springer: Singapore, 2021; pp. 287–391. ISBN 978-981-336-275-8. [Google Scholar]
- Rattan, R. Mineral Nutrition of Plants. In Soil Science: An Introduction; Rattan, R.K., Katyal, J.C., Dwivedi, B.S., Sarkar, A.K., Bhattacharyya, T., Tarafdar, J.C., Kukal, S.S., Eds.; Indian Society of Soil Science: New Delhi, India, 2015. [Google Scholar]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on Metabolism: New Knowledge Gained from Multi-Level Analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Mineral Nutrition. In Plant Physiology, 3rd ed.; Sinauer Publishers: Sunderland, MA, USA, 2002. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition, 5th Edn. Rashtriya Printers, Delhi, First Indian Reprint. Ann. Bot. 2006, 93, 479–480. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological Functions of Mineral Macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hänsch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Kusunoki, M. Mono-Manganese Mechanism of the Photosytem II Water Splitting Reaction by a Unique Mn4Ca Cluster. Biochim. Biophys. Acta BBA-Bioenerg. 2007, 1767, 484–492. [Google Scholar] [CrossRef][Green Version]
- Broschat, T. Iron Deficiency in Palms. Available online: https://edis.ifas.ufl.edu/publication/EP265 (accessed on 31 May 2022).
- Lui, G.; Simonne, E.; Li, Y. Nickel Nutrition in Plants. HS1191 UF/IFAS Extension. Available online: https://edis.ifas.ufl.edu/publication/HS1191 (accessed on 31 May 2022).
- Hebbern, C.A.; Laursen, K.H.; Ladegaard, A.H.; Schmidt, S.B.; Pedas, P.; Bruhn, D.; Schjoerring, J.K.; Wulfsohn, D.; Husted, S. Latent Manganese Deficiency Increases Transpiration in Barley (Hordeum vulgare). Physiol. Plant. 2009, 135, 307–316. [Google Scholar] [CrossRef]
- Chanu, N.B.; Alice, A.K.; Thokchom, A.; Singh, M.C.; Chanu, N.T.; Singh, Y.D. Engineered Nanomaterial and Their Interactions with Plant–Soil System: A Developmental Journey and Opposing Facts. Nanotechnol. Environ. Eng. 2021, 6, 36. [Google Scholar] [CrossRef]
- Morgan, J.B.; Connolly, E.L. Plant-Soil Interactions: Nutrient Uptake. Nat. Educ. Knowl. 2013, 4, 2. [Google Scholar]
- Lesellier, E.; Lefebvre, T.; Destandau, E. Recent Developments for the Analysis and the Extraction of Bioactive Compounds from Rosmarinus Officinalis and Medicinal Plants of the Lamiaceae Family. TrAC Trends Anal. Chem. 2021, 135, 116158. [Google Scholar] [CrossRef]
- Ahmed, F. Antioxidant Activity of Ricinus Communis. Org. Med. Chem. Int. J. 2018, 5, 107–112. [Google Scholar] [CrossRef]
- de la Luz Cádiz-Gurrea, M.; Sinan, K.I.; Zengin, G.; Bene, K.; Etienne, O.K.; Leyva-Jiménez, F.J.; Fernández-Ochoa, Á.; del Carmen Villegas-Aguilar, M.; Mahomoodally, M.F.; Lobine, D.; et al. Bioactivity Assays, Chemical Characterization, ADMET Predictions and Network Analysis of Khaya Senegalensis A. Juss (Meliaceae) Extracts. Food Res. Int. 2021, 139, 109970. [Google Scholar] [CrossRef]
- Sinan, K.I.; Martinović, L.S.; Peršurić, Ž.; Pavelić, S.K.; Etienne, O.K.; Mahomoodally, M.F.; Sadeer, N.B.; Zengin, G. Novel Insights into the Biopharmaceutical Potential, Comparative Phytochemical Analysis and Multivariate Analysis of Different Extracts of Shea Butter Tree-Vitellaria Paradoxa C. F. Gaertn. Process Biochem. 2020, 98, 65–75. [Google Scholar] [CrossRef]
- Tlili, N.; Sarikurkcu, C. Bioactive Compounds Profile, Enzyme Inhibitory and Antioxidant Activities of Water Extracts from Five Selected Medicinal Plants. Ind. Crops Prod. 2020, 151, 112448. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Cör, D.; Simonovska, J.; Knez, Ž.; Kavrakovski, Z.; Rafajlovska, V. Extraction Techniques and Analytical Methods for Characterization of Active Compounds in Origanum Species. Molecules 2020, 25, 4735. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Rai, M.; Kosalec, I. Promising Antimicrobials from Natural Products; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-83503-3. [Google Scholar]
- Maran, S.; Yeo, W.W.Y.; Lim, S.-H.E.; Lai, K.-S. Plant Secondary Metabolites for Tackling Antimicrobial Resistance: A Pharmacological Perspective. In Antimicrobial Resistance; Kumar, V., Shriram, V., Paul, A., Thakur, M., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 153–173. ISBN 9789811631191. [Google Scholar]
- Anand, S.; Bharadvaja, N. Potential Benefits of Nutraceuticals for Oxidative Stress Management. Rev. Bras. Farmacogn. 2022, 32, 211–220. [Google Scholar] [CrossRef]
- Iyer, A.; Guerrier, L.; Leveque, S.; Bestwick, C.S.; Duncan, S.H.; Russell, W.R. High Throughput Method Development and Optimised Production of Leaf Protein Concentrates with Potential to Support the Agri-Industry. J. Food Meas. Charact. 2022, 16, 49–65. [Google Scholar] [CrossRef]
- Stangoulis, J.C.R.; Knez, M. Biofortification of Major Crop Plants with Iron and Zinc-Achievements and Future Directions. Plant Soil 2022, 474, 57–76. [Google Scholar] [CrossRef]
- DeClercq, V.; Nearing, J.T.; Sweeney, E. Plant-Based Diets and Cancer Risk: What Is the Evidence? Curr. Nutr. Rep. 2022, 11, 354–369. [Google Scholar] [CrossRef]
- Setapar, S.; Ahmad, A.; Jawaid, M. Nanotechnology for the Preparation of Cosmetics Using Plant-Based Extracts. A Volume in Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-822967-5. [Google Scholar]
- Ugoeze, K.C.; Odeku, O.A. Herbal Bioactive–Based Cosmetics. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–226. ISBN 978-0-12-824385-5. [Google Scholar]
- Reque, P.M.; Brandelli, A. Encapsulation of Probiotics and Nutraceuticals: Applications in Functional Food Industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Ducrocq, M.; Morel, M.-H.; Anton, M.; Micard, V.; Guyot, S.; Beaumal, V.; Solé-Jamault, V.; Boire, A. Biochemical and Physical–Chemical Characterisation of Leaf Proteins Extracted from Cichorium Endivia Leaves. Food Chem. 2022, 381, 132254. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagan, A.; Lim, L.-T.; Ali, A. Plant Protein Foods; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-91205-5. [Google Scholar]
- Santamaría-Fernández, M.; Lübeck, M. Production of Leaf Protein Concentrates in Green Biorefineries as Alternative Feed for Monogastric Animals. Anim. Feed Sci. Technol. 2020, 268, 114605. [Google Scholar] [CrossRef]
- Ewy, M.W.; Patel, A.; Abdelmagid, M.G.; Mohamed Elfadil, O.; Bonnes, S.L.; Salonen, B.R.; Hurt, R.T.; Mundi, M.S. Plant-Based Diet: Is It as Good as an Animal-Based Diet When It Comes to Protein? Curr. Nutr. Rep. 2022, 11, 337–346. [Google Scholar] [CrossRef]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-Based Diets for Kidney Disease: A Guide for Clinicians. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021, 77, 287–296. [Google Scholar] [CrossRef]
- Kalyniukova, A.; Holuša, J.; Musiolek, D.; Sedlakova-Kadukova, J.; Płotka-Wasylka, J.; Andruch, V. Application of Deep Eutectic Solvents for Separation and Determination of Bioactive Compounds in Medicinal Plants. Ind. Crops Prod. 2021, 172, 114047. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]
- Koprivova, A.; Kopriva, S. Plant Secondary Metabolites Altering Root Microbiome Composition and Function. Curr. Opin. Plant Biol. 2022, 67, 102227. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- El-Ramady, H.; Törős, G.; Badgar, K.; Llanaj, X.; Hajdú, P.; El-Mahrouk, M.E.; Abdalla, N.; Prokisch, J. A Comparative Photographic Review on Higher Plants and Macro-Fungi: A Soil Restoration for Sustainable Production of Food and Energy. Sustainability 2022, 14, 7104. [Google Scholar] [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of Bioactive Plant Secondary Metabolites through in Vitro Technologies—Status and Outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef] [PubMed]
- Fazili, M.A.; Bashir, I.; Ahmad, M.; Yaqoob, U.; Geelani, S.N. In Vitro Strategies for the Enhancement of Secondary Metabolite Production in Plants: A Review. Bull. Natl. Res. Cent. 2022, 46, 35. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Zhu, P.; Nasser, M.I.; Zhang, X.; Zhao, M. Implication of Gut Microbiota in Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 5394096. [Google Scholar] [CrossRef] [PubMed]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Murthy, H.N.; Paek, K.Y. Health Benefits of Underutilized Vegetables and Legumes. In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Paek, K.Y., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–36. ISBN 978-3-030-57414-7. [Google Scholar]
- Rubatzky, V.E.; Yamaguchi, M. World Vegetables: Principles, Production, and Nutritive Values; Chapman & Hall: New York, UK, 1999; ISBN 978-0-8342-1687-7. [Google Scholar]
- Bermejo, J.; Leon, J. Neglected Crops: 1492 from a Different Perspective; FAO Plant Production and Protection Series, No 26; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- Oshodi, A.A.; Ipinmoroti, K.O.; Adeyeye, E.I.; Hall, G.M. Amino and Fatty Acids Composition of African Yam Bean (Sphenostylis Stenocarpa) Flour. Food Chem. 1995, 53, 1–6. [Google Scholar] [CrossRef]
- Yamaguchi, M. World Vegetables; Van Nostrand Reinhold: New York, NY, USA, 1983. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Plant Names: Common Names, Scientific Names, Eponyms, Synonyms and Etymology; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Sridhar, K.; Niveditha, V.R. Nutritional and Bioactive Potential of Coastal Sand Dune Wild Legume Canavalia Maritima (Aubl.) Thou.—An Overview. Indian J. Nat. Prod. Resour. 2014, 5, 107–120. [Google Scholar]
- Dansi, A.; Vodouhè, R.; Azokpota, P.; Yedomonhan, H.; Assogba, P.; Adjatin, A.; Loko, Y.L.; Dossou-Aminon, I.; Akpagana, K. Diversity of the Neglected and Underutilized Crop Species of Importance in Benin. Sci. World J. 2012, 2012, e932947. [Google Scholar] [CrossRef]
- Shivashankar, G.; Kulkarni, R. Lablab Purpureus. In Plant Resources of Southeast Asia (PROSEA); Van Der Maesen, L.J.G., Somaatmadja, S., Eds.; No. 1, Pulses; 1 Health Benefits of Underutilized Vegetables and Legumes 31; PUDOC: Wageningen, The Netherlands, 1992; pp. 48–50. [Google Scholar]
- Bhartiya, A.; Aditya, J.; Kant, L. Nutritional and Remedial Potential of an Underutilized Food Legume Horsegram (Macrotyloma Uniflorum): A Review. J. Anim. Plant Sci. 2015, 53, 1–6. [Google Scholar]
- Lambein, F.; Travella, S.; Kuo, Y.-H.; Van Montagu, M.; Heijde, M. Grass Pea (Lathyrus Sativus L.): Orphan Crop, Nutraceutical or Just Plain Food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K.; Venkatachalam, M. Fractionation and Biochemical Characterization of Moth Bean (Vigna Aconitifolia L.) Proteins. LWT-Food Sci. Technol. 2007, 40, 600–610. [Google Scholar] [CrossRef]
- Chhikara, N.; Devi, H.R.; Jaglan, S.; Sharma, P.; Gupta, P.; Panghal, A. Bioactive Compounds, Food Applications and Health Benefits of Parkia Speciosa (Stinky Beans): A Review. Agric. Food Secur. 2018, 7, 46. [Google Scholar] [CrossRef]
- Morton, J.F. Pito (Erythrina Berteroana) and Chipilin (Crotalaria Longirostrata), (Fabaceae) Two Soporific Vegetables of Central America. Econ. Bot. 1994, 48, 130–138. [Google Scholar] [CrossRef]
- Katoch, R. Nutritional Potential of Rice Bean (Vigna Umbellata): An Underutilized Legume. J. Food Sci. 2013, 78, C8-16. [Google Scholar] [CrossRef]
- Schulke, E. A review on the nutritive value and toxic aspects of leucaena leucocephala. Trop. Anim. Prod. 1979. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi1x9Po3-X4AhWFmVYBHRjbCKsQFnoECAYQAQ&url=https%3A%2F%2Fwww.cipav.org.co%2FTAP%2FTAP%2FTAP42%2F4_2_1.pdf&usg=AOvVaw01pzLRZy-ovwC_LdKb1A6u (accessed on 31 May 2022).
- Assad, R.; Reshi, Z.A.; Jan, S.; Rashid, I. Biology of Amaranths. Bot. Rev. 2017, 83, 382–436. [Google Scholar] [CrossRef]
- Read, B.E. Famine foods listed in the Chiu Huang Pen Ts’ao, giving their identity, nutritional values and notes on their preparation. In Famine Foods Listed in the Chiu Huang Pen Is’ao Giving Their Identity Nutritional Values and Notes on Their Preparation; The Henry Lester Institute of Medical Research: Shanghai, Beijing, 1977. [Google Scholar]
- Wright, C. Mediterranean Vegetables: A Cook’s Compendium of All the Vegetables from The World’s Healthiest Cuisine, with More than 200 Recipes; Harvard Common Press: Boston, MA, USA, 2012; ISBN 978-1-55832-775-7. [Google Scholar]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Robinson, R.W.; Decker-Walters, D.S. Cucurbits; CAB International: Oxon, UK; New York, NY, USA, 1997; ISBN 978-0-85199-133-7. [Google Scholar]
- Mathew, B. The Smaller Bulbs; Batsford BT: London, UK, 1987. [Google Scholar]
- Austin, D.F. Water Spinach (Ipomoea Aquatica, Convolvulaceae): A Food Gone Wild. Ethnobot. Res. Appl. 2007, 5, 123–146. [Google Scholar] [CrossRef]
- Swarna, J.; Lokeswari, T.S.; Smita, M.; Ravindhran, R. Characterisation and Determination of in Vitro Antioxidant Potential of Betalains from Talinum Triangulare (Jacq.) Willd. Food Chem. 2013, 141, 4382–4390. [Google Scholar] [CrossRef]
- Shah, F.H.; Kim, S.J. Therapeutic Role of Medicinal Plant Extracts and Bioactive Compounds in Osteoarthritis. Adv. Tradit. Med. 2022. [Google Scholar] [CrossRef]
- Phillipson, J.D. Phytochemistry and Medicinal Plants. Phytochemistry 2001, 56, 237–243. [Google Scholar] [CrossRef]
- Min, G.-Y.; Park, J.-M.; Joo, I.-H.; Kim, D.-H. Inhibition Effect of Caragana Sinica Root Extracts on Osteoarthritis through MAPKs, NF-ΚB Signaling Pathway. Int. J. Med. Sci. 2021, 18, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Lim, M.J.; Lee, K.M.; Oh, E.; Shin, Y.S.; Kim, S.; Kim, J.S.; Yun, S.P.; Kang, L.-J. Safflower Seed Extract Attenuates the Development of Osteoarthritis by Blocking NF-ΚB Signaling. Pharmaceuticals 2021, 14, 258. [Google Scholar] [CrossRef]
- Panter, K.E.; Welch, K.D.; Gardner, D.R.; Green, B.T. Poisonous Plants: Effects on Embryo and Fetal Development. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 223–234. [Google Scholar] [CrossRef]
- Adnyana, I.K.; Sukandar, E.Y.; Setiawan, F.; Christanti, Y. Efficacy and Safety O-Desmethyl Quinine Compare to Quinine for Nocturnal Leg Cramp. J. Med. Sci. 2013, 13, 819–823. [Google Scholar] [CrossRef]
- Kothari, P.; Tripathi, A.K.; Girme, A.; Rai, D.; Singh, R.; Sinha, S.; Choudhary, D.; Nagar, G.K.; Maurya, R.; Hingorani, L.; et al. Caviunin Glycoside (CAFG) from Dalbergia Sissoo Attenuates Osteoarthritis by Modulating Chondrogenic and Matrix Regulating Proteins. J. Ethnopharmacol. 2022, 282, 114315. [Google Scholar] [CrossRef]
- Russo, P.; Frustaci, A.; Del Bufalo, A.; Fini, M.; Cesario, A. Multitarget Drugs of Plants Origin Acting on Alzheimer’s Disease. Curr. Med. Chem. 2013, 20, 1686–1693. [Google Scholar] [CrossRef]
- Chen, D.-H.; Zheng, G.; Zhong, X.-Y.; Lin, Z.-H.; Yang, S.-W.; Liu, H.-X.; Shang, P. Oroxylin A Attenuates Osteoarthritis Progression by Dual Inhibition of Cell Inflammation and Hypertrophy. Food Funct. 2021, 12, 328–339. [Google Scholar] [CrossRef]
- Rozov-Ung, I.; Mreyoud, A.; Moore, J.; Wilding, G.E.; Khawam, E.; Lackner, J.M.; Semler, J.R.; Sitrin, M.D. Detection of Drug Effects on Gastric Emptying and Contractility Using a Wireless Motility Capsule. BMC Gastroenterol. 2014, 14, 2. [Google Scholar] [CrossRef]
- Simera, M.; Poliacek, I.; Jakus, J. Central Antitussive Effect of Codeine in the Anesthetized Rabbit. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 184–188. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.H.; Kim, Y.S.; Cho, K.Y.; Kim, K.J. Unexpected Effect of Fluorine in Diels-Alder Reaction of 2-Fluoroacrolein with Thebaine. Bull. Korean Chem. Soc. 1990, 11, 178–179. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, Z.; Wu, B.; Gong, S.; Chen, B. Small Molecule Natural Compound Targets the NF-κB Signaling and Ameliorates the Development of Osteoarthritis. J. Cell. Physiol. 2021, 236, 7298–7307. [Google Scholar] [CrossRef]
- Yan, J.; Ni, B.; Sheng, G.; Zhang, Y.; Xiao, Y.; Ma, Y.; Li, H.; Wu, H.; Tu, C. Rhoifolin Ameliorates Osteoarthritis via Regulating Autophagy. Front. Pharmacol. 2021, 12, 661072. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.L.; Lam, S.D.; Che Lah, W.A.; Ahmad, A.; Rinklebe, J.; Sonne, C.; Peng, W. Integration of Environmental Metabolomics and Physiological Approach for Evaluation of Saline Pollution to Rice Plant. Environ. Pollut. 2021, 286, 117214. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, J.F. A Revision of Sarcotheca Bl. and Dapania Korth. (Oxalidaceae). Blumea Biodivers. Evol. Biogeogr. Plants 1967, 15, 519–543. [Google Scholar]
- U.S. Patent 20130123106; Gandhi, P.T. Novel Nicotine Derivatives 2013. USPTO (United States Patent and Trademark Office): Alexandria, VA, USA, 16 May 2013.
- Lu, M.-K.; Shih, Y.-W.; Chang Chien, T.-T.; Fang, L.-H.; Huang, H.-C.; Chen, P.-S. α-Solanine Inhibits Human Melanoma Cell Migration and Invasion by Reducing Matrix Metalloproteinase-2/9 Activities. Biol. Pharm. Bull. 2010, 33, 1685–1691. [Google Scholar] [CrossRef]
- Morrow, W.J.W.; Yang, Y.-W.; Sheikh, N.A. Immunobiology of the Tomatine Adjuvant. Vaccine 2004, 22, 2380–2384. [Google Scholar] [CrossRef]
- Onwurah, N.; Eke, I.; Anaga, A. Antiulcer Properties of Aqueous Extract of Talinum Triangulare Leaves in Experimentally Induced Gastric Ulceration in Mice. Asian J. Pharm. Biol. Res. 2013, 3, 4–7. [Google Scholar]
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant Nutrition for Human Nutrition: Hints from Rice Research and Future Perspectives. Mol. Plant 2020, 13, 825–835. [Google Scholar] [CrossRef]
- Mena, P.; Angelino, D. Plant Food, Nutrition, and Human Health. Nutrients 2020, 12, 2157. [Google Scholar] [CrossRef]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and Human Health: Current Status and Future Needs. Air Soil Water Res. 2020, 13, 117862212093444. [Google Scholar] [CrossRef]
- Stein, A.J. Global Impacts of Human Mineral Malnutrition. Plant Soil 2010, 335, 133–154. [Google Scholar] [CrossRef]
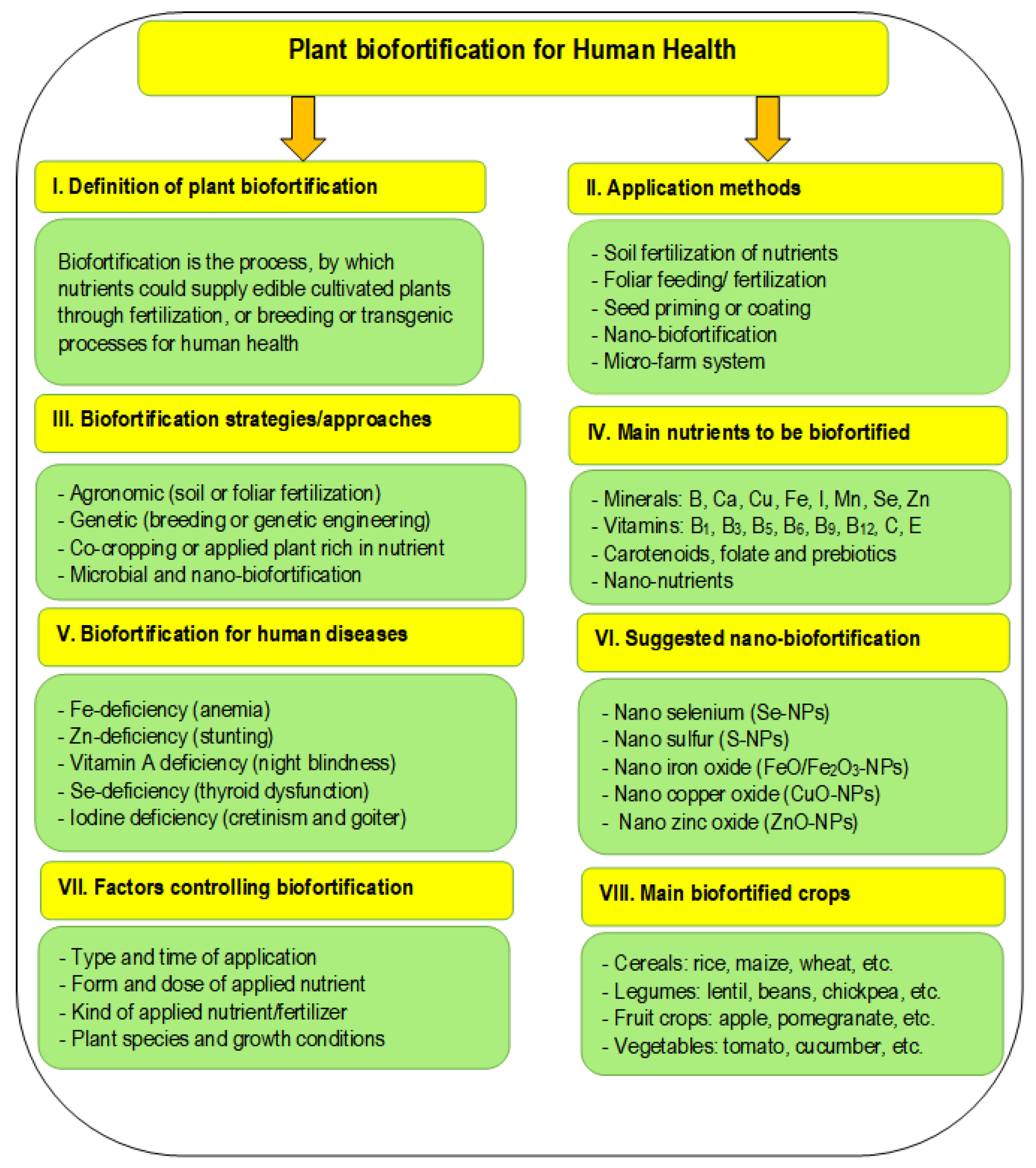
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Kumar, S.; Dikshit, H.K.; Mishra, G.P.; Singh, A. Biofortification of Staple Crops; Springer Nature Singapore Pte Ltd.: Singapore, 2022. [Google Scholar]
- Kumar, S.; Dikshit, H.K.; Mishra, G.P.; Singh, A.; Aski, M.; Virk, P.S. Biofortification of Staple Crops: Present Status and Future Strategies. In Biofortification of Staple Crops; Kumar, S., Dikshit, H.K., Mishra, G.P., Singh, A., Eds.; Springer: Singapore, 2022; pp. 1–30. ISBN 9789811632808. [Google Scholar]
- Schiavon, M.; Nardi, S.; Dalla Vecchia, F.; Ertani, A. Selenium Biofortification in the 21st Century: Status and Challenges for Healthy Human Nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Sharma, D.; Jamra, G.; Singh, U.M.; Sood, S.; Kumar, A. Calcium Biofortification: Three Pronged Molecular Approaches for Dissecting Complex Trait of Calcium Nutrition in Finger Millet (Eleusine Coracana) for Devising Strategies of Enrichment of Food Crops. Front. Plant Sci. 2017, 7, 2028. [Google Scholar] [CrossRef]
- Mishra, G.P.; Dikshit, H.K.; Priti; Kukreja, B.; Aski, M.; Yadava, D.K.; Sarker, A.; Kumar, S. Historical Overview of Biofortification in Crop Plants and Its Implications. In Biofortification of Staple Crops; Kumar, S., Dikshit, H.K., Mishra, G.P., Singh, A., Eds.; Springer: Singapore, 2022; pp. 31–61. ISBN 9789811632792. [Google Scholar]
- Dobermann, A.; Bruulsema, T.; Cakmak, I.; Gerard, B.; Majumdar, K.; McLaughlin, M.; Reidsma, P.; Vanlauwe, B.; Wollenberg, L.; Zhang, F.; et al. Responsible Plant Nutrition: A New Paradigm to Support Food System Transformation. Glob. Food Secur. 2022, 33, 100636. [Google Scholar] [CrossRef]
- Feliciano, D.; Recha, J.; Ambaw, G.; MacSween, K.; Solomon, D.; Wollenberg, E. Assessment of Agricultural Emissions, Climate Change Mitigation and Adaptation Practices in Ethiopia. Clim. Policy 2022, 22, 427–444. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Heckathorn, S.; North, G.; Wang, D.; Zhu, C. Editorial: Climate Change and Plant Nutrient Relations. Front. Plant Sci. 2020, 11, 869. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the Nutritional Quality of Crop Plants under a Changing Climate: Importance and Strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Cracknell, P.; Varotsos, C. Understanding Global Climate Change: Modelling the Climatic System and Human Impacts. CRC Press Is an Imprint of Taylor & Francis Group, LLC, Second Edition Arthur. Available online: https://www.routledge.com/Understanding-Global-Climate-Change-Modelling-the-Climatic-System-and-Human/Cracknell-Varotsos/p/book/9781032001616 (accessed on 31 May 2022).
- Nielsen, R.L.; James, J.J.; Drenovsky, R.E. Functional Traits Explain Variation in Chaparral Shrub Sensitivity to Altered Water and Nutrient Availability. Front. Plant Sci. 2019, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, H. Alteration in Biochemical Constituents and Nutrients Partitioning of Asparagus Racemosus in Response to Elevated Atmospheric CO2 Concentration. Environ. Sci. Pollut. Res. 2022, 29, 6812–6821. [Google Scholar] [CrossRef] [PubMed]
- Kovak, E.; Blaustein-Rejto, D.; Qaim, M. Genetically Modified Crops Support Climate Change Mitigation. Trends Plant Sci. 2022, 27, 627–629. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Mukherjee, S.; Sarkar, B. A Perspective on Biochar for Repairing Damages in the Soil–Plant System Caused by Climate Change-Driven Extreme Weather Events. Biochar 2022, 4, 22. [Google Scholar] [CrossRef]
- Wright, C.Y.; Kapwata, T.; du Preez, D.J.; Wernecke, B.; Garland, R.M.; Nkosi, V.; Landman, W.A.; Dyson, L.; Norval, M. Major Climate Change-Induced Risks to Human Health in South Africa. Environ. Res. 2021, 196, 110973. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, P.; Mahendran, R.; Huang, W.; Gao, Y.; Yang, Z.; Ye, T.; Wen, B.; Wu, Y.; Li, S.; et al. Global Climate Change and Human Health: Pathways and Possible Solutions. Eco-Environ. Health 2022, 1, 53–62. [Google Scholar] [CrossRef]
- Ayi, Q.; Zeng, B.; Yang, K.; Lin, F.; Zhang, X.; van Bodegom, P.M.; Cornelissen, J.H.C. Similar Growth Performance but Contrasting Biomass Allocation of Root-Flooded Terrestrial Plant Alternanthera Philoxeroides (Mart.) Griseb. in Response to Nutrient Versus Dissolved Oxygen Stress. Front. Plant Sci. 2019, 10, 111. [Google Scholar] [CrossRef]
- Xu, H.; Xie, H.; Wu, S.; Wang, Z.; He, K. Effects of Elevated CO2 and Increased N Fertilization on Plant Secondary Metabolites and Chewing Insect Fitness. Front. Plant Sci. 2019, 10, 739. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Jin, J.; Zhang, Q.; Wang, G.; Liu, C.; Wu, J.; Wang, C.; Liu, X. Impact of Elevated CO2 on Seed Quality of Soybean at the Fresh Edible and Mature Stages. Front. Plant Sci. 2018, 9, 1413. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of Elevated CO2 on Nutritional Quality of Vegetables: A Review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Bedeke, S.B. Climate Change Vulnerability and Adaptation of Crop Producers in Sub-Saharan Africa: A Review on Concepts, Approaches and Methods. Environ. Dev. Sustain. 2022, 1–35. [Google Scholar] [CrossRef]
- Vanisree, C.R.; Singh, P.; Jadhav, E.B.; Nair, M.S.; Sankhla, M.S.; Parihar, K.; Awasthi, K.K. Effect of Climate Change and Soil Dynamics on Soil Microbes and Fertility of Soil. In Microbiome under Changing Climate; Elsevier: Amsterdam, The Netherlands, 2022; pp. 437–468. ISBN 978-0-323-90571-8. [Google Scholar]
- Collado-González, J.; Piñero, M.C.; Otalora, G.; Lopez-Marín, J.; del Amor, F.M. Unraveling the Nutritional and Bioactive Constituents in Baby-Leaf Lettuce for Challenging Climate Conditions. Food Chem. 2022, 384, 132506. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Arruda, T.; Campos Bernardes, P.; Robledo Fialho e Moraes, A.; de Fátima Ferreira Soares, N. Natural Bioactives in Perspective: The Future of Active Packaging Based on Essential Oils and Plant Extracts Themselves and Those Complexed by Cyclodextrins. Food Res. Int. 2022, 156, 111160. [Google Scholar] [CrossRef] [PubMed]
- Jamloki, A.; Bhattacharyya, M.; Nautiyal, M.C.; Patni, B. Elucidating the Relevance of High Temperature and Elevated CO2 in Plant Secondary Metabolites (PSMs) Production. Heliyon 2021, 7, e07709. [Google Scholar] [CrossRef]
- Lupitu, A.; Moisa, C.; Gavrilaş, S.; Dochia, M.; Chambre, D.; Ciutină, V.; Copolovici, D.M.; Copolovici, L. The Influence of Elevated CO2 on Volatile Emissions, Photosynthetic Characteristics, and Pigment Content in Brassicaceae Plants Species and Varieties. Plants 2022, 11, 973. [Google Scholar] [CrossRef]
- Yamuangmorn, S.; Jumrus, S.; Jamjod, S.; Sringarm, K.; Arjin, C.; Prom-u-thai, C. Responses of Purple Rice Variety to Light Intensities and Soil Zinc Application on Plant Growth, Yield and Bioactive Compounds Synthesis. J. Cereal Sci. 2022, 106, 103495. [Google Scholar] [CrossRef]
- Ramani, S.; Jayabaskaran, C. Enhanced Catharanthine and Vindoline Production in Suspension Cultures of Catharanthus Roseus by Ultraviolet-B Light. J. Mol. Signal. 2008, 3, 9. [Google Scholar] [CrossRef]
- Regvar, M.; Bukovnik, U.; Likar, M.; Kreft, I. UV-B Radiation Affects Flavonoids and Fungal Colonisation in Fagopyrum Esculentum and F. Tataricum. Open Life Sci. 2012, 7, 275–283. [Google Scholar] [CrossRef]
- Cuadra, P.; Harborne, J.B.; Waterman, P.G. Increases in Surface Flavonols and Photosynthetic Pigments in Gnaphalium Luteo-Album in Response to UV-B Radiation. Phytochemistry 1997, 45, 1377–1383. [Google Scholar] [CrossRef]
- Zu, Y.-G.; Tang, Z.-H.; Yu, J.-H.; Liu, S.-G.; Wang, W.; Guo, X.-R. Different Responses of Camptothecin and 10-Hydroxycamptothecin to Heat Shock in Camptotheca Acuminata Seedlings. Acta Bot. Sin. 2003, 45, 809–814. [Google Scholar]
- Commisso, M.; Toffali, K.; Strazzer, P.; Stocchero, M.; Ceoldo, S.; Baldan, B.; Levi, M.; Guzzo, F. Impact of Phenylpropanoid Compounds on Heat Stress Tolerance in Carrot Cell Cultures. Front. Plant Sci. 2016, 7, 1439. [Google Scholar] [CrossRef]
- Hanson, D.T.; Sharkey, T.D. Effect of Growth Conditions on Isoprene Emission and Other Thermotolerance-Enhancing Compounds. Plant Cell Environ. 2001, 24, 929–936. [Google Scholar] [CrossRef]
- Rosenfeld, H.J.; Aaby, K.; Lea, P. Influence of Temperature and Plant Density on Sensory Quality and Volatile Terpenoids of Carrot (Daucus Carota L.) Root. J. Sci. Food Agric. 2002, 82, 1384–1390. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Son, K.-H.; Lee, J.-H.; Oh, M.-M. Growth Characteristics and Bioactive Compounds of Dropwort Subjected to High CO2 Concentrations and Water Deficit. Hortic. Environ. Biotechnol. 2022, 63, 181–194. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Hagagy, N.; AbdElgawad, H. Establishment of Actinobacteria–Satureja Hortensis Interactions under Future Climate CO2-Enhanced Crop Productivity in Drought Environments of Saudi Arabia. Environ. Sci. Pollut. Res. 2021, 28, 62853–62867. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Okla, M.K.; Al-amri, S.S.; AL-Hashimi, A.; AL-Qahtani, W.H.; Al-Qahtani, S.M.; Abbas, Z.K.; Al-Harbi, N.A.; Abd Algafar, A.; Almuhayawi, M.S.; et al. Effect of Elevated CO2 on Biomolecules’ Accumulation in Caraway (Carum Carvi L.) Plants at Different Developmental Stages. Plants 2021, 10, 2434. [Google Scholar] [CrossRef]
- Selim, S.; Abuelsoud, W.; Al-Sanea, M.M.; AbdElgawad, H. Elevated CO2 Differently Suppresses the Arsenic Oxide Nanoparticles-Induced Stress in C3 (Hordeum Vulgare) and C4 (Zea Maize) Plants via Altered Homeostasis in Metabolites Specifically Proline and Anthocyanin Metabolism. Plant Physiol. Biochem. 2021, 166, 235–245. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Al Jaouni, S.K.; Almuhayawi, S.M.; Selim, S.; Abdel-Mawgoud, M. Elevated CO2 Improves the Nutritive Value, Antibacterial, Anti-Inflammatory, Antioxidant and Hypocholestecolemic Activities of Lemongrass Sprouts. Food Chem. 2021, 357, 129730. [Google Scholar] [CrossRef]
- Qiang, Q.; Gao, Y.; Yu, B.; Wang, M.; Ni, W.; Li, S.; Zhang, T.; Li, W.; Lin, L. Elevated CO2 Enhances Growth and Differentially Affects Saponin Content in Paris Polyphylla Var. Yunnanensis. Ind. Crops Prod. 2020, 147, 112124. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, H.M.; Jeong, H.W.; Oh, M.M.; Hwang, S.J. Growth and Bioactive Compound Content of Glehnia Littoralis Fr. Schmidt Ex Miquel Grown under Different CO2 Concentrations and Light Intensities. Plants 2020, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, M.; Pokharel, S.S.; Li, C.; Parajulee, M.N.; Chen, F.; Fang, W. Effects of Elevated CO2 on Foliar Soluble Nutrients and Functional Components of Tea, and Population Dynamics of Tea Aphid, Toxoptera Aurantii. Plant Physiol. Biochem. 2019, 145, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Mantri, N.; Sun, B.; Jiang, L.; Chen, P.; Jiang, B.; Jiang, Z.; Zhang, J.; Shen, J.; Lu, H.; et al. Effects of Elevated CO2 and Temperature on Gynostemma Pentaphyllum Physiology and Bioactive Compounds. J. Plant Physiol. 2016, 196–197, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics Pollution in the Terrestrial Environments: Poorly Known Diffuse Sources and Implications for Plants. Sci. Total Environ. 2022, 805, 150431. [Google Scholar] [CrossRef] [PubMed]
- Nawab, J.; Ghani, J.; Khan, S.; Xiaoping, W. Minimizing the Risk to Human Health Due to the Ingestion of Arsenic and Toxic Metals in Vegetables by the Application of Biochar, Farmyard Manure and Peat Moss. J. Environ. Manag. 2018, 214, 172–183. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, A.; Palit, D. Morphological and Biochemical Study of Plant Species- a Quick Tool for Assessing the Impact of Air Pollution. J. Clean. Prod. 2022, 339, 130647. [Google Scholar] [CrossRef]
- Khan, M.A.; Ding, X.; Khan, S.; Brusseau, M.L.; Khan, A.; Nawab, J. The Influence of Various Organic Amendments on the Bioavailability and Plant Uptake of Cadmium Present in Mine-Degraded Soil. Sci. Total Environ. 2018, 636, 810–817. [Google Scholar] [CrossRef]
- Elbagory, M.; Farrag, D.K.; Hashim, A.M.; Omara, A.E.-D. The Combined Effect of Pseudomonas Stutzeri and Biochar on the Growth Dynamics and Tolerance of Lettuce Plants (Lactuca Sativa) to Cadmium Stress. Horticulturae 2021, 7, 430. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chatterjee, N.; Sircar, A.; Maikap, S.; Singh, A.; Acharyya, S.; Paul, S. A Comparative Analysis of Heavy Metal Effects on Medicinal Plants. Appl. Biochem. Biotechnol. 2022, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Asgari Lajayer, B.; Ghorbanpour, M.; Nikabadi, S. Heavy Metals in Contaminated Environment: Destiny of Secondary Metabolite Biosynthesis, Oxidative Status and Phytoextraction in Medicinal Plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Haruna, A.; Yahaya, S.M. Recent Advances in the Chemistry of Bioactive Compounds from Plants and Soil Microbes: A Review. Chem. Afr. 2021, 4, 231–248. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, X.; Zhao, Y.; Wang, L.; Cao, K.; Zhang, N.; Gao, Y.; Wang, Z. The Combined Effects of Elevated Atmospheric CO2 and Cadmium Exposure on Flavonoids in the Leaves of Robinia Pseudoacacia L. Seedlings. Ecotoxicol. Environ. Saf. 2021, 210, 111878. [Google Scholar] [CrossRef] [PubMed]
- Lacavé, G.; Soto-Maldonado, C.; Walter, A.; Zúñiga-Hansen, M.; Pérez-Torres, E. Effect of Drought Stress on Bioactives and Starch in Chilean Potato Landraces. Potato Res. 2022, 1–20. [Google Scholar] [CrossRef]
- Esmaili, S.; Tavallali, V.; Amiri, B.; Bazrafshan, F.; Sharafzadeh, S. Foliar Application of Nano-Silicon Complexes on Growth, Oxidative Damage and Bioactive Compounds of Feverfew Under Drought Stress. Silicon 2022, 1–12. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Munné-Bosch, S. Drought-Induced Changes in Flavonoids and Other Low Molecular Weight Antioxidants in Cistus Clusii Grown under Mediterranean Field Conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Kaufman, P.; Warber, S.; Zick, S.; Aaronson, K.; Bolling, S.; Chul Chang, S. Applied Environmental Stresses to Enhance the Levels of Polyphenolics in Leaves of Hawthorn Plants. Physiol. Plant. 2004, 121, 182–186. [Google Scholar] [CrossRef]
- Cho, Y.; Njiti, V.N.; Chen, X.; Lightfoot, D.A.; Wood, A.J. Trigonelline Concentration in Field-Grown Soybean in Response to Irrigation. Biol. Plant. 2003, 46, 405–410. [Google Scholar] [CrossRef]
- Nacif de Abreu, I.; Mazzafera, P. Effect of Water and Temperature Stress on the Content of Active Constituents of Hypericum Brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Karmous, I.; Gammoudi, N.; Chaoui, A. Assessing the Potential Role of Zinc Oxide Nanoparticles for Mitigating Cadmium Toxicity in Capsicum Annuum L. Under In Vitro Conditions. J. Plant Growth Regul. 2022, 1–16. [Google Scholar] [CrossRef]
- Hayat, K.; Zhou, Y.; Menhas, S.; Hayat, S.; Aftab, T.; Bundschuh, J.; Zhou, P. Salicylic Acid Confers Salt Tolerance in Giant Juncao Through Modulation of Redox Homeostasis, Ionic Flux, and Bioactive Compounds: An Ionomics and Metabolomic Perspective of Induced Tolerance Responses. J. Plant Growth Regul. 2022, 41, 1999–2019. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.B.; Niharika; Singh, A.; Amist, N.; Azim, Z.; Bano, C.; Yadav, V.; Yadav, R.K. Salinity-Induced Attenuation in Secondary Metabolites Profile and Herbicidal Potential of Brassica Nigra L. on Anagallis Arvensis L. J. Plant Growth Regul. 2022, 1–16. [Google Scholar] [CrossRef]
- Mogazy, A.M.; Hanafy, R.S. Foliar Spray of Biosynthesized Zinc Oxide Nanoparticles Alleviate Salinity Stress Effect on Vicia Faba Plants. J. Soil Sci. Plant Nutr. 2022, 22, 2647–2662. [Google Scholar] [CrossRef]
- Nouman, W.; Aziz, U. Seed Priming Improves Salinity Tolerance in Calotropis Procera (Aiton) by Increasing Photosynthetic Pigments, Antioxidant Activities, and Phenolic Acids. Biologia 2022, 77, 609–626. [Google Scholar] [CrossRef]
- Farooq, T.; Nisa, Z.U.; Hameed, A.; Ahmed, T.; Hameed, A. Priming with Copper-Chitosan Nanoparticles Elicit Tolerance against PEG-Induced Hyperosmotic Stress and Salinity in Wheat. BMC Chem. 2022, 16, 23. [Google Scholar] [CrossRef]
- Dutta, A.; Sen, J.; Deswal, R. Downregulation of Terpenoid Indole Alkaloid Biosynthetic Pathway by Low Temperature and Cloning of a AP2 Type C-Repeat Binding Factor (CBF) from Catharanthus Roseus (L). G. Don. Plant Cell Rep. 2007, 26, 1869–1878. [Google Scholar] [CrossRef]
- Janas, K.M.; Cvikrová, M.; Pałagiewicz, A.; Szafranska, K.; Posmyk, M.M. Constitutive Elevated Accumulation of Phenylpropanoids in Soybean Roots at Low Temperature. Plant Sci. 2002, 163, 369–373. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Pazouki, L.; Niinemets, Ü. Emissions of Green Leaf Volatiles and Terpenoids from Solanum Lycopersicum Are Quantitatively Related to the Severity of Cold and Heat Shock Treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef]
- Mir, B.A.; Mir, S.A.; Khazir, J.; Tonfack, L.B.; Cowan, D.A.; Vyas, D.; Koul, S. Cold Stress Affects Antioxidative Response and Accumulation of Medicinally Important Withanolides in Withania Somnifera (L.) Dunal. Ind. Crops Prod. 2015, 74, 1008–1016. [Google Scholar] [CrossRef]
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of Low-Temperature Stress on General Phenylpropanoid and Anthocyanin Pathways: Enhancement of Transcript Abundance and Anthocyanin Pigmentation in Maize Seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Sharma, P.; Khanna, V.; Kumari, S. Abiotic Stress Mitigation Through Plant-Growth-Promoting Rhizobacteria. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D.K., Varma, A., Tuteja, N., Eds.; Springe: Singapore, 2016; pp. 327–342. ISBN 978-981-10-2853-3. [Google Scholar]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef] [PubMed]
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline Soils Worldwide: Identifying the Most Promising Areas for Saline Agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Neupane, D.; Adhikari, P.; Bhattarai, D.; Rana, B.; Ahmed, Z.; Sharma, U.; Adhikari, D. Does Climate Change Affect the Yield of the Top Three Cereals and Food Security in the World? Earth 2022, 3, 45–71. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-Growth-Promoting Rhizobacteria to Improve Crop Growth in Saline Soils: A Review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Molnár, Z.; Virág, E.; Ördög, V. Natural Substances in Tissue Culture Media of Higher Plants. Acta Biol. Szeged. 2011, 55, 123–127. [Google Scholar]
- Köninger, J.; Lugato, E.; Panagos, P.; Kochupillai, M.; Orgiazzi, A.; Briones, M.J.I. Manure Management and Soil Biodiversity: Towards More Sustainable Food Systems in the EU. Agric. Syst. 2021, 194, 103251. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Chhaya; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between Phytohormones and Secondary Metabolites in the Drought Stress Tolerance of Crop Plants: A Review. Physiol. Plant. 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary Metabolites in the Drought Stress Tolerance of Crop Plants: A Review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Yin, X.; Yan, X.; Qian, C.; Zhou, S.; Fang, T.; Fan, X.; Gao, Y.; Chang, Y.; Yang, J.; Ma, X.-F. Comparative Transcriptome Analysis to Identify Genes Involved in Terpenoid Biosynthesis in Agriophyllum Squarrosum, a Folk Medicinal Herb Native to Asian Temperature Deserts. Plant Biotechnol. Rep. 2021, 15, 369–387. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Wangchuk, P. Plant Alkaloids: Classification, Isolation and Drug Development; Swamy, M., Patra, J., Rudramurthy, G., Eds.; Taylor & Francis Ltd.: Boca Raton, FL, USA, 2019; pp. 131–137. ISBN 978-0-367-11172-4. [Google Scholar]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Arora, K.; Rai, M.K.; Sharma, A.K. Tissue Culture Mediated Biotechnological Interventions in Medicinal Trees: Recent Progress. Plant Cell Tissue Organ Cult. PCTOC 2022, 1–22. [Google Scholar] [CrossRef]
- Mishra, V.K.; Bajpai, R.; Chaturvedi, R. Androgenic Haploid Plant Development via Embryogenesis with Simultaneous Determination of Bioactive Metabolites in Cambod Tea (Camellia Assamica Ssp. Lasiocalyx). Plant Cell Tissue Organ Cult. PCTOC 2022, 148, 515–531. [Google Scholar] [CrossRef]
- Keshavan, B.; Santosh Srinivas, N.; Muthu Tamizh, M.; Vairamani, M.; Pachaiappan, R. In Vitro Elicitation of Camptothecin by Challenging with Biotic Elicitors in Nothapodytes Nimmoniana (J.Graham) Mabb. S. Afr. J. Bot. 2022, 144, 325–331. [Google Scholar] [CrossRef]
- Escrich, A.; Almagro, L.; Moyano, E.; Cusido, R.M.; Bonfill, M.; Hosseini, B.; Palazon, J. Improved Biotechnological Production of Paclitaxel in Taxus Media Cell Cultures by the Combined Action of Coronatine and Calix[8]Arenes. Plant Physiol. Biochem. 2021, 163, 68–75. [Google Scholar] [CrossRef]
- Rind, N.A.; Rind, K.H.; Dahot, M.U.; Faiza, H.; Aksoy, Ö.; Rind, K.H.; Shar, A.H.; Ullah, H.; Jatoi, A.H. Production of Limonoids Through Callus and Cell Suspension Cultures of Chinaberry (Melia Azedarach L.). Bangladesh J. Bot. 2021, 50, 301–309. [Google Scholar] [CrossRef]
- Farjaminezhad, R.; Garoosi, G. Improvement and Prediction of Secondary Metabolites Production under Yeast Extract Elicitation of Azadirachta Indica Cell Suspension Culture Using Response Surface Methodology. AMB Express 2021, 11, 43. [Google Scholar] [CrossRef]
- Farjaminezhad, R.; Garoosi, G. Prediction of the Effect of Chitosan on Cell Suspension Culture of Azadirachta Indica by Response Surface Methodology. Plant Cell Tissue Organ Cult. PCTOC 2021, 146, 323–337. [Google Scholar] [CrossRef]
- Modarres, M.; Esmaeilzadeh Bahabadi, S.; Taghavizadeh Yazdi, M.E. Enhanced Production of Phenolic Acids in Cell Suspension Culture of Salvia Leriifolia Benth. Using Growth Regulators and Sucrose. Cytotechnology 2018, 70, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Ashokhan, S.; Othman, R.; Abd Rahim, M.H.; Karsani, S.A.; Yaacob, J.S. Effect of Plant Growth Regulators on Coloured Callus Formation and Accumulation of Azadirachtin, an Essential Biopesticide in Azadirachta Indica. Plants 2020, 9, 352. [Google Scholar] [CrossRef]
- Sarmadi, M.; Karimi, N.; Palazón, J.; Ghassempour, A.; Mirjalili, M.H. Improved Effects of Polyethylene Glycol on the Growth, Antioxidative Enzymes Activity and Taxanes Production in a Taxus Baccata L. Callus Culture. Plant Cell Tissue Organ Cult. PCTOC 2019, 137, 319–328. [Google Scholar] [CrossRef]
- Thilip, C.; Mehaboob, V.M.; Varutharaju, K.; Faizal, K.; Raja, P.; Aslam, A.; Shajahan, A. Elicitation of Withaferin-A in Hairy Root Culture of Withania Somnifera (L.) Dunal Using Natural Polysaccharides. Biologia 2019, 74, 961–968. [Google Scholar] [CrossRef]
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of Glucosinolates, Phenolic Compounds and Associated Gene Expression Profiles of Hairy Root Cultures in Turnip (Brassica Rapa Ssp. Rapa). 3 Biotech 2016, 6, 175. [Google Scholar] [CrossRef]
- Moharrami, F.; Hosseini, B.; Sharafi, A.; Farjaminezhad, M. Enhanced Production of Hyoscyamine and Scopolamine from Genetically Transformed Root Culture of Hyoscyamus Reticulatus L. Elicited by Iron Oxide Nanoparticles. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 104–111. [Google Scholar] [CrossRef]
- Gabr, A.M.M.; Mabrok, H.B.; Ghanem, K.Z.; Blaut, M.; Smetanska, I. Lignan Accumulation in Callus and Agrobacterium Rhizogenes-Mediated Hairy Root Cultures of Flax (Linum Usitatissimum). Plant Cell Tissue Organ Cult. PCTOC 2016, 126, 255–267. [Google Scholar] [CrossRef]
- El-Ramady, H.; Faizy, S.; Amer, M.M.; Elsakhawy, T.; Omara, A.E.-D.; Eid, Y.; Brevik, E.C. Management of Salt-Affected Soils: A Photographic Mini-Review. Environ. Biodivers. Soil Secur. 2022, 6, 61–79. [Google Scholar] [CrossRef]
- El-Ramady, H.; Brevik, E.C.; Elsakhawy, T.; Omara, A.-D.; Amer, M.; Abowaly, M.; El-Henawy, A.; Prokisch, J. Soil and Humans: A Comparative and A Pictorial Mini-Review. Egypt. J. Soil Sci. 2022, 62, 2. [Google Scholar] [CrossRef]
- Brevik, E.C.; Omara, A.E.-D.; Elsakhawy, T.; Amer, M.; Fawzy, Z.F.; El-Ramady, H.; Prokisch, J. The Soil-Water-Plant-Human Nexus: A Call for Photographic Review Articles. Environ. Biodivers. Soil Secur. 2022, 6, 117–131. [Google Scholar] [CrossRef]
- Fawzy, Z.F.; El-Ramady, H.; Omara, A.E.-D.; Elsakhawy, T.; Bayoumi, Y.; Shalaby, T.A.; Prokisch, J. From Farm-to-Fork: A pictorial Mini Review on Nano-Farming of Vegetables. Environ. Biodivers. Soil Secur. 2022; 6, in press. [Google Scholar]













| Nutrient Element | Nutrient Symbol | Uptake Form | Nutrient Biological Functions in Plants [Ref.] | Deficiency Symptoms of Nutrients [Ref.] |
|---|---|---|---|---|
| Nitrogen | N | NH4+ and NO3− | Constituent of amides, amino acids, proteins, nucleic acids, nucleotides, coenzymes, chlorophyll, etc. [47] | Inhibits plant growth; yellowing or chlorosis of leaves due to a collapse in chloroplasts [48] |
| Phosphorus | P | H2PO4−; HPO42− | Constituent of nucleic acids and lipid membranes, ATP, etc. [49] | Dark greenish-purple leaves, with necrotic spots and malformed [49] |
| Potassium | K | K+ | Controls more than 60 enzymes, mainly of photosynthesis and respiration [50] | Chlorosis of older leaves; shorter internodes in stems; inhibiting protein synthesis [49] |
| Calcium | Ca | Ca2+ | Ca-pectate is main constituent of cell wall; controls elongation and division of cells; activates many enzymes [47] | Small and younger leaves; deformed and chlorotic, bitter pit (apple); black heart (celery) [50] |
| Magnesium | Mg | Mg2+ | Component of chlorophyll and polyribosomes; enzyme cofactor [51] | Chlorosis of intervein and streaked or patchy effects on leaves [50] |
| Sulfur | S | SO42− | Component of amino acids (i.e., cysteine, cystine, and methionine), CoA and vitamins (biotin; thiamine), and glucosides in onions [44] | Interveinal chlorosis. S-deficiency chlorosis in all leaves at the same time; yellowish-green [49] |
| Boron | B | H2BO3− | Involved in many processes: proteins synthesis, respiration, sugars transport metabolism of RNA, plant hormones, and carbohydrate [52] | Mainly appears on younger leaves; malformed and bluish-green; retaining flowers, forming of pollen [47] |
| Copper | Cu | Cu2+ | Essential respiration and photosynthesis of mitochondria; component of major enzymes Cu-Zn-SOD [52] | Necrosis, spots at tips of younger leaves, white tips, die back, and reclamation disease [49] |
| Chlorine | Cl | Cl− | Essential for osmoregulation and photosynthesis; increases resistance to plant diseases (rice; barely; corn) [53] | Leaf chlorosis, curling of leaves, plant wilting, and restricted branching in root system [52] |
| Iron | Fe | Fe2+ | Essential for respiration; assimilation of N, mitochondria, photosynthesis; hormones biosynthesis; cytochromes; Fe-containing proteins (haem) [52] | Interveinal chlorosis from younger leaves; leaf margins and veins remain green; stunted growth in palms leads to meristem death [54] |
| Nickel | Ni | Ni+2 | Essential for prokaryotic enzymes such as hydrogenases, dehydrogenases; component in urease enzyme [55] | Leaf-tip necrosis; nitrate in leaves accumulation; Ni deficiency in pecan called mouse-ear leaves [55] |
| Manganese | Mn | Mn2+ | Exists in several plant cell enzymes; involves many enzymes (i.e., lyases, hydrolases); proteins Mn-SOD [56] | Interveinal chlorosis in dicots; smallest leaf veins remain green; speckled yellow in sugar beet [47] |
| Molybdenum | Mo | MoO4− | Essential for N-assimilation, phytohormone biosynthesis S-metabolism; controls N-assimilation enzymes [52] | In young plants: dwarfed plants, mottling, grey tinting, cupping and flaccid leaves [44] |
| Zinc | Zn | Zn2+ | Component of synthesis protein enzymes; essential catalytic for more than 300 enzymes, Zn-Cu-SOD [47] | Interveinal chlorosis; internodes short, younger shoots; smaller leaves; malformed, rosetted [50] |
| Period Studied | CO2 (μmol mol−1) | CH4 (ppb Volume) | N2O (ppb Volume) |
|---|---|---|---|
| 1800 | 280.0 | 0.80 | 288 |
| 2017 | 405.0 | 1.72 | 325 |
| 2022 | 420.23 | 1.90 | 334 |
| Studied Plant | Climate Change Factor | Plant Bioactive Compound and Its Response | Ref. |
|---|---|---|---|
| Purple rice (Oryza sativa L.) | Low light intensity | Shading increased total anthocyanin and total phenol compounds | [167] |
| Catharanthus roseus (L.) G. Don | Ultraviolet-B (UV-B) irradiation for 5 min | Increased alkaloids (catharanthine, vindoline) | [168] |
| Fagopyrum esculentum Moench | Three treatments of UV-B | Increased phenolics (quercetin, catechi, rutin) | [169] |
| Gnaphalium luteoalbum L. | Two different levels of irradiance UV-B | Increased phenolics (flavonoids: calycopterin and 3′-methoxycalycopterin) | [170] |
| Camptotheca acuminata Decne. | Heat stress (from 34 to 46 °C at 2 °C intervals) | Increased alkaloids (10-hydroxycamptothecin) | [171] |
| Daucus carota L. | Heat stress (incubated at 44 °C) | Decreased terpenoids (α-terpinolene) | [172] |
| Quercus rubra L. | Heat stress (at 20/14 and 32/24 °C) | Increased terpenoids (isoprene, 2-methyl-1,3-butadiene) | [173] |
| Daucus carota L. | Heat stress (18 and 21 °C) | Decreased terpinolene (α-terpinolene with increasing growth temperature) | [174] |
| Dropwort (Oenanthe stolonifera L.) | Elevated CO2 at 600 and 1000 μmol mol−1 | Total phenolics/cyanidin/antioxidant capacity of plantlets increased by high eCO2 | [175] |
| Asparagus racemosus Willd. | Elevated CO2 400, 600, and 800 μmol mol−1 | Elevated CO2 increased the content of total sugars and proteins in leaves and roots | [152] |
| Summer savory (Satureja hortensis L.) | Elevated CO2 (620 µmol mol−1) | Nutrients (K, Ca, P, Mg) and polyphenols were enhanced by eCO2 under drought stress | [176] |
| Caraway (Carum carvi L.) | Elevated CO2 at 400 and 620 µmol mol−1 | Higher CO2 enhanced content of phenolic compounds and flavonoids | [177] |
| Barley (Hordeum vulgare L.) and maize (Zea mays L.) | eCO2 level (620 μmol mol−1) | Barley accumulated anthocyanins, but total phenolics and flavonoids accumulated in maize under As2O3-NP stress and elevated CO2 | [178] |
| Two species of lemon-grass | Elevated CO2 (620 μmol mol−1) | eCO2 increased level of primary and secondary metabolites such as amino acids and phenolics | [179] |
| Paris polyphylla var. yunnanensis | Elevated CO2 (800 μmol mol−1) | A high-CO2 environment increased the diosgenin content and thus total saponin | [180] |
| Glehnia littoralis Fr. Schmidt ex Miquel | Elevated CO2 (500, 1500 µmol·mol−1) under 3 light intensities | Higher light intensities (300) induced higher content of total saponin and chlorogenic acid, whereas no significant effect from eCO2 | [181] |
| Tea (Camellia sinensis L.) | Elevated CO2 at 406 and 770 μmol mol−1 | Elevated CO2 significantly increased the polyphenols and theanine in tea seedlings | [182] |
| Gynostemma pentaphyllum (Thunb.) Makino | Elevated CO2 at 360 and 720 μmol mol−1 | Elevated CO2 led to decreased accumulation of total phenolics and flavonoids in leaves | [183] |
| Studied Plant | Bioactive Product(s) | The Most Important Finding in the Study | Ref. |
|---|---|---|---|
| Nothapodytes nimmoniana (J. Graham) Mabb. | Camptothecin (CPT) | Cell suspension culture using 5 biotic elicitors (i.e., chitin, chitosan, glutathione, pullulan, and jasmonic acid); the best was chitin (11.48-fold) | [231] |
| Taxus × media Rehder | Paclitaxel (PTX) | Cell culture: using coronatine and calix [8]-arenes as an elicitor to produce PTX as an anticancer agent | [232] |
| Chinaberry (Melia Azedarach L.) | Limonoid | Cell suspension culture for production of bioactive was highest by 141.7 µg/ml | [233] |
| Neem (Azadirachta indica A. Juss.) | Azadirachtin, squalene, and mevalonic acid | Cell suspension culture using chitosan and yeast extract as elicitors; bioactives depended on the used elicitor | [234] |
| Neem (Azadirachta indica A. Juss.) | Azadirachtin, squalene, and mevalonic acid | Cell suspension culture: bioactives depended on response surface methodology (i.e., central composite design and Box–Behnken design) | [235] |
| Salvia leriifolia Benth. | Phenolic acids: cafeic and salvianolic acid B | Cell cultures for max. production of cafeic and salvianolic acid B at the 15th day of the cultivation cycle | [236] |
| Neem (Azadirachta indica A. Juss.) | Azadirachtin and squalene | Callus culture: PGRs (TDZ and 2,4-D) promoted accumulation and the color of bioactives | [237] |
| Taxus baccata L. | Taxanes (paclitaxel and 10-deacetyl baccatin III) | Callus culture: under drought stress (PEG 6000 1, 2, 3, 4, 6%), highest contents of 10-deacetyl baccatin III and taxol at 2 and 3% PEG, resp. | [238] |
| Withania somnifera L. Dunal | Withaferin-A | Hairy root culture using A. rhizogenes and natural polysaccharides (as elicitors) | [239] |
| Brassica rapa subsp. pekinensis (Lour.) Kitam. | Glucosinolates (GLS) and carotenoid (CAR) | Hairy root culture: total GSL, CAR content was 2.7–57.88 μmol/g DW and 467.66 mg kg−1 DW, respectively | [240] |
| Hyoscyamus reticulatus L | Tropane alkaloids: (hyoscyamine and scopolamine) | Hairy root culture using A. rhizogenes and elicited by Fe3O4-NPs at different doses (0.45, 0.9, 1.8, and 3.6 g L−1) | [241] |
| Flax (Linum usitatissimum L.) | Lignan (e.g., secoiso-lariciresinol diglucoside) | Hairy root culture using A. rhizogenes, which had an inhibition effect on the proliferation of human breast cancer under cell line | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ramady, H.; Hajdú, P.; Törős, G.; Badgar, K.; Llanaj, X.; Kiss, A.; Abdalla, N.; Omara, A.E.-D.; Elsakhawy, T.; Elbasiouny, H.; et al. Plant Nutrition for Human Health: A Pictorial Review on Plant Bioactive Compounds for Sustainable Agriculture. Sustainability 2022, 14, 8329. https://doi.org/10.3390/su14148329
El-Ramady H, Hajdú P, Törős G, Badgar K, Llanaj X, Kiss A, Abdalla N, Omara AE-D, Elsakhawy T, Elbasiouny H, et al. Plant Nutrition for Human Health: A Pictorial Review on Plant Bioactive Compounds for Sustainable Agriculture. Sustainability. 2022; 14(14):8329. https://doi.org/10.3390/su14148329
Chicago/Turabian StyleEl-Ramady, Hassan, Peter Hajdú, Gréta Törős, Khandsuren Badgar, Xhensila Llanaj, Attila Kiss, Neama Abdalla, Alaa El-Dein Omara, Tamer Elsakhawy, Heba Elbasiouny, and et al. 2022. "Plant Nutrition for Human Health: A Pictorial Review on Plant Bioactive Compounds for Sustainable Agriculture" Sustainability 14, no. 14: 8329. https://doi.org/10.3390/su14148329
APA StyleEl-Ramady, H., Hajdú, P., Törős, G., Badgar, K., Llanaj, X., Kiss, A., Abdalla, N., Omara, A. E.-D., Elsakhawy, T., Elbasiouny, H., Elbehiry, F., Amer, M., El-Mahrouk, M. E., & Prokisch, J. (2022). Plant Nutrition for Human Health: A Pictorial Review on Plant Bioactive Compounds for Sustainable Agriculture. Sustainability, 14(14), 8329. https://doi.org/10.3390/su14148329











