Biochemical Methane Potential and Kinetic Parameters of Goat Manure at Various Inoculum to Substrate Ratios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Biomethane Potential Assays
2.3. Theoretical Maximum Methane Yield
2.4. Process Efficacy, Simulation, and Data Analysis
3. Results
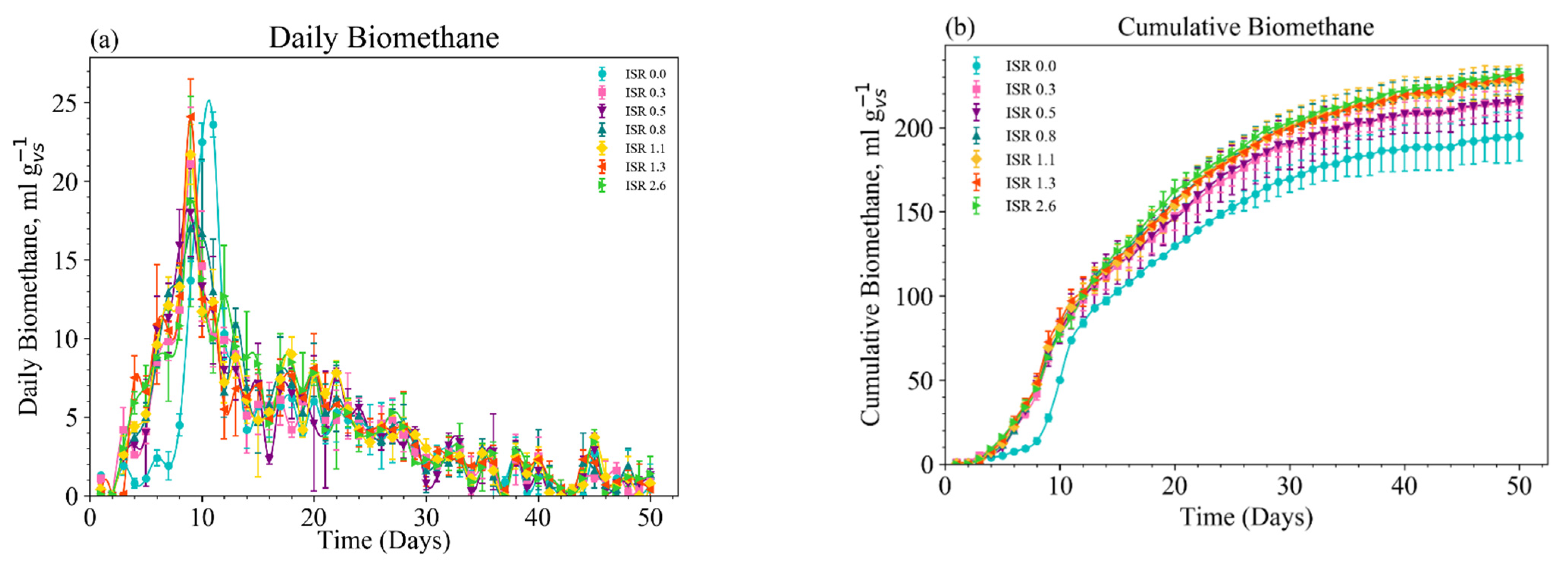
3.1. Daily Biomethane Production
3.2. Cumulative Biomethane
3.3. Methane Production Kinetics
3.4. Biodegradability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USEPA Livestock Anaerobic Digester Database AgSTAR: Biogas Recovery in the Agriculture Sector. Available online: https://www.epa.gov/agstar/livestock-anaerobic-digester-database (accessed on 11 August 2021).
- Rabalais, N.N.; Turner, R.E.; Wiseman, W.J. Gulf of Mexico Hypoxia, A.K.A. “The Dead Zone”. Annu. Rev. Ecol. Syst. 2002, 33, 235–263. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef] [Green Version]
- Daniel, T.C.; Sharpley, A.N.; Lemunyon, J.L. Agricultural Phosphorus and Eutrophication: A Symposium Overview. J. Environ. Qual. 1998, 27, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, A.; Ammon, C.; Schumacher, B.; Stinner, W.; Herrmann, C.; Schneider, M.; Weinrich, S.; Fischer, P.; Amon, T.; Amon, B. Methane emissions from the storage of liquid dairy manure: Influences of season, temperature and storage duration. Waste Manag. 2021, 121, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Nørring, N.P.; Jørgensen, E. Eutrophication and agriculture in Denmark: 20 years of experience and prospects for the future. Eutrophication Coast. Ecosyst. 2009, 629, 65–70. [Google Scholar] [CrossRef]
- He, Z.; Pagliari, P.H.; Waldrip, H.M. Applied and Environmental Chemistry of Animal Manure: A Review. Pedosphere 2016, 26, 779–816. [Google Scholar] [CrossRef]
- Arndt, C.; Leytem, A.; Hristov, A.; Zavala-Araiza, D.; Cativiela, J.; Conley, S.; Daube, C.; Faloona, I.; Herndon, S. Short-term methane emissions from 2 dairy farms in California estimated by different measurement techniques and US Environmental Protection Agency inventory methodology: A case study. J. Dairy Sci. 2018, 101, 11461–11479. [Google Scholar] [CrossRef] [Green Version]
- Howard, A.; Botlaguduru, V.S.V.; Du, H.; Kommalapati, R.R.; Huque, Z. Measurements and Comparative Air Quality Analysis of a Goat Farm Operation. Trans. ASABE 2019, 62, 1723–1733. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, C.A.; Vera-Infanzón, R.R.; Rao, I.M. Predicting methane emissions, animal-environmental metrics and carbon footprint from Brahman (Bos indicus) breeding herd systems based on long-term research on grazing of neotropical savanna and Brachiaria decumbens pastures. Agric. Syst. 2020, 184, 102892. [Google Scholar] [CrossRef]
- Xu, Z.; Li, G.; Huda, N.; Zhang, B.; Wang, M.; Luo, W. Effects of moisture and carbon/nitrogen ratio on gaseous emissions and maturity during direct composting of cornstalks used for filtration of anaerobically digested manure centrate. Bioresour. Technol. 2020, 298, 122503. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xiao, Z.; Zhang, G.; Wang, A.; Li, Z.; Liu, Y.; Wang, H.; Zeng, Q.; Liang, Y.; Zou, D. Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J. Anal. Appl. Pyrolysis 2018, 132, 82–93. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total. Environ. 2020, 728, 138224. [Google Scholar] [CrossRef] [PubMed]
- USDA. Sheep and Goats. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/shep0120.pdf (accessed on 21 August 2021).
- Miller, B.A.; Lu, C.D. Current status of global dairy goat production: An overview. Asian-Australas. J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- USDA. Anaerobic Digestion & Organic Waste Management Project. Available online: https://farmandenergyinitiative.org/projects/biodigesters/ (accessed on 15 September 2021).
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Moriarty, K.; Milbrandt, A.; Lewis, J.; Schwab, A. 2017 Bioenergy Industry Status Report. 2017. Available online: https://www.nrel.gov/docs/fy20osti/75776.pdf (accessed on 18 September 2021).
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; De La Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.-C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.S.-K.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Kommalapati, R.R. Optimizing anaerobic co-digestion of goat manure and cotton gin trash using biochemical methane potential (BMP) test and mathematical modeling. SN Appl. Sci. 2021, 3, 724. [Google Scholar] [CrossRef]
- Kaur, H.; Kommalapati, R.R. Effect of Inoculum Concentration and Pretreatment on Biomethane Recovery from Cotton Gin Trash. J. Agric. Sci. 2021, 13, 15. [Google Scholar] [CrossRef]
- Gujer, W.; Zehnder, A.J.B. Conversion Processes in Anaerobic Digestion. Water Sci. Technol. 1983, 15, 127–167. [Google Scholar] [CrossRef]
- Raposo, F.; De La Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. A mathematical model for dynamic simulation of anaerobic digestion of complex substrates: Focusing on ammonia inhibition. Biotechnol. Bioeng. 1993, 42, 159–166. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef]
- Koch, K.; Lippert, T.; Drewes, J.E. The role of inoculum’s origin on the methane yield of different substrates in biochemical methane potential (BMP) tests. Bioresour. Technol. 2017, 243, 457–463. [Google Scholar] [CrossRef]
- Ohemeng-Ntiamoah, J.; Datta, T. Perspectives on variabilities in biomethane potential test parameters and outcomes: A review of studies published between 2007 and 2018. Sci. Total Environ. 2019, 664, 1052–1062. [Google Scholar] [CrossRef]
- Cao, L.; Keener, H.; Huang, Z.; Liu, Y.; Ruan, R.; Xu, F. Effects of temperature and inoculation ratio on methane production and nutrient solubility of swine manure anaerobic digestion. Bioresour. Technol. 2020, 299, 122552. [Google Scholar] [CrossRef] [PubMed]
- Okoro-Shekwaga, C.K.; Suruagy, M.V.T.; Ross, A.; Camargo-Valero, M.A. Particle size, inoculum-to-substrate ratio and nutrient media effects on biomethane yield from food waste. Renew. Energy 2020, 151, 311–321. [Google Scholar] [CrossRef]
- Posmanik, R.; Kim, A.H.; Labatut, R.A.; Usack, J.G.; Angenent, L.T. Granular sludge is a preferable inoculum for the biochemical methane potential assay for two complex substrates. Bioresour. Technol. 2020, 309, 123359. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M. APHA: Standard Methods for the Examination of Water and Wastewater; Centennial Edition; APHA, AWWA, WEF: Washington, DC, USA, 2005. [Google Scholar]
- Angelidaki, I.; Alves, M.M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Orangun, A.; Kaur, H.; Kommalapati, R. Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste. Energies 2021, 14, 1952. [Google Scholar] [CrossRef]
- Buswell, A.M.; Mueller, H.F. Mechanism of Methane Fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Boyle, W. Energy recovery from sanitary landfills—A review. Microb. Energy Convers. 1977, 119–138. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas Production by Co-Digestion of Goat Manure with Three Crop Residues. PLoS ONE 2013, 8, e66845. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Siddhu, M.A.H.; Amin, F.R.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Methane production through anaerobic co-digestion of sheep dung and waste paper. Energy Convers. Manag. 2018, 156, 279–287. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Møller, H.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef] [PubMed]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef]
- Wahid, R.; Romero-Guiza, M.; Moset, V.; Møller, H.B.; Fernández, B. Improved anaerobic biodegradability of wheat straw, solid cattle manure and solid slaughterhouse by alkali, ultrasonic and alkali-ultrasonic pre-treatment. Environ. Technol. 2020, 41, 997–1006. [Google Scholar] [CrossRef]
- Lauwers, J.; Appels, L.; Thompson, I.P.; Degrève, J.; Van Impe, J.F.; Dewil, R. Mathematical modelling of anaerobic digestion of biomass and waste: Power and limitations. Prog. Energy Combust. Sci. 2013, 39, 383–402. [Google Scholar] [CrossRef] [Green Version]
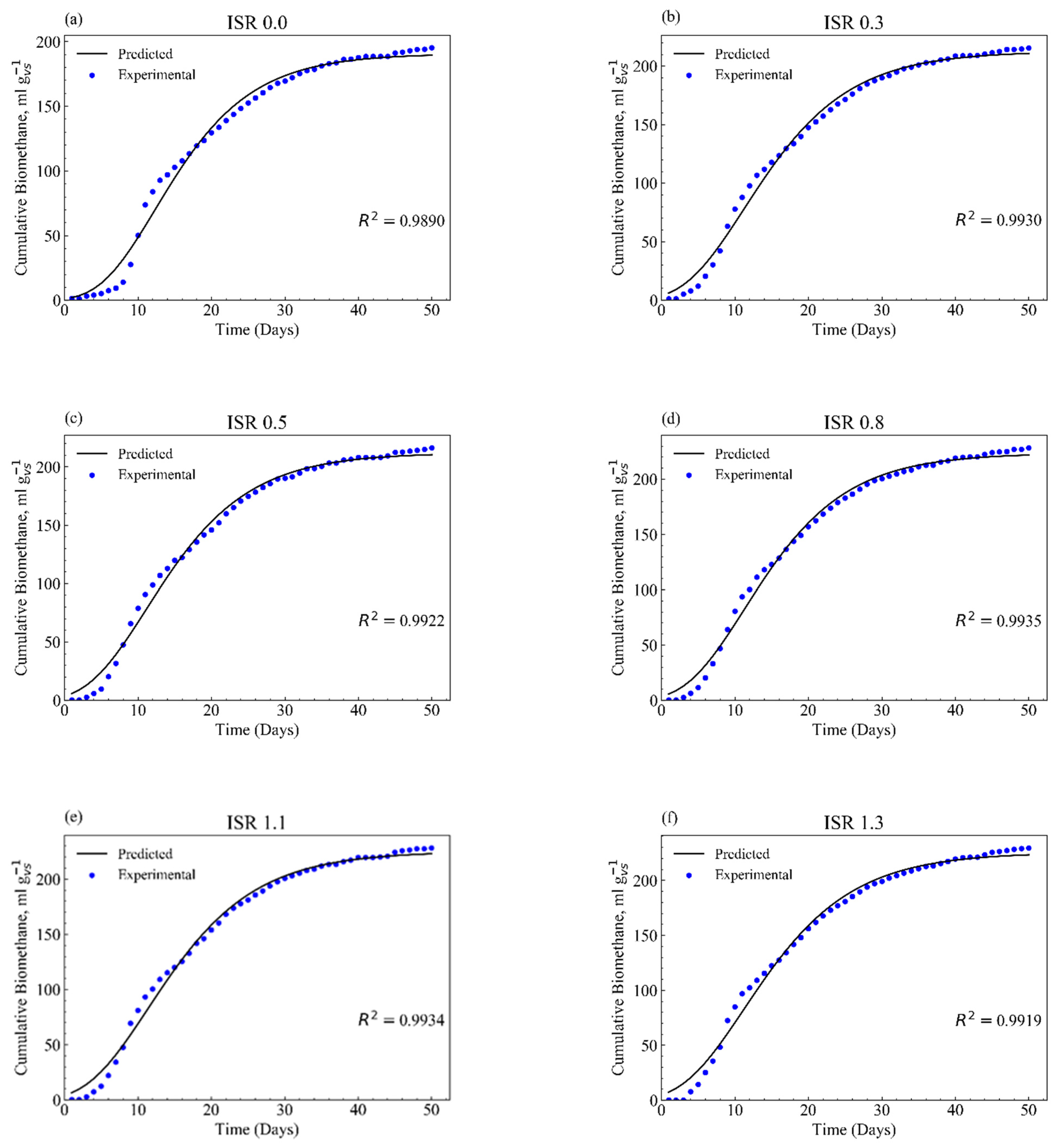

| ISR | P0 1 | E 2 | Rm 3 | λ 4 | R2 | rRMSE 5 | BD (%) |
|---|---|---|---|---|---|---|---|
| 0 | 186.6 ± 19.3 b | 191.8 ± 17.6 b | 9.8 ± 0.6 a | 5.2 ± 0.4 a | 0.988 ± 0.001 b | 0.536 | 65.6 ± 1.6 b |
| 0.3 | 211.0 ± 7.0 a | 214.3 ± 7.8 a | 9.9 ± 0.9 a | 3.4 ± 0.2 bc | 0.992 ± 0.002 a | 1.250 | 73.3 ± 0.7 a |
| 0.5 | 206.5 ± 8.2 a | 214.9 ± 9.0 a | 10.2 ± 1.2 a | 3.7 ± 0.1 b | 0.991 ± 0.002 a | 3.644 | 73.7 ± 0.8 a |
| 0.8 | 220.1 ± 9.4 a | 225.9 ± 8.9 a | 10.7 ± 0.5 a | 3.6 ± 0.3 bc | 0.992 ± 0.003 a | 5.355 | 77.4 ± 0.8 a |
| 1.1 | 218.6 ± 11.2 a | 222.1 ± 12.0 a | 10.0 ± 1.3 a | 3.5 ± 0.1 bc | 0.994 ± 0.002 a | 2.222 | 77.4± 0.9 a |
| 1.3 | 224.2 ± 3.5 a | 222.8 ± 2.2 a | 10.2 ± 0.2 a | 3.2 ± 0.4 c | 0.993 ± 0.000 a | 9.083 | 78.3 ± 0.2 a |
| 2.6 | 223.6 ± 4.9 a | 229.9 ± 2.7 a | 10.9 ± 0.8 a | 3.7 ± 0.3 bc | 0.994 ± 0.001 a | 5.99 | 78.7 ± 0.2 a |
| LSD 6 | 18.7 | 18.7 | 1.6 | 0.7 | 0.002 | - | 5.9 |
| p | ˂0.0001 | ˂0.0001 | ˂0.0001 | ˂0.0001 | ˂0.0001 | - | <0.0001 |
| Parameter | GM | Inoculum |
|---|---|---|
| Proximate analysis | ||
| Moisture (%) | 35.9 | 97.2 ± 0.3 |
| VS (%) | 52.8 | 1.5 ± 0 |
| Ash (%) | 11.3 | 1.5 ± 0.4 |
| Ultimate analysis | ||
| N (%-TS) | 1.7 | - |
| C (%-TS) | 35.5 | - |
| H (%-TS) | 6.0 | - |
| O (%-TS) | 56.2 | |
| S (%-TS) | 0.5 | - |
| C/N | 20.9 | - |
| Compositional analysis | ||
| Cellulose + Hemi-cellulose (%-TS) | 72.4 | - |
| Lignin (%-TS) | 17.6 | - |
| Elemental Formula | C295.8H603.0O351.0N12.6S1.7 | - |
| Chemical analysis | ||
| pH | 7.8 ± 0.3 | |
| Alkalinity (CaCO3, mg L−1) | 5265.0 ± 106.1 | |
| VFA (CH3COOH, mg L−1) | 2795.0 ± 63.3 | |
| NO3−-N (mg L−1) | 9.0 ± 1.4 | |
| PO4−-(mg L−1) | 992.0 ± 4.2 | |
| NH4+-N (mg L−1) | 715.5 ± 154.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, H.; Kommalapati, R.R. Biochemical Methane Potential and Kinetic Parameters of Goat Manure at Various Inoculum to Substrate Ratios. Sustainability 2021, 13, 12806. https://doi.org/10.3390/su132212806
Kaur H, Kommalapati RR. Biochemical Methane Potential and Kinetic Parameters of Goat Manure at Various Inoculum to Substrate Ratios. Sustainability. 2021; 13(22):12806. https://doi.org/10.3390/su132212806
Chicago/Turabian StyleKaur, Harjinder, and Raghava R Kommalapati. 2021. "Biochemical Methane Potential and Kinetic Parameters of Goat Manure at Various Inoculum to Substrate Ratios" Sustainability 13, no. 22: 12806. https://doi.org/10.3390/su132212806
APA StyleKaur, H., & Kommalapati, R. R. (2021). Biochemical Methane Potential and Kinetic Parameters of Goat Manure at Various Inoculum to Substrate Ratios. Sustainability, 13(22), 12806. https://doi.org/10.3390/su132212806






