1. Introduction
In the investigation procedures concerning the causes of the largest transport disasters with the most serious consequences recorded in the first two decades of the 21st century, only the mixing of diesel fuel—used in vehicles—with ammonium nitrate (AN) has been taken into account to assess the power of the explosion [
1]. A brief description of these events is provided below.
Barracas (Spain) 9 March 2004
A passenger car collided with a truck carrying 25 tons of AN on the highway near the city of Barracas. The fuel leaking from the vehicles ignited and mixed with the transported material. An explosion occurred, as a result of which two people died, and five were injured. A crater 5 m deep and about 30 m in diameter was created on the road by the explosion. No violations of safety regulations during the transport were found; the post-incident report only stated that AN is explosive when exposed to high temperatures and that the risk of reaction is greatly increased if mixed with fuels, such as diesel [
2].
Mihăilești (Romania) 24 May 2004
On the international route E85, about 70 km from Bucharest, a truck carrying 23 tons of AN overturned for unknown reasons. Soon after the accident, fire spread to the trailer. About 20 min after the accident, two professional fire departments from Buzău arrived at the scene. During the firefighting operation, there was a small explosion in the driver’s cabin, but the firefighters did not cease the action, and the security services did not remove the onlookers and the TV crew. As a result of the second explosion, 18 people died and 13 people were injured. A crater 6.5 m deep and 14 m in diameter was created on the site [
3].
Monclova (Mexico) 10 September 2007
On a local road, during an overtaking maneuver, a pickup driver lost control over the vehicle and collided head-on with a truck carrying 22 tons of ANFO. As a result of the collision and a leakage of fuel from the ignited vehicles, the fuel mixed with the transported material. After approx. 40 min, the vehicles exploded. Twenty-seven people were killed, and an estimated 250 were injured. The explosion left a crater 3 m deep and 20 m in diameter.
According to a report issued by Orica (the world’s largest supplier of commercial explosives and blasting systems), the incident’s aftermath was due to the insufficient emergency response, which involved:
- –
The ineffective securing of the area by the truck driver and his premature leaving of the scene;
- –
The failure to establish a connection with both SETIQ (Emergency Transportation System for the Chemical Industry) and with Orica;
- –
No danger zone established by the rescuers;
- –
Too much distance from the station of the rescue services to the accident site;
- –
The unfavorable time of the event (Sunday evening);
- –
The failure of the emergency telephone staff to provide information about the incident to SETIQ and the federal police (only the federal police have the authority to coordinate actions)—only local rescuers were notified;
- –
The rescuers’ failure to recognize and understand the meaning of UN/ADR number plates (lack of appropriate training) [
4].
In all the described cases—as well as in most spectacular catastrophes caused by the explosion of AN mixed with hydrocarbons or other organic or inorganic additives—the activation energy originated from fires. Therefore, this study focuses on the rate of fire development and the associated temperature increase over time (from 2 to 50 °C/min). There may also be other causes of an AN mixture explosion (such as static electricity), and the explosion strength and range may vary due to changing environmental conditions, such as pressure, temperature, and humidity. The polymers selected for the experiments are the materials which the packaging for the transport and storage of AN-based fertilizers is made of. In each of the measurements carried out in open containers, the process proceeded without a sudden increase in pressure, and there was no ignition of pure AN with a detonation potential observed.
With regard to the post-incident investigations in the aforementioned cases, one crucial aspect potentially influencing the range and size of the explosion—even if AN does not mix with diesel fuel—has not been taken into account, i.e., the packaging in which the AN or fertilizers with a significant amount of AN are transported. It invariably contains polyolefins.
Polyolefins are the main group of synthetic plastics and include the most popular materials for the production of packaging: polyethylene (PE) and polypropylene (PP). They have no smell or taste, do not dissolve in water, and are non-toxic, chemically resistant, easy to process, light, and cheap, which has contributed to their extraordinary popularity. In terms of production volume (gradually increasing), they outclass all other plastics [
5]. Polyolefins are characterized by a high calorific value: PE—40 MJ/kg, PP—44 MJ/kg, which is comparable to diesel fuel.
Table 1 shows a comparison of the calorific values of the materials treated as high-energy fuels.
Section 5 of the safety data sheets—concerning firefighting measures—contains information on the following topics: firefighting measures applicable in the event of a fire which involves chemicals; possible hazards related to chemicals in the event of a fire (such as the risk of hazardous combustion products or the risk of a vapor cloud explosion). The AN-related characteristics provided in this particular data sheet section on various manufacturers provide various methods of rescue and recommended extinguishing agents (even for the same AN concentration), which are very often mutually exclusive. Most of the post-incident analyses have been carried out on the basis of exceptionally spectacular and tragic events where the amount of AN involved was counted in hundreds or thousands of Mg. The only examined case of an AN explosion at the end user’s location where the AN amount did not exceed 5 Mg was the one in Saint-Romain-en-Jarez in France in 2003 [
7]. In the post-fire proceedings, the French investigators based their analyses on general engineering knowledge without conducting laboratory (or full-scale) tests for the cause of an explosion of such strength (significantly exceeding the explosion scale of AN alone = 0.32 TNT per Mg, while the mixture from the apple crates made of polymers—stored in the same room as the AN—formed a mixture very similar to ANFO in chemical composition—the equivalent of 0.74 TNT per Mg) [
7]. Following the conclusions presented in the final report after the events in Saint-Romain-en-Jarez, laboratory tests were carried out using mixtures of AN with various polymers which may have been stored nearby as fertilizers—e.g., as packaging—within the same rooms in farm buildings.
The article presents research results and conclusions which may be useful in planning a rescue operation. This applies to activities carried out in livestock buildings or, more broadly, the closed facilities that play the role of multifunctional rooms on farms. Usually, fertilizers are stored there with other materials, as well as equipment used in agriculture, including devices powered by hydrocarbon fuels such as diesel oil, gasoline, and vegetable oils.
For the chemical release consequences risk assessment and the likelihood of the occurrence of such effects as fires or explosions, certain parameters have been defined to simulate various scenarios. In the event of a fire hazard, the basic condition for its emergence is the so-called fire triangle, i.e., the simultaneous presence of four factors: an oxygen source or an oxidizer that supports combustion, heat that allows the mixture to ignite, fuel that supports combustion, and a chemical reaction between the fuel and the oxygen source. Each of these factors can be described with different parameters. In the case of fuel, these are, i.a., its moisture, physicochemical parameters, and flammability. For ignition, we can distinguish, e.g., its energy, temperature, and source. The elimination or significant weakening of at least one of these factors makes it possible to extinguish the flames and smother a fire [
8]. In the event of an explosion, the situation is more complicated. Compared to fire, there is a much greater increase in pressure and heat flux, released in a much shorter time. The explosion nature may vary depending on the dynamic conditions around a combustible mixture. However, for the explosive course of a chemical reaction—compared to a fire—the presence of two additional factors is crucial: the homogeneity of the mixture and its confinement, which translate into the possibility of pressure increase [
9].
Laboratory tests do not faithfully reproduce the real-scale events because the conditions in which the experiments are conducted are relatively stable (e.g., the temperature rise is controlled and the amounts of material are reproducible, as are the proportions and the method of mixing the samples) and may not take into account many key parameters. This is usually due to their nature—successive variable factors are isolated in order to assess their impact on the studied phenomenon. They are only intended to indicate trends and potential development opportunities in the event of a road incident and to point out areas in which safety standards for the transport of given materials—which in specific conditions (e.g., fire) may mix and cause additional hazards not present in normal conditions—should be considered. For this reason, in order to correctly assess the risk and plan a rescue strategy, it is necessary to compare the results obtained on the micro and macro scale. This will allow not only the assessment of the process development in a less controlled environment but also the observation of the additional accompanying phenomena.
2. Materials and Methods
A series of thermal analyses of mixtures of AN with PP and PE were carried out [
10,
11,
12]. Such a choice was influenced by the aforementioned accident in Saint-Romain-en-Jarez, where French investigators pointed to the direct contact of molten PE boxes with stored AN as the reason for the ANFO-analogous material creation. It should be noted that the AN was stored in intermediate bulk containers made of PP, the properties of which could also contribute to the creation of material similar to ANFO. The analysis of this influence of polymer addition on the decomposition process was carried out using differential scanning calorimetry (DSC), taking into account several different process parameters, such as the mixture composition and the heating rate of the samples. As the transported sacks and containers are usually placed on wooden pallets, it was also decided to investigate the behavior of the mixture of AN with the most commonly used wood, i.e., pine. For the risk assessment in transport—in the opinion of the authors—this material should be taken into account, because each Euro-pallet is made of approx. 20 kg of dry wood.
Due to the high sensitivity of the equipment, as well as the risk of its damage in the case of the occurrence of an explosion, the mass of the tested sample needed to be relatively small [
13]—about 10 mg. The sample materials were crushed to transfer the size and geometry from the real scale to the microscale. To avoid irreversible changes in the structure of the tested materials due to the heat released during friction, the materials were mechanically disintegrated in an atmosphere of liquid nitrogen [
14]. The samples were fractionated with sieves. AN is a highly hygroscopic material, and any dampness could have a significant impact on the experiment results [
15]. Hence, the 0.1–0.29 mm fraction was placed in a chamber dryer for 24 h, at 25 °C and 25 kPa below atmospheric pressure, and in a desiccator under nitrogen for another 48 h.
The components were weighed with an accuracy of ±0.01 mg. A sample of 10.00 ± 0.15 mg was placed in a 25 µL analytical aluminum vessel with a lid with a hole of 0.3 mm in diameter. Thanks to the opening in the upper part of the vessel, the released gases were removed, which minimized the pressure increase inside the vessel. Thus, the obtained results reflected to a greater extent the influence of the temperature itself on the sample decomposition [
15]. The halves of the vessel were permanently pressed together to avoid the risk of their separation. The DSC 3500 Sirius-NETZSCH apparatus was used for the analysis, in the range of 20–400 °C in an air atmosphere with a constant flow of 50 mL/min. The sample material was heated linearly in the standard way for this type of analysis—10 °C/min. When the total registered heat effect of the exergonic transformation (ΔH) was less than a minute (for a scanning speed of 10 °C/min), the processes were treated as explosive reactions.
In the second stage of the research, the scale and the amount of material used in the experiments were increased 840 times. It was, however, still a laboratory scale. An AN mixture with an additive content of 20% and a sample weight of 4.2 g was prepared according to the procedure described for the DSC analysis. The resulting mixture was thoroughly mixed and placed in an open ceramic crucible used to calcinate the samples. Next, the vessel was placed in an oven in the arrangement shown in the pictures: an open electric heater and a thermocouple to monitor the temperature of the heating surface; the sample temperature was measured with an infrared thermometer. The measurement was performed in a fume hood due to the emission of toxic gases (
Figure 1).
3. Results
3.1. Results of DSC Analysis
In the laboratory of the Lodz University of Technology, a series of tests were carried out to determine the explosive potential of AN with PP and PE in the adopted proportions of 20% and 50%. A more detailed analysis is presented in the first article in the series [
10], and the following articles present an analysis of threats to other popular polymers, such as nylon or polyvinyl chloride [
11,
12].
Figure 2,
Figure 3 and
Figure 4 show the DSC analysis results for the tested AN and PP mixtures.
AN behavior during heating is characteristic of its physicochemical properties. It is a polymorphic material, which means that in certain temperature ranges it occurs under certain crystalline forms. The DSC analysis results presented in
Figure 1 make it possible to identify five different processes that AN undergoes when heated in the range of 20–400 °C. The first three of them are the aforementioned crystallographic transformations—successively, for about 40 °C (transition from orthorhombic δ to orthorhombic γ form), 85 °C (transition from orthorhombic γ to trigonal form), and 126 °C (transition from trigonal to cubic form). Another transformation—melting—takes place at about 169 °C, while at a temperature of about 281 °C the initiation of a thermal decomposition can be observed. All the described processes are endothermic, which means that under the analyzed conditions (i.e., at atmospheric pressure), heat is taken from the environment during their course. Numerous studies [
16] indicate, however, that in conditions of increased pressure, the thermal decomposition of AN may be exothermic. The thermal decomposition of pure PP is exothermic, but the process is slow and poses no risk of explosion. Different behavior can be observed when the materials in question are mixed.
During the decomposition of the mixture containing 20% by mass of PP, the rapid release of significant amounts of heat (−1310 J/g) can be observed in a short period. Such a reaction is potentially explosive and constitutes a significant risk from the fire safety point of view. An additional factor that affects safety is the reduction in the decomposition reaction initiation temperature compared to the decomposition of pure AN—it decreases from 281 to 270 °C. In practice, this means that the decomposition of the analyzed mixture is completed before the decomposition of AN even begins.
The effect of increasing the amount of PP in the mixture to 50% is shown in
Figure 3. The decomposition reaction initiation temperature does not change significantly, but the greater polymer amount results in less heat in the main decomposition reaction—the thermal effect of the reaction decreases from −1310 J/g up to −757 J/g. Moreover, the decomposition reaction of the excess polypropylene can be observed at higher temperatures. It should be remembered that such a reaction is still potentially dangerous and may be explosive in the case of, e.g., increased pressure or the presence of impurities.
Figure 4 shows the effect of reducing the heat supply rate to the sample from 2 to 10 °C/min—to simulate an indirect heating process, for example through a wall separating the mixture from fire. In this case, too, much less heat was observed. Moreover, a noticeably lower decomposition reaction initiation temperature was observed, which in this case is 226.4 °C. Although this reaction is not potentially explosive, it is still exothermic at low temperatures. Its heat may initiate other chemical processes.
A similar analysis was also carried out for a mixture of AN and PE. Most of the observations and conclusions between the samples with these polymers are quite similar. The significant differences include a much lower melting point of pure PE compared to PP (about 125 °C versus 155 °C) and a higher temperature of its decomposition (about 215 °C versus 190 °C). In the case of the 20% mixture, some differences can be observed at a noticeably lower temperature of the decomposition reaction initiation as decomposition begins at 257 °C (
Figure 5). The amount of energy released during decomposition is higher for PE; it is −1496 J/g.
Increasing the amount of polymer in the mixture results in a noticeably different behavior of the mixture. The temperature of the decomposition reaction initiation is reduced to 233 °C, and the enthalpy value of the decomposition reaction is much lower (−968 J/g). Additional analysis was also carried out for PE—the behavior of the mixture containing 8% of the polymer was tested. This is the amount that corresponds to the organic oil content of ANFO. While the course of the reaction itself is quite similar to the mixture containing 20% of PE, the enthalpy of the reaction is much lower, and it proceeds noticeably more slowly. The nature of its course indicates a lower explosion risk.
3.2. Thermal Decomposition of the Mixture in the Macroscale
During the performed DSC analyses, the potentially explosive decomposition reactions were observed. In each of the tested samples, a rapid release of significant amounts of energy was observed, which clearly indicates a significant danger during the decomposition reaction of these spontaneously formed mixtures. Because several conditions must be met to initiate an explosive reaction, it was decided to check whether the described reactions would also be potentially dangerous on a larger scale.
The first analysis was performed on a mixture with PP. Due to the design of the stove and the lack of mixing of the sample, the first signs of sample melting were observed on the walls of the vessel (
Figure 6a). Visible gas evolution was observed when the temperature increased by about 20 °C. Further heating led to the deposition of a brown residue on the walls of the ceramic vessel. As the temperature increased, the emission of smoke became more and more intense. The beginning of the boiling of the sample could also be observed—a thick and sticky foam appeared on its surface, which led to an increase in the sample volume (
Figure 6b). With further heating, the intensification of the above-mentioned phenomena and the beginning of sample carbonization were observed. The molten sample was brown. When the sample was completely melted, it gave forth an intensely white smoke with an unpleasant odor. Then, intense boiling of the sample was observed, as well as the phenomenon of the ceramic vessel boiling over (
Figure 6c).
After boiling over, there was a sudden appearance of the flame and the scattering of the burning foamed material from the dish. The effect, resembling burning rocket fuel, lasted at least a few seconds, with the flame several dozen centimeters high (
Figure 7a). When the process was over, the burning of the sample remains could be observed for a long time (
Figure 7b).
The course of the reaction with wood dust, despite the promising results of the DSC analysis, was not as impressive. During heating, the sample slowly melted and released increasing amounts of thick white smoke with an unpleasant odor. Further smoke intensification occurred when the sample began to boil (
Figure 8a), and a thin, slightly brown-colored foam began to form on its surface. When the amount of foam began to increase rapidly, the liquid material was dispersed over a large area when the large bubbles burst (
Figure 8b). In the event of contact with a hot heating plate, the rapid formation of small flames with a characteristic noise occurred. However, the sample itself did not ignite. With further heating, the intensity of foam formation gradually decreased. It is worth paying attention to the black cover next to the stove, on which the material that spread during decomposition is clearly visible.
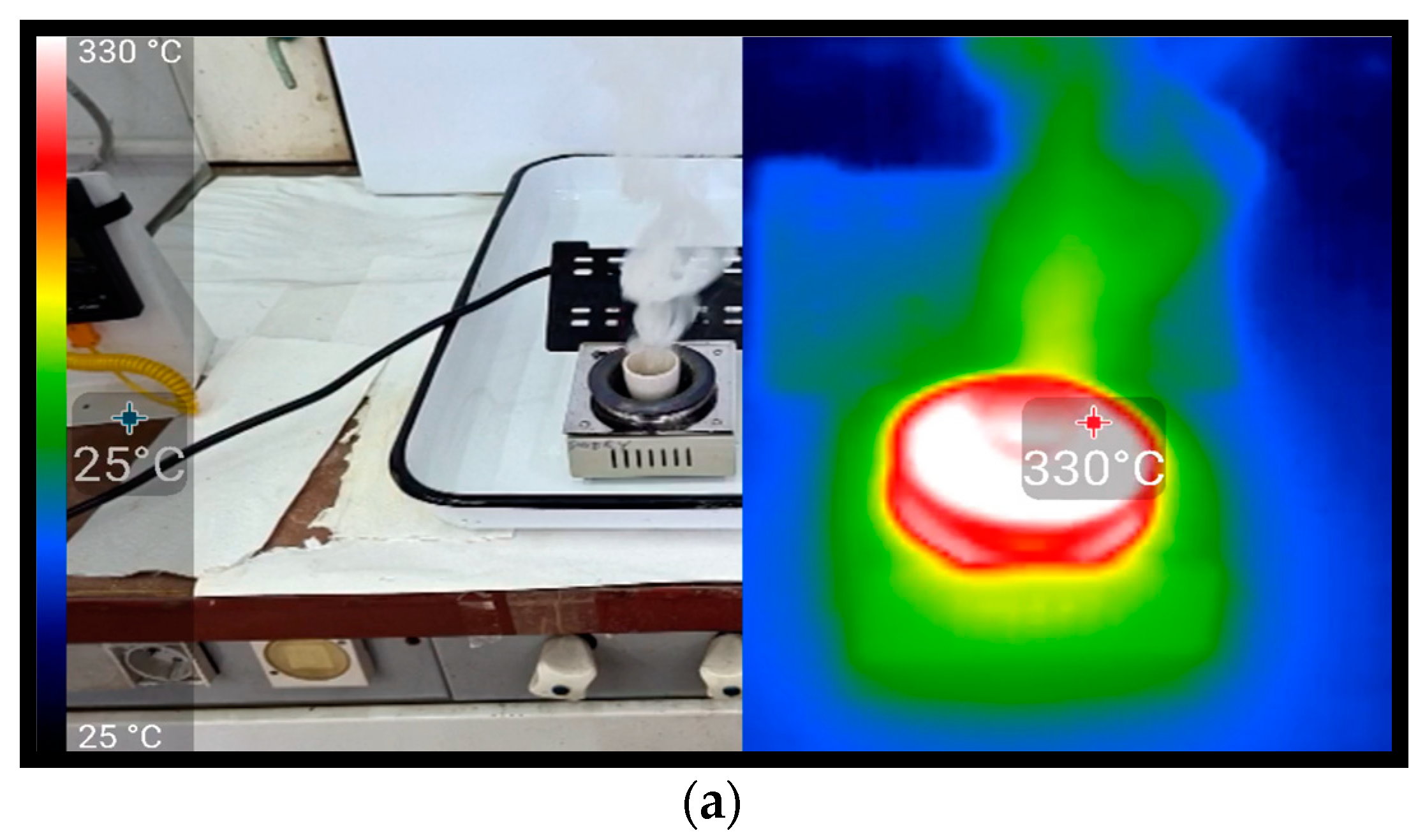
Another larger-scale experiment was carried out for a mixture with PE. In this case, during the reaction, a thermal imaging camera was also used to investigate the possibility of early fire detection. The observations were quite similar to those recorded for PP; the significant difference was a slightly more liquid consistency of the foam formed during the sample boiling. Despite similar observations during the sample giving forth smoke and boiling, the very nature of the sample’s explosion was significantly different.
As in the case of the mixture with PP, a gradual intensification of smoke production was observed along with the increase in sample boiling intensity (
Figure 9a). At one point, the emission of a very dense white smoke was observed, which was also visible on the infrared camera (
Figure 9b). An explosion of much lower force than in the case of a mixture with PP was observed (
Figure 9c); however, in this case, the dispersion of both the liquid mixture and the flames was noticed. Subsequently, there was no smoke production, but slow foam formation was observed for a short time. After a while, the smoke began to emerge again (
Figure 9d), and then, another explosion occurred (
Figure 9e). The situation repeated several times, and each time the outbreak was less and less intense.
The conducted experiments on a larger scale confirm the possibly explosive course of the mixture decomposition reaction for mixtures with molten polymers. The factor that determines this course may be the high viscosity of the molten mixture, which does not allow a large amount of gases to flow out freely from its volume. This may result in a pressure increase in its volume. Other important observations include the formation of a large amount of unpleasant-smelling gases. They can lead to a local pressure increase in confined spaces, which may result in an exothermic decomposition of the AN itself. Moreover, these gases can adversely affect the occupants of the given facility and—in the case of poor ventilation—significantly reduce the visibility and hinder the firefighting operation. Another factor that increases the risk of fire spreading is the burning mixture dispersion, as shown in
Figure 7a. In the case of a mixture with wood dust, no explosion was observed, but the amount of gas released suggests that heating such a mixture in a confined space may lead to an increase in the pressure and an intensification of this reaction.
3.3. Risk Estimation in Real Conditions Based on Model Calculations
The greater the amount of material used, the more the experimental observations correspond to what is going to be observed in the event of a real accident. Conducting field tests seems necessary due to the significant probability of an explosion of mixtures of polymers with AN produced as a result of a fire, having much greater energy than that of pure AN. It will also allow the observation of the phenomena accompanying the process of thermal decomposition and the verification of the methods of early detection and prevention of fire and explosion.
Thus, it is necessary to enlarge the danger zones in the planned rescue operations. A relatively small amount of AN and the polymer from which the apple crates were made in France (2003) caused much greater damage over a much larger area than would have occurred in the case of uncontaminated AN. To estimate the fire zones range, mathematical models based on formulas are used. In some countries, such as Poland, they can be found in implementing acts, which include regulations [
17,
18].
The formulas in Annex 2 to [
17] make it possible to determine the value of the shock wave overpressure (P
f) as a function of the distance between the wavefront (L) and the explosion site, using the hexogen equivalent (G) for the calculations. The value of the pressure increase can be determined empirically (by model tests) or, as is more practical in local emergencies, calculated according to the formula:
where the symbols stand for:
Pf—shock wave overpressure [kPa],
L—distance from the explosion site [m],
G—hexogen equivalent of the explosive [kg].
Around the location of the explosive (or a mixture that spontaneously arises as a result of atypical phenomena), explosion hazard zones are designated, which are divided—due to the expected pressure of the shock wave caused by the explosion—according to:
- (1)
direct-shock wave overpressure over 250 kPa,
- (2)
close-shock wave overpressure from 35 to 250 kPa,
- (3)
indirect-shock wave overpressure from 5 to 35 kPa,
- (4)
far-shock wave overpressure up to 5 kPa.
In turn, Ref [
18] applies to rooms where an explosive mixture may develop, formed from such an amount of the released combustible gases, vapors, mists, or dust that the explosion could cause—within the room—an increase in pressure exceeding 5 kPa. Such interiors are defined as potentially explosive environments. The annex to the aforementioned regulation sets out guidelines for determining the pressure increase in a room, which could be caused by an explosion.
The pressure increase in the room Δ
P (in Pa) caused by an explosion involving combustible materials (not belonging to homogeneous combustible gases or vapors with particles composed of carbon, hydrogen, oxygen, nitrogen, and halogen atoms) is determined by the equation:
where:
—maximum mass of combustible materials forming an explosive mixture that can be released in the investigated room (kg);
—heat of combustion (J/kg);
—normal atmospheric pressure, i.e., 101,325 Pa;
= 0.17 for combustible gases and raised combustible dust;
—room airspace volume, which is the difference between the volume of the room and the volume of the installations, equipment, closed packages, etc., located within (m3);
—air density at temperature T, i.e., 1.2 kg/m3;
—specific heat of air, i.e., 1.01 · 103 J/kgK;
—room temperature under normal operating conditions, i.e., 293 K.
The analysis results of the real, full-scale accident included in the report by the French investigators [
7] are the basis for such an assessment. It is stated in the report that a mixture—spontaneously formed in fire conditions—with a specificity similar to ANFO has the TNT equivalent of 0.74. This means that the effect described in the report resulted from the explosion of 400–700 kg of AN and a molten PE and PP crates mixture. For pure AN—having a TNT equivalent of 0.32—this would amount to 900–1600 kg for a comparable blast force.
Based on the findings of the French investigators and the experiments carried out on a laboratory scale, it appears that the amount of material and its percentage composition are not the only factors affecting the explosion strength. It also depends on the material heating rate. When the material temperature increase is 2 °C/min, the temperature of the decomposition initiation is much lower, and the decomposition process itself is completely different to when the increase is more dynamic (10 °C/min). It means that—in a rapidly developing fire—the effects of initiating an explosion of AN and its mixtures with polymers can be completely different, as can the observable behavior of the material before the explosion. Using the Saint-Romain-en-Jarez example of how the actual fire development ended with an explosion—from the point of view of the person managing the rescue operation, the situation is definitely less favorable when the fire develops in a different part of the building to where the AN and polymers are located. This should have a significant impact on the tactical intention and the designation of the danger zones scope. The conclusions from the laboratory tests can prove the thesis that molten PP combined with AN—constituting a potentially explosive mixture—is a much more dangerous combination than a similar mixture containing wood dust; in such a case, no violent reactions causing a significant increase in pressure occur, and heavy smoke would be the only danger on the real scale. In studies with PE, instead of one large explosion, the occurrence of several smaller ones appeared, sequentially—at similar time intervals. This could lead to the formation of further primers and the spread of fire with a significant likelihood of secondary explosions.
4. Discussion
The conducted research shows that some of the commonly used plastics—mixed with AN or AN-based fertilizers—pose a genuine threat. The combination of these materials may lead to a significant increase in the fire dynamics or to an explosion. Taking the conducted experiments into account, the question arises as to whether it is worth considering replacing PP (the material used for the production of flexible intermediate bulk containers) and PE (the inserts for flexible intermediate bulk containers) and successively replacing the traditional packaging with bags made of safer materials. The analyses results and research concerning the materials stored in farm buildings suggest that further laboratory research should be carried out. In the event of an explosion of AN mixed with materials based on hydrocarbons or other additives which increase the explosion strength, a suspicion of intentional human action always occurs until a detailed analysis is performed [
19]. Furthermore, attention should always be paid to how the reaction initiation occurs. In the described accident in Saint-Romain-en-Jarez, it occurred due to the heat coming from an external fire. However, the reaction may also take place due to mechanical causes, such as an explosion of a smaller charge or other material, strong light, electric current, a strike, or contact with other chemicals [
20]. AN contamination with powdered metal or metal increases the force of an explosion in a significant way [
21]. Moreover, nanostructured ferrites MFe
2O
4 (M = Cu, Co, Mg, Zn) act as catalysts in the decomposition of AN [
22].
Even though the explosive reaction does not necessarily have to take place in the entire volume and mass of all the materials involved, the conducted experiments show that explosive mixtures of polymers and AN—in various proportions, concentrations, and temperature rates—might be easily created, resulting either from deliberate action or incautious storage of fertilizer in farm utility rooms [
23].
The very formation of a primer-like material may lead to a further, strongly exothermic decomposition reaction of the resulting mixture or even of the AN itself. Thus, this aspect is worth deeper analysis and experiments, preceded by on-site inspections in utility buildings. From the point of view of the rescue services’ operational activities, the fact of storing the mentioned group of polymers together with AN in one room should be treated with extreme caution, and the risk of explosion should be taken into account in the operational action plan. In some situations, the head of the rescue operation may decide to conduct a defensive fire attack only to prevent the fire from spreading to neighboring buildings. What is also important is that an AN and polymer mixture in contact with water or steam may intensify the exothermic reaction and thus increase the risk of explosion [
24].
In the majority of the available safety data sheets,
Section 5 (concerning rules of conduct in the case of fire) emphasizes that burning AN should not be extinguished with large amounts of water. The problem of determining the proportion of water mass to AN mass still remains. It is not stated anywhere, and the term „large amounts” is extremely imprecise.
Among all the tested AN and plastics mixtures, the explosion was most commonly observed in mixtures containing 20% plastics. The rates of heat energy release are in this case significantly higher than for the pure components of the mixture alone. The charcoal addition as an ANFO component was investigated in [
25,
26]. The effect of chemically bound carbon on the properties of thermal decomposition of AN was investigated in [
27]. Even though the issue turns out to be more complex, in the event of a fire in objects containing these materials there is a possibility of an explosion.
The results of the laboratory tests do not provide a complete answer as to the scale of threats during rescue operations. They do, however, determine some potential possibilities of the behavior of AN-based fertilizers and various polymers mixtures.
Table 2 shows the decomposition enthalpy depending on the heating rate (pure AN or its mixture with polypropylene in different percentages).
Table 2 and
Figure 2,
Figure 3 and
Figure 4 show that AN + 20% PP is the most explosive configuration, where during the sample decomposition a rapid release of 1310 kJ/kg of energy is emitted in an exothermic reaction. The important information is, however, that for each of the case studies, as well as for the other polymers [
10,
11,
12], the nature of the decomposition changed from endothermic—for pure AN—to exothermic for mixtures with polymers or dusts. Moreover, this decomposition was often violent and resulted in the release of significant amounts of heat into the environment. The results of the DSC analyses allow the determination of this amount and the temperature at the beginning of the mixture decomposition, which is extremely valuable information for rescue teams. However, it is worth noting that—as the macroscale tests suggest—the DSC analysis itself, despite the considerable usefulness of its results, is not sufficient to accurately describe a real fire scenario. For this purpose, it is necessary to perform additional tests that will enable taking into account other factors influencing the behavior of the mixture. Based on the tests performed on a macroscale, the ability of the given configuration to create conditions of increased pressure seems to be of key importance. Due to the large amount of smoke produced and the sensitivity of AN to overpressure, it may be necessary to conduct further research in this regard. Additionally, it is worth paying attention to other observed aspects. Thick white smoke produced during decomposition can significantly reduce the field of view of fire crews, and its irritating and unpleasant smell indicates the need for additional protective measures and medical equipment for both the crew and the civilian casualties.
Laboratory tests with the use of a mixture of AN and powdered PP with different percentages show that an explosive reaction may occur depending on the proportion of both materials. Even a small amount of polymer in AN transport or storage may—due to friction—lead to the initiation of an explosion. The main difference between the particular materials is the energy released during their decomposition, the rate of release, and the temperature at which decomposition occurs. It is not possible to determine what amounts of polymers get mixed with AN in practice, because it depends on how the material is handled during transport or during storage, in what weather conditions it is transported, how it is protected against the effects of moisture, the ambient temperature and, finally, the friction of packages against each other due to shocks during reloading or the transport itself. However, there is a high probability, or even a violent presumption, that such a polymer addition appears even in tightly closed packages and constitutes a material initiating an AN explosion when exposed to high temperature in fire conditions. The rate of heating also depends on the distance from the fire, the materials burned, and how hermetic the room is, which has a significant impact on the local pressure increase in fire conditions.
5. Conclusions
The conducted experiments also made it possible to draw conclusions from the observation of an AN mixture decomposition course, which may improve the effectiveness and safety in managing the rescue and firefighting operation. An important aspect—when it comes to making operational decisions—is the variable intensity of the smoke production during sample decomposition. In the case of explosive reactions, a gradual increase in the intensity of smoke production was observed, and then—just before the explosion—a short break in its emission was noted. By the obtained experimental results in conjunction with the infrared measurement of the material temperature and the smoke itself (which has been confirmed by larger-scale studies), it will be possible to observe significant changes in the fire development and thus enable a flexible approach to the tactics or—in the event of an increased risk of explosion—the immediate withdrawal of rescuers from the danger zone. In addition, the appearance and behavior of the molten mixture constitute important information. Explosions of much greater force were observed for the molten material covered with dense and sticky foam. Such visual information obtained from a reconnaissance team or a drone will reduce the risk of endangering the health and the lives of firefighters.
For the incident commander, each piece of information that gives a chance to identify potential threats is valuable. Interviews with the owners of facilities or—in the case of transport—with drivers, can provide information on what admixtures may potentially be in the direct vicinity of the AN, which—in turn—will affect the implementation of appropriate tactics. Not only polymers, but also metal filings and dusts and their compounds have a significant impact on the operation development. Their influence on the course of the reaction with AN was investigated by researchers from the National University of La Plata in Argentine. A repair shop or other plant where metal processing is carried out—located in the same facility or immediate vicinity—can significantly influence the type of hazards, as well as the violence of the reaction. Therefore, knowledge about the substances, which, according to the research may impact the force of the explosion or the rate of fire development, should be popularized in the firefighting community, not only in fire schools, but also in courses for the commanders of volunteer fire departments. The latter are particularly frequent in rural areas where the AN—stored in the form of fertilizers and its various pollutants—explosion risk is most likely, and volunteers are most often the first to reach such scenes. It would also be advisable to conduct a social campaign in rural areas about the potential dangers of storing various types of common materials on farms in the same buildings as fertilizers.
The conducted laboratory tests are to provide initial research material to estimate the time that—in certain conditions—is at the rescuers’ disposal between the fire occurrence and the potential spontaneous formation of an explosive in the fire environment. This may increase the safety of rescuers and bystanders in the event of fires in facilities containing polyethylene, polypropylene [
10], polyurethane [
11], or other polymers, such as polyamides, polyvinyl chloride, polystyrene, poly(methyl methacrylate) [
12], dusts of organic origin [
28], silicon dioxide [
29], and even soybean oil [
30], as well as other components which have the potential to generate—in an uncontrolled manner—an explosive similar to ANFO. This similarity is based not only on the analogous chemical structure of the given organic substance in the mixture, but also on the comparable course of the decomposition reaction.
Self-generated ANFO analogues can, after all, be obtained deliberately, as in the mining of explosives which contain polymers. A double effect is thus achieved: increased explosive force as well as plastics utilization. This type of research was carried out in Poland, at the University of Science and Technology (AGH) [
31]. In the event of road accidents, the main explosive may arise from the transported AN and a leakage from the unsealed fuel tank of the vehicle. Polymer additives may get into the fire-heated plastic finishing elements or vehicle equipment as well as the collective packaging in which the AN is transported. All of these materials, according to the research carried out in the AGH laboratory, increase the explosion power of the thus enriched ANFO.