Suitability of Selected Plant Species for Phytoremediation: A Case Study of a Coal Combustion Ash Landfill
Abstract
:1. Introduction
- -
- to identify the species of plants with the highest phytostabilization capacity (defined by bioaccumulation and translocation factors).
- -
- to determine the influence of the location of plant growth on the landfill on phytostabilization.
- -
- Phytostabilization is influenced by the species (i).
- -
- Phytostabilization is influenced by the location on the slope (ii).
- -
- Phytostabilization is influenced by the interaction of species x location (iii).
2. Materials and Methods
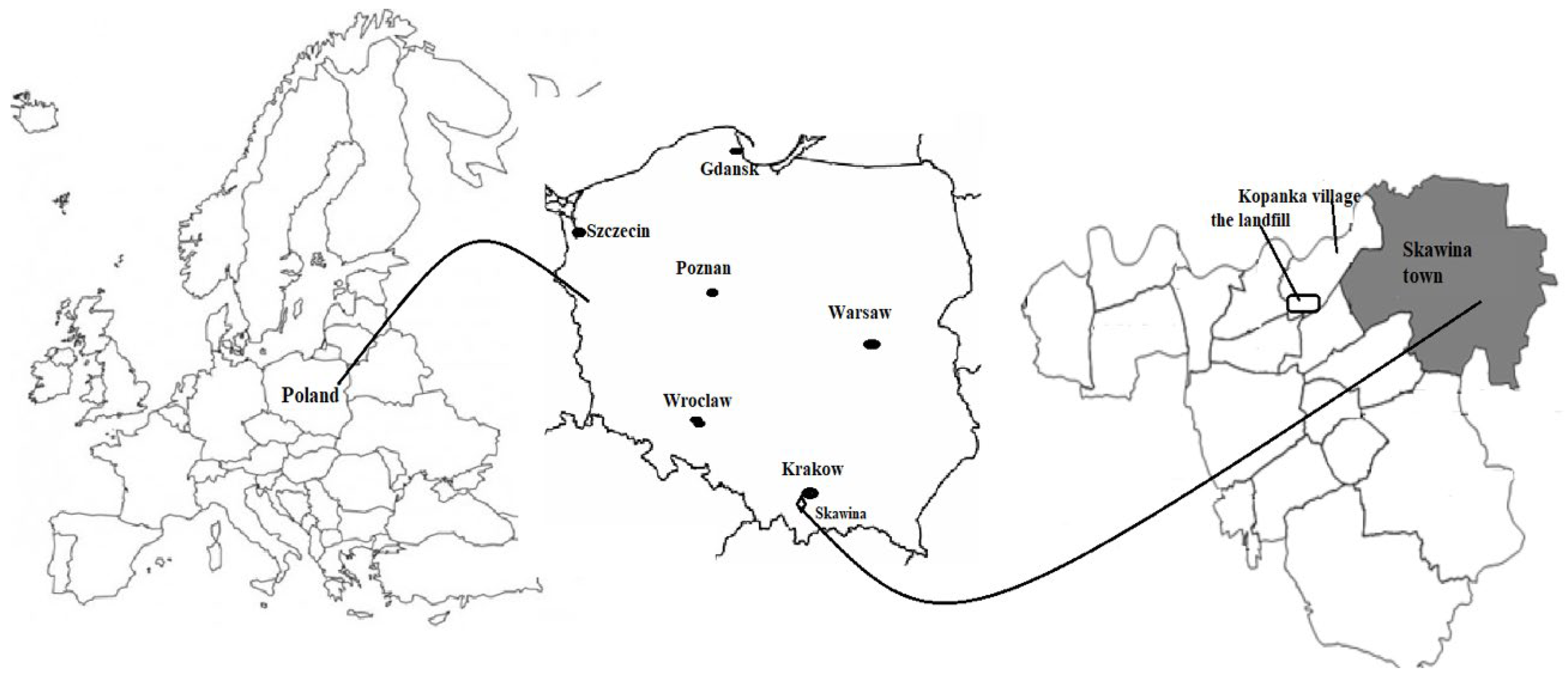
2.1. Study Site Description
2.2. Plant and Soil Sampling
2.3. Chemical Analyses
2.4. Assessment Methods
3. Results and Discussion
3.1. Results and General Assessment
3.2. Detailed Assessment
3.2.1. Influence of Species (Regardless of Location) on Metal Phytostabilization
3.2.2. Influence of the Interaction of Species and Location on Phytostabilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. EU Energy in Figures Statistical Pocketbook. 2018. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/pocketbook_energy_2017_web.pdf (accessed on 29 April 2022).
- Samanli, S.; Celik, H.; Oney, O.; Can, Y. A review on improvement of coal-fired power plants and environmental benefits of ash utilization. Int. J. Global Warm. 2017, 11, 67–86. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Hirajima, T.; Petrus, H.T.B.M.; Oosako, Y.; Nonaka, M.; Sasaki, K.; Ando, T. Recovery of cenospheres from coal fly ash using a dry separation process: Separation estimation and potential application. Int. J. Min. Process. 2010, 95, 18–24. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilization of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- PCSO. Polish Central Statistical Office. Statistics Poland. Environment. 2021. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2020,1,21.html (accessed on 29 April 2022).
- Pandey, V.C.; Singh, J.S.; Singh, R.P.; Singh, N.; Yunus, M. Arsenic hazards in coal fly ash and its fate in Indian scenario. Resour. Conserv. Recycl. 2011, 55, 819–835. [Google Scholar] [CrossRef]
- Gruchot, A.; Szwalec, A.; Mundała, P. Chemical and geotechnical properties of ash-slag mixture from ‘Czajka’ landfill near Tarnow. Environ. Prot. Nat. Res. 2013, 24, 63–67. [Google Scholar]
- Bhattacharyya, S.; Donahoe, R.J.; Patel, D. Experimental study of chemical treatment of coal fly ash to reduce the mobility of priority trace elements. Fuel 2009, 88, 1173–1184. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Jambhulkar, H.P. Restoration of fly ash dump through biological interventions. Environ. Monit. Assess. 2008, 139, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J. Reclamation and revegetation of fly ash disposal sites–challenges and research needs. J. Environ. Manag. 2009, 90, 43–53. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Dyguś, K.H. The role of plants in experimental biological reclamation in a bed of furnace waste from coal-based energy. Ecol. Eng. 2015, 16, 8–22. [Google Scholar] [CrossRef]
- Técher, D.; Laval-Gilly, P.; Bennasroune, A.; Henry, S.; Martinez-Chois, C.; D’Innocenzo, M.; Falla, J. An appraisal of Miscanthus × giganteus cultivation for fly ash revegetation and soil restoration. Ind. Crop. Prod. 2012, 36, 427–433. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Baum, C.; Niedojadło, J.; Dahm, H. Promotion of mycorrhiza formation and growth of willows by the bacterial strain Sphingomonas sp. 23 L on fly ash. Biol. Fert. Soils 2009, 45, 385–394. [Google Scholar] [CrossRef]
- Jambhulkar, H.P.; Juwarkar, A.A. Assessment of bioaccumulation of heavy metals by different plant species grow non fly ash dump. Ecotoxicol. Environ. Saf. 2009, 72, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Jaiswal, S. Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: A field study from Santaldih thermal power plant, West Bengal, India. Environ. Monit. Assess. 2008, 136, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C. Invasive species based efficient green technology for phytoremediation of fly ash deposits. J. Geoch. Explor. 2012, 123, 13–18. [Google Scholar] [CrossRef]
- Woch, M.W.; Radwańska, M.; Stanek, M.; Łopata, B.; Stefanowicz, A.M. Relationships between waste physicochemical properties, microbial activity and vegetation at coal ash and sludge disposal sites. Sci. Total Environ. 2018, 642, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Szwalec, A.; Mundała, P.; Kędzior, R. Cadmium, lead, zinc and copper content in herbaceous plants overgrowing furnace waste landfill. Environ. Prot. Nat. Res. 2013, 24, 33–37. [Google Scholar]
- Chakraborty, R.; Mukherjee, A. Mutagenicity and genotoxicity of coal fly ash water leachate. Ecotoxicol. Environ. Saf. 2008, 72, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jari, S.; Mitrović, M.; Pavlović, P. Ecological potential of plants for phytoremediation and ecorestoration of fly ash deposits and mine wastes. Front. Environ. Sci. 2018, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- DoEP. Department of Environmental Protection of Skawina Power Plant, Characteristics of the Combustion Waste Landfill; DoEP: Warsaw, Poland, 2012; (Typescript In Polish, Not Published). [Google Scholar]
- Kędzior, R.; Szwalec, A.; Mundała, P.; Skalski, T. Ground beetle (Coleoptera, Carabidae) life history traits as indicators of habitat recovering process in postindustrial areas. Ecol. Eng. 2020, 142, 105615. [Google Scholar] [CrossRef]
- Ostrowska, A.; Gawliński, S.; Zczubiałka, Z. Methods of Analysis and Evaluation of Soil and Plant Properties. A Catalogue; Institute of Environmental Protection: Warsaw, Poland, 1991. (In Polish) [Google Scholar]
- Carter, M.R.; Gregorich, E.G. (Eds.) Soil Sampling and Methods of Analysis, 2nd ed.; Canadian Society of Soil Science: Pinawa, MB, Canada, 2021; Available online: https://www.niordc.ir/uploads%5C86_106_Binder1.pdf (accessed on 29 April 2022).
- Li, M.S.; Luo, Y.P.; Su, Z.Y. Heavy metal concentrations in soils and plant accumulation in a restored manganese mine land in Guangxi. South China. Environ. Pollut. 2007, 147, 168–175. [Google Scholar] [CrossRef]
- Marchiol, L.; Fellet, G.; Boscutti, F.; Montella, C.; Mozzi, R.; Guarino, C. Gentle remediation at the former ‘Pertusola Sud’ Zinc Smelter: Evaluation of native species for phytoremediation purposes. Ecol. Eng. 2013, 53, 343–353. [Google Scholar] [CrossRef]
- Nannoni, F.; Rossi, S.; Protano, G. Potentially toxic element contamination in soil and accumulation in maize plants in a smelter area in Kosovo. Environ. Sci. Pollut. Res. 2016, 23, 11937–11946. [Google Scholar] [CrossRef] [PubMed]
- Stanislawska-Glubiak, E.; Korzeniowska, J.; Kocn, A. Effect of peat on the accumulation and translocation of heavy metals by maize grown in contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 4706–4714. [Google Scholar] [CrossRef] [PubMed]
- Galal, T.M.; Shehata, H.S. Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol. Indic. 2015, 48, 244–251. [Google Scholar] [CrossRef]
- Boechat, C.L.; Pistóia, V.C.; Gianelo, C.; de Oliveira-Camargo, F.E. Accumulation and translocation of heavy metal by spontaneous plants growing on multi-metal-contaminated site in the Southeast of Rio Grande do Sul state. Brazil. Environ. Sci. Pollut. Res. 2016, 23, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals in Soil; Blackie and Son Ltd.: London, UK, 1990. [Google Scholar]
- Szwalec, A.; Lasota, A.; Kędzior, R.; Mundała, P. Variation in heavy metal content in plants growing on a zinc and lead tailings dump. Appl. Ecol. Environ. Res. 2018, 16, 5081–5094. [Google Scholar] [CrossRef]
- Lehmann, C.; Rebele, F. Assessing the potential cadmium phytoremediation with Calamagrostis epigejos: A pot experiment. Int. J. Phytorem. 2004, 6, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Szöcs, E.; Schäfer, R.B. Ecotoxicology is not normal. A comparison of statistical approaches for analysis of count and proportion data in ecotoxicology. Environ. Sci. Pollut. Res. 2015, 22, 13990–13999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, B.; Edell, A.; Edson, Y.; Edwin, P. A phytoremediation approach using Calamagrostis ligulata and Juncus imbricatus in Andean wetlands of Peru. Environ. Monit. Assess. 2013, 185, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Dmuchowski, W.; Gozdowski, D.; Brągoszewska, P.; Baczewska, A.H.; Suwara, I. Phytoremediation of zinc contaminated soils using silver birch (Betula pendula Roth). Ecol. Eng. 2014, 71, 32–35. [Google Scholar] [CrossRef]
- Bielecka, A.; Królak, E. Selected features of Canadian goldenrod that predispose the plant to phytoremediation. Ecol. Eng. 2019, 20, 88–93. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, K.; Singh, R.P.; Singh, B. Naturally growing Saccharum munja on the fly ash lagoons: A potential ecological engineer for the revegetation and stabilization. Ecol. Eng. 2012, 40, 95–99. [Google Scholar] [CrossRef]
- Pandey, V.C. Suitability of Ricinus communis L. cultivation for phytoremediation of fly ash disposal sites. Ecol. Eng. 2013, 57, 336–341. [Google Scholar] [CrossRef]
- Da Conceição Gomes, M.A.; Hauser-Davis, R.A.; de Souza, A.N.; Vitória, A.P. Metal phytoremediation: General strategies. genetically modified plants and applications in metal nanoparticle contamination. Ecotoxicol. Environ. Saf. 2016, 134, 133–147. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Nandhini, S. Bioavailability of metals in fly ash and their bioaccumulation in naturally occurring vegetation: A pilot scale study. Environ. Monit. Assess. 2006, 116, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Haigh, M.; Walkington, H. Phytoremediation: Metal decontamination of soils after the sequential forestation of former opencast coal land. Sci. Total Environ. 2019, 656, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, L.; Scebba, F.; Tognetti, R. Heavy metal accumulation and growth responses in poplar clones Eridano (Populus deltoides × maximowiczii) and I-214 (P. × euramericana) exposed to industrial waste. Environ. Exp. Bot. 2004, 52, 79–88. [Google Scholar] [CrossRef]
- Hassinen, V.; Vallinkoski, V.M.; Issakainen, S.; Tervahauta, A.; Kärenlampi, S.; Servomaa, K. Correlation of foliar MT2b expression with Zn and Zn concentrations in hybrid aspen (Populus tremula × tremuloides) grown in contaminated soil. Environ. Pollut. 2009, 157, 922–930. [Google Scholar] [CrossRef]
- De Oliveira, V.H.; Tibbett, M. Tolerance, toxicity and transport of Cd and Zn in Populus trichocarpa. Environ. Exp. Bot. 2018, 155, 281–292. [Google Scholar] [CrossRef]
- Joshi, R.C. Fly ash-production, variability and possible complete utilization. In Proceedings of the Indian Geotechnical Conference—2010, GEOtrendz, Mumbai, India, 16–18 December 2010; pp. 103–111. Available online: https://gndec.ac.in/~igs/ldh/conf/2010/articles/v012.pdf (accessed on 29 April 2022).
- Armesto, L.; Merino, J.L. Characterization of some coal combustion solid residues. Fuel 1999, 78, 613–618. [Google Scholar] [CrossRef]
- Levandowski, J.; Kalkreuth, W. Chemical and petrographical characterization of feed coal, fly ash and bottom ash from the Figueira Power Plant, Paraná, Brazil. Int. J. Coal Geol. 2009, 77, 269–281. [Google Scholar] [CrossRef]
- Maiti, D.; Pandey, V.C. Metal remediation potential of naturally occurring plants growing on barren fly ash dumps. Environ. Geochem. Health 2021, 43, 1415–1426. [Google Scholar] [CrossRef]
- Pandey, V.C.; Sahu, N.; Singh, D.P. Physiological profiling of invasive plant species for ecological restoration of fly ash deposits. Urban For. Urban Green. 2020, 54, 126773. [Google Scholar] [CrossRef]
- Pandey, V.C. Assisted phytoremediation of fly ash dumps through naturally colonized plants. Ecol. Eng. 2015, 82, 1–5. [Google Scholar] [CrossRef]
- Malhotra, S.; Mishra, V.; Karmakar, S.; Sharma, R.S. Environmental predictors of indole acetic acid producing Rhizobacteria at fly ash dumps: Nature-based solution for sustainable restoration. Front. Environ. Sci. 2017, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
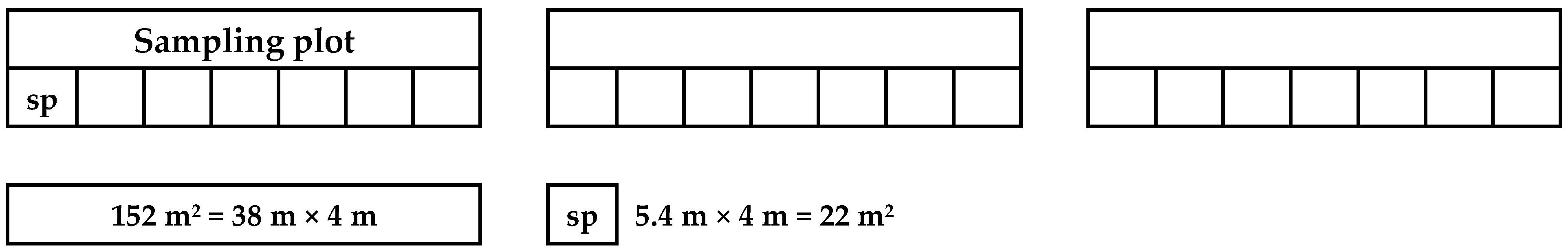


| Cd | Pb | Zn | Cu | pH | |
|---|---|---|---|---|---|
| Range, Mean mg·kg−1 d.m. and RSD % | |||||
| Range | 0.19–0.37 | 17.23–36.98 | 79.65–147.25 | 28.01–63.86 | 7.1–7.9 |
| Mean | 0.292 | 24.664 | 117.019 | 48.348 | 7.4 |
| RSD | 16 | 30 | 21 | 28 | 3 |
| Metal/Plant Species | Black Locust | Silver Birch | European Aspen | Common Oak | Common Reed | European Goldenrod | Wood Small Reed | |
|---|---|---|---|---|---|---|---|---|
| Range, Mean mg·kg−1 d.m., RSD % | ||||||||
| Cd | Range | 0.069–0.326 | 0.149–0.650 | 0.262–1.058 | 0.253–0.578 | 0.028–0.052 | 0.047–0.590 | 0.028–0.120 |
| Mean | 0.199 | 0.327 | 0.565 | 0.384 | 0.037 | 0.230 | 0.074 | |
| RSD | 46 | 60 | 57 | 31 | 26 | 103 | 44 | |
| Pb | Range | 0.970–1.416 | 1.448–2.410 | 1.404–1.906 | 1.778–2.044 | 0.380–0.470 | 0.550–1.250 | 0.207–0.340 |
| Mean | 1.169 | 1.897 | 1.686 | 1.893 | 0.422 | 0.921 | 0.265 | |
| RSD | 11 | 16 | 10 | 4 | 6 | 28 | 16 | |
| Zn | Range | 28.924–53.618 | 139.156–169.340 | 148.852–222.294 | 83.764–105.050 | 8.210–19.580 | 28.470–62.780 | 11.050–15.220 |
| Mean | 37.532 | 156.525 | 185.913 | 93.774 | 14.949 | 40.374 | 12.722 | |
| RSD | 25 | 5 | 12 | 6 | 27 | 30 | 8 | |
| Cu | Range | 4.106–7.362 | 4.350–6.776 | 6.534–7.920 | 5.130–6.506 | 0.880–2.870 | 3.110–6.700 | 0.810–1.120 |
| Mean | 5.290 | 5.900 | 7.022 | 5.851 | 1.841 | 4.667 | 0.931 | |
| RSD | 24 | 10 | 5 | 7 | 37 | 27 | 8 | |
| Metal | Species | Common Reed | European Goldenrod | Wood Small-Reed |
|---|---|---|---|---|
| Data | Mean, Range mg·kg−1 d.m., RSD % | |||
| Cd | Range | 0.038–0.150 | 0.195–1.240 | 0.046–0.900 |
| Mean | 0.084 | 0.665 | 0.525 | |
| RSD | 45 | 62 | 67 | |
| Pb | Range | 0.670–1.450 | 0.770–1.980 | 2.800–4.450 |
| Mean | 1.071 | 1.241 | 3.373 | |
| RSD | 25 | 34 | 17 | |
| Zn | Range | 14.500–32.780 | 30.680–50.850 | 71.550–110.170 |
| Mean | 22.096 | 38.904 | 91.321 | |
| RSD | 28 | 13 | 12 | |
| Cu | Range | 2.300–8.630 | 10.36–17.23 | 5.630–7.750 |
| Men | 6.171 | 12.945 | 6.562 | |
| RSD | 42 | 19 | 9 | |
| A/sp. | BCFCd | dif. | B/sp. | BCFZn | dif. | E/sp | TFCd | dif. | ||
| c.r. | 0.134 | a | w.s.r. | 0.131 | a | w.s.r. | 0.102 | a | ||
| w.s.r. | 0.267 | a | c.r. | 0.149 | a | c.r. | 0.534 | b | ||
| b.l. | 0.711 | b | b.l. | 0.394 | a | e.g. | 0.618 | b | ||
| e.g. | 0.766 | b | e.g. | 0.420 | a | |||||
| s.b. | 1.087 | c | c.o. | 0.950 | b | F/sp. | TFZn | dif. | ||
| c.o. | 1.312 | c | s.b. | 1.587 | c | w.s.r. | 0.141 | a | ||
| e.a. | 1.938 | d | e.a. | 1.935 | c | c.r. | 0.683 | b | ||
| e.g. | 1.022 | c | ||||||||
| C/sp. | BCFPb | dif. | D/sp. | BCFCu | dif. | |||||
| w.s.r. | 0.012 | a | w.s.r. | 0.017 | a | G/sp. | TFPb | dif. | ||
| c.r. | 0.019 | b | c.r. | 0.033 | a | w.s.r. | 0.079 | a | ||
| e.g. | 0.038 | c | e.g. | 0.084 | b | c.r. | 0.425 | b | ||
| b.l. | 0.050 | d | b.l. | 0.095 | b,c | e.g. | 0.785 | c | ||
| e.a. | 0.075 | e | s.b. | 0.105 | c | |||||
| c.o. | 0.083 | f | c.o. | 0.105 | c | H/sp. | TFCu | dif. | ||
| s.b. | 0.085 | f | e.a. | 0.126 | d | w.s.r. | 0.143 | a | ||
| c.r. common reed; w.s.r. wood small-reed; b.l. black lotus; e.g. European goldenrod; s.b. silver birch; c.o. common oak; e.a. European aspen | c.r. | 0.315 | b | |||||||
| e.g. | 0.356 | b | ||||||||
| A/sp. x l. | BCFCd | dif. | E/sp. x l. | TFCd | dif. | |
| e.g. x t. | 0.156 | a | c.r. x b. | 0.232 | a | |
| e.g. x m. | 0.340 | a | e.g. x b. | 0.471 | a | |
| s.b. x b. | 0.741 | b | c.r. x t. | 0.744 | b | |
| e.a. x t. | 0.932 | b | e.g. x t. | 1.002 | c | |
| c.o. x b. | 1.107 | b | ||||
| e.a. x m. | 1.629 | c | F/sp. x l. | TFZn | dif. | |
| c.o. x t. | 1.691 | c | c.r. x b. | 0.615 | a | |
| e.g. x b. | 1.800 | c | c.r. x t. | 0.830 | a,b | |
| s.b. x t. | 1.862 | c | e.g. x m. | 0.853 | b | |
| e.a. x b. | 3.252 | d | e.g. x b. | 1.264 | c | |
| B/sp. x l. | BCFPb | dif. | G/sp. x l. | TFPb | dif. | |
| e.a. x t. | 0.043 | a | w.s.r. x b. | 0.075 | a | |
| s.b. x t. | 0.044 | a | w.s.r. x t. | 0.076 | a | |
| c.o. x t. | 0.056 | b | w.s.r. x m. | 0.086 | a | |
| e.a. x b. | 0.094 | c | c.r. x b. | 0.314 | b | |
| c.o. x b. | 0.105 | d | e.g. x m. | 0.537 | c | |
| s.b. x b. | 0.106 | d | c.r. x t. | 0.600 | c | |
| e.g. x b. | 0.710 | c | ||||
| C/sp. x l. | BCFZn | dif. | e.g. x t. | 1.107 | d | |
| c.o. x t. | 0.676 | a | ||||
| c.o. x b. | 1.018 | a,b | H/sp. x l. | TFCu | dif. | |
| e.a. x t. | 1.119 | b | w.s.r. x b. | 0.129 | a | |
| s.b. x t. | 1.122 | b | w.s.r. x t. | 0.167 | a | |
| s.b. x b. | 1.891 | c | e.g. x m. | 0.310 | b | |
| e.a. x b. | 2.452 | d | e.g. x t. | 0.368 | c | |
| e.g. x b. | 0.391 | c | ||||
| D/sp. x l. | BCFCu | dif. | ||||
| c.r. x b. | 0.017 | a | l. location; b base; m middle; t top; sp. species; x interaction. c.r. common reed; w.s.r. wood small-reed; b.l. black loctus; e.g. European goldenrod; s.b. silver birch; c.o. common oak; e.a. European aspen. | |||
| c.r. x m. | 0.043 | b | ||||
| e.g. x m. | 0.064 | c | ||||
| b.l. x t. | 0.074 | c | ||||
| e.g. x b. | 0.118 | d | ||||
| b.l. x b. | 0.130 | d | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwalec, A.; Mundała, P.; Kędzior, R. Suitability of Selected Plant Species for Phytoremediation: A Case Study of a Coal Combustion Ash Landfill. Sustainability 2022, 14, 7083. https://doi.org/10.3390/su14127083
Szwalec A, Mundała P, Kędzior R. Suitability of Selected Plant Species for Phytoremediation: A Case Study of a Coal Combustion Ash Landfill. Sustainability. 2022; 14(12):7083. https://doi.org/10.3390/su14127083
Chicago/Turabian StyleSzwalec, Artur, Paweł Mundała, and Renata Kędzior. 2022. "Suitability of Selected Plant Species for Phytoremediation: A Case Study of a Coal Combustion Ash Landfill" Sustainability 14, no. 12: 7083. https://doi.org/10.3390/su14127083
APA StyleSzwalec, A., Mundała, P., & Kędzior, R. (2022). Suitability of Selected Plant Species for Phytoremediation: A Case Study of a Coal Combustion Ash Landfill. Sustainability, 14(12), 7083. https://doi.org/10.3390/su14127083







