Abstract
The article presents the results of an investigation of water composition and quality in sprinkler installations, as well as the influence of temperature changes on the corrosion process. The physical and chemical components of the water were measured to ascertain the influence of its properties on the corrosion process in a wet pipe sprinkler system operating in significantly changing ambient temperature conditions. The article presents the results of measurements of the wall thickness of galvanized pipes and changes in the chemical composition of water occurring under the influence of variable ambient temperature. The range and variability of temperatures corresponded to the seasons of the year: spring and summer in a temperate climate. Changes in the corrosive aggressiveness of water were assessed using the Langelier saturation index (LSI), the Ryznar stability index (RSI), and the general acidity intensity index (I). The tests revealed that the tap water used demonstrated strong corrosive properties for galvanized pipes. The calculated indices showed the tendency of water to cause corrosion (−1.1 > LSI < 1.0; RSI = 7.1–12.8). The chemical parameters that significantly influenced the corrosion of galvanized pipes are chloride (Cl−), sulphate (SO42−), and bicarbonate (HCO3−) ions. An important factor contributing to the intensification of corrosion is the roughness and heterogeneity of the pipe surface.
1. Introduction
Wet pipe sprinkler systems are often made of galvanized steel pipes. Modern galvanized steel pipes are inexpensive and recyclable, which makes them economical [1]. This material is used for both hose and sprinkler pipes. Zinc coatings protect the steel by providing a physical barrier and cathodic protection for the steel under the zinc coating. It is assumed that galvanized pipes have sufficient corrosion resistance for the reliable operation of the installation over the expected service life [2,3]. In the corrosion process, zinc acts as a sacrificial anode, protecting the steel, which acts as the cathode. This material does not, therefore, require additional protective coatings [4].
Galvanized pipes may already be subject to corrosion while in storage. This type of corrosion is called “white corrosion” and is strictly dependent on the humidity of the environment in which the pipes are stored. As a result of the uneven action of ambient moisture, corrosion stains form on the inner surfaces of the pipes. These stains are white in color, hence the name white corrosion. In installations made of galvanized pipes, zinc reacts with moisture or stagnant water. As a result of the processes taking place, the following compounds may be formed: zinc oxide (ZnO), zinc hydroxide (Zn (OH)2), alkali zinc carbonate ((ZnCO3)2 × [Zn(OH)2]3), as well as hydrated compounds containing zinc sulfate (ZnSO4). White corrosion products deposit on the surface of the pipes, forming a powder tarnish. The white powder may form a thick, wide, irregular area on the inside of the pipe. While white corrosion products can seriously affect the appearance of the pipes, it is not harmful to long-term corrosion resistance. The use of galvanized pipes leads to the formation of an additional internal layer of corrosion products. This layer may have protective properties, but it may also lead to accelerated corrosion processes with the formation of corrosion products, which, in turn, may affect the hydraulic performance of the pipes [5] and, in the case of sprinklers, may present a risk for the closure of the sprinkler nozzle [6].
The factors contributing to the corrosion of galvanized pipes include: water temperature, roughness of internal surfaces, the condition of the welding seam, quality of water in the pipes, quality of workmanship and preparation of pipes for operation.
In the case of fire protection systems that are permanently filled with water, the water temperature may change with variations in the ambient temperature [7]. Sprinkler systems located under the ceiling of protected spaces are often exposed to significant seasonal changes in ambient temperature [7,8]. This especially applies to the protected space in facilities such as warehouses or shopping malls. An increase in the ambient temperature causes an increase in the temperature of the water filling the pipe network. The increase in water temperature promotes chemical reactions in the installations, which increases the susceptibility to pocket formation in the zinc coating. Anode areas very often form within the ruptured pockets. Temperature also influences the cathode reaction of decreasing oxygen concentration dissolved in water. This causes an increase in the surface thickness of the zinc oxide layer, which has semiconductor properties. Accordingly, the susceptibility to pitting corrosion increases significantly as water temperature increases. Oxygenation of water confined in a fire protection installation is not uniform. As the distance from the water source increases, the concentration of dissolved oxygen in water decreases.
Regardless of the water temperature, roughness is a factor conducive to the destruction of the inner surface of galvanized pipes. Due to its heterogeneity, the rough surface is more prone to the formation of local anodes. The properties and concentration of anions in stagnant water in the fire protection system exert a great influence on the partial anode reaction. Chloride, nitrate, and sulphate ions that migrate to the anode in the electric field of the corrosion cell accelerate the corrosion process. In this case, the increase in water temperature also accelerates the intensity of the destruction of the zinc coating.
The care with which installation occurs has a great influence on the phenomena taking place inside the pipe network [9]. Installation-related contamination, e.g., solid particles resulting from pipe threading or contaminants that remain in the pipes after their storage at the construction site, can also lead to corrosion, both chemical and biochemical.
During periodic inspections of fire protection installations, local blockages or reductions in the effective cross-section of pipes are found [10]. These result from the deposited material caused by chemical changes occurring in stagnant water, as well as being direct products of chemical and biological corrosion.
In the case of fire protection installations that are constantly filled with stagnant water due to the requirement that they always be ready to use (quick activation in the case of a fire), unfavorable phenomena may occur inside the pipes. The scale of undesirable phenomena that can be observed inside the pipes of fire protection installations is influenced not only by the pipe material itself, but also by the water quality and changing environmental conditions. The corrosion process occurs under certain conditions of the chemical composition of the water. The inner wall of a galvanized steel pipe is usually more susceptible to corrosion due to the environment of flowing water (so-called internal corrosion) than the outer wall exposed to the weather conditions [11]. In many water distribution systems, changes occur due to pitting corrosion on the internal surfaces of pipelines. Zinc corrosion in flowing water is a complex process controlled largely by water chemistry and temperature. Relatively small differences in the chemical composition of the water can cause relatively significant changes in the products and the corrosion rate [12]. The zinc coating protects the galvanized pipe. When corrosion occurs, high concentrations of zinc and iron appear in the water which can be deposited at the water outlet. The zinc coating on the steel pipe may contain lead, copper, cadmium, chromium, aluminum, bar, and other impurities. The presence of these metals in the pipe material, which is subject to corrosion, may result in the release of trace metals [13]. Damage to galvanized pipes may result from the loss of the inner protective layer. According to European Standards [14], certain conditions influence the damage of galvanized steel pipes. The main factors influencing the corrosion process concern the composition of the water [14]. The influence on pitting corrosion of increased concentrations of certain ions in water, such as chloride, nitrate, and sulphate, is determined by the relationship presented by the equation:
where [Cl−]—concentration of chloride ions
[NO3−]—concentration of nitrate ions (V)
[SO42−]—concentration of sulphate (VI) ions
[HCO3−]—concentration of nitrate ions (V).
Pitting corrosion is unlikely if the S1 value is below 0.5. Pitting corrosion is very likely for S values above 3.
This article attempts to determine the effect of changes in the quality of water in galvanized steel pipes on the changes in the internal surface of the pipes in conditions of changing ambient temperature. Such a comprehensive approach to the analysis of the influence of temperature and water quality on the process of corrosion in large industrial installations has not been presented so far. The analyses of research results included different corrosion indices, which allowed a precise estimation of the factors influencing the corrosion process in sprinkler installations made with galvanized pipes.
2. Research
The tests were carried out on DN50 galvanized steel pipes, stored in a roofed, unheated room [1]. The pipes were stored for one year. As a result of the influence of moisture inside the pipes, white corrosion appeared (Figure 1).

Figure 1.
Photo of the inside of the pipe with visible irregular areas of white corrosion.
The pipes were divided into 0.5 m long pipes (Figure 2). Seventeen pipes were prepared, the length and weight of which were marked. On the outer surface of the pipes, the three wall thickness measurement points were marked in red (Figure 2). This marking guaranteed that the measurement of the wall thickness of the pipe was at exactly the same points before and after the experiment. Then they were filled with tap water and closed with tight plugs. In the tap water sample, called starting water, the physical and chemical indicators characterizing the quality of the water used for the experiment with galvanized pipes were determined.

Figure 2.
0.5 m samples of galvanized pipes.
2.1. Assumptions/Scheme of the Experiment
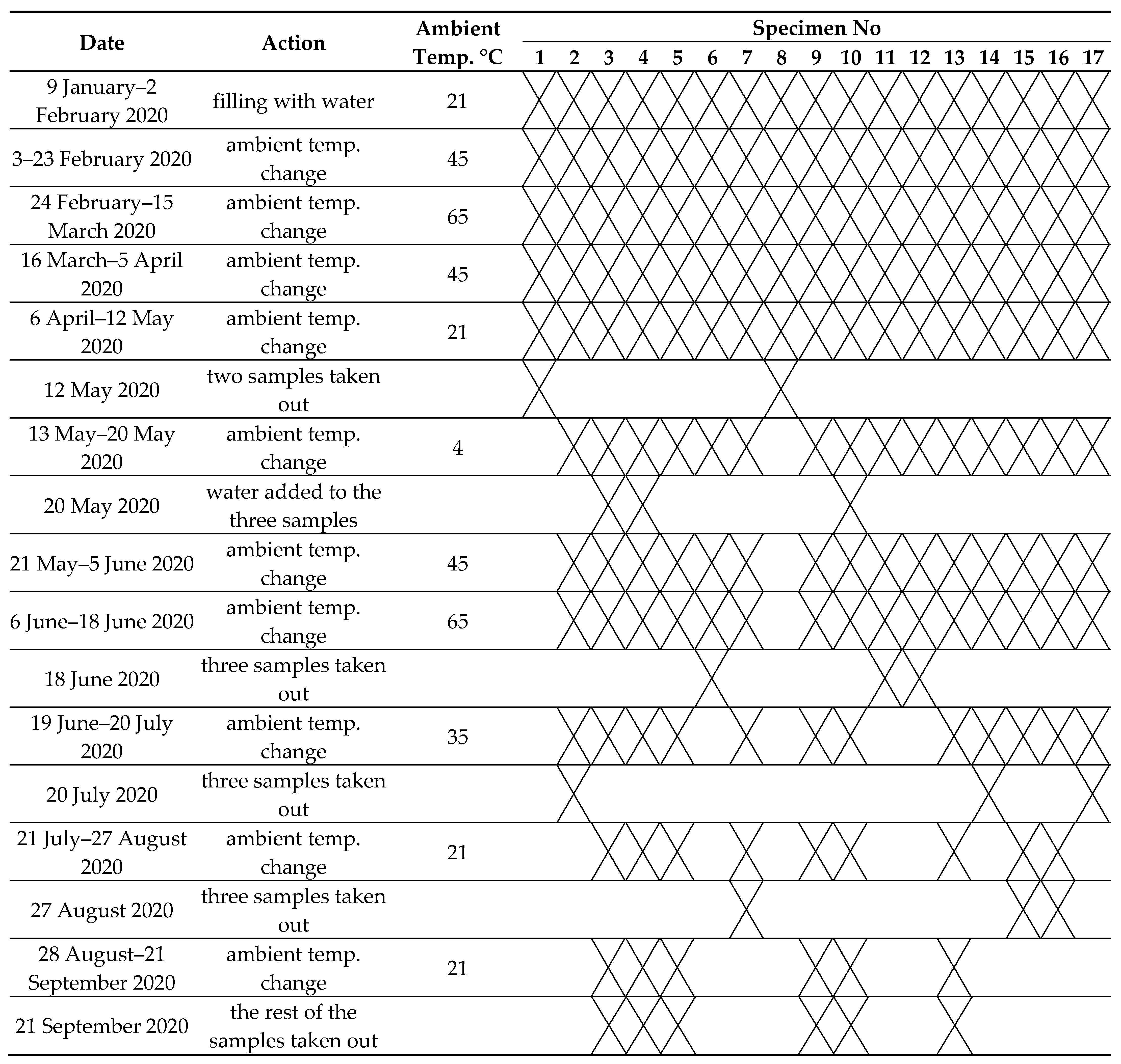
Due to the working environment of fire protection systems characterized by, among other things, significant temperature fluctuations, the prepared pipes filled with water were subjected to periodic, controlled changes in ambient temperatures. For this purpose, 17 pipes filled with water were placed in the oven for a certain period of time. The temperature variation range that was achievable was from 5 °C above ambient temperature to 300 °C. The temperature in the oven was monitored continuously. Temperature changes were recorded automatically. The temperature was recorded with an accuracy of ±0.5 °C. The average temperature recorded during the experiment was used for further analysis. The schedule of environmental temperature changes, the duration of the changing temperatures, and the sequence of taking samples of water placed in numbered pipes are presented in Table 1.

Table 1.
Test schedule.
In the case of wet pipe sprinkler systems, it is very important to maintain the required constant water pressure inside these systems. In practice, it involves having to periodically add water into the pipe network. Therefore, in the study, three samples were selected, which were supplemented with fresh water (samples 3, 4, 10; see Table 1).
2.2. Wall Thickness Measurements
In the experiment, the thickness of the walls of galvanized pipes was measured. For this purpose, three points were marked on the prepared pipes (Figure 2). At each of these points, the wall thickness was measured three times at the beginning of the tests. To measure the wall thickness, an ultrasound thickness gauge Sono type M610 (Metrison Co., Mościska, Poland) was used, which measures to an accuracy of 0.1 mm. Additionally, each pipe was weighed with a laboratory scale before being filled with water. The weight was determined with an accuracy of 0.1 g.
After the planned period of the experiment, the pipes were emptied of water, dried and weighed, and the wall thickness was measured again. To ensure the reliability of the obtained measurements, the wall thickness was measured three times at exactly the same points, the same as before the experiment. The result of the measurements before and after the test are summarized in Table 2. The observations showed changes on the inner surfaces of the pipes. The 17 pipes used in the experiment were covered with white corrosion products.

Table 2.
Summary of the obtained measurement results.
2.3. Water Chemistry Test
Eighteen water samples were subjected to chemical analysis, one with tap water collected at the beginning of the test in January, and 17 water samples from galvanized pipes filled with tap water. The samples were characterized by general water quality parameters and selected metals. The analytical scope included the following basic parameters: pH, conductivity, dissolved substances, alkalinity, hardness, calcium, magnesium, chlorides, sulphates (VI), phosphates, nitrates (V), and ammonium nitrogen. Iron, zinc, and copper ions were determined in the tested samples. The water quality results are presented in the form of graphs in order to visually present the collected data during the study period. The water composition was tested in accordance with ISO procedures for chemical analyses. The content of iron, copper, and zinc in the specimens was determined with means of FAAS (flame atomic absorption spectrometry). All reagents used in the analysis were analytical grade.
For the analysis of the results, the parameters were grouped according to their chemical nature.
- -
- a group of indicators characterizing salinity-conductivity, chlorides, sulphates, calcium, magnesium, total hardness
- -
- a group of indicators characterizing acidification (acidification)-pH, alkalinity
- -
- a group of indicators characterizing the corrosiveness of water-the content of free carbon dioxide (total acidity of water), reaction, carbonate hardness of water, total alkalinity of water, content of bicarbonates, dissolved substances.
- -
- a group of indicators characterizing the biogenic conditions (nutrients)-ammonium nitrogen, nitrate nitrogen (V), phosphate nitrogen.
- -
- metals: concentrations of iron, zinc, and copper.
Physico-chemical parameters were determined in tap water, marked as starting water. The results of these analyses are presented in Table 3. The results obtained allowed the changes that occurred in the water composition during the experiment to be evaluated.

Table 3.
Physico-chemical parameters of tap water used to fill galvanized pipes.
The starting tap water was used to test the corrosivity of 17 pipes. The pipes (17) were filled with starting water, closed tightly, and stored in the same temperature conditions until water samples were taken for testing. The analysis of the quality of water from the pipes was performed at monthly intervals, from May to September. In May, the quality of starting water and samples numbered 1 and 8 were examined. Three samples were analyzed on 6, 11, 12 June; on 2, 14, and 17 July; and on 7, 15, 16 August. In September, water was analyzed from six pipes marked with the following numbers: 3, 4, 5, 9, 10, 13.
The starting water was of good quality, in accordance with the MoH regulation of 2017 and in accordance with the World Health Organization (WHO) standards for drinking water [15]. The water had a pH = 7.35, and the specific conductivity was 475 µS/cm. The water contained 299 mg/L of solutes. The alkalinity of the water was low, 3.2 mmol/L. The concentration of calcium (52 mg/L) and magnesium (17.8 mg/L) indicates that the water was hard (185 mg CaCO3/L). In tap water, chloride (128 mg/L) and sulphate (VI) (75 mg/L) ions were below the value specified in the 2017 regulation. There were no zinc and copper ions in the water, and the iron concentration was below the permissible concentration (0.2 mg/L) for drinking water and for household purposes.
The tested water from the pipes was alkaline. The range of pH values throughout the research cycle was from 6.8 to 9.8. The concentrations of nitrate and phosphate also remained constant. The other parameters changed significantly during the experiment. From May to September, the concentration of chloride and sulphate ions increased (Figure 3) and the alkalinity value decreased, which indicates an increased content of bicarbonate ions (Figure 4). The stability of one type of ion and the slight change of others resulted in a slight difference in the amount of solutes (Figure 4). In the first month of the research (May), the water was of the same acid-alkaline pH as the starting water. The pH of the water after a month of being in the pipes did not change and ranged from 7.34 to 7.38. Moreover, the content of calcium and magnesium was at the same level after a month, which means that the hardness of the water did not change either. A high concentration of silica (26 mg/L) was found in the water. In the second month of the research, water from three pipes was analyzed. Large changes in water quality were observed. In the water tested, the pH (8.3–9.5) increased significantly in the alkaline direction. The amount of dissolved substances reached the value of over 400 mg/L. The concentration of phosphates and magnesium ions increased, especially in pipe 6, in which the concentration of zinc was also high (59 mg/L). Calcium, chloride, and sulphate ions, as well as silica, remained at a similar level, like they did in May (Figure 3). The water did not contain free CO2, and the concentration of nitrates was below 0.01 mg/L. The lowest pH was recorded in July (6.82–7.73), in August in three pipes the pH was stable (8.23). In September, the water from the pipes had a pH that ranged from 7.42 to 8.33. The conductivity did not change significantly during the tests. The highest value of specific conductivity was typical for tap water. The water in the pipes from May to September did not change significantly in terms of this parameter, reaching the average value of 317 µS/cm and the range from 269 to 368 µS/cm (Figure 4). The remaining parameters were less stable. Depending on the pipe, the content of bicarbonate varied, reaching the highest values in May (268 mg/L) in pipe 1 (Figure 4). In the following months, the concentration of HCO3−ion decreased, reaching values, in September, of 24.2 mg/L in pipe 10 and 9.76 mg/L in pipe 4. From July, trace amounts of nitrates and phosphates, as well as ammoniacal nitrogen and silica, were observed in the waters. The concentrations of calcium, magnesium, and sulphates did not change during this period (Figure 3). In contrast, the chloride concentration decreased from 130 mg/L to an average of 68 mg/L (Figure 3). From May to September, the concentration of dissolved iron decreased to 0.07 mg/L. On the other hand, the concentration of zinc and copper was high throughout the research period. In the case of copper, the concentration was highest in September. Iron in the water samples was found at low concentrations throughout the study period (Figure 5).
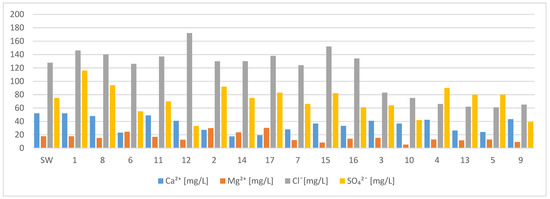
Figure 3.
Concentrations of base ions in water from 17 pipes. SW—start water.
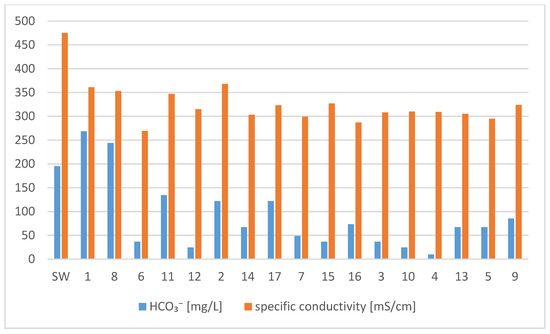
Figure 4.
Content of bicarbonate and specific conductivity in water from 17 pipes. SW—start water.
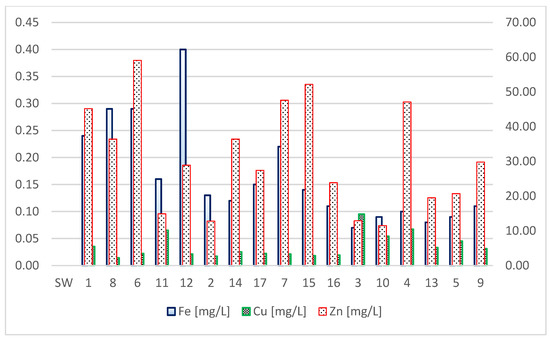
Figure 5.
Metal concentrations in the starting water and water from 17 pipes. SW—start water.
3. Research Results
The obtained measurements of the mass and wall thickness of the pipes before the test and after their completion are summarized in Table 2. On the basis of the results obtained, it was noticed that two pipes increased in weight, and in the case of three pipes, a change in wall thickness was noticed (Table 4). For a more detailed analysis of the observed changes, wall fragments were taken and analyzed under a microscope. Images with a magnification of four times were obtained.

Table 4.
Magnitude of the observed changes of the tubes before and after the end of the experiment.
Measurements showed that there were changes in wall thicknesses in samples 5 and 12. The change in wall thickness in sample 8 is within the measurement error. On the other hand, mass measurements showed changes in samples 4 and 15. In order to illustrate the processes taking place in galvanized steel pipes as a result of long-term accumulation of water and temperature changes, fragments of walls were cut out of the pipes, and then microscopic observations of the inner surface of the pipes were made. Photos of selected samples are shown in Figure 6.
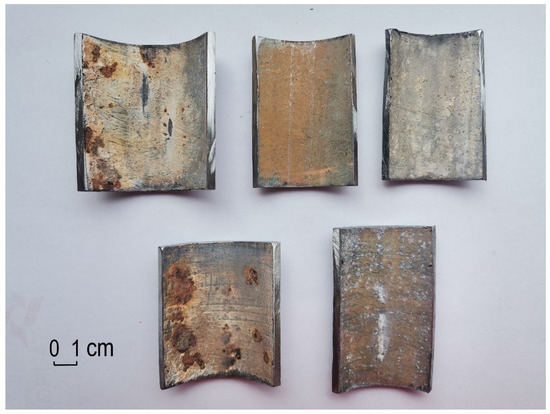
Figure 6.
Photo of the inner surface of pipe fragments, 4, 5, 8, 12, and 15 from the left.
Specific changes are noticeable on the inner surface of the analyzed sections of galvanized pipes:
- -
- Pipe 4: visible white coating of the pipe surface with local small corrosion pits.
- -
- Pipe 5: the entire surface is evenly covered with a rusty tarnish.
- -
- Pipe 8: the entire surface is evenly covered with a white tarnish.
- -
- Pipe 12: the surface is covered with a white tarnish with point pitting.
- -
- Pipe 15: surface covered with a rusty tarnish, locally visible white corrosion centers in the form of spots and streaks.
The internal walls of the steel pipes show that the most advanced corrosion process occurred in pipe 4 (Figure 7a) and pipe 12 (Figure 8a), and these appeared locally, within the seam, as corrosion centers in the form of pitting rusty surfaces with clear boundaries. These changes are characteristic of pipes carrying water at a temperature above 35 °C and occur in places where the zinc coating is discontinued. At the beginning of the process, as a result of hydrogen evolution and adsorption on the surface of the zinc-iron phases, pockets are formed; the coating separates from the steel surface, and anode regions take shape. The speed of this process is greatly influenced by the water temperature and water stagnation in the pipes, which favor the formation of local anodes.
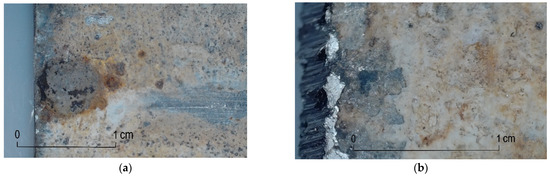
Figure 7.
Corrosion changes on the inner surface: (a) pipe 4, visible pitting with clear boundaries; (b) pipe 4, visible layer of zinc and white corrosion.

Figure 8.
Corrosion changes on the inner surface: (a) pipe 12, visible pitting with clear boundaries; (b) pipe 15, internal surface of the pipe covered with rust and a layer of white tarnish.
In the sample taken from pipe 5 (Figure 9a), a rusty tarnish was observed on the analyzed inner surface of the pipe, with no visible local corrosion centers. In the sample taken from the pipe 15 (Figure 8b), apart from the rusty tarnish, a white corrosion streak is visible on the surface. This type of change occurs in galvanized pipes during the early operation of the pipe installation; when a passive layer of zinc oxide and carbonate is not formed, the result would be tightness and corrosion resistance in an aqueous environment, and the pipes would exhibit a high pH. Initially, there are spot changes in the form of a white tarnish, which in the long run may cover the entire surface of the pipe with a white tarnish. This type of corrosion change is extremely dangerous for steel, because in a short time (several months) it can lead to a complete loss of zinc from the surface of the steel pipe and the formation of a rusty tarnish. This phenomenon can be observed in Figure 9b, which shows the sample of pipe 8: the inner surface of the pipe is covered with a white tarnish, on which single rust spots of small dimensions are visible.
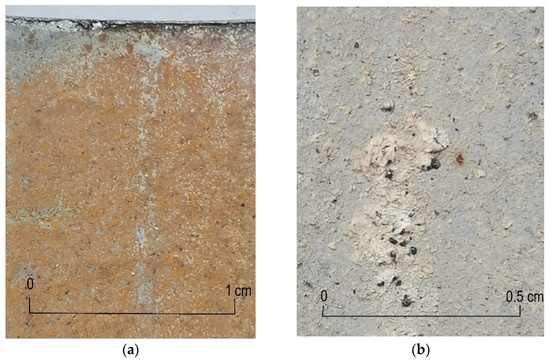
Figure 9.
Corrosion changes on the inner surface: (a) pipe 5, even surface coating with a rusty tarnish; (b) pipe 8, the inner surface of the pipe is covered with a white tarnish.
The assessment of water corrosivity is a difficult issue. The corrosion process depends on many factors. The material from which the system is made may show corrosive properties, and the water in the system may accelerate or inhibit the corrosion process. It follows that the corrosion of the material and the corrosivity of the water should be considered in correlation.
One of the basic indicators of water corrosivity is the acid aggressiveness intensity indicator (I). It is based on the carbonate-calcium balance in water, described by the reaction equation:
where the values in brackets are the amounts of bound and aggressive carbon dioxide, in mg CO2/dm3.
CaCO3 + H2O + CO2 = Ca(HCO3)2
It indicates the required amount of CO2 providing the balance between dissolved bicarbonate and undissolved calcium carbonate. The content of aggressive and bound carbon dioxide expressed in the equation is taken into account:
AI = [CO2]2 [CO2zw] + [CO2agr]
It indicates the required amount of CO2 providing the balance between dissolved bicarbonate and undissolved calcium carbonate. The content of aggressive and bound carbon dioxide expressed in the equation is taken into account:
AI = [CO2]2 [CO2zw] + [CO2agr]
Water is aggressive when the I value is greater than 1.0.
Another indicator that describes the corrosivity of water is the Langelier Saturation Index (LSI) [16]. It is an indicator of the possibility of scale formation in the water. If a CaCO3 precipitate is formed in the water, the corrosivity of the water is low and the corrosion process will occur to a small extent. The LSI index depends on the alkalinity of the water and calcium hardness expressed as mg/L CaCO3, as well as on the total dissolved solids content (TDS mg/L), pH and water temperature. LSI is expressed as the relationship:
LSI = pH − pHs,
The pHs value depends on the above-mentioned water indicators.
If LSI < 0 it means that the water is unsaturated with CaCO3 and contains aggressive CO2. A protective layer of CaCO3 will not be formed on the surface of the pipes transporting water and a corrosive process will occur. If LSI = 0, then the water is corrosive because the water is in equilibrium with CaCO3, and no protective layer will be formed on the surface of the material. For water supersaturated with CaCO3, the LSI index > 0. There is no aggressive carbon dioxide in the water, and a protective layer of CaCO3 may form. The water is not aggressive.
The measure of the influence of the actual pH, the concentration of calcium, bicarbonate, TDS, and temperature forms another index called the Ryznar Stability Index (RSI) [16], expressed as:
RSI = 2(pHs) − pH
In this equation, a value below 5 indicates water instability, and a CaCO3 sediment forming capacity, and thus the corrosivity of the water, is lowered. Within 5–7, the depleting CaCO3 does not form a sediment, the water is corrosive, and sub-sediment corrosion may occur. For the RSI over 7, the water is stable and tends to corrode.
Conducted research indicates the tendency of water to strong corrosion in galvanized pipes. The results of measurements and data of physico-chemical properties of raw underground water, as well as the corrosion indicators, were calculated (Figure 10 and Figure 11). The investigated tap water revealed strong corrosive impact towards galvanized steel pipes, as evidenced by corrosive aggressiveness indices: LSI~−0.1, RSI~9.3, S1~1.0, I~40 IL = 5.9, IL = 6.0. Such water is extremely aggressive to fittings and devices in sprinkler systems and during the water storage (i.e., in time of retention in pipes).
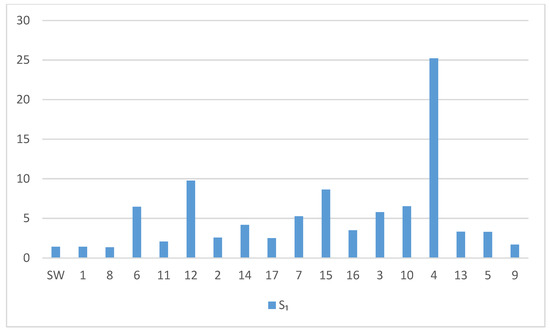
Figure 10.
S1 water corrosivity index. SW—start water.
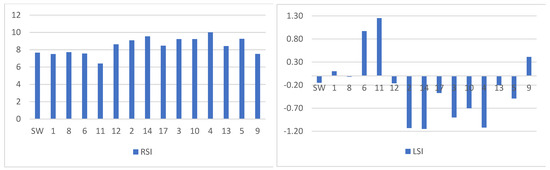
Figure 11.
Ryznar Stability Index (RSI) and Langelier Saturation Index (LSI). SW—start water.
The presented indices have a very strong relation to the presence of carbon dioxide in water, which cannot always be found in stable forms. Unbound carbon dioxide can mostly be found in an unstable, aggressive form, which reacts with the installations’ natural components, such as carbonate salt (i.e., ZnCO3 produces a well soluble form Zn(HCO3)2 that, when in contact with water, causes pits in the surface layer). This phenomenon occurred in the tested pipes. Bicarbonates are present in acidic, neutral and slightly alkaline water. The pH in investigated specimens ranged between 6.82 and 9.5. The acid-base variability of water depended on many factors, mainly temperature and pipes’ surface roughness. The materials destruction process is slow. Over time the changes and loss in its surface layer becomes noticeable, leading to galvanic corrosion as a consequence. These processes may be accelerated by substances causing salinity, especially highly dissociated salts.
The LSI and RSI indices allow only a qualitative assessment of the corrosivity of water in closed systems. These indices do not take other indicators into account, such as chloride and sulphate (VI) ions and dissolved oxygen, high concentrations of which make water corrosive.
The second group of corrosive indices are the indices revealing salinity of water regardless of the carbonate balance. Larson-Skold index, depending on the concentration of corrosive ions (Cl− and SO42−) and the ability to form protecting layer by bicarbonates HCO3−, is described with the formulation:
where the concentrations are in mmol/l; this formulation describes the corrosiveness of water in relation to cast iron and steel pipes [17]. The water is not corrosive in the case of IL < 0.2. When 0.2 > IL < 1, the water varies from very slight to average corrosivity. The water is highly corrosive when IL > 1 [18]. In the presented research results, the IL index is between 1 and 16, which indicates the high tendency of water to corrosivity. Withers showed that when IL > 1.2, the concentration of chlorides and sulphates may disrupt the formation of a layer of natural protective sediments. Concentrations of chlorides and sulphates differed highly in tests (Figure 3), achieving the highest values in waters tested in June and July, 130 mgCl/l on average. The sulphate concentrations were less fluctuate in July (95 mg/L) and September (90 mg/L), representing maximum values.
With regard to carbon steel, galvanized steel, copper and copper alloys, and acid-resistant stainless steels, dependencies have been developed that take into account corrosive water quality indicators [19]. The relationship is expressed by an equation including the influence of nitrogen form (V) in water on corrosion:
Water is corrosive when it contains more than 0.1 g/m3 of oxygen, and the ratio of the sum of the concentrations (mol/m3) of Cl−, NO3− and SO42− ions to the basicity expressed in mol/dm3 is greater than 0.5. Regarding galvanized steel, there is a probability of point corrosion of the zinc layer when S1 > 3. Factors increasing the risk of pitting corrosion are: LSI < −0.5, Ca2+ ion concentration <20 g/m3, alkalinity “m” < 2 mol/m3, dissolved CO2 concentration > 1 mol/m3, and Cu2+ ion concentration > 0.065 g/m3. With a high content of NO3− ions in the water, there is a risk of selective zinc corrosion (inter-crystalline corrosion). This type of corrosion can occur when the dependency occurs:
S2 = ([Cl−] + 2[SO42−])/[NO3−] < 2
The concentration of nitrates (V) in water in the presented results was very low (below 0.1 mg/L). This does not indicate inter-crystalline corrosion due to the nitrates (V) presence. However, high concentration of chlorides and sulphates may cause the pitting corrosion.
The obtained LSI and RSI values indicate an ambiguous aggressiveness of the water in the pipes (Figure 11). In the case of tap water, the LSI value was −0.09, which indicates its corrosivity. The LSI index was below zero for water since July in most pipes, which means that the water is unsaturated with CaCO3 and contains aggressive CO2. No protective layer of CaCO3 will be formed on the surface of pipes containing water, and a corrosive process will occur.
The Ryznar Stability Index (RSI) relates to the saturation of water in CaCO3, the tendency to deposit formation, and the corrosivity of the water. RSI values above 7 for 16 water samples indicate their stability and corrosivity due to the lack of CaCO3 precipitation and the formation of a protective carbonate layer. The RSI values were consistent with the calculated LSI values. Only in pipe 11, from which the water was taken in June, the Langelier and Ryznar indices indicate the precipitation of CaCO3 in the form of a protective layer against corrosion.
The calculated values of the S1 index (Figure 10) for the starting water and for the water samples from 17 pipes exceeded the value of 1, which indicates the probability of pitting corrosion on the surface of galvanized pipes. S1 values clearly increased during the experiment, reaching the highest value (25) for the water sample taken from pipe 4.
In the assessment of the impact of water on the material from which the installations are made, the proportions of the concentration of aggressive ions present in the water as basic components are important. These include chloride, bicarbonate, and calcium ions. The values of simple indices of these ions, calculated as the concentration quotient expressed in mg/dm3, should obtain the following values:
[HCO3−]/[Cl−] < 1
[Ca2+]/[ HCO3−] > 2
[Ca2+]/[Cl−] < 1.5
For the first correlation, the corrosive value was shown by tap water (1.53) and water from pipes 5, 9, and 13. The concentration ratio of calcium and bicarbonate ions for most of the samples was below 2, which indicates the aggressiveness of the water. Only in pipe 4 did the water contain many more calcium ions as compared to bicarbonate ions. On the other hand, the value of the proportion of calcium ions to chloride ions was below 1.5 for all tested water samples.
The obtained values of concentration of basic water indicators (Ca2+, Mg2+, Cl−, SO42−, HCO3−) did not reveal the dependence on temperature. The increase in temperature of these ion concentrations was not observed.
The tests did not show any significant changes in the values of the LSI and RSI indices depending on the temperature (Figure 12). As shown in Figure 12, the time of the water retention in the pipes had a greater impact on the corrosion process. The index values in the final period of the research were the highest. The index values confirmed the lack of influence of temperature on the corrosion of galvanized pipes used in fire installations.
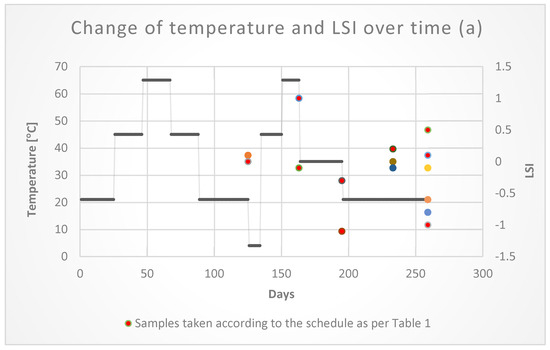
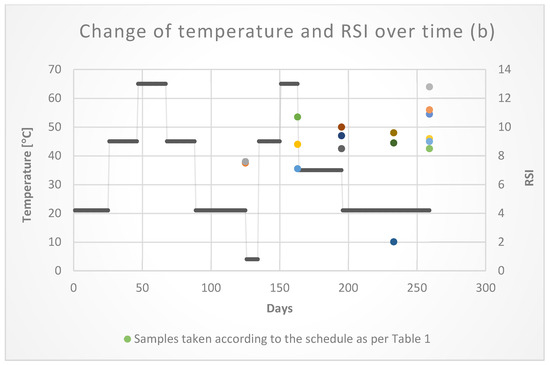
Figure 12.
Temperature changes LSI (a) and RSI (b) in time.
4. Conclusions
The tap water used in the tests to fill the pipes made of galvanized steel was characterized by high alkalinity and a high concentration of bicarbonates as well as chlorides and sulphates, which resulted in very strong corrosive aggressiveness towards the pipe material. This was confirmed by the calculated values of the commonly used corrosivity indices, depending on the carbonate balance and the concentration of acid ions (Cl− and SO42−). The experiment showed that when storing the water in galvanized steel pipes, the quality of the water used should be taken into account. An important factor limiting corrosion in fire protection systems is the material of the installation. The high porosity of the pipes accelerates the process of corrosion, especially pitting corrosion, in the presence of high concentrations of acid ions.
When assessing the corrosivity of water, various indicators should be analyzed and their synergy in the dissolution processes of the steel surface layer should be taken into account. In the conducted research, a comprehensive analysis of the water composition was carried out, assessing the effect of carbonate balance (LSI and RSI indices) and acid components (IL and S indices) and the proportions of ion concentrations on corrosion. The need to use different parameters is due to the fact that the water used in the installations has varying compositions, and the indices take into account different water quality parameters: pH, hardness, alkalinity, concentration of CO2, Ca, Cl, or SO42−, and NO3−. An important result of the conducted tests is to show that the corrosion process is significantly influenced by the quality of water and the quality of the material of the installation in which the water is transported or periodically stored, while the influence of temperature is insignificant. Materials used for sprinkler installations should be checked for their resistance to moisture. Galvanized steel pipes should be stored in optimal conditions to prevent the appearance of corrosion.
Author Contributions
Conceptualization, A.M. (Agnieszka Malesińska) and A.M. (Agnieszka Machowska); methodology, A.M. (Agnieszka Malesińska), A.M. (Agnieszka Machowska), and M.W.; formal analysis, M.W.; investigation, S.B. and P.P.; laboratory resources, M.W.; writing—original draft preparation, S.B. and G.B.; writing—review, and editing, S.B. and V.F.; supervision, M.W., and A.M. (Agnieszka Malesińska). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data has not been archived in the cloud, but it is possible to make them available after contact by e-mail agnieszka.malesinska@pw.edu.pl.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Chatterjee, B. Hot Dip Galvanizing. Hot Dip Galvaniz. Today 2006, 3, 33–37. [Google Scholar]
- Wilmot, B.; Thompson, R.; Barnett, W. Corrosion of hot dip galvanized piping used for re-circulating mine water. Hot Dip Galvaniz. 2006, 3, 33–37. [Google Scholar]
- Bae, C.-H.; Park, N.-S.; Park, S.-Y.; Lee, H.-D.; Hong, S.-H. Assessment of galvanized steel pipes for water service inbuildings by direct diagnosis method. J. Water Supply Res. Technol. 2007, 56, 335–342. [Google Scholar] [CrossRef]
- Andrianov, A. Pitting corrosion of galvanized pipes in hot water supply systems. In Proceedings of the XXIV International Scientific Conference “Construction the Formation of Living Environment”, Moscow, Russia, 22–24 April 2021. [Google Scholar]
- Malesińska, A.; Rogulski, M.; Puntorieri, P.; Barbaro, G.; Kowalska, B. Displacements of the pipe system caused by a transient phenomenon using the dynamic forces measured in the laboratory. Meas. Control 2018, 51, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Brzezinska, D.; Bryant, P.; Markowski, A.S. An Alternative Evaluation and Indicating Methodology for Sustainable Fire Safety in the Process Industry. Sustainability 2019, 11, 4693. [Google Scholar] [CrossRef] [Green Version]
- Stagrum, A.E.; Andenæs, E.; Kvande, T.; Lohne, J. Climate Change Adaptation Measures for Buildings—A Scoping Review. Sustainability 2020, 12, 1721. [Google Scholar] [CrossRef] [Green Version]
- Gałaj, J.; Drzymała, T.; Pełech, A.; Šukys, R. Analysis of the impact of water flow rate of selected Turbo type nozzle on the distribution of sprinkling intensity. In Proceedings of the 13th International Conference Modern Building Materials, Structures and Techniques, Vilnius, Lithuania, 16–17 May 2019. [Google Scholar]
- Malesińska, A.; Kubrak, M.; Rogulski, M.; Puntorieri, P.; Fiamma, V.; Barbaro, G. Water Hammer Simulation in a Steel Pipeline System with a Sudden Cross Section Change Journal of Fluids Engineering. Trans. ASME 2021, 143, 4050728. [Google Scholar]
- Huang, Y.-H.; Shen, T.S. An Article on Green Firefighting Equipment in Taiwan. Sustainability 2021, 13, 12421. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Fang, L.; Wu, Z.; Chen, H. Corrosion analysis of a steel drinking water pipe in an indoor environment. Mater. Perform. 2012, 51, 62–65. [Google Scholar]
- American Galvanizers Association. Hot-Dip Galvanizing for Corrosion Protection of Steel Products; American Galvanizers Association: Englewood, CO, USA, 2000. [Google Scholar]
- Hill, C.P.; Cantor, A.F. Internal Corrosion Control in Water Distribution Systems; Manual of Water Supply Practices—M58; American Water Works Association: Denver, CO, USA, 2011. [Google Scholar]
- EN 12502-3:2005; Protection of Metallic Materials Against Corrosion—Guidance on the Assessment of Corrosion Likelihood in Water Distribution and Storage Systems—Part 3: Influencing Factors for Hot Dip Galvanised Ferrous Materials. European Committee for Standardization: Brussels, Belgium, 2005.
- World Health Organization (WHO). Guidelines for Drinking-Water Quality; WHO: Genewa, Switzerland, 2011.
- Vasconcelos, H.C.; Fernández-Pérez, B.M.; González, S.; Souto, R.M.; Santana, J.J. Characterization of the Corrosive Action of Mineral Waters from Thermal Sources: A Case Study at Azores Archipelago, Portugal. Water 2015, 7, 3515–3530. [Google Scholar] [CrossRef]
- Withers, A. Options for recarbonation, remineralisation and disinfection for desalination plants. Desalination 2005, 179, 11–24. [Google Scholar] [CrossRef]
- Delion, N.; Mauguin, G.; Corsin, P. Importance and impact of post treatment on design and operation of SWRO plants. Desalination 2004, 165, 323–334. [Google Scholar] [CrossRef]
- Zehra, S.; Mobin, M.; Aslam, J. Environmentally Sustainable Corrosion Inhibitors. In Fundamentals and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–23. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).