1. Introduction
Numerous studies have confirmed that our systems of consumption and production are simply unsustainable [
1]. Environmental eco-sustainability is closely linked to the problem of pollution [
2,
3,
4,
5]. The latter involves many aspects and researchers have aimed at counteracting it by acting synergistically on several fronts, for example through the study of new techniques and processes [
6,
7,
8,
9,
10,
11] with the aid of innovative materials that can be advantageously used for waste treatment [
12,
13,
14,
15,
16,
17]. Another important research front that aims to prevent pollution is the production of environmentally friendly materials with a low environmental impact deriving from their use of natural raw materials [
18,
19,
20,
21,
22,
23,
24]. In fact, in recent years, much study and research has been developed on this aspect, making the eco-sustainability of materials a real need, in search of multifunctional materials that, in addition to their classic functions, are able to reduce pollutants in the environment [
25,
26,
27,
28,
29]. Environmental protection also includes the recycling of materials [
30,
31,
32,
33,
34,
35,
36,
37]. The linear economic model, which consists in transforming raw materials into products which, after their use or consumption, are directly eliminated, determines not only an increase in pollution and in the production of waste, but also in the global competition for natural resources [
38,
39,
40,
41,
42,
43].
One of the most active sectors in the research of new materials is certainly that of construction. The construction sector, in particular, is of great importance is the construction of panels that are used in different ways such as for interspaces, in thermal and acoustic insulation, etc.
Many natural materials have been extensively investigated for the production of insulating panels such as: pine bark, bamboo fibers, hemp etc. The data reported in the literature confirm the possibility of using natural materials, with satisfactory thermal insulation characteristics and above all the advantage of obtaining products mainly of natural origin [
44,
45,
46,
47].
The literature also reports numerous studies on the use of waste materials for the preparation of panels with the advantage of contributing to environmental sustainability. [
48,
49,
50,
51].
Polylaminate containers, more commonly known as Tetra Pak containers, represent the most used packaging in the world [
52,
53,
54]. Their number, which is around 200 billion units produced per year, means that the global disposal of these containers is no longer negligible [
55,
56]. The polylaminate container is one of the most popular containers in the world for its countless possibilities of use and for its cost-effectiveness.
There are two main types of polylaminate containers and they are distinguished by the presence or absence of aluminum [
57]. The first type consists of two layers of polyethylene and one of cardboard. The polyethylene serves to isolate the container from external agents and allows a greater preservation of the contents. The cardboard gives a consistency to the containers. A second type is made up of six layers: four layers of polyethylene, one of cardboard and one of aluminum. In this case, the aluminum layer makes it possible to carry out a UHT treatment on the content, prolonging its conservation. The presence of aluminum improves insulation from the outside. The polylaminate containers can have different shapes (parallelepipeds, prisms, cubes, etc.) and also countless uses. This is mainly due to the possibility of hosting ready meals, sauces, etc. in addition to milk and water. They are therefore multifunctional containers. Polyethylene acts as a waterproofer, protecting the container and contents from external moisture and as a binder for various materials. Paper and aluminum act as barriers against the effects of light, oxygen and gases and provide stability [
58]. Multi-laminate container companies have started incorporating recycled wood into their carton production system and are also trying to recycle the containers placed on the market by making rolls of paper and aluminum with a naturally lower quality than the original. However, the main problem with polylaminate containers remains their disposal and recycling. In fact, there are few useful systems for the complete recycling of the container [
57]. In most cases, the containers are disposed of with non-recyclable waste. Few studies propose the reuse of Tetra Pak containers [
59]. In 2013 Rogari proposed the use of Tetra Pak containers for the insulation of walls, wherein the containers are simply joined and placed side by side.
This work aims to study a new strategy, called the thermal method, for the reuse of disused polylaminate containers for the construction of panels that can be used in construction and in particular as insulating panels. This method is proposed primarily in order to establish a way to use only recycled material, without the addition of chemical or other binders.
2. Materials and Methods
The experimentation was developed through three main consecutive phases:
Procurement and processing of disused polylaminate containers
Application and optimization of the thermal method
Analysis of the characteristics of the product obtained
2.1. Procurement and Processing of Disused Polylaminate Containers
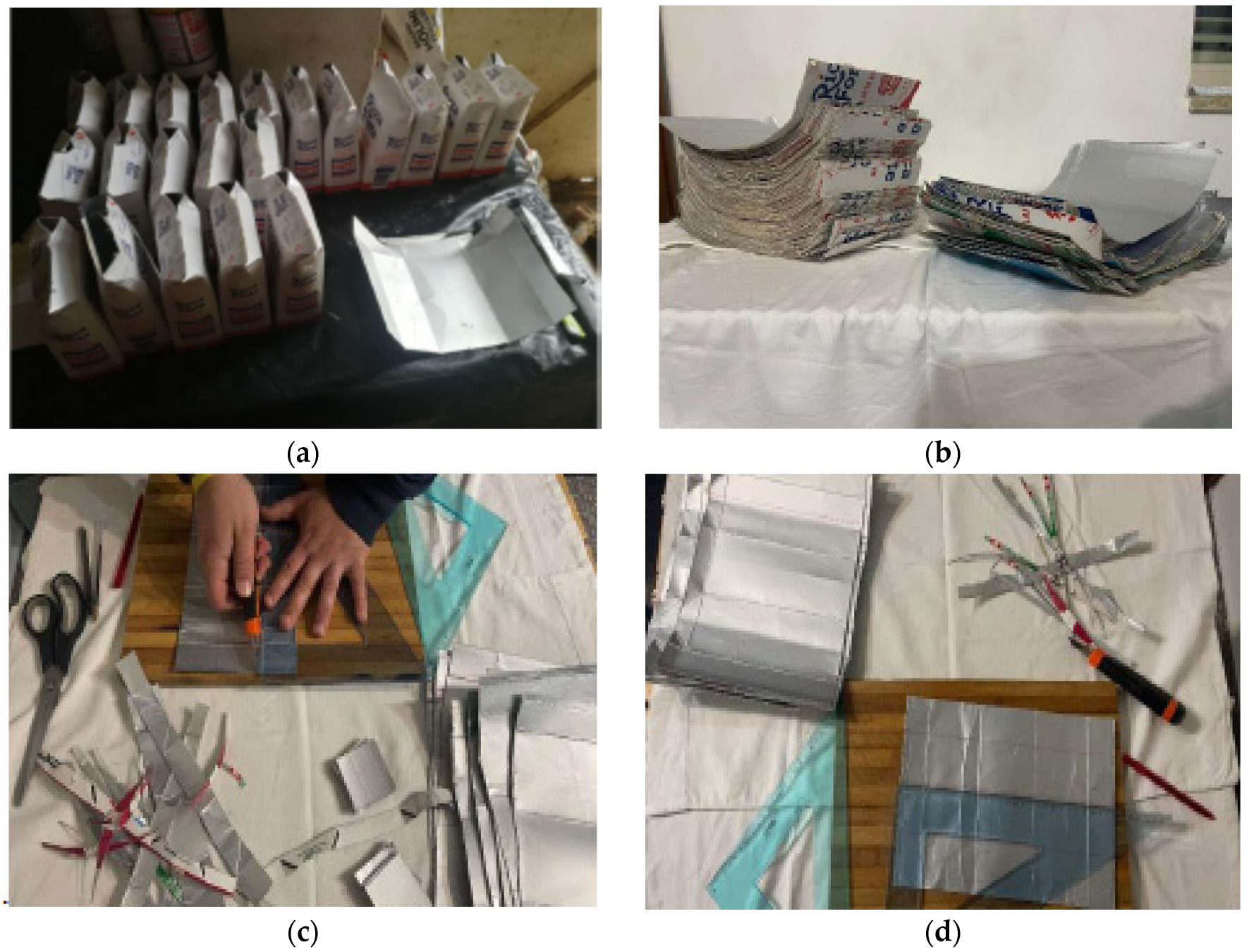
The disused polylaminate containers (Tetra Pak), necessary for the experimentation, were found in catering companies and local canteens. Particular attention was paid to the choice of containers in order to obtain a mostly homogeneous sample with similar characteristics. For this reason, only containers intended for storing milk were used. These containers belong to the type that contains 6 layers: 4 of polyethylene, 1 of cardboard and 1 of aluminum. Once the containers were found, they were processed with the aim of obtaining an easily usable material from the point of view of assembly. In particular, they were washed with running water, opened, their upper and lower parts eliminated, and they were cut out in order to have sheets of 5 × 5 cm size. Finally, these sheets were washed again with a common degreaser and dried at room temperature (
Figure 1).
2.2. Application and Optimization of the Thermal Method
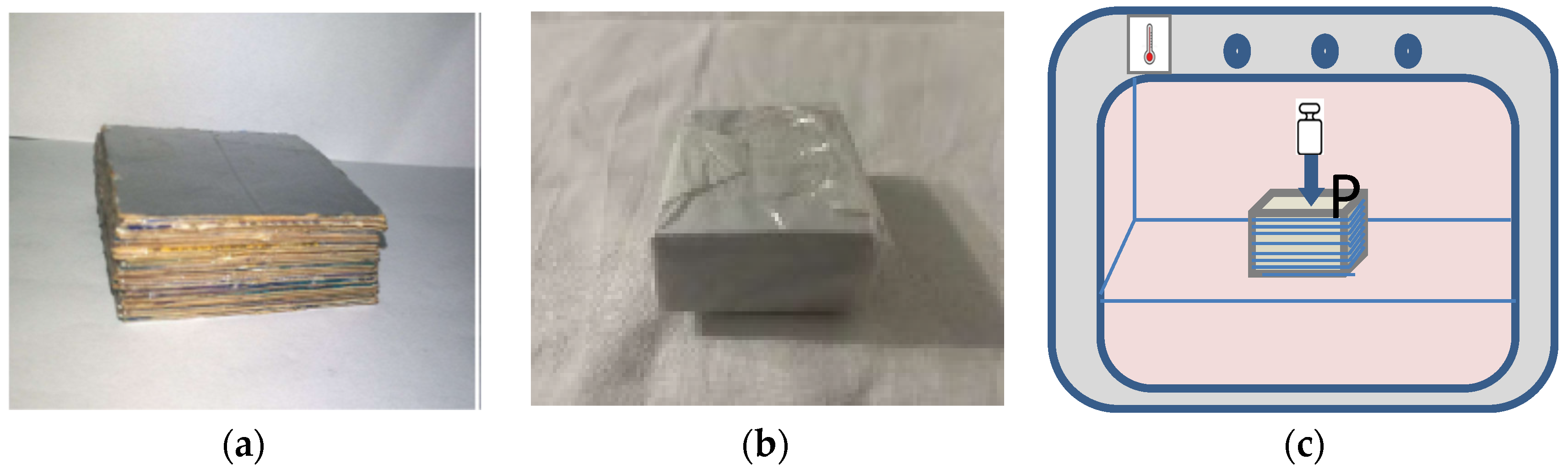
The specimens were obtained by assembling a programmed number of polylaminate sheets using a new thermal method illustrated below.
- -
Initially, different 5 × 5 cm polylaminate sheets were superimposed so that the aluminum face of the upper layer always overlapped the cardboard face of the lower layer (
Figure 2a).
- -
All the sheets were initially blocked, to avoid slipping between them, by wrapping them in common baking paper (
Figure 2b).
- -
The block of polylaminate sheets was placed in a thermo-ventilated oven at a predetermined temperature and time and subjected to different pressure by applying weights to it (
Figure 2c).
- -
After the heat treatment, the specimens were removed from the oven and the weights were kept on them until cooling. After cooling, the parchment baking paper with which they were kept compact, was removed.
In order to identify the best experimental conditions, parameters such as number of sheets, temperature, time and pressure were made to vary.
Table 1 shows the different values of each parameter (such as time, temperature, pressure, number of sheets) that were used to find the best experimental conditions for the preparation of the specimens.
2.3. Analysis of the Characteristics of the Product Obtained
The specimens obtained were subsequently characterized on three main characteristics considered important for the purposes of the product to be made. The characteristics investigated were thermal conductivity, fire resistance and water resistance. The detailed tests are provided in the following paragraphs for better discursive continuity.
3. Results
3.1. Preliminary Tests: Feasibility of the Thermal Method
The first tests were carried out in more drastic conditions in order to test the feasibility of the thermal method. In particular, each specimen was characterized by 45 superimposed polylaminate sheets, subjected to a temperature of 220 °C, to a pressure of 16.09 × 104 N/m2 and for a time of 30 min.
The thermal method, used for the preparation of prototypes of panels for construction, immediately proved to be encouraging. The samples obtained showed a high availability for assembly and the different sheets were well adhered to each other. A decrease in thickness after heat treatment, of about 13.5%, due to good compaction and also a decrease in weight of about 54.76% due to the loss of moisture present in the sheets before treatment, were highlighted. The results obtained show that this method is very effective especially because it does not require the use of a binder, which is very important both from an economic and an environmental point of view. As is known, Tetra Packs have polyethylene coatings on their external surfaces and it is precisely these polyethylene films that allow self-gluing of the Tetra Pack sheets with heat only and pressure. The polyethylene softens and acquires adhesive capacity at the temperature used. Finally, the pressure optimizes the adhesion and compaction of the different sheets.
3.2. Optimization of the Thermal Method
Once the feasibility of the method was verified, the next step was to optimize it. The goal was both to identify the optimal operating conditions to obtain a material with characteristics and properties suitable for its use, and to reduce costs by trying to reconcile a lowering of temperature and times. The quality of the sample was determined by its degree of compaction, this being a fundamental and decisive characteristic for a predetermined use of the final material.
Then, a scale to distinguish the different degrees of compaction was used as shown in
Table 2.
In particular, to optimize the method, four operational variables were taken into consideration such as time, temperature, pressure and number of sheets. The investigation was carried out in a systematic way taking into consideration the specimens with the number of sheets equal to 12, 24 and 36. On each of this set of samples the times and the temperature were made to vary, maintaining from time to time a value of constant pressure equal to 1.96, 3.97, 7.85 and 11.77 [×104 N/m2]. All the specimens that showed a good compaction (green dot) underwent a decrease, compared with the specimen before the heat treatment, of the thickness of about 13/14%, and a weight loss of about 55/60%.
3.2.1. Test Samples with Twelve Sheets of Polylaminate
The following
Figure 3, show the degree of compaction relative to specimens made up of 12 sheets of polylaminate as a function of time, pression and temperature.
In particular, at a temperature of 100 °C, regardless of the times and pressure values, the specimens composed of 12 sheets exhibit a red behavior, i.e., the sheets are not able to bind together, and therefore the treatment at this temperature is always ineffective to obtain an adequate compaction. The green behavior zone, i.e., sheets perfectly connected to each other, extends linearly increasing time and temperature. Above a pressure value of 7.85 × 104 N/m2 there are no more variations, making it clear that the pressure action has reached its maximum. The ideal sample, consisting of 12 sheets, was obtained under the following experimental conditions: T = 180 °C; t = 10 min; p = 7.84 × 104 N/m2, which presents a compromise between an excellent degree of compaction and optimized temperatures and times.
3.2.2. Specimens with Twenty-Four Sheets of Polylaminate
Figure 4 shows the degree of compaction of specimens prepared with 24 sheets of polylaminate.
By increasing the number of sheets it is possible to see how the yellow zone begins to grow while remaining a detachment zone between the red zone and the green zone. Compared with the previous test it can clearly be seen that the green area decreases at the expense of the growth of the yellow and red areas. As the sheets grow, the trend is that of an increase in the red zone and a decrease in the green zone. Also, in this case no variations are observed for pressure values higher than 7.85 × 104 N/m2. The ideal sample, consisting of 24 sheets, was obtained under the following experimental conditions: T = 180 °C; t = 15 min; p = 7.84 × 104 N/m2, which presents a compromise between an excellent degree of compaction and optimized temperatures and times.
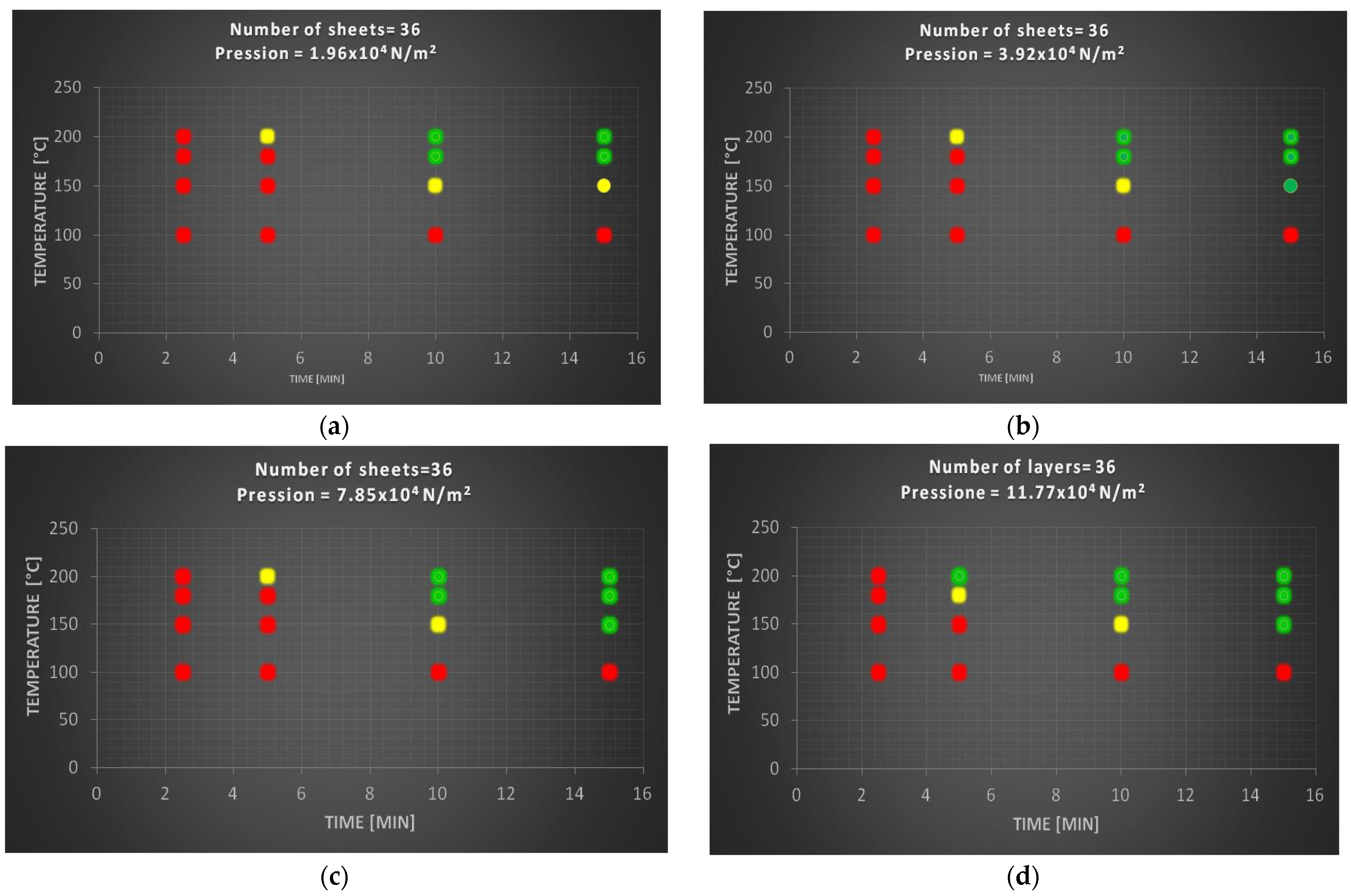
3.2.3. Specimens with Thirty-Six Sheets of Polylaminate
Figure 5 shows the degree of compaction of specimens prepared with 36 sheets.
The latest test confirms the trend already seen in the previous tests that, as the number of sheets increases, independently of the other experimental variables, there is a reduction in the green area, replaced, in fact, with a red zone. A greater thickness presupposes higher times and temperatures so that the innermost layers of polyethylene can soften and lead to sufficient adhesion of the sheets. Also, in the latter case there is no variation above the pressure value of 7.85 × 104 N/m2.
The ideal specimen consisting of 36 sheets was identified at a higher temperature and pressure than in the previous cases, although these more drastic conditions allow for lower times.
In particular, the ideal sample, consisting of 36 sheets, was obtained under the following experimental conditions: T = 200 °C; t = 15 min; p = 7.85 × 104 N/m2
3.3. Characterization of the Material
After the optimization of the thermal method, as a function of parameters such as temperature, time, pressure and number of sheets, the best specimens obtained were characterized.
3.3.1. Thermal Conductivity
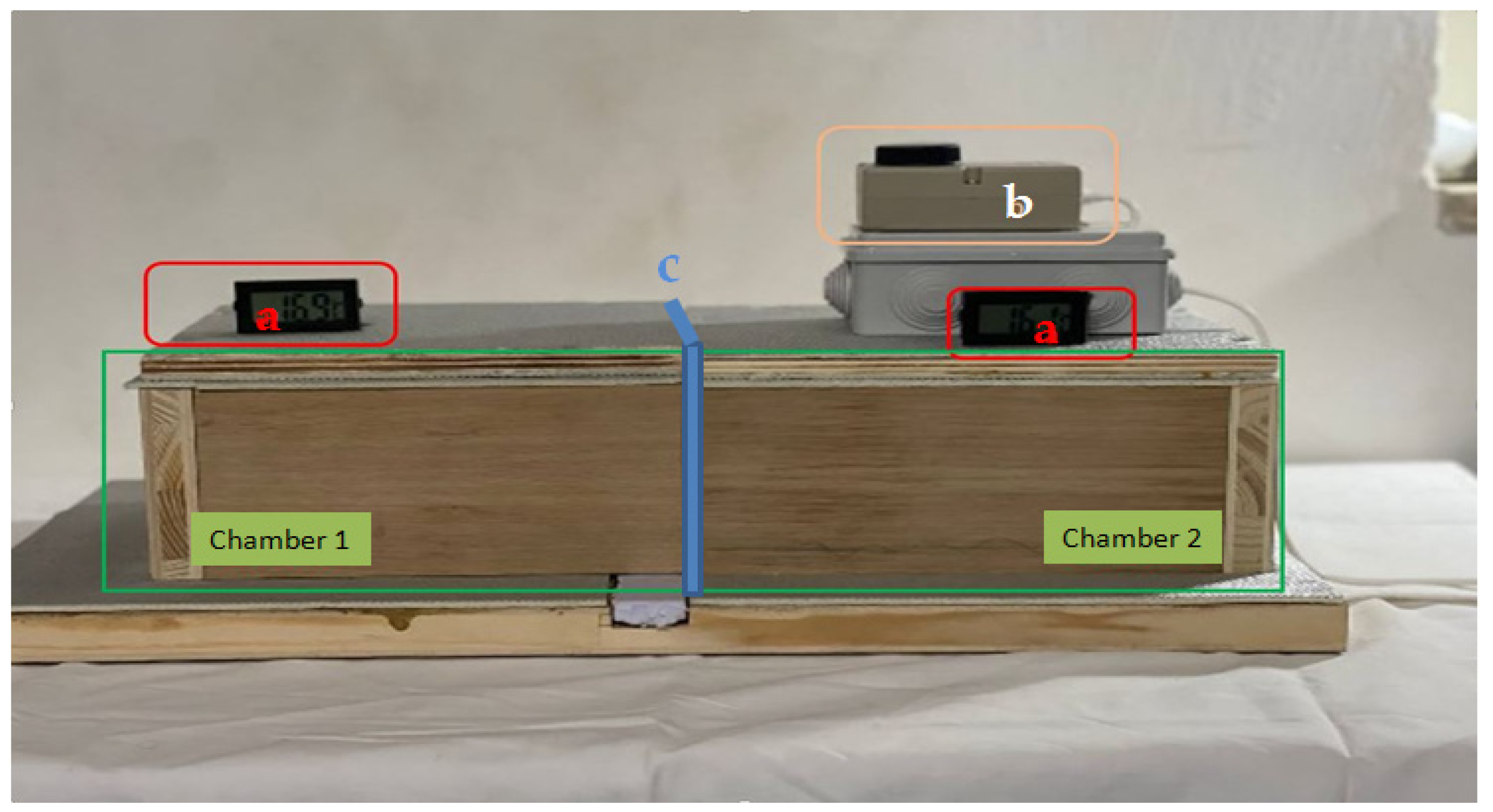
The thermal conductivity was measured by building and using a system consisting of two thermally insulated chambers and interposing the material to be tested between them. To better isolate the internal environment from the external one, the two chambers were internally lined with heat-reflecting panels. A heating system was placed in chamber 2. Both chambers were equipped with a thermometer in order to record the variations in temperature and therefore the transmission of heat through the material undergoing characterization (
Figure 6).
The tests were conducted not only on the polylaminate panel but also on two other materials, such as chipboard and polyethylene panel. The aim was to be able to compare the characteristics of the prepared polylaminate panel with those of other materials known for their insulating characteristics.
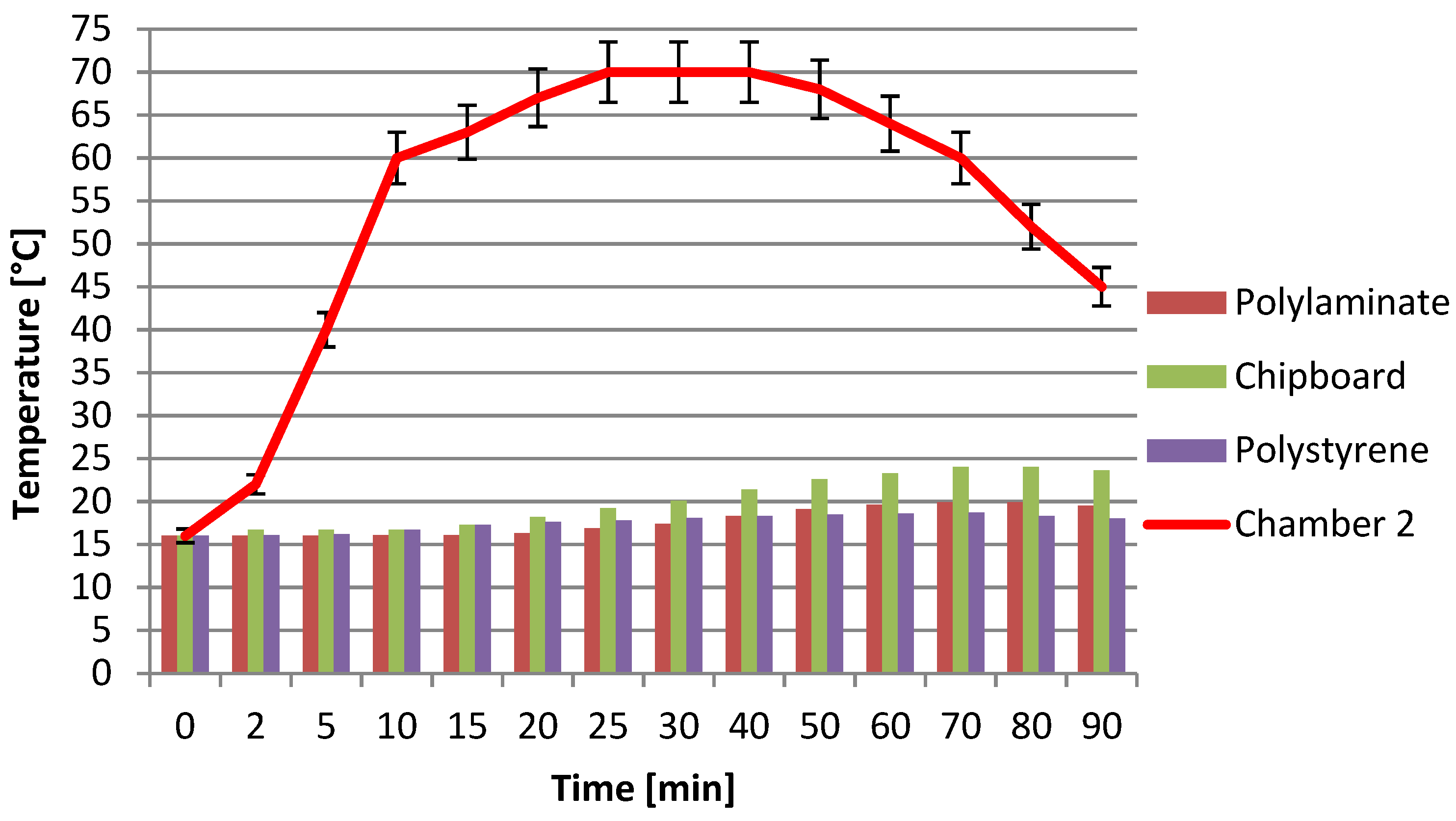
In particular, the specimens of the three different materials: chipboard panel, polystyrene panel and polylaminate panel have a size of 21 × 13 cm and a thickness of 2, 3 and 1 cm respectively. The difference in thickness is due to having wanted to consider the comparison with panels with typical commercial characteristics used as insulators. During the test, the temperatures in chamber 1 were measured as the heating in chamber 2 increased, as a function of time. In particular, in the first 30 min the temperature was brought to the maximum value of 70 °C and kept at this temperature for another 30 min with a subsequent cooling phase of a further 30 min.
Figure 7 shows the temperatures recorded in the two chambers as the material interposed between the two chambers varies and as a function of time.
From the previous graph it is clear that the material under study, the polylaminate panel, shows a high insulating behavior that is comparable with the usual materials recognized as insulating. The temperatures reached in chamber 1 of the device, thanks to the transmission of heat from chamber 2 through the three different materials, are very similar and are, though with small differences, also in favor of the polylaminate panel despite the thickness of the latter being lower. Taking into consideration the ratio R, given by the temperature difference between the two chambers with respect to the thickness of the panel (R=ΔTchamber2-Tchamber1/panel thickness), it is possible to have a comparison parameter between the materials considered, all conditions being equal. The higher this parameter, the higher the insulating action of the panel. Taking as a reference, 30 min of heating, in which the maximum temperature in chamber 2 is reached, the corresponding values of this ratio for the three materials analyzed are 17.3, 24.95 and 52.6 [°C/cm] respectively for the chipboard, polystyrene and polylaminate. The results obtained show, with respect to the same thickness, an R value of the polylaminate panel of about double that of polyethylene and about three times that of chipboard.
3.3.2. Fire Resistance Tests
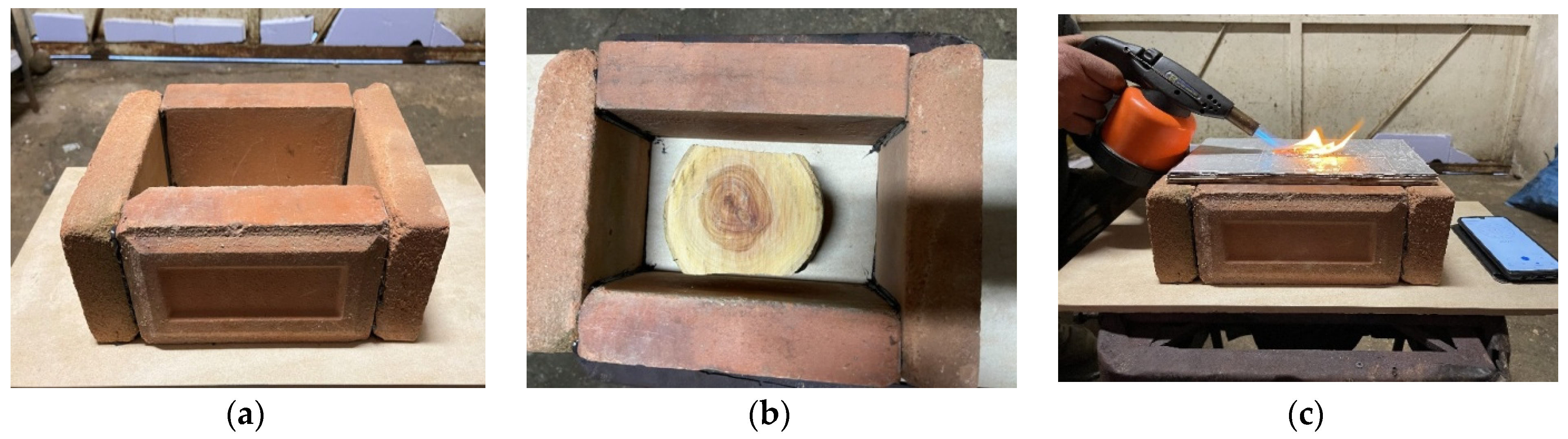
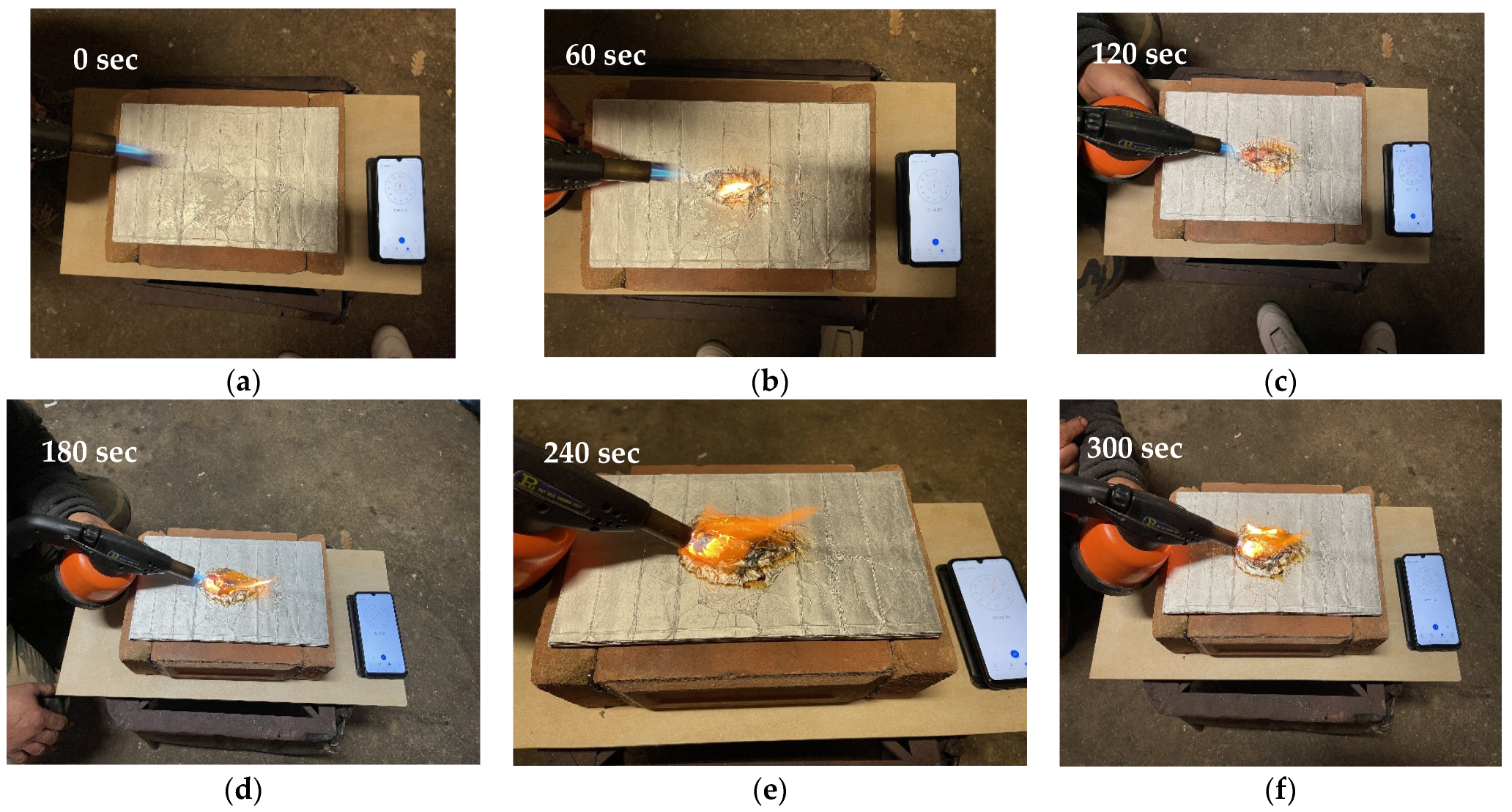
For the fire test, a panel obtained from 24 sheets of polylaminate was used at a temperature of 180 °C, a time of 15 min and a pressure of 7.84 × 104 N/m2. The dimensions of the specimen for this test were necessarily increased and were 10 × 20 cm. The test was inspired by the UNI 8457 standard.
In particular, through the use of refractory bricks, a container was built (
Figure 8a), inside which a small piece of wood was placed (
Figure 8b) and the panel was placed on the upper part of the container (
Figure 8c).
Subsequently, the panel was subjected to the action of a blowtorch placed at a distance of 10 cm, with an inclination of about 45° with respect to the panel. After 5 min the flames were extinguished and the state of the panel and the stub inside the container was assessed to assess any expansion of the flames.
Figure 9 shows the images of the specimen subjected to fire resistance tests after different exposure times.
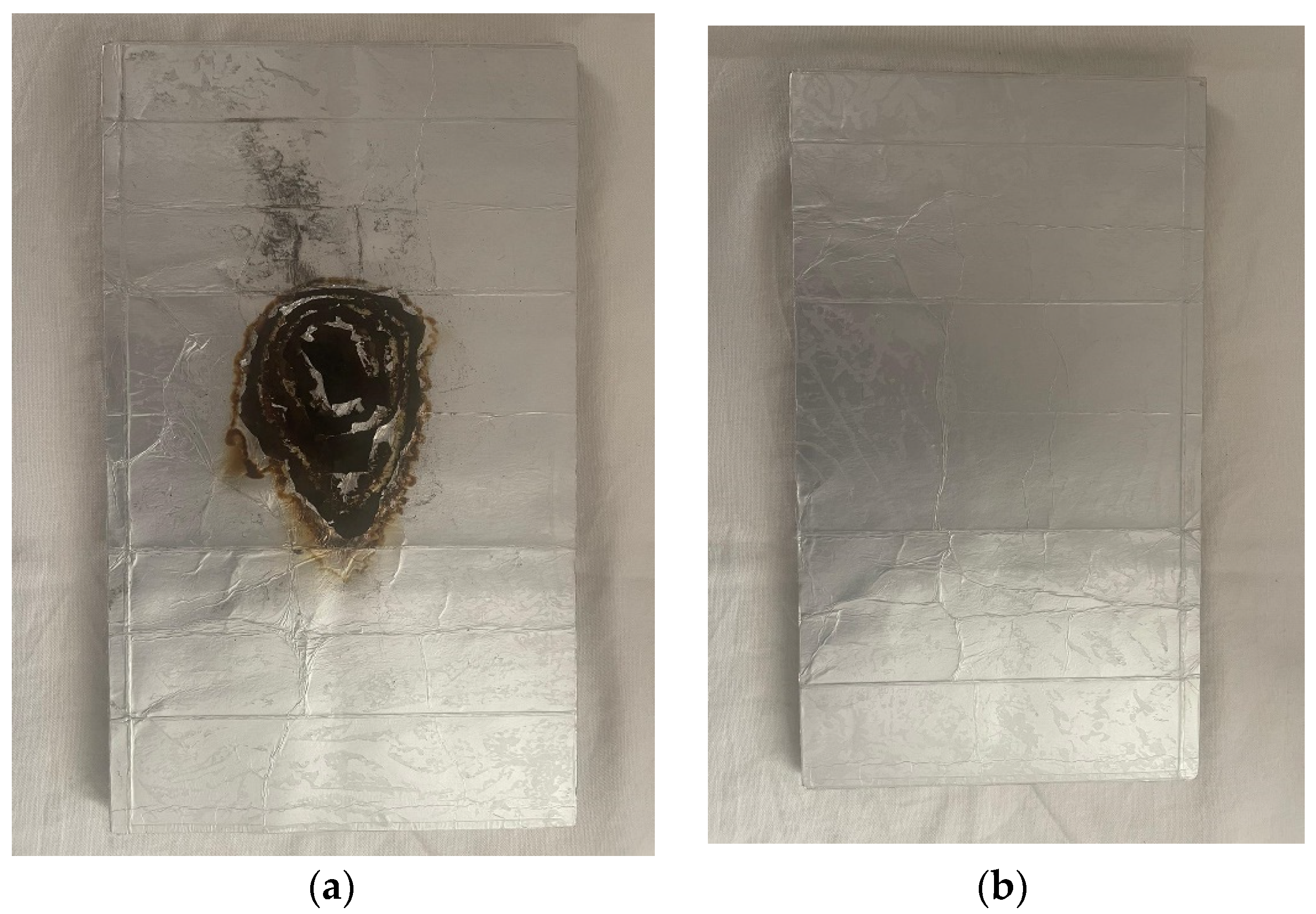
From the images above it can be seen that the panel has excellent flame resistance. In fact, it resisted the spread of the flame well, preventing it from rapidly spreading inside. As can be seen from
Figure 10a, after 300 s of flame exposure only the part subject to direct contact with the flame burned while the rest remained in a normal state (
Figure 10b).
The material also proved to be very resistant to vertical flame propagation, the trunk inside the brick container did not undergo any changes, while the panel inside was not even very hot to the touch.
The polylaminate panel therefore shows a high resistance to flame (RF) and after 300 sec of exposure it is only superficially altered.
This result is certainly satisfactory when compared with polystyrene or chipboard which, as is known, have a flame resistance (RF) of a few seconds [
60,
61].
In conclusion, it can be safely stated that the material has excellent flame resistance due to the aluminum sheets which, during the exposure phase, act as barriers preventing propagation by creating a sort of protective layer that slows the propagation of the flame. Furthermore, the flame resistance is also favored by the high compaction of the material which, unlike for example other materials such as chipboard, being instead very porous, favor the spread of flames.
3.3.3. Resistance to Water
The water resistance was conducted by immersing the samples completely in water at room temperatures for programmed time intervals. After each time interval, three specimens were removed from the water and left to dry for 72 h at room temperature. Subsequently the samples were observed to verify the alterations suffered during the wet–dry cycle.
To carry out the test, three 5 × 5 × 1 cm specimens were used, which were made following the characteristics of the ideal specimen, identified in the previous phases. In particular, the specimens used were prepared with 24 sheets and under the following experimental conditions: T = 180 °C; t = 15 min; p = 7.84 × 104 N/m2.
Table 3 shows the characteristics of the specimens, observed as a function of the immersion time in water.
As can be seen from the observation notes shown in
Table 3, the polylaminate panel remains unchanged after immersion in water for one hour. This means that the infiltration of water, despite the presence of cardboard layers, is not very fast. After one hour of immersion, the specimens begin to show small swellings at the corners that become increasingly greater as the hours of immersion increase, although despite this, they retain their shape after 12 h (
Figure 11).
It can therefore be deduced, in the light of the results obtained, that in its upper and lower faces the specimen is waterproof while on its contour the specimen is more vulnerable to infiltration by liquids, in particular water in this case. Once exposed for long periods exceeding six hours, the specimen begins to have losses also in the mechanical characteristics of compactness. A solution to overcome this problem could be to coat and waterproof the lateral contours, where the material is not protected, by polyethylene film.
4. Conclusions
The results obtained allow us to draw the following conclusions:
The thermal method studied in this research proved to be feasible for the preparation of insulating panels from polylaminate sheets obtained from recycled packing. The method does not involve the use of chemical additives but takes advantage of the presence of polyethylene films present on the surface of the recycled polylaminate sheets. The latter during the treatment phases, following high temperatures, soften and subsequently cool, allowing the adhesion of the sheets which is also favored by the combination with pressure.
The main characteristics and qualities of the produced panels are:
Insulating properties: the material has been shown to have low thermal conductivity. With the same thickness of the panels, the results obtained show that the thermal insulation of the polylaminate is approximately double that of polyethylene and triple that of chipboard.
Reaction to fire: the panel, even if placed in contact with an open flame, did not catch fire and did not disintegrate quickly but ignited slowly and only in correspondence with the flame contact area. This highlights the fact that, despite the high presence of cardboard inside the panel, the spread of the flame is not rapid.
Compared with other materials such as polyethylene or chipboard for which a low flame resistance of a few seconds is known, the polylaminate panel showed a high resistance to flame and in fact did not burn and remained almost intact even after 300 s of expose to flame.
Water resistance: the polylaminate panel remains unchanged after immersion in water for a time of one hour. This means that the infiltration of water, despite the presence of cardboard sheets, is not very fast. However, this property can be easily improved by applying a waterproofing layer to the product.
Since the polylaminate sheets obtained from recycled packing could generally have a maximum size of 20 × 30 cm, the production of a panel of larger dimensions, that is with dimensions typical of an insulating panel used for construction, presupposes two important aspects to take into consideration: the overlapping of the sheets and the areas of surface discontinuity between the sheets.
The first aspect, that is the overlapping of the sheets, should be carried out simply by overlapping the different sheets one on top of the other in a staggered way. This would not lead to particular variations.
The other aspect is the areas of discontinuity between the juxtaposition of one sheet and another, which would be generated on the front and rear surfaces and which could represent areas of weakness with respect to any infiltration of water. All this could be easily overcome by using any waterproofing material that would prevent any infiltration of water in the areas of discontinuity, that is, both on the surfaces and on the edges. A water-proofer, for example, could be a common resin. Another option could be the application of a superficial thin aluminum or PVC film over the entire panel which would also increase the appearance of the panel from an aesthetic point of view. However, it is necessary to emphasize that the waterproofing of the panel is not always essential, bearing in mind that, from the results obtained, the panels show an acceptable resistance to water infiltration. Furthermore, for an assumed use as insulating panels, their most probable location should be in the cavities between walls, where there is hardly any contact with water.
Therefore, the preliminarily tested method is promising and above all simple, economical and does not use chemicals that act as binders. Furthermore, the insulating panels that can be made could be destined for green building, thus contributing to the circular economy and environmental sustainability. Discarded polylaminate containers that in most cases are destined for landfill could have a new life.
Author Contributions
Conceptualization, P.D.L. and G.B.; methodology, P.D.L. and G.B.; validation, P.D.L. and G.B.; formal analysis, G.B.; investigation, G.B.; data curation, G.B.; writing—review and editing, P.D.L.; supervision, P.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Furkan, S. Linear Economy Versus Circular Economy: A Comparative and Analyzer Study for Optimization of Economy for Sustainability. Visegr. J. Bioecon. Sustain. Dev. 2017, 6, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006, 56, 707–708. [Google Scholar] [CrossRef]
- Mazza, S.; Aiello, D.; Macario, A.; De Luca, P. Vehicular emission: Estimate of air pollutants to guide local political choices. Environments 2020, 7, 37. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil–plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- De Raffele, G.; Aloise, A.; De Luca, P.; Vuono, D.; Tagarelli, A.; Nagy, J.B. Kinetic and thermodynamic effects during the adsorption of heavy metals on ETS-4 and ETS-10 microporous materials. J. Porous Mater. 2016, 23, 389–400. [Google Scholar] [CrossRef]
- De Luca, P.; Foglia, P.; Siciliano, C.; Nagy, J.B.; Macario, A. Water contaminated by industrial textile dye: Study on decolorization process. Environments 2019, 6, 101. [Google Scholar] [CrossRef] [Green Version]
- Teng, Q.; Zhang, D.; Yang, C. A review of the application of different treatment processes for oily sludge. Environ. Sci. Pollut. Res. 2021, 28, 121–132. [Google Scholar] [CrossRef]
- De Luca, P.; Chiodo, A.; Macario, A.; Siciliano, C.; Nagy, J.B. Semi-Continuous Adsorption Processes with Multi-Walled Carbon Nanotubes for the Treatment of Water Contaminated by an Organic Textile Dye. Appl. Sci. 2021, 11, 1687. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, Y.; Zhu, Y.; Zhang, M.; Yu, Y.; Guo, Y.; Zhou, T. Efficient Treatment of Mature Landfill Leachate with a Novel Composite Biological Trickle Reactor Developed Using Refractory Domestic Waste and Aged Refuse. J. Clean. Prod. 2021, 305, 127194. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill Leachate Treatment: Review and Opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Poulsen, T.G.; Salituro, A.; Tedeschi, A.; Vuono, D.; Kònya, Z.; Madaràsz, D.; Nagy, J.B. Evaluation and comparison of the ammonia adsorption capacity of titanosilicates ETS-4 and ETS-10 and aluminotitanosilicates ETAS-4 and ETAS-10. J. Therm. Anal. Calorim. 2015, 122, 1257–1267. [Google Scholar] [CrossRef]
- Mojoudi, N.; Mirghaffari, N.; Soleimani, M.; Shariatmadari, H.; Belver, C.; Bedia, J. Phenol adsorption on high microporous activated carbons prepared from oily sludge—Equilibrium, kinetic and thermodynamic studies. Sci. Rep. 2019, 9, 19352. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Nagy, J.B. Treatment of Water Contaminated with Reactive Black-5 Dye by Carbon Nanotubes. Materials 2020, 13, 5508. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, C.; Macario, A.; Siciliano, C.; Nagy, J.B.; De Luca, P. Adsorption of reactive blue 116 dye and reactive yellow 81 dye from aqueous solutions by multi-walled carbon nanotubes. Materials 2020, 13, 2757. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Siciliano, C.; Macario, A.; Nagy, J.B. The Role of Carbon Nanotube Pretreatments in the Adsorption of Benzoic Acid. Materials 2021, 14, 2118. [Google Scholar] [CrossRef]
- Yang, H.; Shen, K.; Fu, P.; Zhang, G. Preparation of a novel carbonaceous material for Cr(VI) removal in aqueous solution using oily sludge of tank bottom as a raw material. J. Environ. Chem. Eng. 2019, 7, 102898. [Google Scholar] [CrossRef]
- Rasmussen, S.C. From parkesine to celluloid: The birth of organic plastics. Angew. Chem. Int. Ed. 2021, 60, 8012–8016. [Google Scholar] [CrossRef]
- Faruk, O.; Bledski, A.K.; Fink, H.P.; Sain, M. Progress report on natural fiber reinforced composites. Macromol. Mater. Eng. 2014, 299, 9–26. [Google Scholar] [CrossRef]
- De Luca, P.; Roberto, B.; Vuono, D.; Siciliano, C.; Nagy, J.B. Preparation and optimization of natural glues based on Larice pine. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012071. [Google Scholar] [CrossRef]
- Kumar, R.; Haq, M.I.U.; Raima, A.; Anand, A. Industrial applications of natural fibre-reinforced polymer composites—Challenges and opportunities. Int. J. Sustain. Eng. 2018, 12, 212–220. [Google Scholar] [CrossRef]
- Karimah, A.; Ridho, M.R.; Munawar, S.S.; Adi, D.S.; Damayant, R.; Subiyanto, B.; Fatriasari, W.; Fudkoli, A. A review on natural fibers for development of eco-friendly bio-composites: Characteristics and utilizations. J. Mater. Res. Technol. 2021, 13, 2442–2458. [Google Scholar] [CrossRef]
- Cicala, G.; Tosto, C.; Latteri, A.; La Rosa, A.D.; Blanco, I.; Elsabbagh, A.; Russo, P.; Ziegmann, G. Green composites based on blends of polypropylene with liquid wood reinforced with hemp fibers: Thermomechanical properties and the effect of recycling cycle. Materials 2017, 10, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiciotto, L.; Rothenberg, G. Biodegradable plastics: Standards, policies, and impacts. ChemSusChem 2021, 14, 56–72. [Google Scholar] [CrossRef]
- Chakraborty, I.; Chatterjee, K. Polymers and Composites Derived from Castor Oil as Sustainable Materials and Degradable Biomaterials: Current Status and Emerging Trends. Biomacromolecules 2020, 21, 4639–4662. [Google Scholar] [CrossRef]
- Choina, J.; Duwensee, H.; Flechsig, G.-U.; Kosslick, H.; Morawski, A.W.; Tuan, V.A.; Schulz, A. Removal of hazardous pharmaceutical from water by photocatalytic treatment. Cent. Eur. J. Chem. 2010, 8, 1288–1297. [Google Scholar] [CrossRef]
- De Luca, P.; Candamano, S.; Macario, A.; Crea, F.; Nagy, J.B. Preparation and characterization of plasters with photodegradative action. Buildings 2018, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- De Luca, P.; Chiodo, A.; Nagy, J.B. Activated ceramic materials with deposition of photocatalytic titano-silicate micro-crystals. WIT Trans. Ecol. Environ. 2011, 154, 155–165. [Google Scholar]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Asaro, L.; Gratton, M.; Seghar, S.; Hocine, N.A. Recycling of rubber wastes by devulcanization. Resour. Conserv. Recycl. 2018, 133, 250–262. [Google Scholar] [CrossRef]
- Mohammadhosseini, H.; Alyousef, R.; Tahir, M.M. Towards Sustainable Concrete Composites through Waste Valorisation of Plastic Food Trays as Low-Cost Fibrous Materials. Sustainability 2021, 13, 2073. [Google Scholar] [CrossRef]
- Mastali, M.A.; Dalvand, A.R.; Sattarifard, A. The impact resistance and mechanical properties of reinforced self-compacting concrete with recycled glass fibre reinforced polymers. J. Clean. Prod. 2016, 124, 312–324. [Google Scholar] [CrossRef]
- Mohammadhosseini, H.; Alyousef, R.; Lim, N.H.A.S.; Tahir, M.M.; Alabduljabbar, H.; Mohamed, A.M.; Samadi, M. Waste metalised film food packaging as low cost and ecofriendly fibrous materials in the production of sustainable and green concrete composites. J. Clean. Prod. 2020, 258, 120726. [Google Scholar] [CrossRef]
- Santucci, V.; Fiore, S. Recovery of Waste Polyurethane from E-Waste. Part II. Investigation of the Adsorption Potential for Wastewater Treatment. Materials 2021, 14, 7587. [Google Scholar] [CrossRef]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef]
- Mwanza, B.G.; Mbohwa, C.; Telukdarie, A. Strategies for the Recovery and Recycling of Plastic Solid Waste (PSW): A Focus on Plastic Manufacturing Companies. Procedia Manuf. 2018, 21, 686–693. [Google Scholar] [CrossRef]
- White, P. Building a sustainability strategy into the business. Corp. Gov. 2009, 9, 386–394. [Google Scholar] [CrossRef]
- Scalenghe, R. Resource or waste? A perspective of plastics degradation in soil with a focus on end–of–life options. Heliyon 2018, 4, e00941. [Google Scholar] [CrossRef] [Green Version]
- Klöpffer, W. Life-Cycle based methods for sustainable product development. Int. J. Life Cycle Assess. 2003, 8, 157–159. [Google Scholar] [CrossRef]
- Rimano, M.; Simboli, A.; Taddeo, R.; Raggi, A. Life Cycle Approaches for the Environmental Impact Assessment of Organizations: Defining the State of the Art. Adm. Sci. 2019, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Stahel, W.R. Circular Economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieder, M.; Rashid, A. Towards circular economy implementation: A comprehensive review in context of manufacturing industry. J. Clean. Prod. 2016, 115, 36–51. [Google Scholar] [CrossRef]
- Kain, G.; Tudor, E.M.; Barbu, M.-C. Bark Thermal Insulation Panels: An Explorative Study on the Effects of Bark Species. Polymers 2020, 12, 2140. [Google Scholar] [CrossRef]
- Kain, G.; Lienbacher, B.; Barbu, M.-C.; Richter, K.; Petutschnigg, A. Larch (Larix decidua) bark insulation board: Interactions of particle orientation, physical–mechanical and thermal properties. Holz Roh. Werkst. 2018, 76, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Bekhta, P.; Sedliačik, J.; Kačík, F.; Noshchenko, G.; Kleinoví, A. Lignocellulosic waste fibers and their application as a component of urea-formaldehyde adhesive composition in the manufacture of plywood. Eur. J. Wood Wood Prod. 2019, 77, 495–508. [Google Scholar] [CrossRef]
- Madurwar, M.V.; Ralegaonkar, R.V.; Mandavgane, S.A. Application of agro-waste for sustainable construction materials: A review. Constr. Build. Mater. 2013, 38, 872–878. [Google Scholar] [CrossRef]
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A review of unconventional sustainable building insulation materials. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Binici, H.; Eken, M.; Dolaz, M.; Aksogan, O.; Kara, M. An environmentally friendly thermal insulation material from sunflower stalk, textile waste and stubble fibres. Constr. Build. Mater. 2014, 51, 24–33. [Google Scholar] [CrossRef]
- Binici, H.; Aksogan, O. Eco-friendly insulation material production with waste olive seeds, ground PVC and wood chips. J. Build. Eng. 2016, 5, 260–266. [Google Scholar] [CrossRef]
- Danihelova, A.; Nemec, M.; Gergel, T.; Gejdos, M.; Gordanova, J.; Scesny, P. Usage of Recycled Technical Textiles as Thermal Insulation and an Acoustic Absorber. Sustainability 2019, 11, 2968. [Google Scholar] [CrossRef] [Green Version]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Wikström, F.; Williams, H.; Venkatesh, G. The influence of packaging attributes on recycling and food waste behaviour—An environmental comparison of two packaging alternatives. J. Clean. Prod. 2016, 137, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Martinho, G.; Pires, A.; Portela, G.; Fonseca, M. Factors affecting consumers’ choices concerning sustainable packaging during product purchase and recycling. Resour. Conserv. Recycl. 2015, 103, 58–68. [Google Scholar] [CrossRef]
- Zawadiak, J.; Wojciechowski, S.; Piotrowski, T.; Krypa, A. Tetra Pak Recycling—Current Trends and New Developments. Am. J. Chem. Eng. 2017, 5, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Foschi, E.; Bonoli, A. The commitment of packaging industry in the framework of the european strategy for plastics in a circular economy. Adm. Sci. 2019, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Yuhui, M. Changing Tetra Pak: From Waste to Resource. Sci. Prog. 2018, 101, 161–170. [Google Scholar] [CrossRef]
- Corzani, C.; Siboni, V. In brick o in bag in box, il vino è garantito. Agricoltura 2005, 87–88. Available online: http://www.crpv.it/doc/5209/brick05.pdf (accessed on 1 June 2022).
- Rogora, A.; Lo Bartolo, D. Costruire Alternativo; Wolters Kluwer Italia: Milan, Italy, 2013. [Google Scholar]
- Salasinska, K.; Mizera, K.; Celiński, M.; Kozikowski, P.; Borucka, M.; Gajek, A. Thermal properties and fire behavior of polyethylene with a mixture of copper phosphate and melamine phosphate as a novel flame retardant. Fire Saf. J. 2020, 115, 103137. [Google Scholar] [CrossRef]
- Lin, Z.; Yanchun, F. Flame-Retardant Wood Composites Based on Immobilizing with Chitosan/Sodium Phytate/Nano-TiO2-ZnO Coatings via Layer-by-Layer Self-Assembly. Coatings 2020, 10, 296. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
 Insufficient;
Insufficient;  Partial;
Partial;  Total.
Total.
 Insufficient;
Insufficient;  Partial;
Partial;  Total.
Total.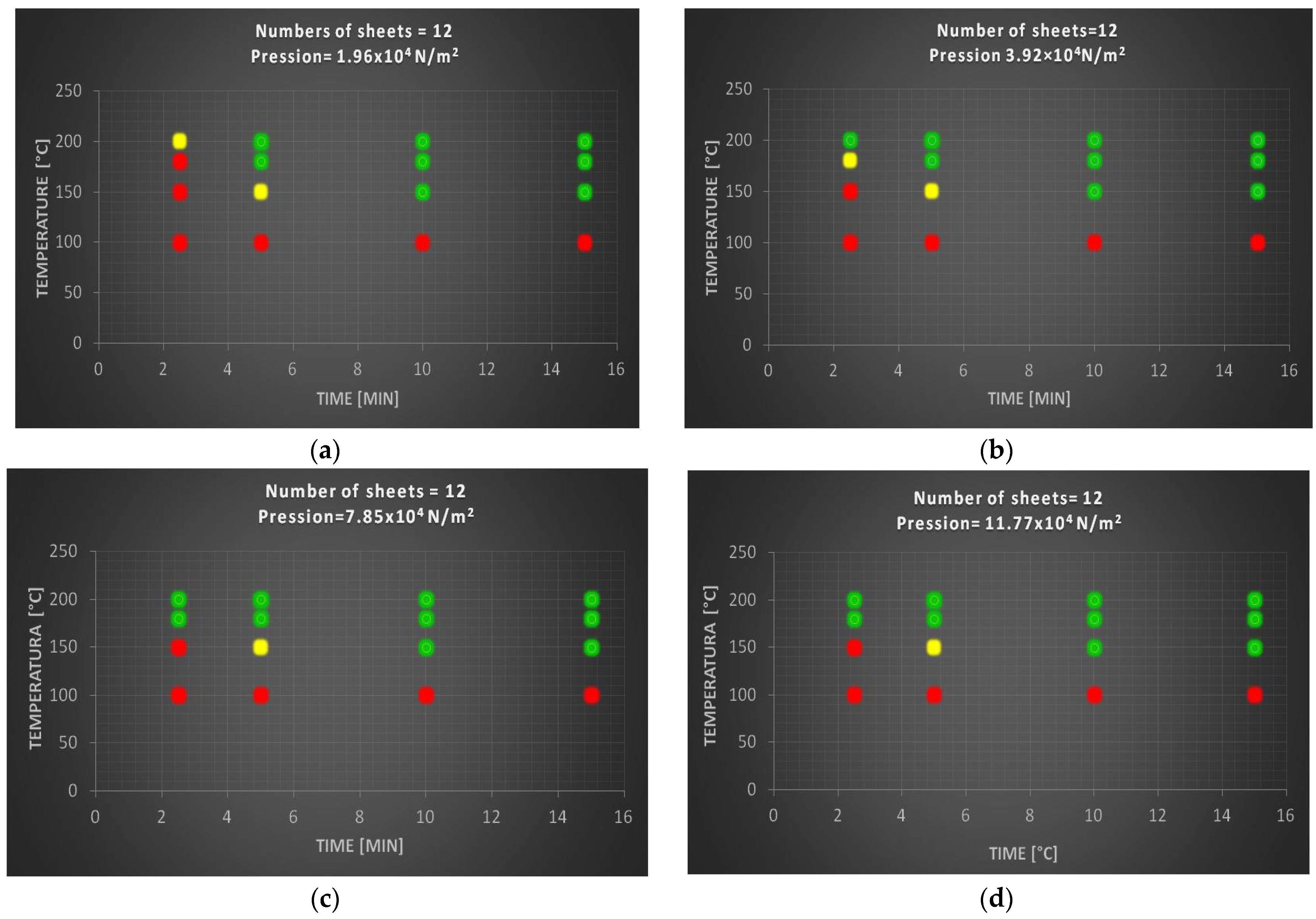
 Insufficient;
Insufficient;  Partial;
Partial;  Total.
Total.
 Insufficient;
Insufficient;  Partial;
Partial;  Total.
Total.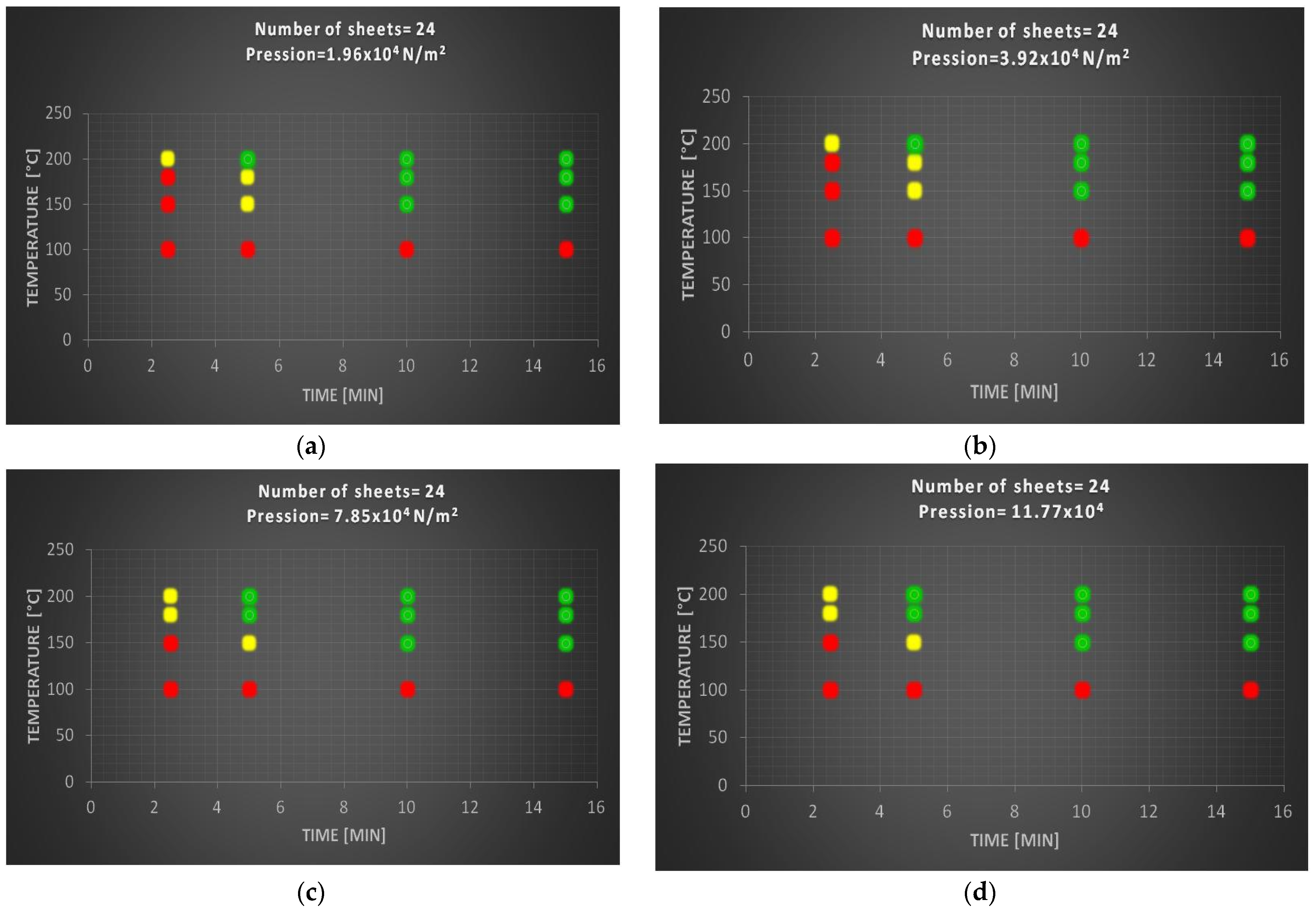
 Insufficient;
Insufficient;  Partial;
Partial;  Total.
Total.
 Insufficient;
Insufficient;  Partial;
Partial;  Total.
Total.