A Pantropical Overview of Soils across Tropical Dry Forest Ecoregions
Abstract
1. Introduction
2. Materials and Methods
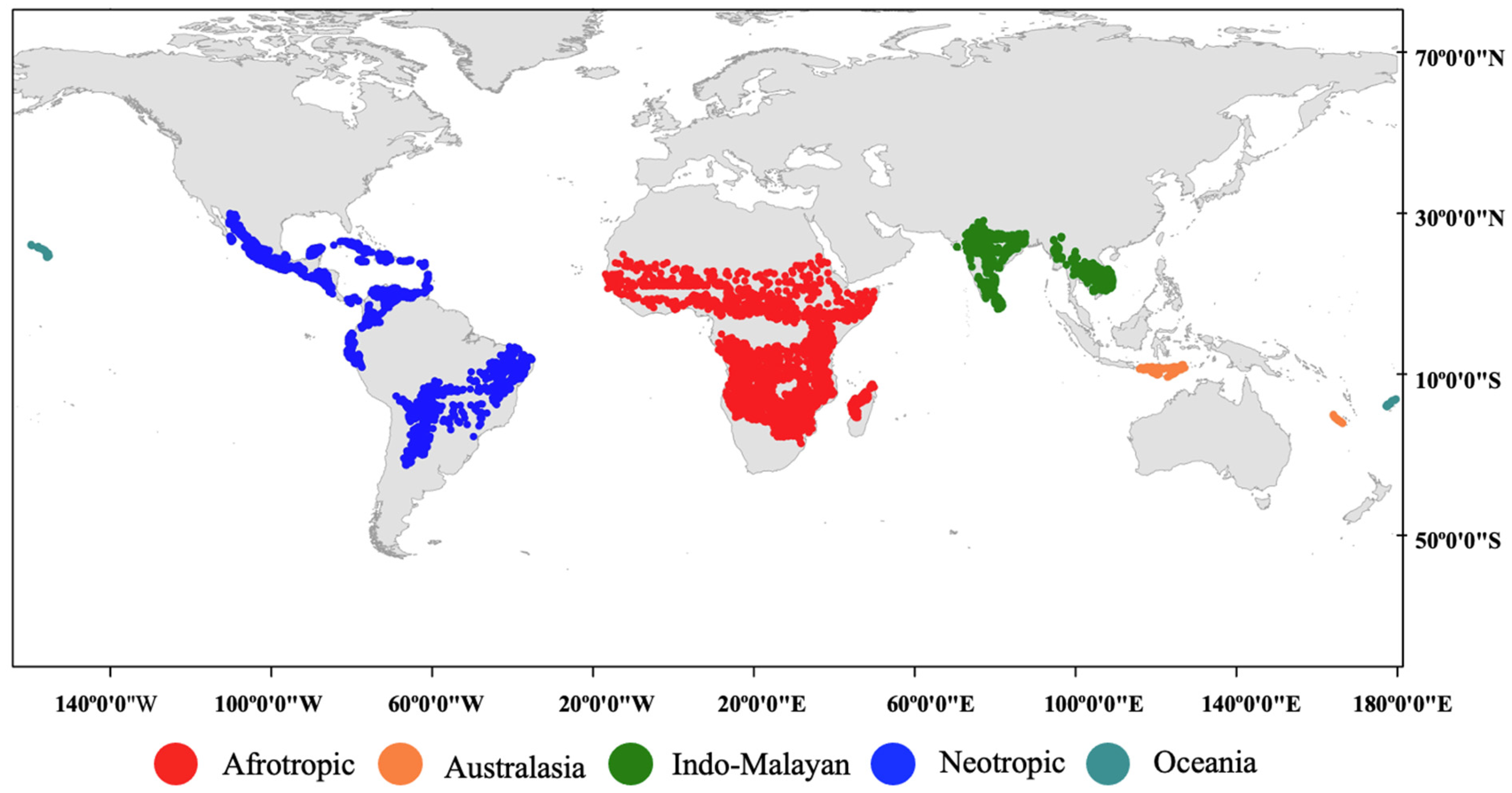
2.1. Selection of Ecoregions
2.2. Selection of Sample Points
2.3. Climatic and Edaphic Metrics
2.4. Data Analysis
3. Results
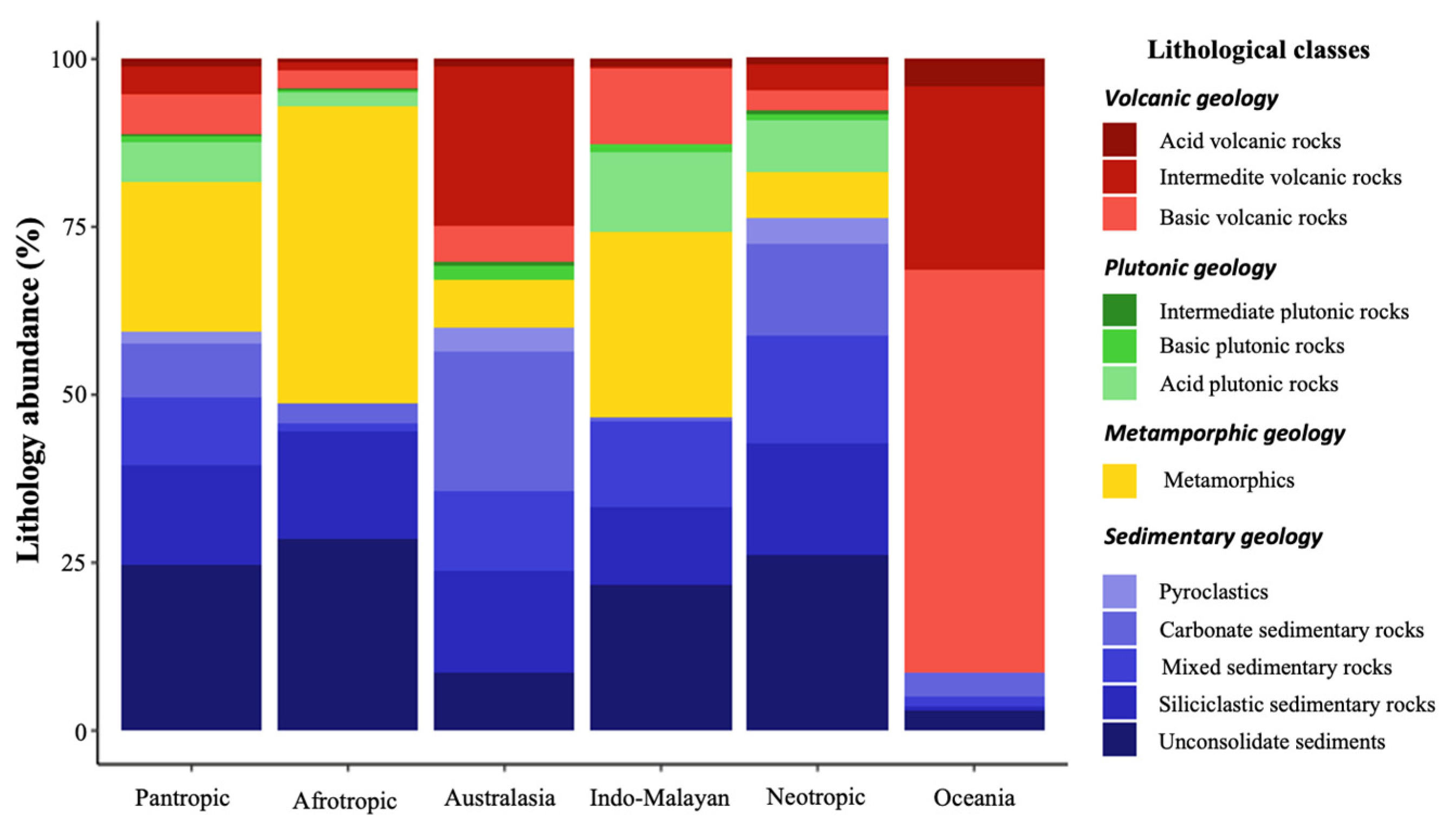
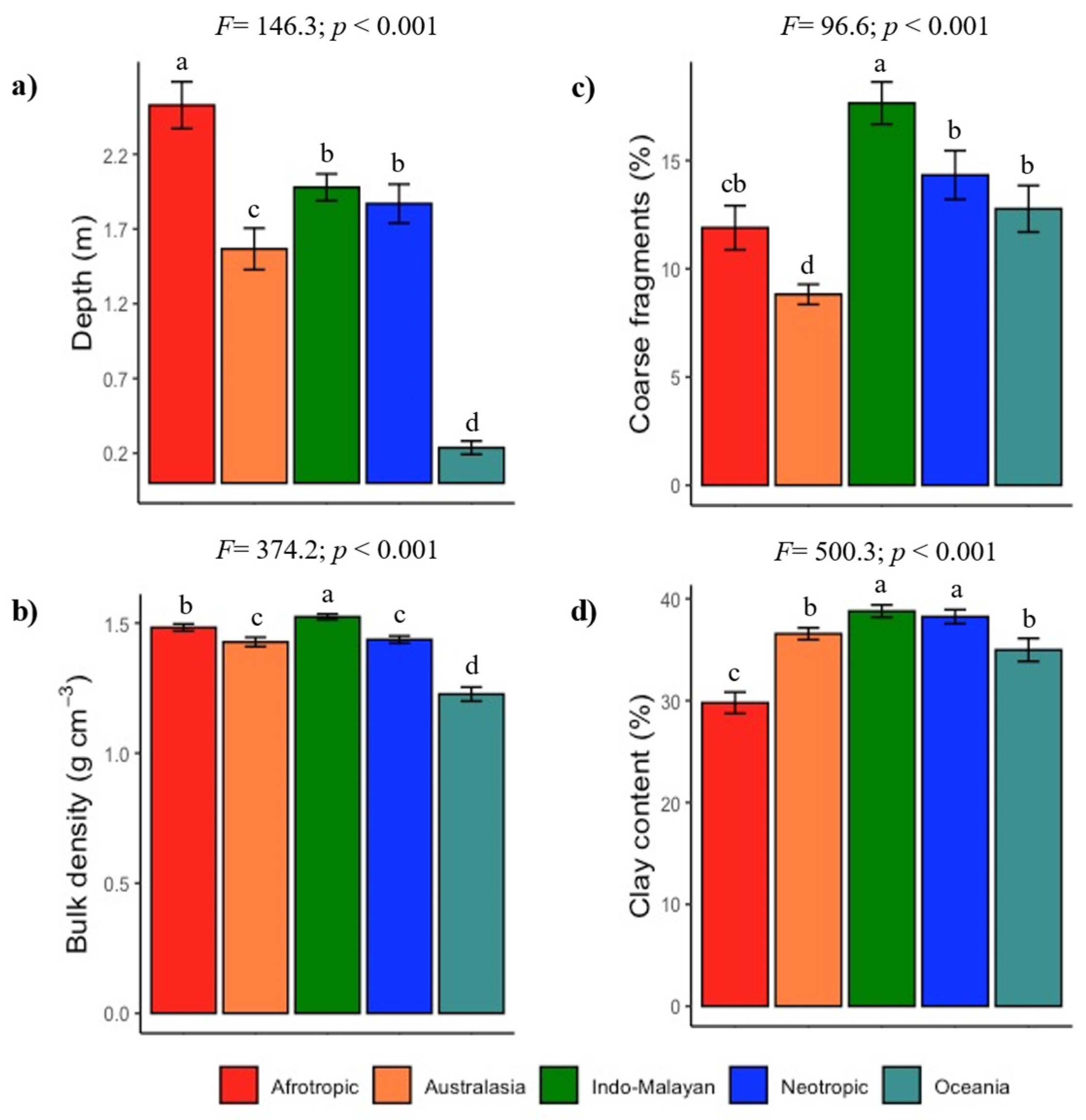
3.1. Global Lithological Classes and Soils
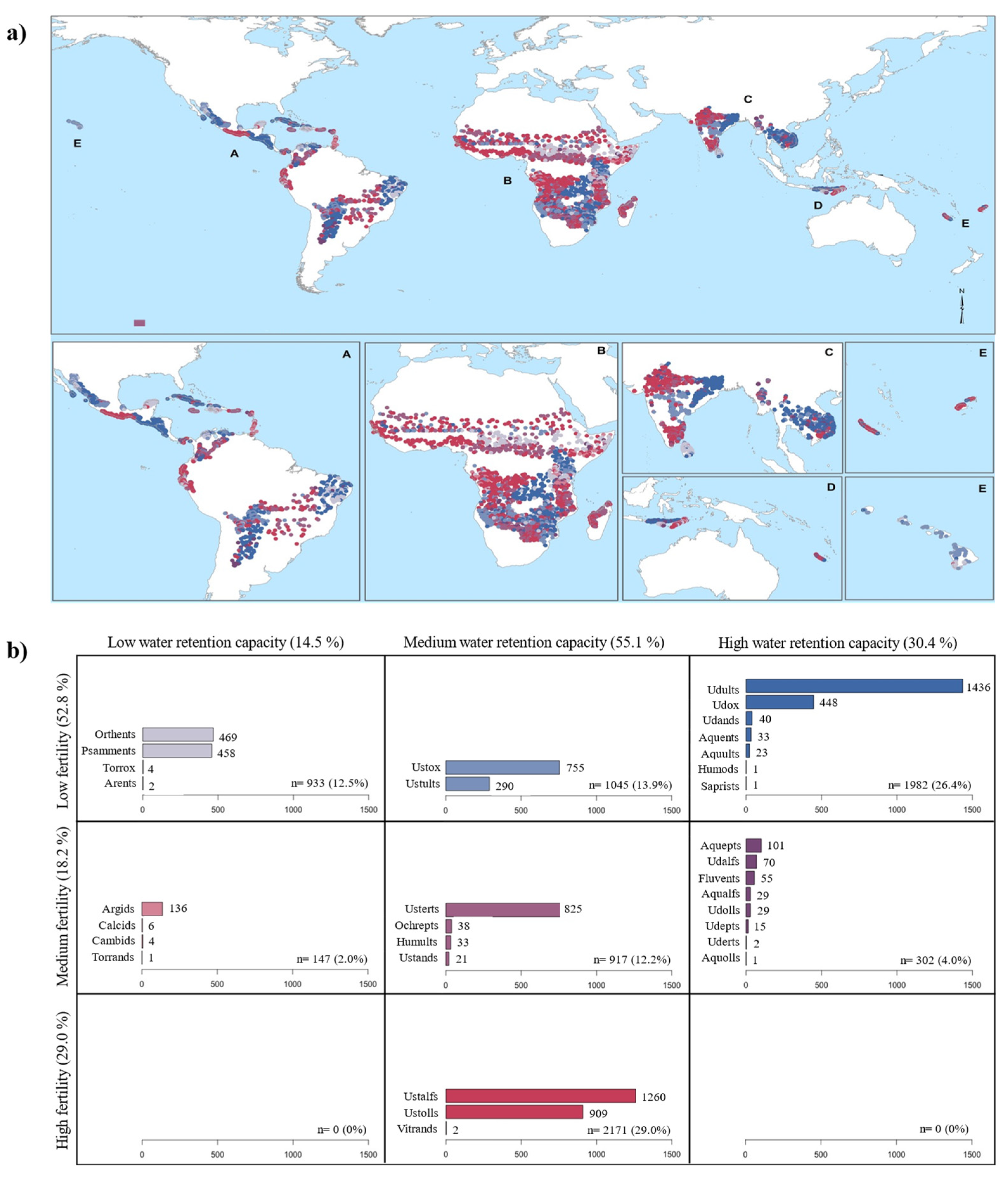
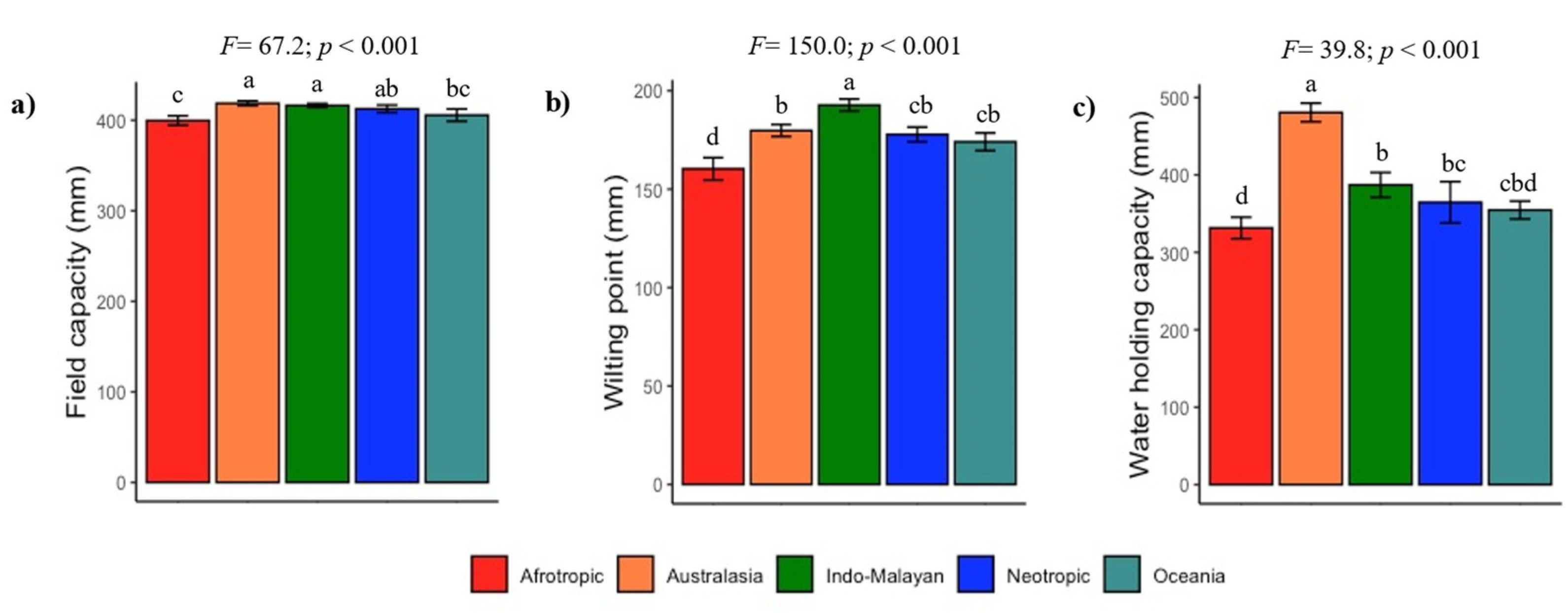
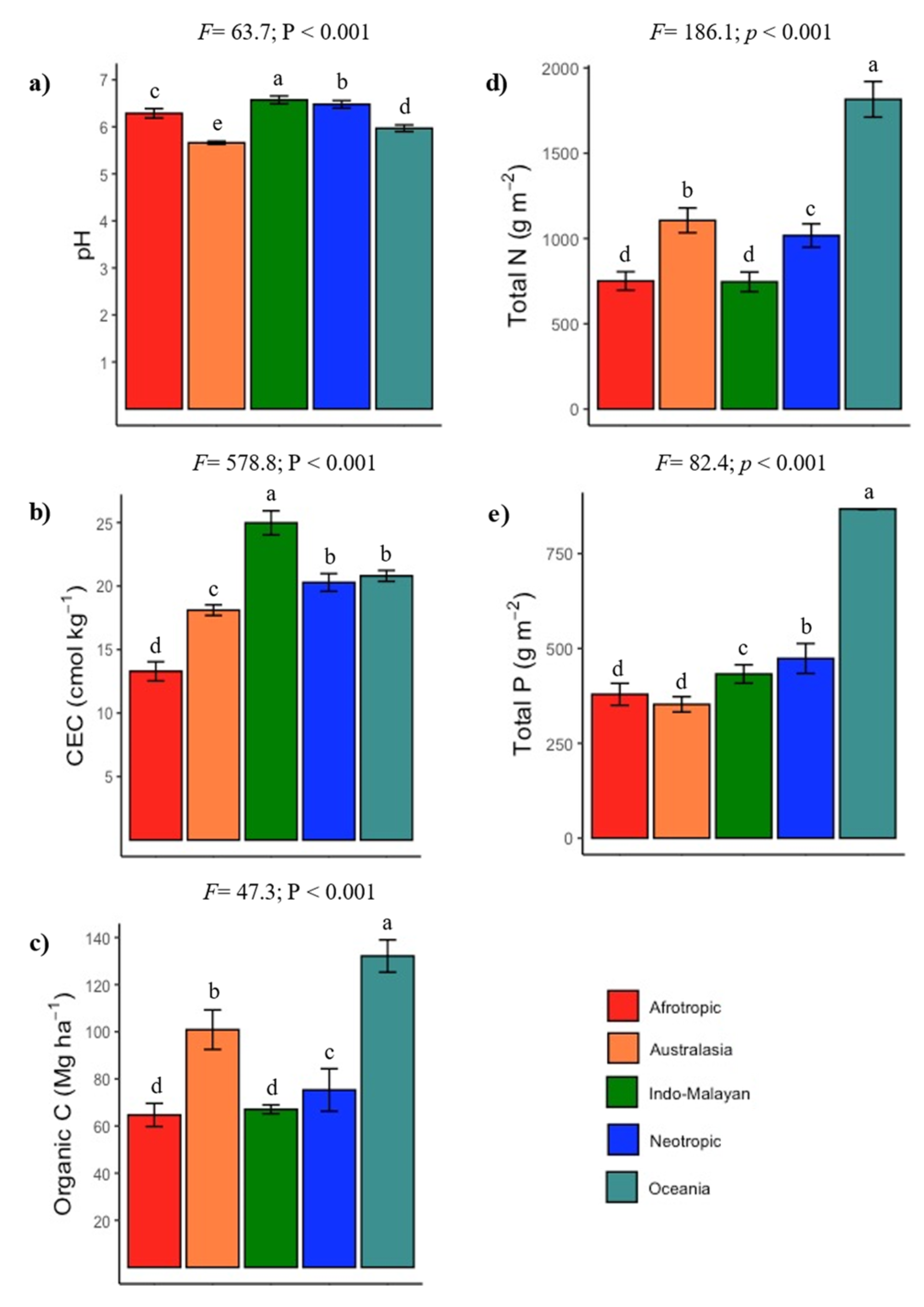
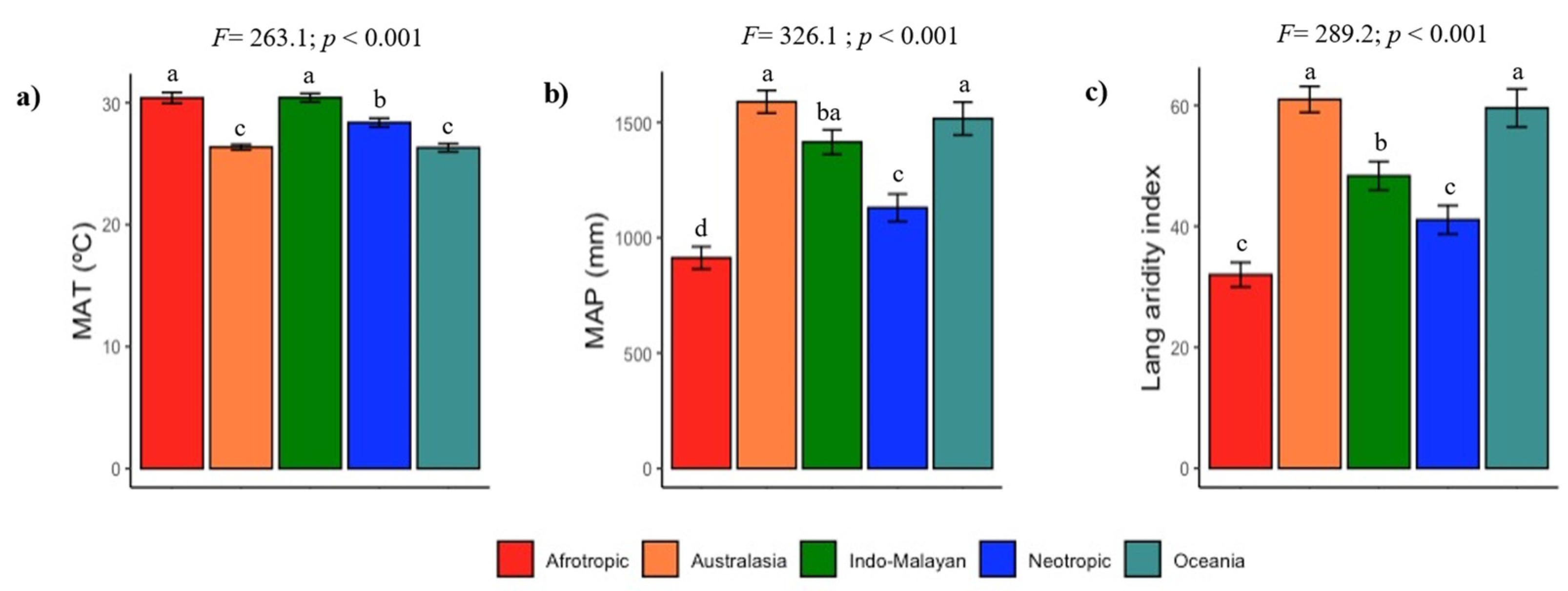
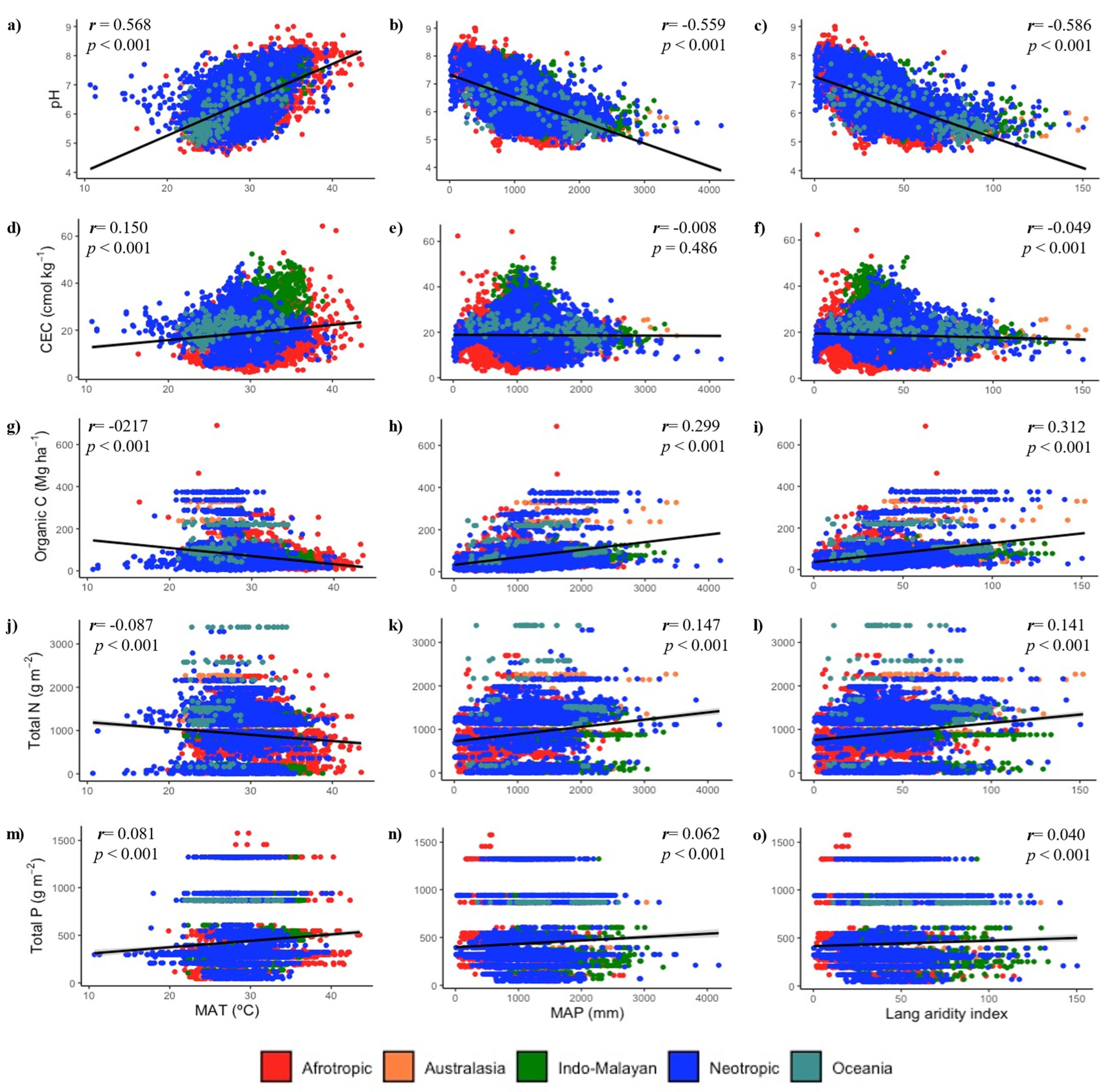
3.2. Climate and Soil Fertility Relationships
4. Discussion
4.1. Soil Diversity in the Tropical Dry Forest Biome
4.2. Are Tropical Dry Forest Soils Fertile?
4.3. Vulnerability of Tropical Dry Forest Soils to Disturbances
4.4. Vulnerability of Soil C, N, and P to Ongoing Climate Change: Some Clues Derived from the Relationship between Soil Fertility and Climate in TDF Ecoregions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEC | cation exchange capacity |
| MAP | mean annual precipitation |
| MAT | mean annual temperature |
| PC | principal component |
| PCA | principal component analysis |
| TDF | tropical dry forest |
References
- Richter, D.D.; Babbar, L.I. Soil diversity in the tropics. Adv. Ecol. Res. 1991, 21, 315–389. [Google Scholar] [CrossRef]
- Buol, S.W.; Southard, R.J.; Graham, R.C.; McDaniel, P.A. Soil Genesis and Classification, 6th ed.; Wiley-Blackwell: Chichester, UK, 2011; ISBN 978-0-8138-0769-0. [Google Scholar]
- Singh, J.S.; Chaturvedi, R.K. Tropical Dry Deciduous Forest: Research Trends and Emerging Features; Springer: New York, NY, USA, 2017; ISBN 978-981-10-7259-8. [Google Scholar]
- Delgado-Baquerizo, M.; Reich, P.B.; Bardgett, R.D.; Eldridge, D.J.; Lambers, H.; Wardle, D.A.; Reed, S.C.; Plaza, C.; Png, G.K.; Neuhauser, S.; et al. The influence of soil age on ecosystem structure and function across biomes. Nat. Commun. 2020, 11, 4721. [Google Scholar] [CrossRef] [PubMed]
- Pennington, R.T.; Lewis, G.P.; Ratter, J. Neotropical Savannas and Seasonally Dry Forests; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780849329876. [Google Scholar]
- Binkley, D.; Fisher, R.F. Ecology and Management of Forest Soils, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; ISBN 9781118422342. [Google Scholar]
- Murphy, P.G.; Lugo, A.E. Ecology of tropical dry forest. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Dexter, K.G.; Pennington, R.T.; Oliveira-Filho, A.T.; Bueno, M.L.; Silva de Miranda, P.L.; Neves, D.M. Inserting tropical dry forests into the discussion on biome transitions in the tropics. Front. Ecol. Evol. 2018, 6, 104. [Google Scholar] [CrossRef]
- Waring, B.G.; De Guzman, M.E.; Du, D.V.; Dupuy, J.M.; Gei, M.; Gutknecht, J.; Hulshof, C.; Jelinski, N.; Margenot, A.J.; Medvigy, D.; et al. Soil biogeochemistry across Central and South American tropical dry forests. Ecol. Monogr. 2021, 92, e01453. [Google Scholar] [CrossRef]
- Townsend, A.R.; Asner, G.P.; Cleveland, C.C. The biogeochemical heterogeneity of tropical forests. Trends Ecol. Evol. 2008, 23, 424–431. [Google Scholar] [CrossRef]
- Hofhansl, F.; Chacón-Madrigal, E.; Fuschslueger, L.; Jenking, D.; Morera-Beita, A.; Plutzar, C.; Silla, F.; Andersen, K.M.; Buchs, D.M.; Dullinger, S.; et al. Climatic and edaphic controls over tropical forest diversity and vegetation carbon storage. Sci. Rep. 2020, 10, 5066. [Google Scholar] [CrossRef]
- Campo-Alves, J. Nutrient availability and fluxes along a toposequence with tropical dry forest. Agrociencia 2003, 37, 211–219. [Google Scholar]
- Waring, B.G.; Adams, R.; Branco, S.; Powers, J.S. Scale-dependent variation in soil nitrogen cycling and soil fungal communities along gradients of forest composition and age in regenerating tropical dry forests. New Phytol. 2016, 209, 845–854. [Google Scholar] [CrossRef]
- Pajares, S.; Campo, J.; Bohannan, B.J.M.; Etchevers, J.D. Environmental controls on the soil microbial communities in a seasonally dry tropical forest. Appl. Environ. Microbiol. 2018, 84, e00342-18. [Google Scholar] [CrossRef]
- Rivero-Villar, A.; Ruíz-Suárez, G.; Templer, P.H.; Souza, V.; Campo, J. Nitrogen cycling in tropical dry forests is sensitive to changes in rainfall regimen and nitrogen deposition. Biogeochemistry 2021, 153, 283–302. [Google Scholar] [CrossRef]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Corona-Núñez, R.O.; Campo, J.; Williams, M. Aboveground carbon storage in tropical dry forest plots in Oaxaca, Mexico. Forest Ecol. Manag. 2018, 409, 202–214. [Google Scholar] [CrossRef]
- Maass, J.M. Conversion of tropical dry forest to pasture and agriculture. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, MA, USA, 1995; pp. 399–422. ISBN 978-0-52143-514-7. [Google Scholar]
- García-Oliva, F.; Jaramillo, V.J. Impact of anthropogenic transformation of seasonally dry tropical forests on ecosystem biogeochemical processes. In Seasonally Dry Tropical Forests: Ecology and Conservation; Dirzo, R., Young, H.S., Mooney, H.A., Ceballos, G., Eds.; Island Press: Washington, DC, USA, 2011; pp. 159–172. ISBN 978-1-59726-704-5. [Google Scholar]
- Figueroa, D.; Ortega-Fernández, P.; Abbruzzini, T.F.; Rivero-Villar, A.; Galindo, F.; Chávez-Vergara, B.; Etchevers, J.; Campo, J. Effects of land use change from native forest to livestock on soil C, N and P dynamics along a rainfall gradient in Mexico. Sustainability 2020, 12, 8656. [Google Scholar] [CrossRef]
- Pérez-Lombardini, F.; Mancera, K.F.; Suzán, G.; Campo, J.; Solorio, J.; Galindo, F. Assessing sustainability in cattle silvopastoral systems in the Mexican tropics using the SAFA framework. Animals 2021, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Baccini, A.; Walker, W.; Carvalho, L.; Farina, M.; Houghton, R.A. Response to Comment on “Tropical forests are a net carbon source based on aboveground measurements of gain and loss”. Science 2019, 363, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Siyum, Z.G. Tropical dry forest dynamics in the context of climate change: Syntheses of drivers, gaps, and management perspectives. Ecol. Process. 2020, 9, 25. [Google Scholar] [CrossRef]
- Baer, S.G. Nutrient dynamics as determinants and outcomes of restoration. In Foundations of Restoration Ecology, 2nd ed.; Palmer, M.A., Zedler, J.B., Falk, D.A., Eds.; Island Press: Washington, DC, USA, 2016; pp. 333–364. ISBN 978-1-61091-697-4. [Google Scholar]
- Larkin, D.J.; Bruland, G.L.; Zedler, J.B. Heterogeneity theory and ecological restoration. In Foundations of Restoration Ecology, 2nd ed.; Palmer, M.A., Zedler, J.B., Falk, D.A., Eds.; Island Press: Washington, DC, USA, 2016; pp. 271–300. ISBN 978-1-61091-697-4. [Google Scholar]
- Campo, J.; Vázquez-Yanes, C. Effects of nutrient limitation on aboveground carbon dynamics during tropical dry forest regeneration in Yucatán, Mexico. Ecosystems 2004, 7, 311–319. [Google Scholar] [CrossRef]
- Gamboa, A.M.; Hidalgo, C.; de Leon, F.; Etchevers, J.D.; Gallardo, J.F.; Campo, J. Nutrient addition differentially affects soil carbon sequestration in secondary tropical dry forests: Early- vs. late-succession stages. Restor. Ecol. 2010, 18, 252–260. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Chen, Y., Goldfarb, L., Gomis, M.I., Matthews, J.B.R., Berger, S., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Hirota, M.; Holmgren, M.; Van Nes, E.H.; Scheffer, M. Global resilience of tropical forest and savanna to critical transitions. Science 2011, 334, 232–235. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M.J.S. What controls the distribution of tropical forest and savanna? Ecol. Lett. 2012, 15, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Dinerstein, E. The Global 200: Priority ecoregions for global conservation. Ann. Missouri. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Powers, J.S.; Marín-Spiotta, E. Ecosystem processes and biogeochemical cycles in secondary tropical forest succession. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 497–519. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. Global Ecological Zones for FAO Forest Reporting: 2010 Update; FAO: Rome, Italy, 2012.
- NASA Earth Observations. Available online: https://neo.sci.gsfc.nasa.gov/ (accessed on 23 April 2021).
- Climatic Hazards Center-UC Santa. Available online: https://www.chc.ucsb.edu/about (accessed on 7 October 2021).
- Numerical Terradynamic Simulation Group (NTSG)-University of Montana. Available online: https://www.ntsg.umt.edu/ (accessed on 14 October 2021).
- Oak Ridge National Laboratory Distributed Active Archive Center (ORNL DAAC), Composition of the Geological Map of the World. Available online: https://ccgm.org/en/home/168-lithological-map-of-the-world-9782917310250.html (accessed on 23 September 2021).
- Biogeochemical Dynamics NASA. Available online: https://daac.ornl.gov/ (accessed on 23 September 2021).
- SoilGrids. Available online: https://soilgrids.org/ (accessed on 23 April 2021).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Afifi, A.; May, S.; Donatello, R.A.; Clark, V.A. Practical Multivariate Analysis, 6th ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1138702226. [Google Scholar]
- Muller-Landau, H.C.; Cushman, K.C.; Arroyo, E.E.; Martinez Cano, I.; Anderson-Teixeira, K.J.; Backiel, B. Patterns and mechanisms of spatial variation in tropical forest productivity, woody residence time, and biomass. New Phytol. 2021, 229, 3065–3087. [Google Scholar] [CrossRef]
- Hartmann, J.; Moosdorf, N. The new global lithological map database GLiM: A representation of rock properties at the Earth surface. Geochem. Geophys. Geosys. 2012, 13, Q12004. [Google Scholar] [CrossRef]
- Medvigy, D.; Wang, G.; Zhu, Q.; Riley, W.J.; Trierweiler, A.M.; Waring, B.G.; Xu, X.; Powers, J.S. Observed variation in soil properties can drive large variation in modelled forest functioning and composition during tropical forest secondary succession. New Phytol. 2019, 223, 1820–1833. [Google Scholar] [CrossRef]
- Porder, S.; Vitousek, P.M.; Chadwick, O.A.; Chamberlain, C.P.; Hilley, G.E. Uplift, erosion, and phosphorus limitation in terrestrial ecosystems. Ecosystems 2007, 10, 159–171. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 4th ed.; Academic Press: London, UK, 2020; ISBN 978-0-12-814608-8. [Google Scholar]
- Hou, E.; Wen, D.; Jiang, L.; Luo, X.; Kuang, Y.; Lu, X.; Chen, C.; Allen, K.T.; He, X.; Huang, X.; et al. Latitudinal patterns of terrestrial phosphorus limitation over the globe. Ecol. Lett. 2021, 24, 1420–1431. [Google Scholar] [CrossRef]
- Gei, M.G.; Powers, J.S. Nutrient cycling in tropical dry forests. In Tropical Dry Forests in the Americas: Ecology, Conservation and Management; Sánchez-Azofeifa, G.A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 141–156. ISBN 978-1-4665-1200-9. [Google Scholar]
- Chaturvedi, R.K.; Raghubanshi, A.S. Soil water availability influences major ecosystem processes in tropical dry forest. Int. J. Hydrol. 2018, 2, 14–15. [Google Scholar] [CrossRef][Green Version]
- Vitousek, P.M.; Sanford, R.L. Nutrient cycling in moist tropical forest. Ann. Rev. Ecol. Syst. 1986, 17, 137–167. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Mooney, H.A.; Ceballos, G. Seasonally Dry Tropical Forests Ecology and Conservation; Island Press: Washington, DC, USA, 2011; ISBN 9781597267038. [Google Scholar]
- Hasnat, G.N.T.; Hossain, M.K. Global overview of tropical dry forests. In Handbook of Research on the Conservation and Restoration of Tropical Dry Forests; Bhadouria, R., Srivastava, P., Singh, P., Eds.; ISI Global Publisher: New Delhi, India, 2020; pp. 1–23. ISBN 9781799800149. [Google Scholar]
- Austin, A.T.; Vitousek, P.M. Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 1998, 113, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Van Bloem, S.J.; Lugo, A.E.; Murphy, P.G. Regional forest types—Tropical Dry Forests. In Encyclopedia of Forest Science; Elsevier: San Diego, CA, USA, 2004; pp. 1–10. [Google Scholar]
- Pennington, R.T.; Lavin, M.; Oliveira-Filho, A. Woody plant diversity, evolution, and ecology in the tropics: Perspectives from seasonally dry tropical forests. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 437–457. [Google Scholar] [CrossRef]
- Banda-R, K.; Delgado-Salinas, A.; Dexter, K.G.; Linares-Palomino, R.; Oliveira-Filho, A.; Prado, D.; Pullan, M.; Quintana, C.; Riina, R.; Rodriguez, G.M.; et al. Plant diversity patterns in neotropical dry forests and their conservation implications. Science 2016, 353, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Bullock, S.H.; Mooney, H.A.; Medina, E. Seasonally Dry Tropical Forests; Cambridge University Press: Cambridge, MA, USA, 1995; ISBN 978-0-52143-514-7. [Google Scholar]
- Sánchez-Azofeifa, A.; Powers, J.S.; Fernandes, G.W.; Quesada, M. (Eds.) Tropical Dry Forests in the Americas: Ecology, Conservation, and Management; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4665-1200-9. [Google Scholar]
- Schulte, A.; Ruhiyat, D. Soils of Tropical Forest Ecosystems: Characteristics, Ecology and Management; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9783540637072. [Google Scholar]
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Chadwick, O.A.; Derry, L.A.; Vitousek, P.M.; Huebert, B.J.; Hedin, L.O. Changing sources of nutrients during 4 million years of soil and ecosystem development. Nature 1999, 397, 491–497. [Google Scholar] [CrossRef]
- Campo, J.; Maass, M.; de Pablo, L. Weathering in a tropical dry forest of Mexico. Agrociencia 2001, 35, 245–254. [Google Scholar]
- Campo, J.; Maass, J.M.; Jaramillo, V.J.; Martínez-Yrízar, A. Calcium, potassium, and magnesium cycling in a Mexican tropical dry forest ecosystem. Biogeochemistry 2000, 49, 21–36. [Google Scholar] [CrossRef]
- Campo, J.; Maass, M.; Jaramillo, V.J.; Martínez-Yrízar, A.; Sarukhán, J. Phosphorus cycling in a Mexican tropical dry forest ecosystem. Biogeochemistry 2001, 53, 161–179. [Google Scholar] [CrossRef]
- Mayes, M.; Mustard, J.; Melillo, J.; Neill, C.; Nyadzi, G. Going beyond the green: Senesced vegetation material predicts basal area and biomass in remote sensing of tree cover conditions in an African tropical dry forest (miombo woodland) landscape. Environ. Res. Lett. 2017, 12, 085004. [Google Scholar] [CrossRef]
- Chesworth, W. The parent rock effect in the genesis of soil. Geoderma 1973, 10, 215–225. [Google Scholar] [CrossRef]
- Bationo, A.; Lompo, F.; Koala, S. Research on nutrient flows and balances in West Africa: State-of-the-art. Agric. Ecosyst. Environ. 1998, 71, 19–35. [Google Scholar] [CrossRef]
- Birkeland, P.W. Soils and Geomorphology, 3rd ed.; Oxford University Press: New York, NY, USA, 1999; ISBN 9780195078862. [Google Scholar]
- Rasmussen, C.; Heckman, K.; Wieder, W.R.; Keiluweit, M.; Lawrence, C.R.; Berhe, A.A.; Blankinship, J.C.; Crow, S.E.; Druhan, J.L.; Pries, C.E.H.; et al. Beyond clay: Towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 2018, 137, 297–306. [Google Scholar] [CrossRef]
- Lopezaraiza-Mikel, M.; Quesada, M.; Alvarez-Añorve, M.; Ávila-Cabadilla, L.; Martén-Rodríguez, S.; Calvo-Alvarado, J.; Espírito-Santo, M.M.; Fernandes, G.; Sánchez-Azofeifa, A.; de Jesús Aguilar, M. Phenological patterns of tropical dry forests along latitudinal and successional gradients in the Neotropics. In Tropical Dry Forests in the Americas: Ecology, Conservation, and Management; Sánchez-Azofeifa, A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 101–128. ISBN 978-1-4665-1200-9. [Google Scholar]
- Allen, K.; Dupuy, J.M.; Gei, M.G.; Hulshof, C.; Medvigy, D.; Pizano, C.; Salgado-Negret, B.; Smith, C.M.; Trierweiler, A.; Van Bloem, S.J.; et al. Will seasonally dry tropical forests be sensitive or resistant to future changes in rainfall regimes? Environ. Res. Lett. 2017, 12, 023001. [Google Scholar] [CrossRef]
- Brus, J.; Pechanec, V.; Machar, I. Depiction of uncertainty in the visually interpreted land cover data. Ecol. Inf. 2018, 47, 10–13. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. The State of the World’s Forest 2020: Forests, Biodiversity and People; FAO: Rome, Italy, 2020; ISBN 978-92-5-132419-6.
- Verduzco, V.S.; Garatuza-Payán, J.; Yépez, E.A.; Watts, C.J.; Rodríguez, J.C.; Robles-Morua, A.; Vivoni, E.R. Variations of net ecosystem production due to seasonal precipitation differences in a tropical dry forest of northwest Mexico. J. Geophys. Res. Biogeosci. 2015, 120, 2081–2094. [Google Scholar] [CrossRef]
- Mendes, K.R.; Campos, S.; da Silva, L.L.; Mutti, P.R.; Ferreira, R.R.; Medeiros, S.S.; Perez-Marin, A.M.; Marques, T.V.; Ramos, T.M.; Vieira, M.M.D.L.; et al. Seasonal variation in net ecosystem CO2 exchange of a Brazilian seasonally dry tropical forest. Sci. Rep. 2020, 10, 9454. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Durán, S.M.; Hu, K.-T.; Li, H.-J.; Swenson, N.G.; Enquist, B.J. Remotely sensed assessment of increasing chronic and episodic drought effects on a Costa Rican tropical dry forest. Ecosphere 2021, 12, e03824. [Google Scholar] [CrossRef]
- Lyu, M.; Giardina, C.P.; Litton, C.M. Interannual variation in rainfall modulates temperature sensitivity of carbon allocation and flux in a tropical montane wet forest. Glob. Chang. Biol. 2021, 27, 3824–3836. [Google Scholar] [CrossRef]
- Corona-Núñez, R.O.; Campo, J. Climate and socioeconomic drivers of biomass burning and carbon emissions from fires in tropical dry forests: A Pantropical analysis. Glob. Chang. Biol. 2022; in review. [Google Scholar]
- Becknell, J.M.; Vargas, G.G.; Pérez-Aviles, D.; Medvigy, D.; Powers, J.S. Above-ground net primary productivity in regenerating seasonally dry tropical forest: Contributions of rainfall, forest age and soil. J. Ecol. 2021, 109, 3903–3915. [Google Scholar] [CrossRef]
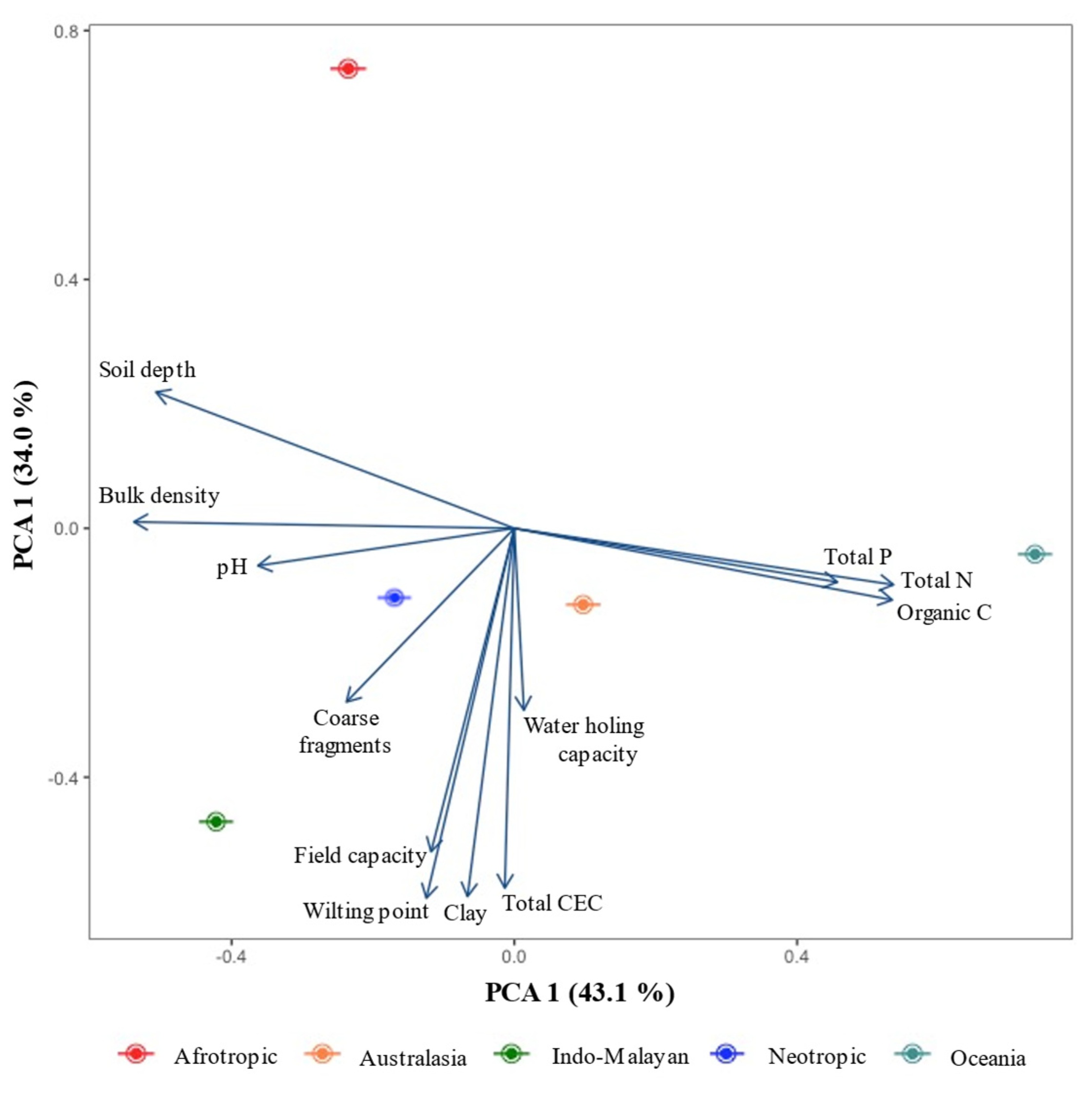
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalue | 5.168 | 4.307 | 2.169 |
| Cumulative variation percent | 43.06 | 76.99 | 98.69 |
| Eigenvectors | |||
| Soil depth | −0.925 | 0.355 | 0.128 |
| Bulk density | −0.982 | 0.017 | 0.162 |
| Coarse fragments | −0.434 | −0.452 | −0.772 |
| Clay content | −0.121 | −0.958 | 0.019 |
| Field capacity | −0.215 | −0.842 | 0.487 |
| Water holding capacity | 0.025 | −0.473 | 0.877 |
| Wilting point | −0.227 | −0.963 | 0.011 |
| pH | −0.662 | −0.097 | −0.732 |
| Cation exchange capacity | −0.025 | −0.936 | −0.334 |
| Organic carbon | 0.976 | −0.187 | −0.101 |
| Total nitrogen | 0.979 | −0.147 | −0.119 |
| Total phosphorus | 0.834 | −0.14 | −0.533 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Villar, A.; de la Peña-Domene, M.; Rodríguez-Tapia, G.; Giardina, C.P.; Campo, J. A Pantropical Overview of Soils across Tropical Dry Forest Ecoregions. Sustainability 2022, 14, 6803. https://doi.org/10.3390/su14116803
Rivero-Villar A, de la Peña-Domene M, Rodríguez-Tapia G, Giardina CP, Campo J. A Pantropical Overview of Soils across Tropical Dry Forest Ecoregions. Sustainability. 2022; 14(11):6803. https://doi.org/10.3390/su14116803
Chicago/Turabian StyleRivero-Villar, Anaitzi, Marinés de la Peña-Domene, Gerardo Rodríguez-Tapia, Christian P. Giardina, and Julio Campo. 2022. "A Pantropical Overview of Soils across Tropical Dry Forest Ecoregions" Sustainability 14, no. 11: 6803. https://doi.org/10.3390/su14116803
APA StyleRivero-Villar, A., de la Peña-Domene, M., Rodríguez-Tapia, G., Giardina, C. P., & Campo, J. (2022). A Pantropical Overview of Soils across Tropical Dry Forest Ecoregions. Sustainability, 14(11), 6803. https://doi.org/10.3390/su14116803






