Abstract
Understanding how environmental adaptations mediate plant and ecosystem responses becomes increasingly important under accelerating global environmental change. Multi-stemmed trees, for example, differ in form and function from single-stemmed trees and may possess physiological advantages that allow for persistence during stressful climatic events such as extended drought. Following the worst drought in Hawaii in a century, we examined patterns of stem abundance and turnover in a Hawaiian lowland dry forest (LDF) and a montane wet forest (MWF) to investigate how multi-stemmed trees might influence site persistence, and how stem abundance and turnover relate to key functional traits. We found stem abundance and multi-stemmed trees to be an important component for climate resilience within the LDF. The LDF had higher relative abundance of multi-stemmed trees, stem abundance, and mean stem abundance compared to a reference MWF. Within the LDF, multi-stemmed trees had higher relative stem abundance (i.e., percent composition of stems to the total number of stems in the LDF) and higher estimated aboveground carbon than single-stemmed trees. Stem abundance varied among species and tree size classes. Stem turnover (i.e., change in stem abundance between five-year censuses) varied among species and tree size classes and species mean stem turnover was correlated with mean species stem abundance per tree. At the plot level, stem abundance per tree is also a predictor of survival, though mortality did not differ between multiple- and single-stemmed trees. Lastly, species with higher mean stem abundance per tree tended to have traits associated with a higher light-saturated photosynthetic rate, suggesting greater productivity in periods with higher water supply. Identifying the traits that allow species and forest communities to persist in dry environments or respond to disturbance is useful for forecasting ecological climate resilience or potential for restoration in tropical dry forests.
Keywords:
tropical dry forest; resprouting; aboveground carbon; persistence; drought; photosynthesis 1. Introduction
Understanding and predicting how plant functional traits can mediate shifts in community structure and ecosystem function becomes increasingly important under accelerating global environmental change [1,2,3]. Resprouting ability, for example, was a common growth form in ancient flora that now occurs across diverse woody plant lineages and many environmental contexts, and contributes to plant fitness and persistence and ecosystem carbon gain in response to disturbance or environmental perturbations [4,5,6,7,8,9]. Multi-stemmed trees are a common component of forests and woodlands globally and differ in form and function from larger single-stemmed trees more typically associated with old-growth forests. At the individual scale, multi-stemmed trees are often shorter, but may have advantages in carbon gain, reproduction, and site persistence [6], whereas at the ecosystem scale, higher levels of resprouting are associated with lower productivity [5]. Investigating the structure and dynamics of multi-stem tree-dominated forests will improve knowledge of their function and vulnerability.
Resprouting in woody species is the emergence of new tissues from buds located in stems, roots, root crown, rhizomes, or lignotubers [7], typically following some external perturbation. Although the location and extent of resprouting is shaped by a variety of evolutionary pressures [10], resprouting commonly occurs after disturbances, such as fire [11,12], windstorms, and hurricanes [13], and after animal browsing [14]. Many dryland ecosystems are dominated by plant species capable of regeneration through resprouting [15], and resprouting species can mediate ecosystem responses to drought stress [9,16]. Resprouting species tend to possess a suite of functional traits related to conservative water use, higher growth rates, and higher belowground biomass allocation [7,9,16,17] relative to non-resprouting species. Indeed, resprouting species are more capable of surviving severe drought and have an increased probability of persistence [18,19]. Thus, resprouting species may dominate in climates with a significant dry season or highly variable precipitation regimes, and the resulting multi-stemmed trees may be of high conservation and restoration value in the face of modern global change.
Tropical dry forests (TDFs) are some of the most endangered ecosystems on the planet [20,21,22], and the forest fragments that remain in Hawaii are of high conservation concern [23]. Most TDFs have distinct wet and dry seasons [24,25], but Hawaiian dry forests tend to be dry (i.e., <100 mm rain per month) all year long [26,27]. Highly aseasonal water availability and strong droughts in Hawaiian dry forests are thought to favor asexual regeneration via resprouting (e.g., basal, root, and/or branch layering). For example, Busby et al. [28] compared dry, mesic, and wet forests on Hawaii Island and found greater resprouting in the dry forest, and concluded that rainfall is likely a controlling factor. On Hawaii Island, a prolonged drought persisted from 2008 to 2013, with two severe droughts occurring in 2010 and 2012. During the 2010 drought, ~40% of land area in Hawaii experienced severe drought for 35 consecutive weeks (http://droughtmonitor.unl.edu, accessed on 25 May 2022 [29]). In this study and in the context of drought, we tested how multi-stemmed trees influence forest structure and differ from single-stemmed trees in their demographic trends and functional traits to illuminate the conservation needs and restoration potential of multi-stem-dominated forests.
In previous work in a lowland dry forest (LDF), we observed an extensive number of multi-stemmed trees in our ForestGEO forest dynamics plot (FDP) in Pālama Nui on Hawaii Island. This included a high abundance of multi-stemmed trees, a high abundance of small diameter stems per hectare, low aboveground biomass [26], and low tree growth (median diameter change over five years was 0.0 cm). Here, we now utilize ForestGEO FDP in Pālama Nui and a second montane wet forest (MWF) FDP in Laupāhoehoe, also on Hawaii Island, to (1) contrast the abundance of multi-stemmed trees and stems between LDF and MWF FDPs, and (2) within the LDF, examine how stem abundance and stem turnover relate to mean species relative growth rates (RGRs), mortality (m), and previously established functional trait modules. We hypothesized that stem abundance and stem turnover (i.e., addition and loss of stems) is a primary mechanism of forest productivity and structural change across tree species and tree size classes. We also hypothesized that multi-stemmed trees and their associated stem abundance would be associated with persistence within the LDF. Lastly, we hypothesized that stem abundance and/or turnover would be associated with other traits that confer advantages in an environment characterized by low year-round water availability. More specifically, we expect stem abundance to be positively correlated with photosynthetic traits (primary production and gas-exchange parameters), such that multi-stemmed trees can achieve high productivity during short periods of high water availability. TDF restoration is increasingly becoming an important priority for nations around the globe [30], yet relatively little attention has been paid to the role of species selection for multi-stemmed traits to support immediate and longer-term restoration goals. Given that ongoing climate change is shifting disturbance regimes in Hawaii, improving knowledge on key characteristics and dynamics of multi-stemmed dominated forests will inform urgent forest conservation and restoration practices.
2. Materials and Methods
2.1. Forest Dynamics Plot Descriptions
The Hawaii Permanent Plot Network (HIPPNET; http://www.hippnet.hawaii.edu/; accessed on 25 May 2022), a member of ForestGEO (https://forestgeo.si.edu/; accessed on 25 May 2022) consists of four forest dynamics plots located across a steep rainfall gradient, and was established in 2008 as a platform to investigate forest and climatic processes in Hawaii. The two plots included in this study, Laupāhoehoe (LAU), a montane wet forest (MWF, established in 2008), and Pālama Nui (PLN, established in 2009), a lowland dry forest (LDF), are 4 hectares in size (Figure 1). The Pālama Nui LDF is an example of one of the world’s most endangered forest types. The FDP is located on the northwest slope of Hualālai Volcano in the district of North Kona (240 m elevation, 19°44′ N, 155°59′ W). Over the last 200 years, much of the LDF in Hawaii has been subjected to grazing and browsing by exotic ungulates, with remnants impacted by wildfire facilitated by non-native grasses [31]. Cumulatively, these disturbance patterns have greatly reduced the native forest from its original extent [32]. Although the area containing the FDP has not been burned or significantly browsed by ungulates, the surrounding area is a matrix of degraded LDF and open grassland. In 2009, a fence and firebreak were installed around the area to protect it from ungulates and fire.

Figure 1.
Plot profile for Pālama Nui, a tropical, lowland dry forest (LDF), located on the leeward (west) side of Hawaii Island, Hawaii. Table displays key site characteristics. Panel (A) attempts to visually describe forest canopy and structure within the LDF. Panel (B) provides three visual examples of the multi-stemmed growth habit through resprouting, a highly dominant trait within the LDF.
The 4 ha Laupāhoehoe FDP (19°55′ N, 155°17′ W) is located within the state-owned Laupāhoehoe Natural Area Reserve section of the Hawaii Experimental Tropical Forest (HETF) on the northeast slope of Mauna Kea volcano. The dominant pre-human contact disturbance regime in this forest type was single- to multiple-tree falls and dieback due to cohort senescence of older Metrosideros polymorpha stands [33,34]. Following contact, large Acacia koa trees were occasionally harvested for traditional canoe building. In modern times, the MWF FDP has no known history of logging [35]. Non-native wild pigs disturb soils while rooting, as well as tree ferns [36], with damage found over large areas. Additional plot description of the LDF and MWF FDPs can be found in Ostertag et al. [26].
2.2. Structure, Growth, Demography
During the establishment and initial census of the two FDPs, all live, native woody plants of ≥1 cm diameter at breast height (DBH measured at 130 cm) were identified, tagged, and mapped within 5 m × 5 m grids installed throughout the 200 m × 200 m plots. In each census, the DBH of each tree was measured and the status (i.e., alive, dead, missing, new) was recorded to examine changes in abundance, tree size, relative growth rates, basal area, and mortality rates. For trees with multiple stems, all stems were counted to determine the total number and diameters of stems ≥1 cm at 130 cm, though only the main stem was individually tracked through time. Census 1 was conducted from December 2007 to May 2009, and Census 2 was conducted from November 2013 to October 2014. Stem turnover was estimated for each individual tree by subtracting initial stem count in census 1 from final stem count in census 2 (the two full censuses for each plot). More detailed methods can be found in Ostertag et al. [26]. From individual plant DBH measured on the main stems throughout censuses, we used the add_tropical_biomass function in the fgeo.biomass (github.com/forestgeo/fgeo.biomass) (accessed on 25 May 2022) and BIOMASS packages to estimate aboveground biomass for all trees using allometric equations for tropical trees [37]. Cumulative biomass for multi-stemmed trees was determined by summing biomass estimates for all stems (similar to [38]). Aboveground carbon storage was estimated (see [39,40]) as:
Carbon = aboveground biomass × 0.50 − aboveground Biomass (AGB, Mg/ha)
Relative growth rates for DBH (RGRdbh) and aboveground biomass (RGRbiom) were calculated as:
where dbh is the diameter at breast height at timepoint t.
RGRdbh: Annual relative growth rate (cm cm−1 year−1): (ln(dbht+1) − ln(dbht))/((datet+1 − datet)/365.25)
RGRbiom: Annual biomass relative growth rate (kg kg−1 year−1): (ln(biomasst+1) − ln(biomasst)/((datet+1 − datet)/365.25)
Annual mortality rate (m; %/year) for all dominant species was determined as:
where N is the total number of alive individuals at timepoint t, and S is the total number of individuals surviving at the next timepoint t + 1.
m = [1 − (N1/N0)(1/∆t)] × 100
Tree fern species were excluded from all analyses, as they differ in growth habit compared to the tree species in the study. Due to the potential for a disproportionate influence of apparent stochasticity in determining the demographics of small populations, species with <15 individuals were excluded from analyses of RGR and m [41,42].
2.3. Functional Trait Modules
All functional trait data were collected by Medeiros et al. [42], who established an extensive suite of traits with specific mechanistic influences on resource acquisition, growth, and stress tolerance that predict species vital rates across wet and dry tropical forests. The 45 functional traits are grouped into functional trait “modules,” representing clusters of traits that co-vary, due to selection, partially independently of other trait clusters [42,43]. We included all seven modules described in Medeiros et al. [42]: (1) stomatal morphology, (2) leaf and wood economics and structure, (3) leaf venation, (4) leaf composition, (5) estimated photosynthesis, (6) whole plant size, and (7) vital rates (i.e., RGRs and m). Comprehensive information regarding functional traits and trait measurement is provided in detail in Medeiros et al. [42].
2.4. Statistical Approach
In the sole comparison of the LDF and MWF, differences in stem abundance between the LDF and MWF were determined using a nested generalized linear model (GLM) with species nested within forest type as a fixed effect. Within the LDF, differences in stem abundance between tree species, tree size classes, and stem habit (i.e., whether an individual tree is multiple or single stemmed) were determined using a GLM. For all GLM models examining differences in stem abundance across forest type, species, tree size classes, and stem habit, a negative binomial model was used to fit the stem abundance data.
Within the LDF, additional models were used to investigate potential interactive effects of species, tree size classes, and stem habit on stem abundance patterns. In the first full model, species, tree size class, and the interaction of species and tree size class were included as fixed effects. In the second full model, species, stem habit, and the interaction between species and stem habit were included as fixed effects. If either full model yielded a significant interaction term, the model was reduced to specifically examine individual fixed effects. If either model yielded a nonsignificant interaction term, that term was removed from the model. The same fixed effects and interaction effects were used to examine how variation in relative growth rates (RGR) differed across species, tree size class, and stem habit. The upper size class, a DBH of 30 cm+, was removed when examining models with interactive effects, as several individuals of only one tree species were in this size class. Similarly, the 11–30 cm size class was removed for all but the two tree species with trees in this class, Diospyros sandwicensis and Santalum paniculatum.
Also within the LDF, patterns in stem turnover (i.e., the addition or loss of stems) were examined for multi-stemmed trees across species and tree size classes. Individual t-tests were used analyze whether stem turnover within a species differed from zero. Differences in stem turnover among tree species were determined using a GLM. A Tukey test at 5% probability was used to determine pairwise differences if a species fixed effect was significant.
To highlight the dynamics of multi-stemmed trees and patterns of structural change, species mean values were determined for stem abundance per tree and stem turnover per tree, and Pearson’s correlation tests were used to determine the strength and direction of their relationship. To test whether stem abundance was related to survival, stem abundance in census 1 was used to predict tree status (i.e., alive or dead) in census 2 using a negative binomial model to fit the stem abundance data. A χ2-test was used to test whether mortality differed among multiple- and single-stem trees between the two census time points.
To test whether stem abundance was related across species to functional traits that confer tolerance to year-round water availability and frequent droughts or to higher vital rates (i.e., RGRs and m), we used Pearson’s correlation tests to determine the relationship of mean stem abundance per tree with traits related to stomatal morphology, leaf and wood economics and structure, leaf venation, leaf composition, estimated photosynthesis, whole plant size, and vital rates (i.e., RGRs and m). All analyses were performed in R [44].
3. Results
3.1. Stem Abundance and Relative Growth Rate across Plots, Species, Tree Size Class, and Stem Habit
Stem abundance patterns contrasted strongly between the LDF and MWF. Total stem abundance was 2.8-fold higher in the LDF (28,384 stems) than the MWF (9966 stems). Similarly, LDF trees had 1.7-fold higher stem abundance per tree than the MWF (LDF: mean ± standard error = 2.29 ± 0.02; MWF: 1.35 ± 0.012; χ2 = 1726, df = 1, p < 0.0001; Figure 2). Multi-stemmed trees also occurred in higher relative abundance for dominant tree species of the LDF than the MWF (Figure 3).
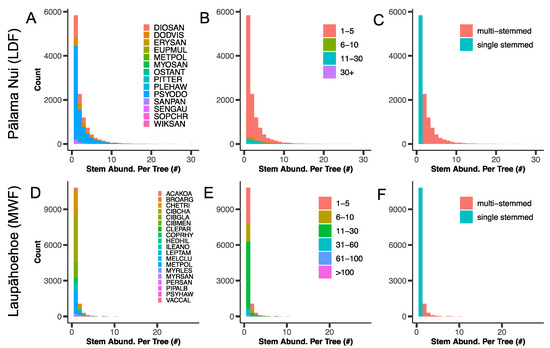
Figure 2.
Distributions of stem abundance by tree species, tree size class, and stem habit across both lowland dry forest (LDF) and montane wet forest (MWF). Panels (A,D) represent total stem abundances (#) for all trees by species. Panels (B,E) represent total stem abundances (#) for all trees by tree size class. Panels (C,F) represent total stem abundance (#) for all trees by stem habit, i.e., whether the tree is a single-stemmed or a multiple-stemmed tree. A full species list can be found in the supplemental information.
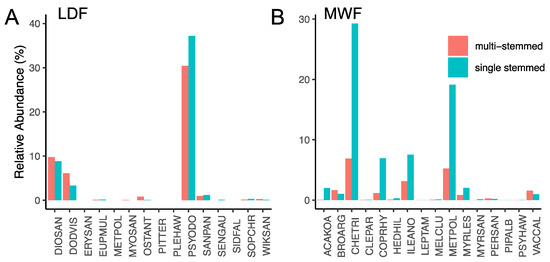
Figure 3.
Relative abundance of multiple-stem and single-stem trees across both lowland dry forest (LDF) and montane wet forest (MWF). Panel (A) represents the relative abundance (%) of multiple-stem and single-stem trees within the LDF. Panel (B) represents the relative abundance (%) of multiple-stem and single-stem trees within the MWF. A full species list can be found in the Supplemental Information.
Within the LDF, differences in mean stem abundance were determined across species and tree size classes, and we tested for their interaction (Table 1). Examining individual fixed effects, we found strong evidence across several species where stem abundance varied by tree size classes, whereas a handful of species show no differences in mean stem abundance (Table S1). The two highly dominant species, Diospyros sandwicensis and Psydrax odoratum, as well as an occasional species, Santalum paniculatum, were the three species whose stem abundance varied by tree size class. Likewise, differences were also detected across species, stem habit, and the interaction between species and stem habit (Table 1). Analyses for individual fixed effects showed that all eight species tested had higher mean stem abundance per tree in multi-stemmed trees compared to single-stemmed trees (Table S2). Stem abundance means across species and tree size class, as well as species and stem habit, can be found in Tables S3 and S4. Furthermore, patterns in relative stem abundance (%, Figure 4A) and relative aboveground carbon (%, Figure 4B) across species and stem habit showed that multi-stemmed trees dominate the LDF.

Table 1.
Full model results testing the interactive effect of species and tree size class and species and stem habit (i.e., multiple stemmed or single stemmed) on stem abundance and relative growth rate of biomass (RGRbiom) (example: stem abundance~species + tree size class + species × tree size class + error). Significant model results are in bold.
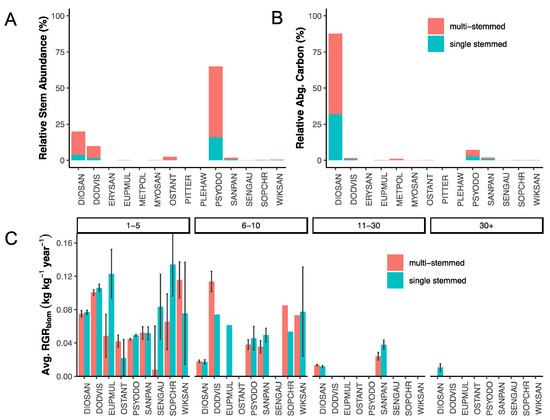
Figure 4.
Multiple-stem and single-stem comparisons within the LDF. Panel (A) highlights species-level patterns in relative stem abundance (%) by stem habit. Panel (B) highlights species-level patterns in relative aboveground carbon (%) by stem habit. Panel (C) highlights species variation in mean values of relative growth rates (RGRbiom) by tree size class. Error bars represent ±1 standard error of the mean. A full species list can be found in the supplemental information.
We also observed differences in mean RGRbiom across species, stem habit, and the interaction between species and stem habit (Table 1, Figure 4C). Individual fixed effects were examined, and we identified mean RGRbiom differences among stem habits within species (Table S5). The two highly dominant species, D. sandwicensis and P. odoratum, had higher RGRbiom in their single-stemmed cohort. At the plot level and across all species, RGRbiom was higher in single- than multiple-stemmed trees (multiple stem: least squares mean ± se = 0.0476 ± 0.001; single stem: 0.0519 ± 0.001; p = 0.005). Of the 10 species with both multiple- and single-stemmed trees, single-stem cohorts had higher RGRbiom than their multiple-stem counterparts for four species (Diospyros sandwicensis, Euphorbia multiformis, Psydrax odoratum, and Sophora chrysophylla), RGRbiom was higher in multi-stemmed cohorts for two species (Dodonaea viscosa and Myoporum sandwicensis), and RGRbiom did not differ for four species (Osteomeles anthyllidifolia, Santalum paniculatum, Senna gaudichaudii, and Wikstroemia sandwicensis) (Table S6).
3.2. Stem Abundance and Turnover as Common Components of the Lowland Dry Forest
Differences in stem turnover among species and among size classes within species provide evidence of an important mechanism for site persistence. We found a mean stem turnover of +0.04 stems per tree among multi-stemmed trees within the LDF between 2009 and 2014 (census 1 and 2, respectively; t = 2.41, df = 5532, p = 0.016). Species showed negative, near zero, and positive mean stem turnover estimates (Table S7), with differences among pairwise combinations of species (χ2 = 308.6, df = 12, p < 0.0001, Table S8), indicating species and size class differences (Table 1 and Table S9). We found a strong negative relationship between species mean stem abundance and species mean turnover (r = −0.62, p = 0.01, Figure 5A). To test this multiple stem-persistence hypothesis, we used stem abundance from our first census (2008–2009) to predict tree status (i.e., whether the tree was alive or dead) in census 2 (2013–2014) and found evidence that trees that were still alive in census 2 had a higher number of stems on average than trees that died between census 1 and census 2 (Figure 5B). Yet, mortality between the two censuses was not statistically different between multiple- and single-stem trees (χ2 = 0.12, df = 1, p = 0.7212).
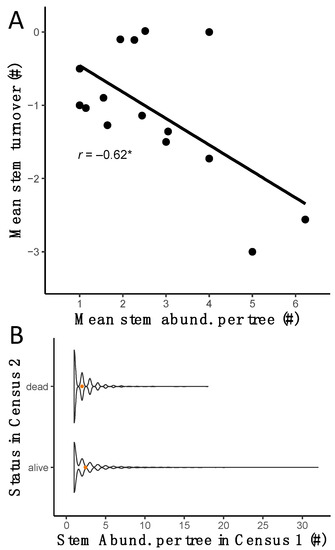
Figure 5.
Temporal patterns relating stem abundance to stem turnover and survival. Panel (A) shows the correlation across species between mean values of stem abundance per tree in census 2 and stem turnover per tree between census 1 and census 2. r represents Pearson’s correlation coefficient; * p < 0.05. Shaded area represents 95% confidence interval. Panel (B) shows a violin plot depicting differences in stem abundance (#) in census 1 by tree status (i.e., alive or dead) in census 2. Orange point and error bars represent mean ± one standard error of the mean.
3.3. Trait–Trait Correlations
Across species, mean stem abundance values were correlated with traits related to photosynthetic productivity and water use per leaf area. We found positive correlations between mean values of stem abundance and estimates of electron transport rate, maximum carboxylation rate, leaf CO2 assimilation rate, and stomatal conductance (Figure 6). No significant correlations were detected between mean stem abundance and species mean estimates of stomatal morphology, leaf and wood economics and structure, leaf venation, leaf composition, whole plant size, and vital rates (i.e., RGRs or m). Likewise, no significant correlations were detected between mean values of stem turnover and any functional trait modules included in this study.
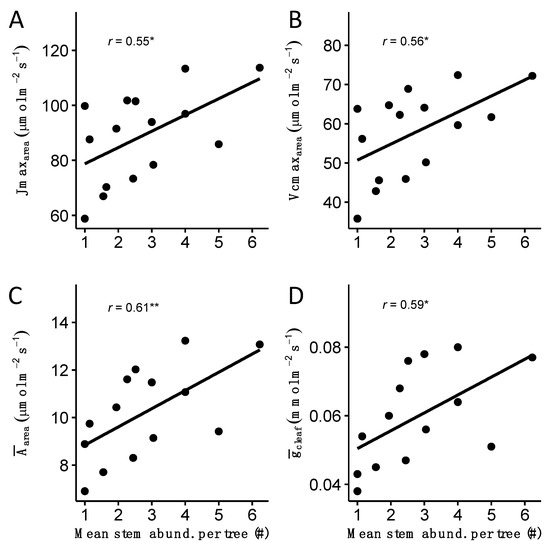
Figure 6.
Functional trait–stem trait correlations. All four panels show the correlation across the 15 species in the LDF between mean values of stem abundance per tree and estimates of electron transport rate (Panel A), maximum carboxylation rate (Panel B), leaf CO2 assimilation rate (Panel C), and stomatal conductance (Panel D). r represents Pearson’s correlation coefficient; * p < 0.05, ** p < 0.01. Shaded area represents 95% confidence interval. Estimated stomatal and photosynthesis traits are publically available and described in depth in [42].
4. Discussion
Utilizing multi-year censusing of forest dynamics data across the most serious drought to impact this region of Hawaii in a century, we examined patterns of stem abundance among Hawaiian LDF and MWF sites to investigate how multi-stemmed trees influence forest structure and site persistence, and how stem abundance and turnover relate to functional traits known to be important for existing in a water-limited system. The LDF had a higher relative abundance of multi-stemmed trees, with a 2.8-fold higher stem abundance and a 1.7-fold higher mean stem abundance per tree than the MWF. Within the LDF, multi-stemmed trees had a higher relative stem abundance and contributed a higher proportion of estimated aboveground carbon (Mg/ha) compared to single-stemmed trees. Likewise, the two dominant species are highly multi-stemmed, suggesting again that the multi-stem habit is an important way of life in the LDF. Estimates of relative growth rates in aboveground biomass (RGRbiom) also varied by species and stem habit, with higher RGRbiom in some multi-stemmed species groups. We also found that trees still alive in census 2 had a higher number of stems on average than trees that died between census 1 and census 2, suggesting an important link between the multi-stemmed growth form and survival. The greater rate of survival of multi-stemmed trees may be in part due to functional trait values that encourage higher rates of stomatal conductance and photosynthesis. Identifying the species and functional traits that confer persistence in response to environmental stress by altering growth strategies provides unique information for forecasting ecological response and potential restoration efforts in response to drought and increasing temperatures in a Hawaiian tropical dry forest.
4.1. Multi-Stemmed Trees Are an Important Structural and Functional Component of the Hawaiian Lowland Dry Forest
Many arid ecosystems are dominated by plants that regenerate by resprouting [15]. When comparing the LDF and MWF, we found the LDF to have ~2.8 times more stems, ~1.7 times more stems per tree, higher relative abundances of multi-stemmed trees, and higher relative aboveground carbon. Such high relative abundance of multi-stemmed trees, high stem abundance, and higher aboveground carbon suggest that limited and aseasonal water availability in the Hawaiian LDF may have selected for functional variation in resprouting ability that allows for ecosystem persistence in response to suboptimal environmental conditions. For example, a high proportion of multi-stemmed trees was in the smallest tree size class (a DBH of 1–5 cm), which might suggest that resprouting contributes to successful establishment and persistence in younger trees (Figure 2B). Cumulatively, these findings match expectations that resprouting trees should dominate in environments with pronounced seasonal drought or with high interannual variability of precipitation [9].
In addition to aseasonal precipitation regimes in the Hawaiian LDF, two severe droughts occurred in 2010 and 2012, during an extended dry period from 2008 to 2013. During the 2010 drought, ~40% of land area in Hawaii experienced severe drought for 35 consecutive weeks (http://droughtmonitor.unl.edu, accessed on 25 May 2022, [45]). During these prolonged droughts and the years immediately following, we described a modest trend of stem accumulation (an average +0.04 stems per tree, or roughly a net gain of ~232 stems), as we found evidence that stem turnover within multi-stemmed trees was positive, even if there might have been substantial loss in biomass during or immediately after the severe drought events. Furthermore, the sensitivity of trees to drought stress depends on structural and physiological adaptations to water limitation and the degree of environmental stress (i.e., the duration and intensity of the water deficit). For example, D. sandwicensis and P. odoratum, the two most abundant and dominant species in the LDF, have on average 3+ stems per tree and tree size classes ranging from 1.7–3.8 and 2.2–3.8 stems per tree, respectively. Therefore, the dominant patterns of stem abundance and observed forest-wide stem accumulation provide optimism that ecosystems dominated by multi-stemmed tree species may possess functional variation necessary to recover from severe environmental disturbances.
4.2. Multiple-Stemmed Trees Possess Traits That Allow for Persistence in Dry Environments
Several studies have uncovered physiological adaptations to limited, aseasonal rainfall and drought in Hawaiian lowland dry forests [27,42]. For example, Medeiros et al. [42] linked functional traits with vital rates and resolved mechanisms of drought tolerance. Similarly, Ware et al. (* in review) described short temporal sensitivities of tree growth to rainfall anomaly in the lowland dry forest, which suggests that dry forest species respond strongly to limited precipitation. In this study, a negative correlation between mean stem abundance and mean stem turnover suggests a potential mechanism for plant persistence in response to drought and recovery from drought. First, trees that possess more than one stem have biomass in auxiliary stems to lose in response to environmental disturbances. Likewise, multi-stemmed trees are better at regenerating quickly after disturbance, where recovery of water use and aboveground carbon to pre-disturbance levels is often quicker in multi-stemmed tree-dominated ecosystems [46,47,48].
Notably, RGRbiom did not differ clearly among multiple- and single-stemmed trees. At the plot level and across all species, RGRbiom was higher in single-stem trees than multiple-stem trees. Yet, of the 10 species with both multiple- and single-stemmed trees, single-stem cohorts had higher RGRbiom than their multiple-stem counterparts for four species (D. sandwicensis, E. multiformis, Psydrax odoratum, and S. chrysophylla), RGRbiom was higher in multi-stemmed cohorts for two species (D. viscosa and M. sandwicensis), and RGRbiom did not differ for four species (O. anthyllidifolia, S. paniculatum, S. gaudichaudii, and W. sandwicensis) (Table S6). These results suggest that at least 66% of the species present in the LDF can regenerate through resprouting, and that RGRbiom patterns among tree size classes and stem habit are species specific (Figure 4C). These results further suggest an interspecific growth–mortality tradeoff, where slow-growing species tend to possess higher rates of survival and faster-growing species have the cost of increased mortality, matching patterns previously established in Hawaii [42] and tropical forests elsewhere [49,50]. Therefore, multi-stemmed trees may, on average, accumulate aboveground biomass more slowly than single-stemmed trees, but the lower productivity is likely associated with a higher probability of survival.
Our findings for stem abundance and the ability to regenerate through resprouting indicate that multi-stemmed species are more capable of surviving severe drought and have an increased probability of persistence [18,19,48]. The strong negative relationship between species mean stem abundance and species mean turnover (Figure 5A) points to a tradeoff for multi-stemmed trees to not only survive and persist through periods of environmental stress, but potentially respond more quickly when ephemeral resource limitations are alleviated. We also determined that trees still alive in census 2 had higher mean stem abundances in census 1 than trees that died between censuses. However, there was no evidence of mortality between the two census time points differing among multiple- and single-stem individuals. These results coupled with a net gain in stems raise the following question: Which functional traits are associated with stem-abundant species that allow them to persist under a near-constant water deficit?
Resprouting species have been shown to have lower photosynthesis, stomatal conductance and transpiration, xylem-specific conductance, and critical xylem cavitation pressure potential (Pcrit), but higher xylem implosion resistance and larger vessel diameter with lower vessel density than non-resprouters [16]. The positive correlations between mean stem abundance and estimates of photosynthesis and stomatal conductance detected in this study do not necessarily contrast with the literature. Medeiros et al. [42] showed higher photosynthesis in the single-stem-dominated Hawaiian MWF, but also lower mortality rates in species with higher estimated photosynthesis within the LDF. We believe these structural trait–physiological trait correlations suggest that tree species with more stems on average can maximize photosynthesis and gas exchange when water is available for uptake. These results, coupled with the unclear RGRbiom patterns, suggest that higher photosynthetic rates in multi-stemmed trees could be compensating for greater belowground biomass allocation. Resprouting trees have been shown to have higher root–shoot ratios [51], which in turn could further promote persistence in response to drought. Our results provide evidence that the LDF, which is dominated by resprouters, possesses some key ecosystem characteristics to avoid mortality, withstand drought, and recover from disturbance more quickly than ecosystems dominated by species without the ability to regenerate through resprouting. Taken together, multi-stemmed trees are ideal candidates to be used for natural regeneration and restoration efforts in tropical dry forests.
4.3. Multi-Stemmed Trees and Implications for Restoration Practice in Tropical Dry Forests
Tropical dry forests are the most threatened terrestrial ecosystems, as nearly all of the remnant TDFs globally are currently experiencing the compounding effects of deforestation-driven fragmentation, degradation, and, in some geographies, desertification [52]. From a conservative perspective, TDFs are often home to high biodiversity that can include high rates of endemism, with many species that are particularly adapted to the extreme environmental conditions, and therefore have high conservation and restoration value. Our results suggest that afforestation efforts and management activities should concentrate on the conservation and restoration of the remnant vegetation, especially resprouting tree species to effectively access locally adapted, drought-tolerant, and persistent phenotypes. Likewise, resprouting after disturbance provides a shortcut for forest recovery, as it eliminates more vulnerable life stages, starting as a shoot [12,15]. An integrated practice of promoting natural regeneration and establishing out-plantings of stem cuttings of multi-stemmed trees could lower potential mortality, increase drought tolerance, and quicken recovery through increased resprouting potential. As such, the impact of increased drought stress in ecosystems dominated by these species could be minimized. To this end, compounding anthropogenic threats are increasing the sensitivity of TDFs to ongoing climate change [53], and improving our knowledge of TDF species and their associated functional variation that allows them to persist in response to environmental stress will improve conservation and restoration outcomes.
5. Conclusions
This study has shown that the multi-stemmed habit in trees is an important characteristic in a Hawaiian lowland dry forest, where stem abundance is related to stem turnover, survival, a suite of physiological traits related to photosynthesis, and aboveground carbon pools. Improving information on the dominant phenotypes in TDFs is important for guiding conservation and restoration practice as dry forest ecosystems experience further global change pressures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su14116779/s1, Species Codes Supplemental; Table S1—Individual fixed effects of DBH size class by species on stem abundance. Table S2—Individual fixed effects of stem habit by species on stem abundance. Table S3—Stem abundance means and standard errors by species and size class. Table S4—Stem abundance means and standard errors by species and stem habit. Table S5—Individual fixed effects of stem habit by species on RGRbiom. Table S6—Relative growth rate (RGR) means and standard errors by species and stem habit. Table S7—One sample T-test on stem turnover by tree species. Table S8—Tukey test of pairwise species comparisons in stem turnover estimates. Table S9—Stem turnover means and standard errors by species and size class.
Author Contributions
Conceptualization, I.M.W. and R.O.; data curation, I.M.W., R.O., L.S., C.D.M., F.I., C.M.L., T.G., G.P.J. and C.S.; formal analysis, I.M.W.; methodology, I.M.W. and R.O.; writing—original draft, I.M.W.; writing—review and editing, I.M.W., R.O., S.C., C.P.G., L.S., C.D.M., C.M.L., T.G., G.P.J. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
Support to establish the HIPPNET plot network came from NSF EPSCoR grants 0554657 and 0903833. Funding support for I.M.W., S.C. and C.P.G. was from the United States Forest Service. Funding support for C.M.L. came from the College of Tropical Agriculture and Human Resources, University of Hawai’i at Mānoa via the USDA National Institute of Food and Agriculture, Hatch and McIntire-Stennis Programs (HAW00132-H, HAW01127-H, HAW00188-M, and HAW01123-M). Funding support for L.S. came from National Science Foundation grant 2017949. Funding support for C.S. came from National Science Foundation Career grant 1943583.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in this study are available via Figshare at the following DOIs: https://doi.org/10.6084/m9.figshare.19945796; https://doi.org/10.6084/m9.figshare.19945634; https://doi.org/10.6084/m9.figshare.19945478.
Acknowledgments
We acknowledge the lands on which this study was conducted, lands that are sacred to Kānaka ‘Ōiwi, the Native Hawaiian people. Following the 1893 overthrow of the Hawaiian Monarchy, many of today’s conservation lands in Hawaii were ceded to the US Federal Government and then the State of Hawaii without consent or compensation to Kānaka ‘Ōiwi or their descendants. With this acknowledgement, we seek to raise awareness of and normalize conversations about this injustice, with the goal of fostering solutions.
Conflicts of Interest
The authors have no conflict of interest to report.
References
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A Framework for Community Interactions under Climate Change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, S.; Mouquet, N.; Thuiller, W.; Ronce, O. Biodiversity and Climate Change: Integrating Evolutionary and Ecological Responses of Species and Communities. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 321–350. [Google Scholar] [CrossRef] [Green Version]
- Woolbright, S.A.; Whitham, T.G.; Gehring, C.A.; Allan, G.J.; Bailey, J.K. Climate Relicts and Their Associated Communities as Natural Ecology and Evolution Laboratories. Trends Ecol. Evol. 2014, 29, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Paula, S.; Kaneakua-Pia, I.N.; Davis, S.D. Towards Understanding Resprouting at the Global Scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef] [Green Version]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a Life History Strategy in Woody Plant Communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Multi-Stemmed Trees in Montane Rain Forests: Their Frequency and Demography in Relation to Elevation, Soil Nutrients and Disturbance. J. Ecol. 2009, 97, 472–483. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a Key Functional Trait: How Buds, Protection and Resources Drive Persistence after Fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeley, J.; Bond, W.; Bradstock, R.; Pausas, J.G.; Rundel, P. Fire in Mediterranean Ecosystems; Ecology, Evolution and Management. In Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: Cambridge, UK, 2011; pp. 1–515. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Harrison, S.P.; Adams, H.D.; Kelley, D.I.; Li, G.; Tissue, D.T.; Dawson, T.E.; Fensham, R.; Medlyn, B.E.; Palmer, A.; et al. Drought and Resprouting Plants. New Phytol. 2015, 206, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Keeley, J.E. Evolutionary Ecology of Resprouting and Seeding in Fire-prone Ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- Bond, W.J.; Wilgen, B.W. Fire and Plants; Springer: New York, NY, USA, 2012; ISBN 978-94-009-1499-5. [Google Scholar]
- Kennard, D.K.; Gould, K.; Putz, F.E.; Fredericksen, T.S.; Morales, F. Effect of Disturbance Intensity on Regeneration Mechanisms in a Tropical Dry Forest. For. Ecol. Manag. 2002, 162, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Van Bloem, S.J.; Murphy, P.G.; Lugo, A.E.; Ostertag, R.; Costa, M.R.; Bernard, I.R.; Colón, S.M.; Mora, M.C. The Influence of Hurricane Winds on Caribbean Dry Forest Structure and Nutrient Pools1. Biotropica 2005, 37, 571–583. [Google Scholar] [CrossRef]
- Fornara, D.A.; Du Toit, J.T. Responses of Woody Saplings Exposed to Chronic Mammalian Herbivory in an African Savanna. Écoscience 2008, 15, 129–135. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. Ecology of Sprouting in Woody Plants: The Persistence Niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Hernández, E.I.; Luis, V.C.; Cochard, H.; Pausas, J.G. Physiological Differences Explain the Co-Existence of Different Regeneration Strategies in Mediterranean Ecosystems. New Phytol. 2014, 201, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Knox, K.J.E.; Clarke, P.J. Nutrient Availability Induces Contrasting Allocation and Starch Formation in Resprouting and Obligate Seeding Shrubs. Funct. Ecol. 2005, 19, 690–698. [Google Scholar] [CrossRef]
- Pratt, R.B.; Jacobsen, A.L.; Hernandez, J.; Ewers, F.W.; North, G.B.; Davis, S.D. Allocation Tradeoffs among Chaparral Shrub Seedlings with Different Life History Types (Rhamnaceae). Am. J. Bot. 2012, 99, 1464–1476. [Google Scholar] [CrossRef] [Green Version]
- West, A.G.; Dawson, T.E.; February, E.C.; Midgley, G.F.; Bond, W.J.; Aston, T.L. Diverse Functional Responses to Drought in a Mediterranean-Type Shrubland in South Africa. New Phytol. 2012, 195, 396–407. [Google Scholar] [CrossRef]
- Wilson, E.O.; Peter, F.M. Tropical Dry Forests the Most Endangered Major Tropical Ecosystem; National Academies Press: Washington, DC, USA, 1988. [Google Scholar]
- Gillespie, T.W.; Lipkin, B.; Sullivan, L.; Benowitz, D.R.; Pau, S.; Keppel, G. The Rarest and Least Protected Forests in Biodiversity Hotspots. Biodivers. Conserv. 2012, 21, 3597–3611. [Google Scholar] [CrossRef]
- Blackie, R.; Baldauf, C.; Gautier, D.; Gumbo, D.; Kassa, H.; Parthasarathy, N.; Paumgarten, F.; Sola, P.; Pulla, S.; Waeber, P.; et al. Tropical Dry Forests: The State of Global Knowledge and Recommendations for Future Research; CIFOR: Bogor, Indonesia, 2014. [Google Scholar]
- Libby, R.; Sato, A.Y.; Alapai, L.; Brawner, W.P.; Carter, Y.Y.; Carter, K.A.; Tomich, K.; Ticktin, T. A Hawaiian Tropical Dry Forest Regenerates: Natural Regeneration of Endangered Species under Biocultural Restoration. Sustainability 2022, 14, 1159. [Google Scholar] [CrossRef]
- Reich, P.B.; Borchert, R. Water Stress and Tree Phenology in a Tropical Dry Forest in the Lowlands of Costa Rica. J. Ecol. 1984, 72, 61–74. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E. Ecology of Tropical Dry Forest. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Ostertag, R.; Inman-Narahari, F.; Cordell, S.; Giardina, C.P.; Sack, L. Forest Structure in Low-Diversity Tropical Forests: A Study of Hawaiian Wet and Dry Forests. PLoS ONE 2014, 9, e103268. [Google Scholar] [CrossRef] [Green Version]
- Sandquist, D.R.; Cordell, S. Functional Diversity of Carbon-Gain, Water-Use, and Leaf-Allocation Traits in Trees of a Threatened Lowland Dry Forest in Hawaii. Am. J. Bot. 2007, 94, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Busby, P.E.; Vitousek, P.; Dirzo, R. Prevalence of Tree Regeneration by Sprouting and Seeding Along a Rainfall Gradient in Hawai’i. Biotropica 2010, 42, 80–86. [Google Scholar] [CrossRef]
- Frazier, A.G.; Giambelluca, T.W. Spatial Trend Analysis of Hawaiian Rainfall from 1920 to 2012. Int. J. Climatol. 2017, 37, 2522–2531. [Google Scholar] [CrossRef]
- Mesa-Sierra, N.; de la Peña-Domene, M.; Campo, J.; Giardina, C.P. Restoring Mexican Tropical Dry Forests: A National Review. Sustainability 2022, 14, 3937. [Google Scholar] [CrossRef]
- Freifelder, R.R.; Vitousek, P.M.; D’Antonio, C.M. Microclimate Change and Effect on Fire Following Forest-Grass Conversion in Seasonally Dry Tropical Woodland1. Biotropica 1998, 30, 286–297. [Google Scholar] [CrossRef]
- Litton, C.M.; Sandquist, D.R.; Cordell, S. Effects of Non-Native Grass Invasion on Aboveground Carbon Pools and Tree Population Structure in a Tropical Dry Forest of Hawaii. For. Ecol. Manag. 2006, 231, 105–113. [Google Scholar] [CrossRef]
- Kellner, J.R.; Asner, G.P. Convergent Structural Responses of Tropical Forests to Diverse Disturbance Regimes. Ecol. Lett. 2009, 12, 887–897. [Google Scholar] [CrossRef]
- Mueller-Dombois, D. Rain Forest Establishment and Succession in the Hawaiian Islands. Landsc. Urban Plan. 2000, 51, 147–157. [Google Scholar] [CrossRef]
- Friday, J.B.; Scowcroft, P.G.; Ares, A. Responses of Native and Invasive Plant Species to Selective Logging in an Acacia Koa-Metrosideros Polymorpha Forest in Hawai’i. Appl. Veg. Sci. 2008, 11, 471–482. [Google Scholar] [CrossRef]
- Murphy, M.J.; Inman-Narahari, F.; Ostertag, R.; Litton, C.M. Invasive Feral Pigs Impact Native Tree Ferns and Woody Seedlings in Hawaiian Forest. Biol. Invasions 2014, 16, 63–71. [Google Scholar] [CrossRef]
- Réjou-Méchain, M.; Tanguy, A.; Piponiot, C.; Chave, J.; Hérault, B. Biomass: An r Package for Estimating above-Ground Biomass and Its Uncertainty in Tropical Forests. Methods Ecol. Evol. 2017, 8, 1163–1167. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Lian, J.; Shen, H.; Cao, H.; Lu, H.; Ye, W. Tree Aboveground Carbon Storage Correlates with Environmental Gradients and Functional Diversity in a Tropical Forest. Sci. Rep. 2016, 6, 25304. [Google Scholar] [CrossRef] [Green Version]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved Allometric Models to Estimate the Aboveground Biomass of Tropical Trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Gosnell, J.S.; Davis, S.L.; Ahumada, J.; Boundja, P.; Clark, D.B.; Mugerwa, B.; Jansen, P.A.; O’Brien, T.G.; Rovero, F.; et al. Carbon Storage in Tropical Forests Correlates with Taxonomic Diversity and Functional Dominance on a Global Scale. Glob. Ecol. Biogeogr. 2014, 23, 563–573. [Google Scholar] [CrossRef]
- Fiske, I.J.; Bruna, E.M.; Bolker, B.M. Effects of Sample Size on Estimates of Population Growth Rates Calculated with Matrix Models. PLoS ONE 2008, 3, e3080. [Google Scholar] [CrossRef]
- Medeiros, C.D.; Scoffoni, C.; John, G.P.; Bartlett, M.K.; Inman-Narahari, F.; Ostertag, R.; Cordell, S.; Giardina, C.; Sack, L. An Extensive Suite of Functional Traits Distinguishes Hawaiian Wet and Dry Forests and Enables Prediction of Species Vital Rates. Funct. Ecol. 2019, 33, 712–734. [Google Scholar] [CrossRef]
- Armbruster, W.; Pélabon, C.; Bolstad, G.; Hansen, T. Integrated Phenotypes: Understanding Trait Covariation in Plants and Animals. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 245. [Google Scholar] [CrossRef] [Green Version]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 April 2022).
- Frazier, A.G.; Giambelluca, T.W.; Diaz, H.F.; Needham, H.L. Comparison of Geostatistical Approaches to Spatially Interpolate Month-Year Rainfall for the Hawaiian Islands. Int. J. Climatol. 2016, 36, 1459–1470. [Google Scholar] [CrossRef] [Green Version]
- Gharun, M.; Turnbull, T.L.; Adams, M.A. Stand Water Use Status in Relation to Fire in a Mixed Species Eucalypt Forest. For. Ecol. Manag. 2013, 304, 162–170. [Google Scholar] [CrossRef]
- Heath, J.T.; Chafer, C.J.; Bishop, T.F.A.; Van Ogtrop, F.F. Post-Fire Recovery of Eucalypt-Dominated Vegetation Communities in the Sydney Basin, Australia. Fire Ecol. 2016, 12, 53–79. [Google Scholar] [CrossRef]
- Kelley, D.I.; Harrison, S.P.; Prentice, I.C. Improved Simulation of Fire–Vegetation Interactions in the Land Surface Processes and EXchanges Dynamic Global Vegetation Model (LPX-Mv1). Geosci. Model Dev. 2014, 7, 2411–2433. [Google Scholar] [CrossRef] [Green Version]
- Philipson, C.D.; Dent, D.H.; O’Brien, M.J.; Chamagne, J.; Dzulkifli, D.; Nilus, R.; Philips, S.; Reynolds, G.; Saner, P.; Hector, A. A Trait-Based Trade-off between Growth and Mortality: Evidence from 15 Tropical Tree Species Using Size-Specific Relative Growth Rates. Ecol. Evol. 2014, 4, 3675–3688. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S.; et al. Functional Traits and the Growth–Mortality Trade-off in Tropical Trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef]
- Nzunda, E.F.; Griffiths, M.E.; Lawes, M.J. Sprouting by Remobilization of Above-Ground Resources Ensures Persistence after Disturbance of Coastal Dune Forest Trees. Funct. Ecol. 2008, 22, 577–582. [Google Scholar] [CrossRef]
- Vieira, D.L.M.; Scariot, A. Principles of Natural Regeneration of Tropical Dry Forests for Restoration. Restor. Ecol. 2006, 14, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Allen, K.; Dupuy, J.M.; Gei, M.G.; Hulshof, C.; Medvigy, D.; Pizano, C.; Salgado-Negret, B.; Smith, C.M.; Trierweiler, A.; Bloem, S.J.V.; et al. Will Seasonally Dry Tropical Forests Be Sensitive or Resistant to Future Changes in Rainfall Regimes? Environ. Res. Lett. 2017, 12, 023001. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).