Impact of Granular Activated Carbon on Anaerobic Process and Microbial Community Structure during Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analytical Methods
2.3. Microbial Community Analysis
3. Results and Discussion
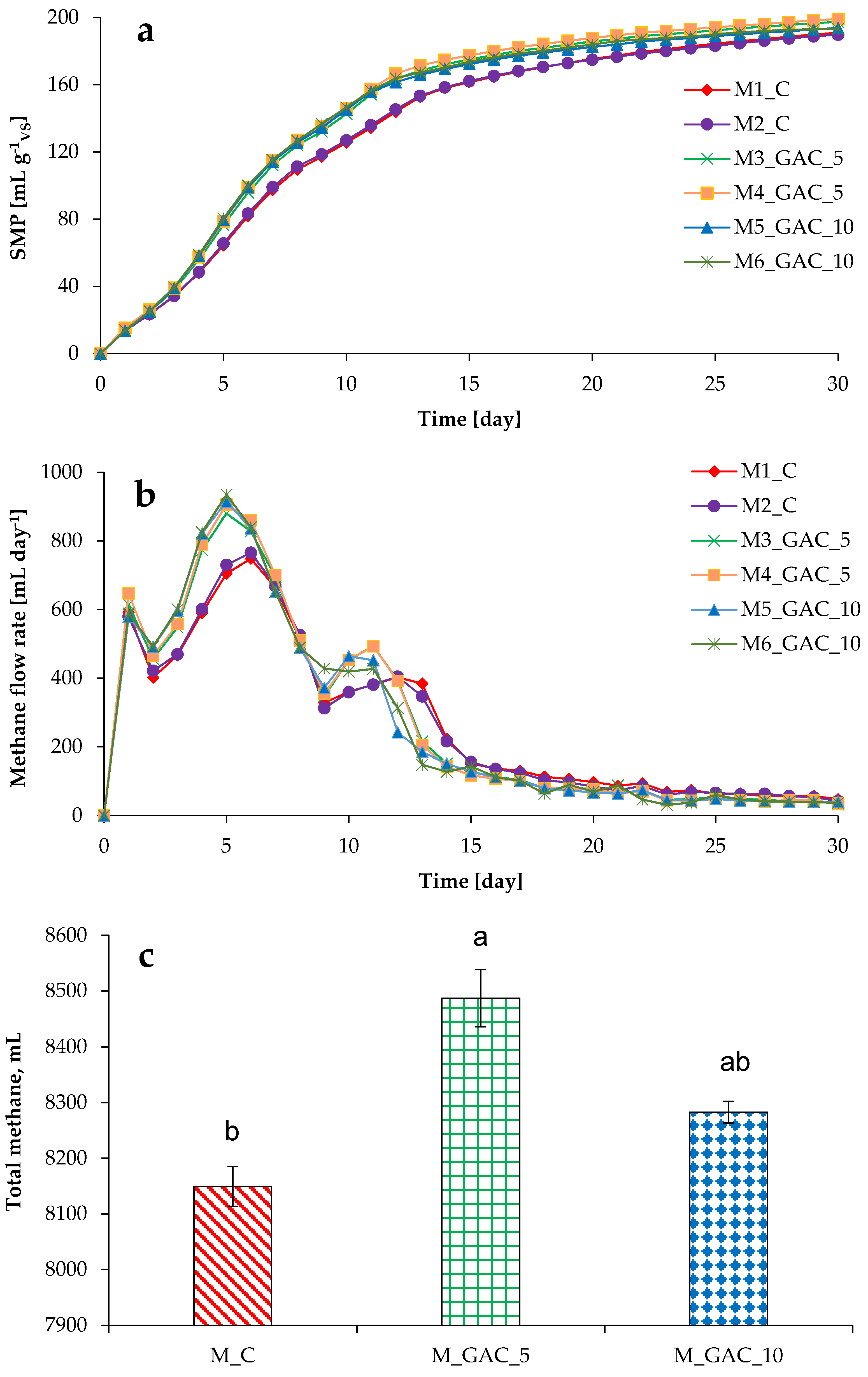
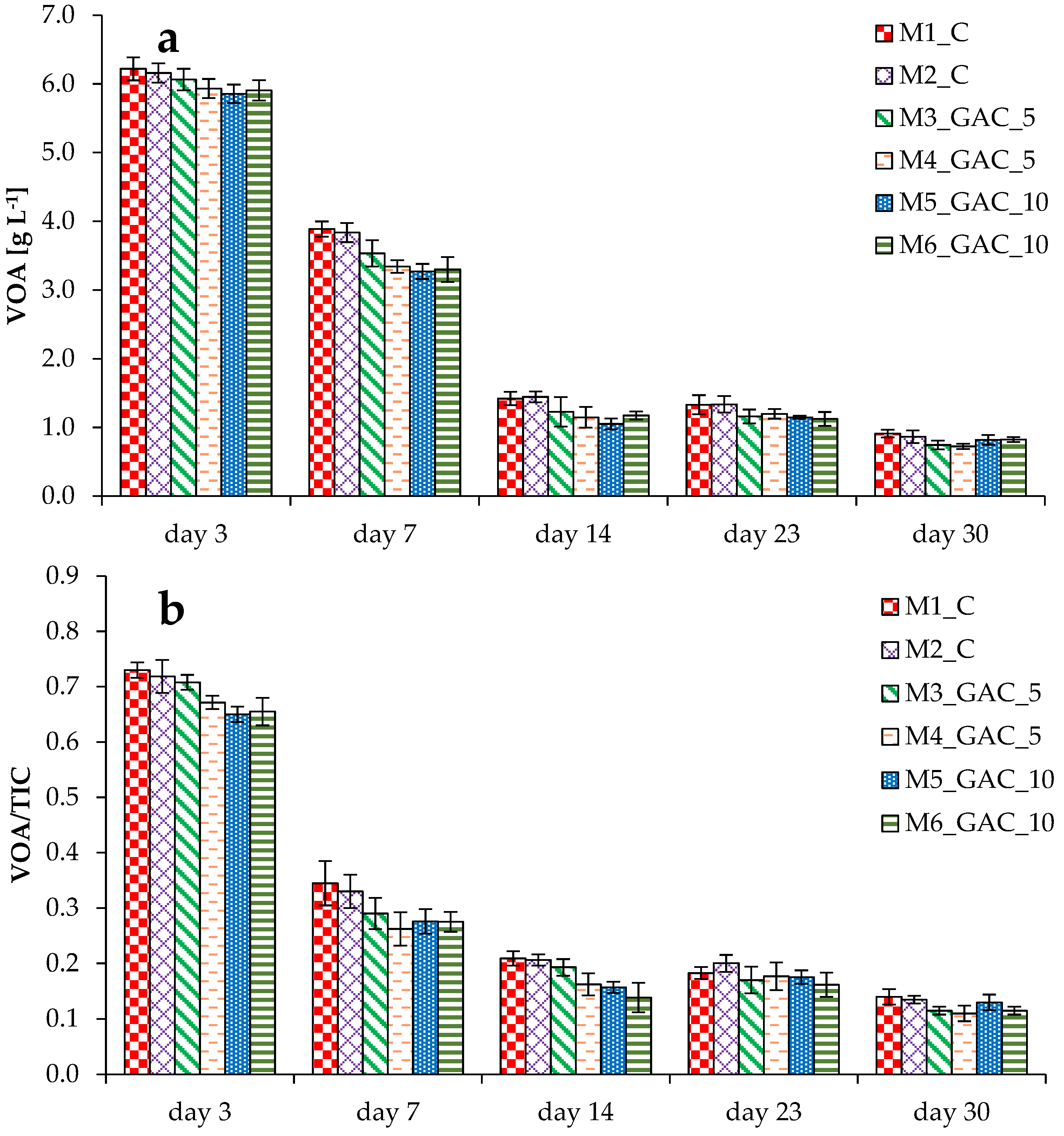
3.1. Process Stability and Methane Production in Mesophilic Experiments
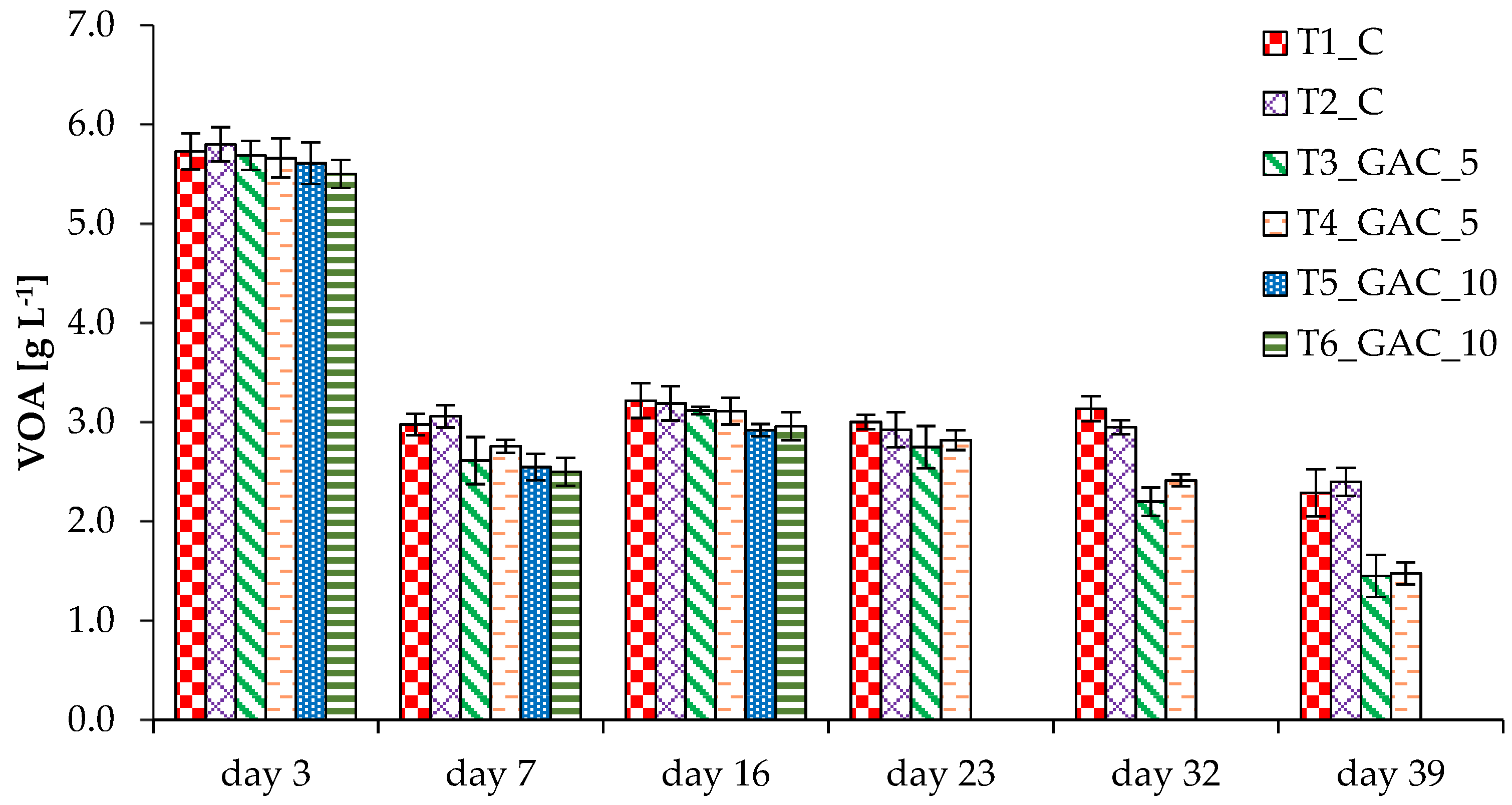
3.2. Process Stability and Methane Production in Thermophilic Experiments
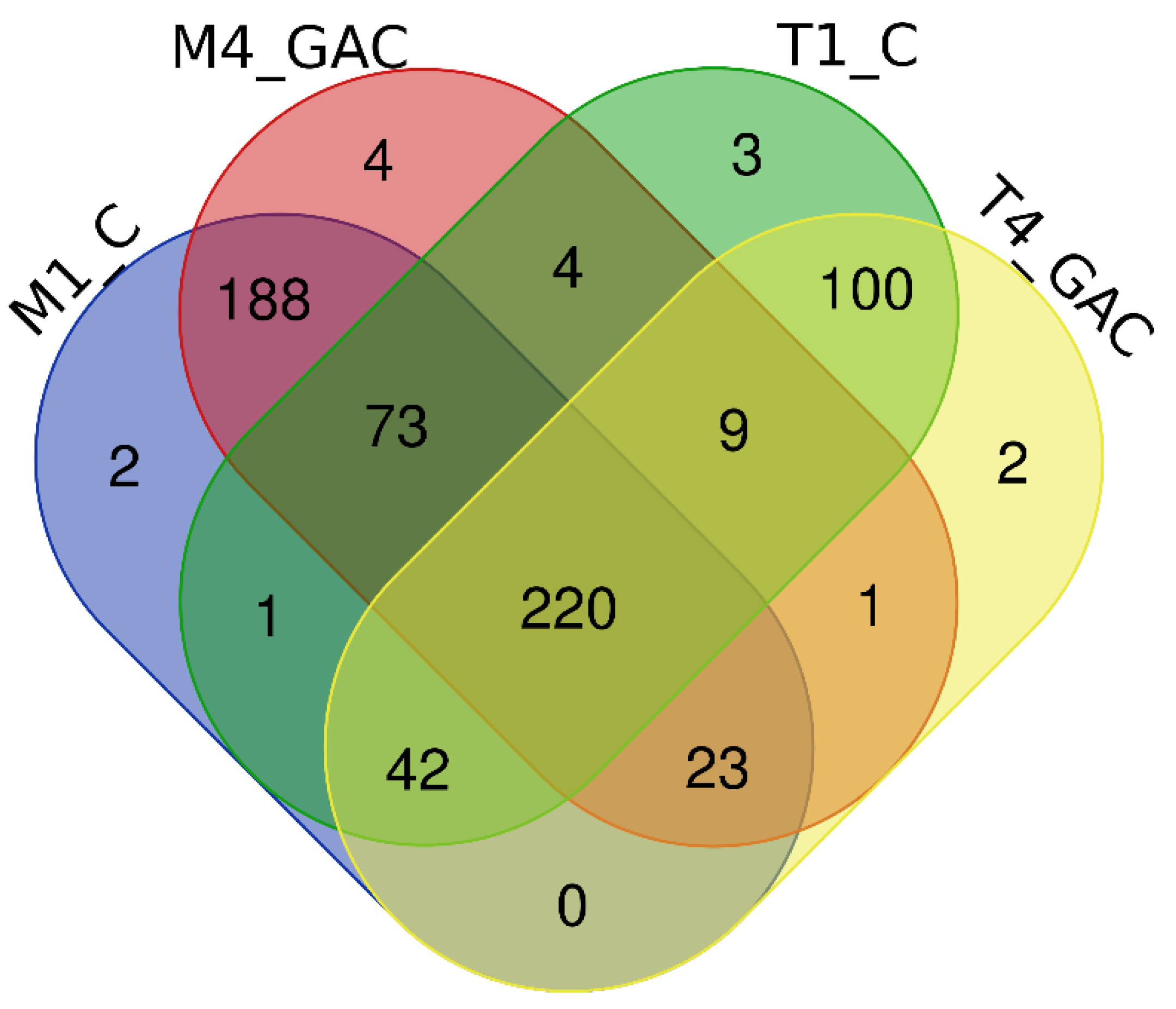
3.3. Description of the Bacterial Community Structure
3.4. Description of the Archaeal Community Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, H.; Zhang, Y.; Zhang, H. Characterization of the microbial communities in rumen fluid inoculated reactors for the biogas digestion of wheat straw. Sustainability 2017, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Westerholm, M.; Moestedt, J.; Schnurer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Maurya, R.; Tirkey, S.R.; Rajapitamahuni, S.; Ghosh, A.; Mishra, S. Chapter 9—Recent advances and future prospective of biogas production. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts, 1st ed.; Hosseini, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 159–178. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Monofermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.A.; Martin, M.A.; Chica, A.F.; Estevez-Pastor, F.S.; Toro-Baptista, E. Effect of microwave pretreatment on semi-continuous anaerobic digestion of sewage sludge. Renew. Energy 2018, 115, 917–925. [Google Scholar] [CrossRef]
- Montalvo, S.; Vielma, S.; Borja, R.; Huilinir, C.; Guerrero, L. Increase in biogas production in anaerobic sludge digestion by combining aerobic hydrolysis and addition of metallic wastes. Renew. Energy 2018, 123, 541–548. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef]
- Shober, A.L.; Maguire, R.O. Manure Management. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: New York, NY, USA, 2018. [Google Scholar]
- Nielsen, H.B.; Angelidaki, I. Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresour. Technol. 2008, 99, 7995–8001. [Google Scholar] [CrossRef]
- Sasaki, K.; Morita, M.; Hirano, S.; Ohmura, N.; Igarashi, Y. Decreasing ammonia inhibition in thermophilic methanogenic bioreactors using carbon fiber textiles. Appl. Microbiol. Biotechnol. 2011, 90, 1555–1561. [Google Scholar] [CrossRef]
- Leverenz, H.; Adams, R.; Hazard, J.; Tchobanoglous, G. Continuous thermal stripping process for ammonium removal from digestate and centrate. Sustainability 2021, 13, 2185. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef]
- Leite, W.; Magnus, B.S.; Guimaraes, L.B.; Gottardo, M.; Belli Filho, P. Feasibility of thermophilic anaerobic processes for treating waste activated sludge under low HRT and intermittent mixing. J. Environ. Manag. 2017, 201, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Kasinski, S. Mesophilic and thermophilic anaerobic digestion of organic fraction separated during mechanical heat treatment of municipal waste. Appl. Sci. 2020, 10, 2412. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.; Liu, T. Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 2003, 53, 43–52. [Google Scholar] [CrossRef]
- Antoni, D.; Zverlov, V.V.; Schwarz, W.H. Biofuels from microbes. Appl. Microbiol. Biotechnol. 2007, 77, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Claes, A.; Melchi, L.; Uludag-Demirer, S.; Demirer, G.N. Supplementation of carbon-based conductive materials and trace metals to improve biogas production from apple pomace. Sustainability 2021, 13, 9488. [Google Scholar] [CrossRef]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.P.; Bernet, N.; Delgenes, J.P.; et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of granular activated carbon on anaerobic co-digestion of sugar beet pulp and distillers grains with solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite triggering enhanced direct interspecies electron transfer: A scavenger for the blockage of electron transfer in anaerobic digestion of high-solids sewage sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Effect of magnetite on anaerobic digestion of distillers grains and beet pulp: Operation of reactors and microbial community dynamics. J. Biosci. Bioeng. 2021, 131, 290–298. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, S.H.; Lim, T.G.; Park, H.D. Effect of inoculum concentration on methanogenesis by direct interspecies electron transfer: Performance and microbial community composition. Bioresour. Technol. 2019, 291, 121881. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Lee, S.H.; Lim, T.G.; Park, J.H.; Kim, B.; Buffiere, P.; Park, H.D. Recent advances in methanogenesis through direct interspecies electron transfer via conductive materials: A molecular microbiological perspective. Bioresour. Technol. 2021, 322, 124587. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana growth in anaerobic digester effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Y.; Tan, D.; Zhao, Z.; Zhao, H.; Quan, X. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion. Bioresour. Technol. 2018, 249, 666–672. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; Gonzalez, R.; Otero, M.; Gomez, X. Enhancing anaerobic digestion of poultry blood using activated carbon. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Shen, N.; Xiao, Y.; Chen, Y.; Sun, F.; Kumar Tyagi, V.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Choi, Y.K.; Kan, E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef]
- Holmes, D.E.; Shrestha, P.M.; Walker, D.J.F.; Dang, Y.; Nevin, K.P.; Woodard, T.L.; Lovley, D.R. Metatranscriptomic evidence for direct interspecies electron transfer between Geobacter and Methanothrix species in methanogenic rice paddy soils. Appl. Environ. Microbiol. 2017, 83, e00223-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, K.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [Green Version]
- Yee, M.O.; Rotaru, A.E. Extracellular electron uptake in Methanosarcinales is independent of multiheme c-type cytochromes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, M.O.; Snoeyenbos-West, O.L.; Thamdrup, B.; Ottosen, L.D.M.; Rotaru, A.E. Extracellular electron uptake by two Methanosarcina species. Front. Energy Res. 2019, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Bagge, E.; Sahlström, L.; Albihn, A. The effect of hygienic treatment on the microbial flora of biowaste at biogas plants. Water Res. 2005, 39, 4879–4886. [Google Scholar] [CrossRef]
- Luo, G.; Fotidis, I.A.; Angelidaki, I. Comparative analysis of taxonomic, functional, and metabolic patterns of microbiomes from 14 full-scale biogas reactors by metagenomic sequencing and radioisotopic analysis. Biotechnol. Biofuels 2016, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.A.; Ulbricht, A.; Neulinger, S.C.; Refai, S.; Waßmann, K.; Künzel, S.; Schmitz, R.A. Immediate effects of ammonia shock on transcription and composition of a biogas reactor microbiome. Front. Microbiol. 2019, 10, 2064. [Google Scholar] [CrossRef]
- Su, L.; Sun, X.; Liu, C.; Ji, R.; Zhen, G.; Chen, M.; Zhang, L. Thermophilic solid-state anaerobic digestion of corn straw, cattle manure, and vegetable waste: Effect of temperature, total solid content, and C/N ratio. Archaea 2020, 2020, 8841490. [Google Scholar] [CrossRef]
- Mulat, D.G.; Huerta, S.G.; Kalyani, D.; Horn, S.J. Enhancing methane production from lignocellulosic biomass by combined steam-explosion pretreatment and bioaugmentation with cellulolytic bacterium Caldicellulosiruptor bescii. Biotechnol. Biofuels 2018, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.D.; Song, M.; Jo, M.; Shin, S.G.; Khim, J.H.; Hwang, S. Growth condition and bacterial community for maximum hydrolysis of suspended organic materials in anaerobic digestion of food waste recycling wastewater. Appl. Microbiol. Biotechnol. 2010, 85, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Mosbaek, F.; Kjeldal, H.; Mulat, D.; Albertsen, M.; Ward, A.J.; Feilberg, A.; Nielsen, J.L. Identification of syntrophic acetate-oxidizing bacteria in anaerobic digesters by combined protein-based stable isotope probing and metagenomics. ISME J. 2016, 10, 2405–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerholm, M.; Müller, B.; Singh, A.; Karlsson Lindsjö, O.; Schnürer, A. Detection of novel syntrophic acetate-oxidizing bacteria from biogas processes by continuous acetate enrichment approaches. Microb. Biotechnol. 2018, 11, 680–693. [Google Scholar] [CrossRef] [Green Version]
- Karakashev, D.; Batstone, D.J.; Trably, E.; Angelidaki, I. Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl. Environ. Microbiol. 2006, 72, 5138–5141. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Müller, B.; Westerholm, M.; Schnürer, A. Syntrophic acetate oxidation in industrial CSTR biogas digesters. J. Biotechnol. 2014, 171, 39–44. [Google Scholar]
- Krieg, N.R.; Ludwig, W.; Euzéby, J.; Whitman, W.B. Phylum XIV. Bacteroidetes phyl. nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Krieg, N.R., Staley, J.T., Brown, D.R., Hedlund, B.P., Paster, B.J., Ward, N., Ludwig, W., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2010; Volume 4, p. 25. [Google Scholar]
- Sun, L.; Pope, P.B.; Eijsink, V.G.H.; Schnürer, A. Characterization of microbial community structure during continuous anaerobic digestion of straw and cow manure. Microb. Biotechnol. 2015, 8, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Treu, L.; Campanaro, S.; Kougias, P.G.; Sartori, C.; Bassani, I.; Angelidaki, I. Hydrogen-fueled microbial pathways in biogas upgrading systems revealed by genome-centric metagenomics. Front. Microbiol. 2018, 9, 1079. [Google Scholar] [CrossRef] [Green Version]
- Schink, B.; Munoz, R. The Family Syntrophomonadaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 371–379. [Google Scholar]
- Breitenstein, A.; Wiegel, J.; Haertig, C.; Weiss, N.; Andreesen, J.R.; Lechner, U. Reclassification of Clostridium hydroxybenzoicum as Sedimentibacter hydroxybenzoicus gen. nov., comb. nov., and description of Sedimentibacter saalensis sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 801–807. [Google Scholar] [PubMed] [Green Version]
- Imachi, H.; Sakai, S.; Kubota, T.; Miyazaki, M.; Saito, Y.; Takai, K. Sedimentibacter acidaminivorans sp. nov., an anaerobic, amino-acid-utilizing bacterium isolated from marine subsurface sediment. Int. J. Syst. Evol. Microbiol. 2016, 66, 1293–1300. [Google Scholar] [CrossRef]
- Lee, J.; Koo, T.; Yulisa, A.; Hwang, S. Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition. J. Environ. Manag. 2019, 241, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y. Conductive Fe3O4 nanoparticles accelerate syntrophic methane production from butyrate oxidation in two different lake sediments. Front. Microbiol. 2016, 7, 1316. [Google Scholar] [CrossRef] [Green Version]
- Froese, A.; Schellenberg, J.; Sparling, R. Enhanced depolymerization and utilization of raw lignocellulosic material by co-cultures of Ruminiclostridium thermocellum with hemicellulose-utilizing partners. Can. J. Microbiol. 2019, 65, 296–307. [Google Scholar] [CrossRef]
- Ravachol, J.; Borne, R.; Meynialsalles, I.; Soucaille, P.; Pages, S.; Tardif, C. Combining free and aggregated cellulolytic systems in the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Biotech. Biofuels 2015, 8, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Rettenmaier, R.; Kowollik, M.L.; Klingl, A.; Liebl, W.; Zverlov, V. Ruminiclostridium herbifermentans sp. nov., a mesophilic and moderately thermophilic cellulolytic and xylanolytic bacterium isolated from a lab-scale biogas fermenter fed with maize silage. Int. J. Syst. Evol. Microb. 2021, 71, 004692. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.M.; Ziganshina, E.E.; Kleinsteuber, S.; Pröter, J.; Ilinskaya, O.N. Methanogenic community dynamics during anaerobic utilization of agricultural wastes. Acta Nat. 2012, 4, 91–97. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Wintsche, B.; Seifert, J.; Carstensen, M.; Born, J.; Kleinsteuber, S. Spatial separation of metabolic stages in a tube anaerobic baffled reactor: Reactor performance and microbial community dynamics. Appl. Microbiol. Biotechnol. 2019, 103, 3915–3929. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- McKay, L.J.; Klingelsmith, K.B.; Deutschbauer, A.M.; Inskeep, W.P.; Fields, M.W. Draft genome sequence of Methanothermobacter thermautotrophicus WHS, a thermophilic hydrogenotrophic methanogen from Washburn Hot Springs in Yellowstone National Park, USA. Microbiol. Resour. Announc. 2021, 10, e01157-20. [Google Scholar] [CrossRef] [PubMed]
- Hassa, J.; Wibberg, D.; Maus, I.; Pühler, A.; Schlüter, A. Genome analyses and genome-centered metatranscriptomics of Methanothermobacter wolfeii strain SIV6, isolated from a thermophilic production-scale biogas fermenter. Microorganisms 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, A.; Einarsson, P.; Schnürer, A.; Sundberg, C.; Ejlertsson, J.; Svensson, B. Impact of trace element addition on degradation efficiency of volatile fatty acids, oleic acid and phenyl acetate and on microbial populations in a biogas digester. J. Biosci. Bioeng. 2012, 114, 446–452. [Google Scholar] [CrossRef] [PubMed]

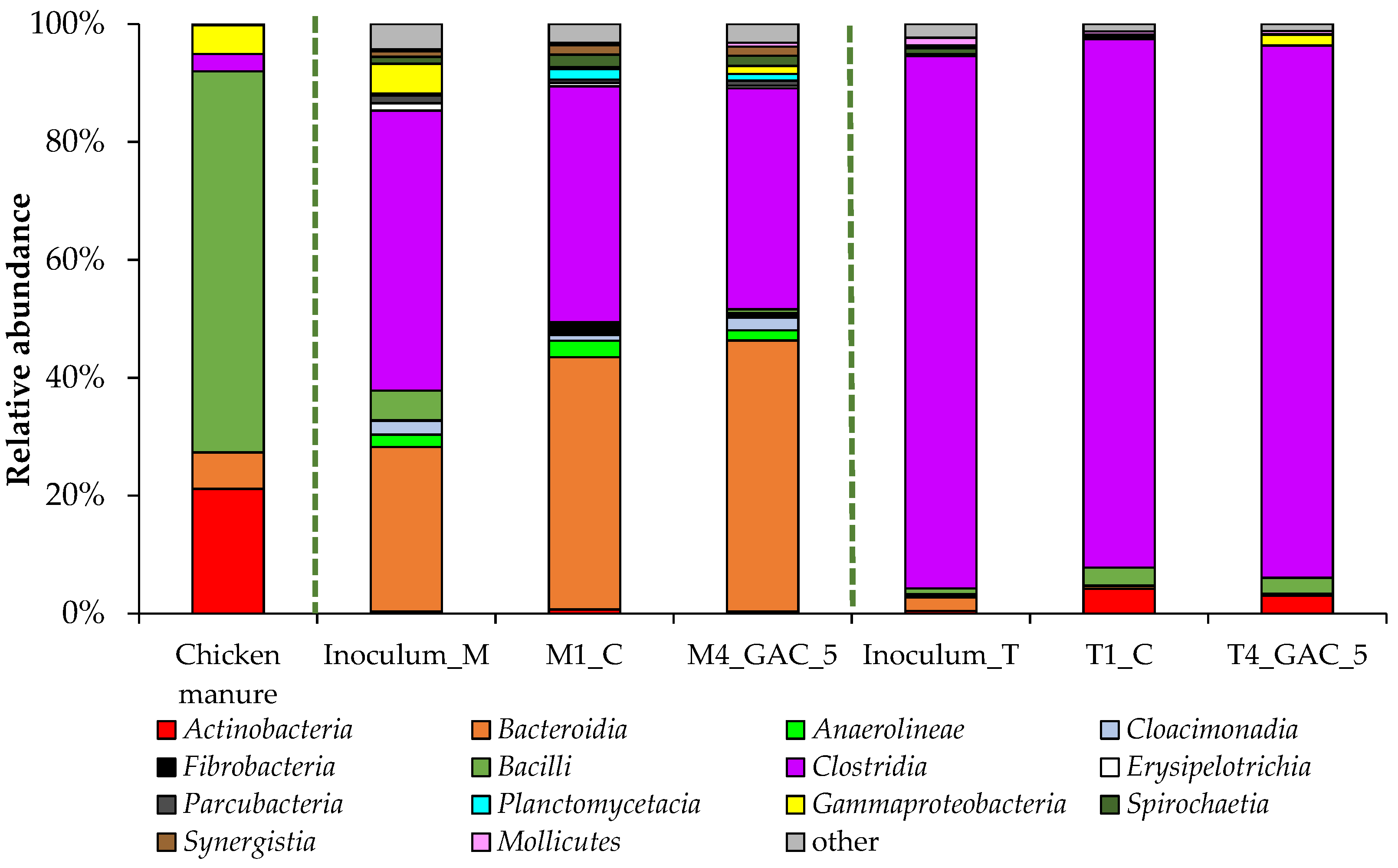

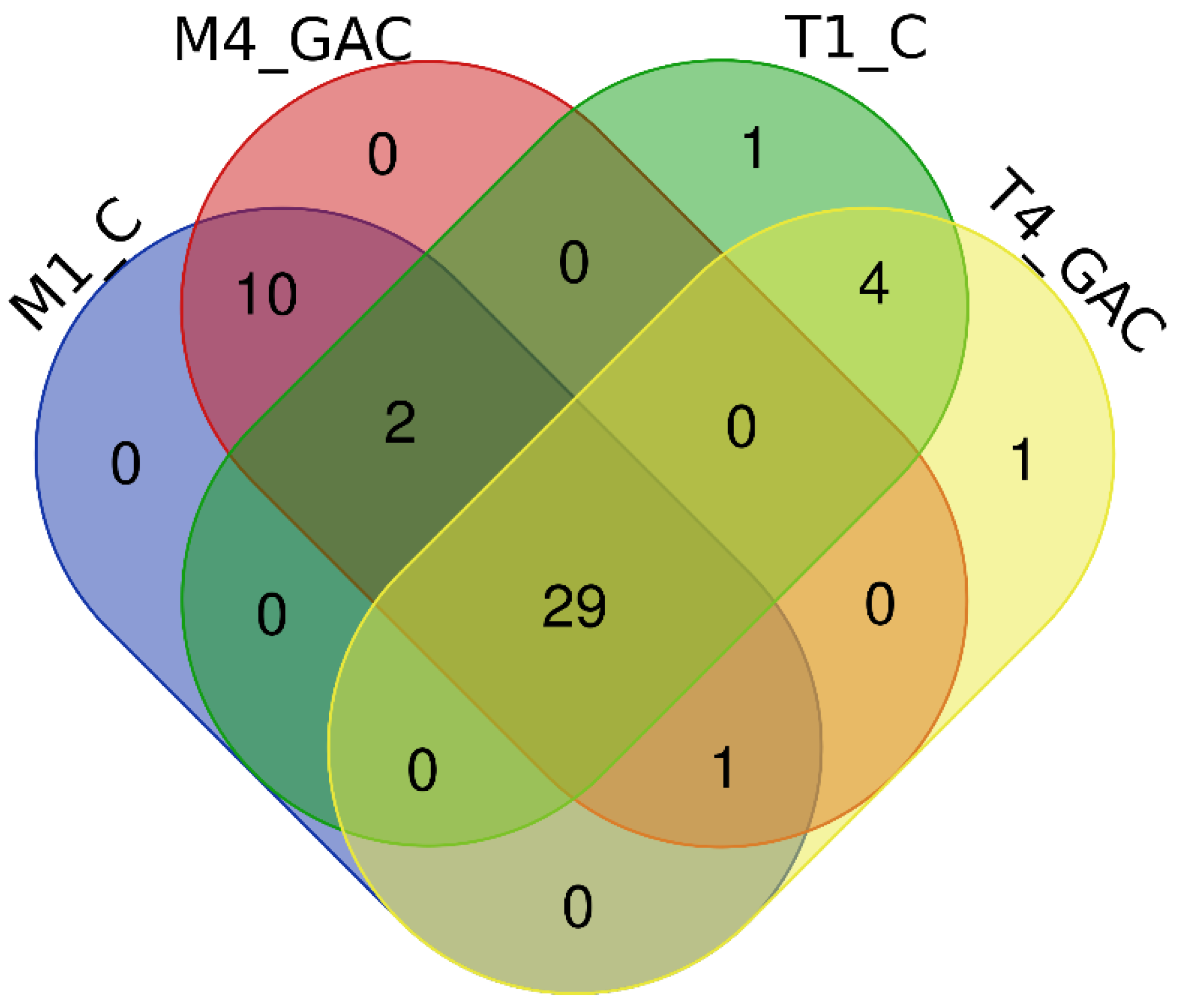

| Reactor | TAN (g L−1) | FAN (mg L−1) | ||
|---|---|---|---|---|
| Day 7 | Day 23 | Day 7 | Day 23 | |
| M1_C | 2.28 ± 0.05 | 2.44 ± 0.16 | 137 ± 3.0 | 118 ± 7.8 |
| M2_C | 2.12 ± 0.06 | 2.55 ± 0.07 | 125 ± 3.3 | 129 ± 3.6 |
| M3_GAC_5 | 2.07 ± 0.14 | 2.35 ± 0.10 | 133 ± 8.8 | 111 ± 4.8 |
| M4_GAC_5 | 2.33 ± 0.18 | 2.65 ± 0.19 | 156 ± 12.0 | 120 ± 8.7 |
| M5_GAC_10 | 2.26 ± 0.16 | 2.62 ± 0.12 | 142 ± 10.1 | 106 ± 4.9 |
| M6_GAC_10 | 2.18 ± 0.14 | 2.47 ± 0.14 | 134 ± 8.7 | 105 ± 6.0 |
| Reactor | TAN (g L−1) | FAN (mg L−1) | ||||
|---|---|---|---|---|---|---|
| Day 7 | Day 16 | Day 32 | Day 7 | Day 16 | Day 32 | |
| T1_C | 2.29 ± 0.09 | 2.14 ± 0.06 | 2.82 ± 0.03 | 560 ± 21.6 | 455 ± 12.5 | 343 ± 3.8 |
| T2_C | 2.67 ± 0.04 | 2.21 ± 0.07 | 2.78 ± 0.11 | 617 ± 14.7 | 404 ± 12.4 | 318 ± 12.2 |
| T3_GAC_5 | 2.57 ± 0.07 | 2.12 ± 0.07 | 2.75 ± 0.14 | 565 ± 14.7 | 359 ± 11.8 | 514 ± 26.5 |
| T4_GAC_5 | 2.66 ± 0.04 | 2.12 ± 0.06 | 2.92 ± 0.11 | 645 ± 3.4 | 426 ± 7.1 | 588 ± 23.0 |
| T5_GAC_10 | 2.75 ± 0.06 | 2.11 ± 0.04 | ND | 638 ± 13.7 | 402 ± 7.6 | ND |
| T6_GAC_10 | 2.59 ± 0.07 | 2.28 ± 0.10 | ND | 580 ± 15.8 | 418 ± 18.1 | ND |
| Sample | Bacteria | Archaea | ||||||
|---|---|---|---|---|---|---|---|---|
| OTUs | Chao1 | Shannon | Simpson | OTUs | Chao1 | Shannon | Simpson | |
| Manure | 220 | 281 | 5.06 | 0.93 | 0 | 0 | - | - |
| Inoculum_M | 523 | 538 | 6.69 | 0.97 | 42 | 42 | 4.09 | 0.91 |
| M1_C | 549 | 563 | 6.41 | 0.97 | 42 | 42 | 2.56 | 0.65 |
| M4_GAC_5 | 522 | 530 | 6.43 | 0.97 | 42 | 42 | 2.65 | 0.67 |
| Inoculum_T | 419 | 431 | 5.43 | 0.91 | 36 | 36 | 3.70 | 0.89 |
| T1_C | 452 | 474 | 5.30 | 0.92 | 36 | 36 | 2.85 | 0.77 |
| T4_GAC_5 | 397 | 430 | 5.13 | 0.90 | 35 | 35 | 2.97 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of Granular Activated Carbon on Anaerobic Process and Microbial Community Structure during Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure. Sustainability 2022, 14, 447. https://doi.org/10.3390/su14010447
Ziganshina EE, Bulynina SS, Ziganshin AM. Impact of Granular Activated Carbon on Anaerobic Process and Microbial Community Structure during Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure. Sustainability. 2022; 14(1):447. https://doi.org/10.3390/su14010447
Chicago/Turabian StyleZiganshina, Elvira E., Svetlana S. Bulynina, and Ayrat M. Ziganshin. 2022. "Impact of Granular Activated Carbon on Anaerobic Process and Microbial Community Structure during Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure" Sustainability 14, no. 1: 447. https://doi.org/10.3390/su14010447
APA StyleZiganshina, E. E., Bulynina, S. S., & Ziganshin, A. M. (2022). Impact of Granular Activated Carbon on Anaerobic Process and Microbial Community Structure during Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure. Sustainability, 14(1), 447. https://doi.org/10.3390/su14010447





