Combination of Coagulation, Adsorption, and Ultrafiltration Processes for Organic Matter Removal from Peat Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Peat Water Characterization
2.2. Membrane Preparation and Characterization
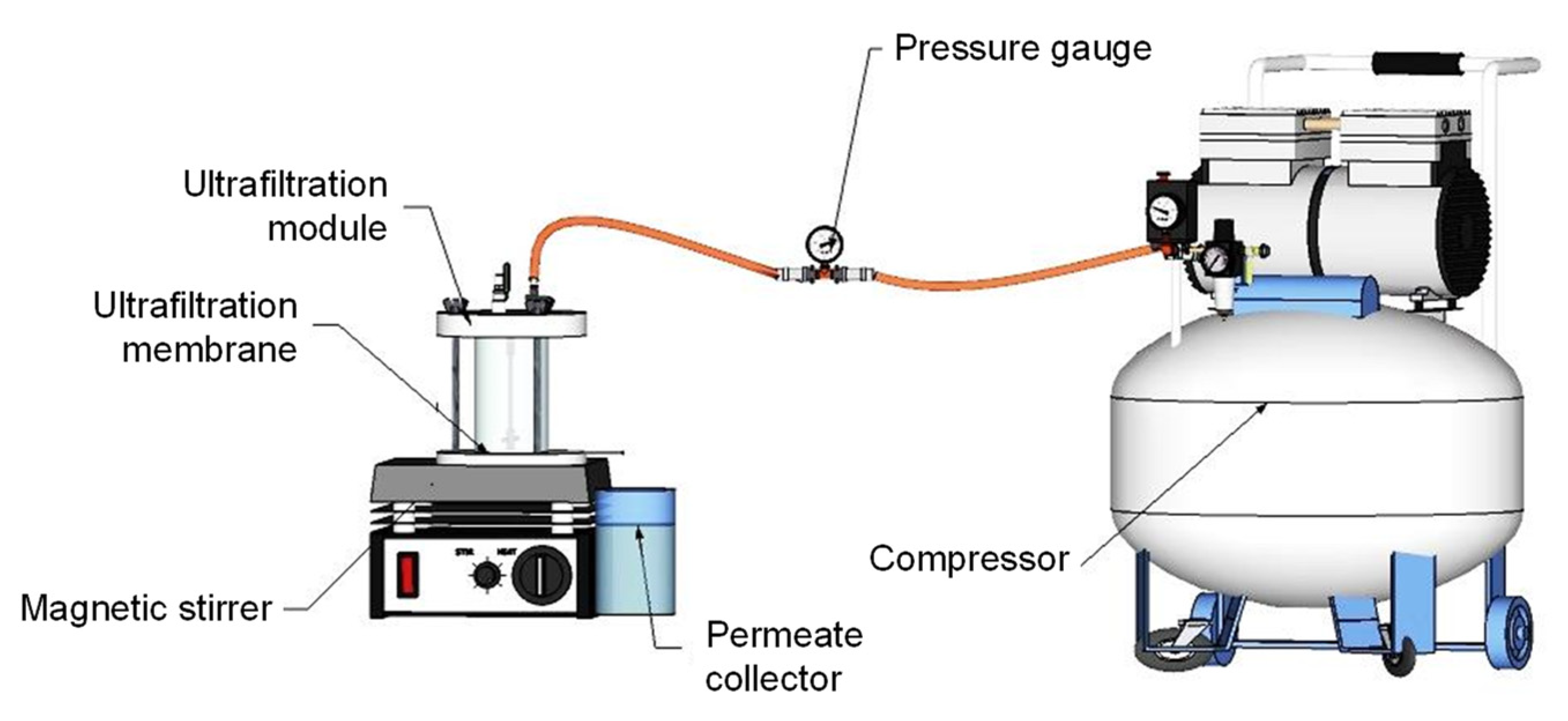
2.3. Coagulation, Adsorption, and Ultrafiltration
3. Results
3.1. Peat Water Characteristics
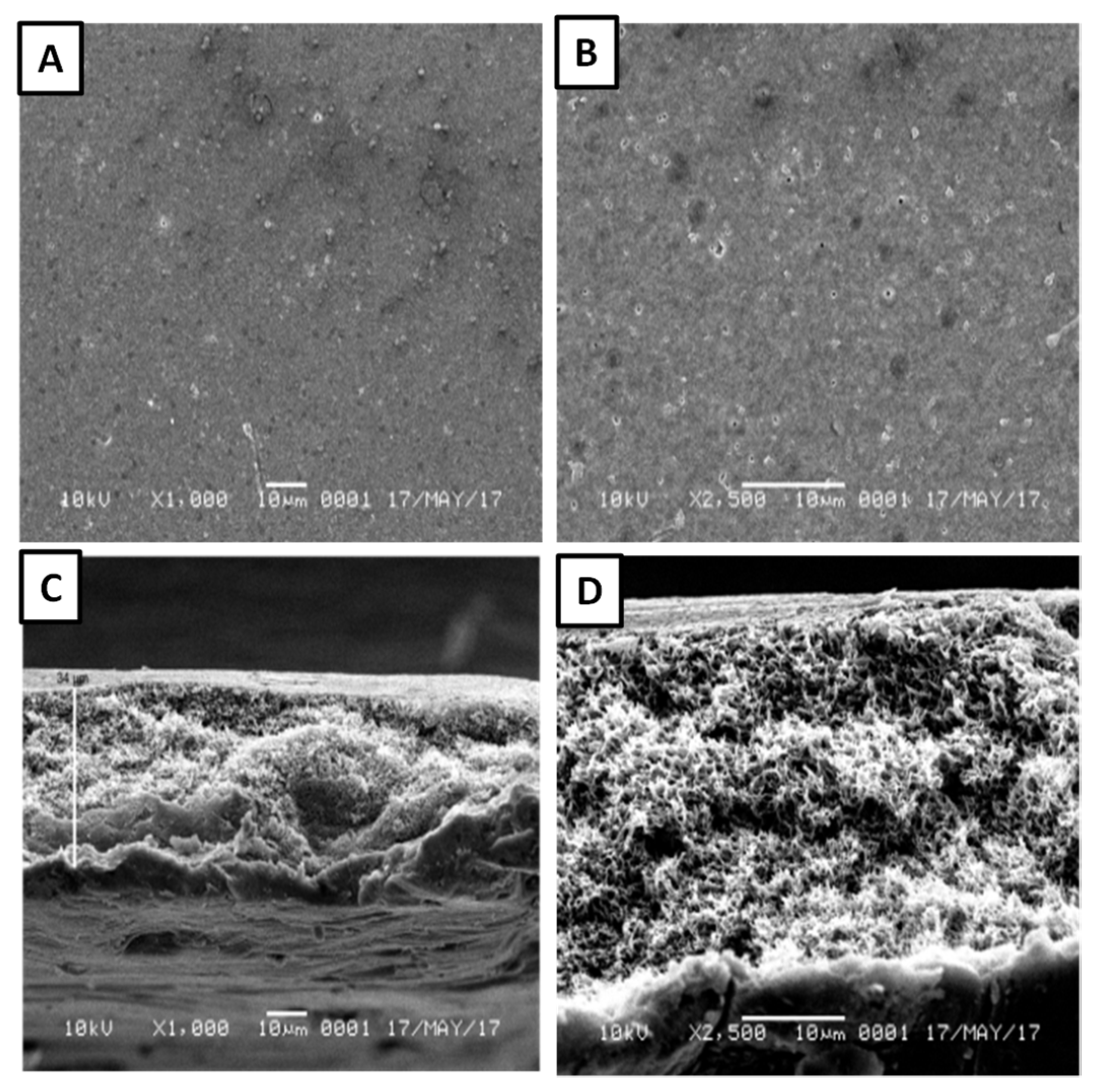
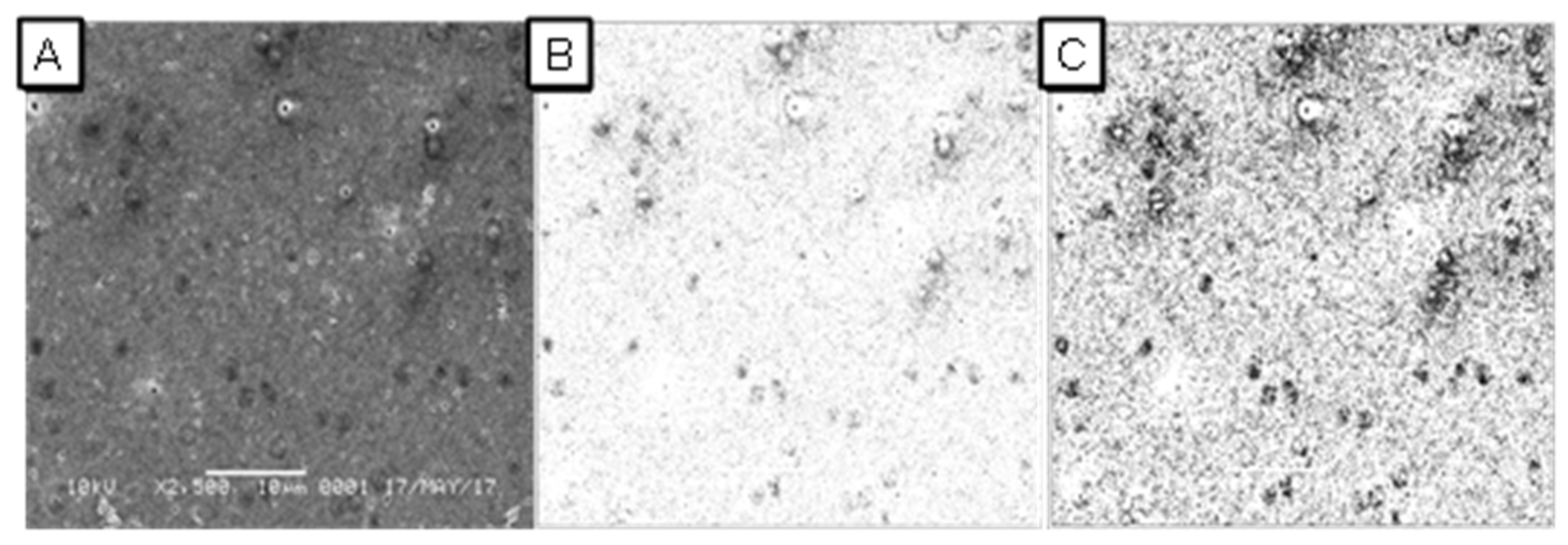
3.2. Characterisation of UF-PSf Membranes
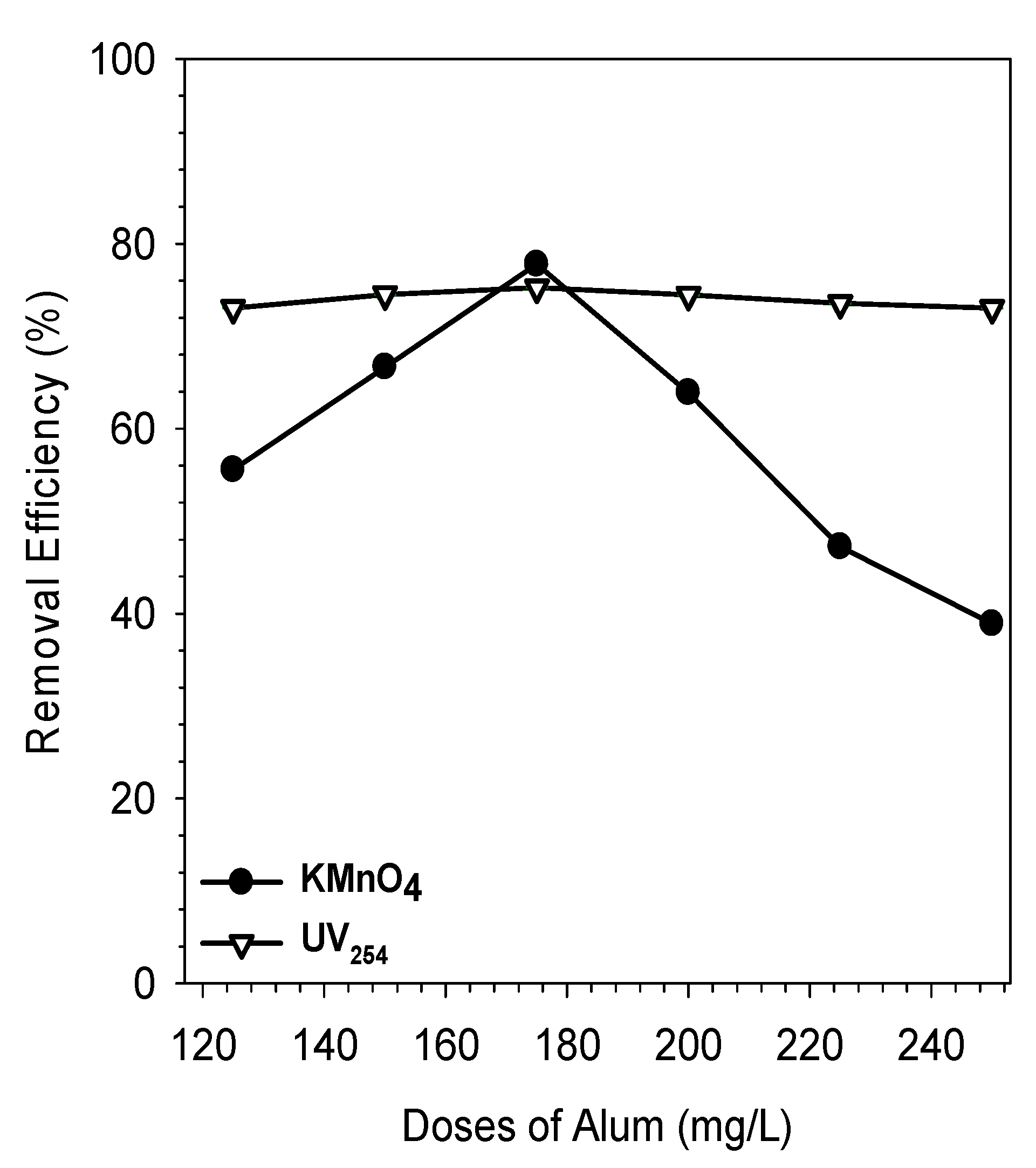
3.3. Coagulation-Flocculation
3.4. Coagulation-Adsorption
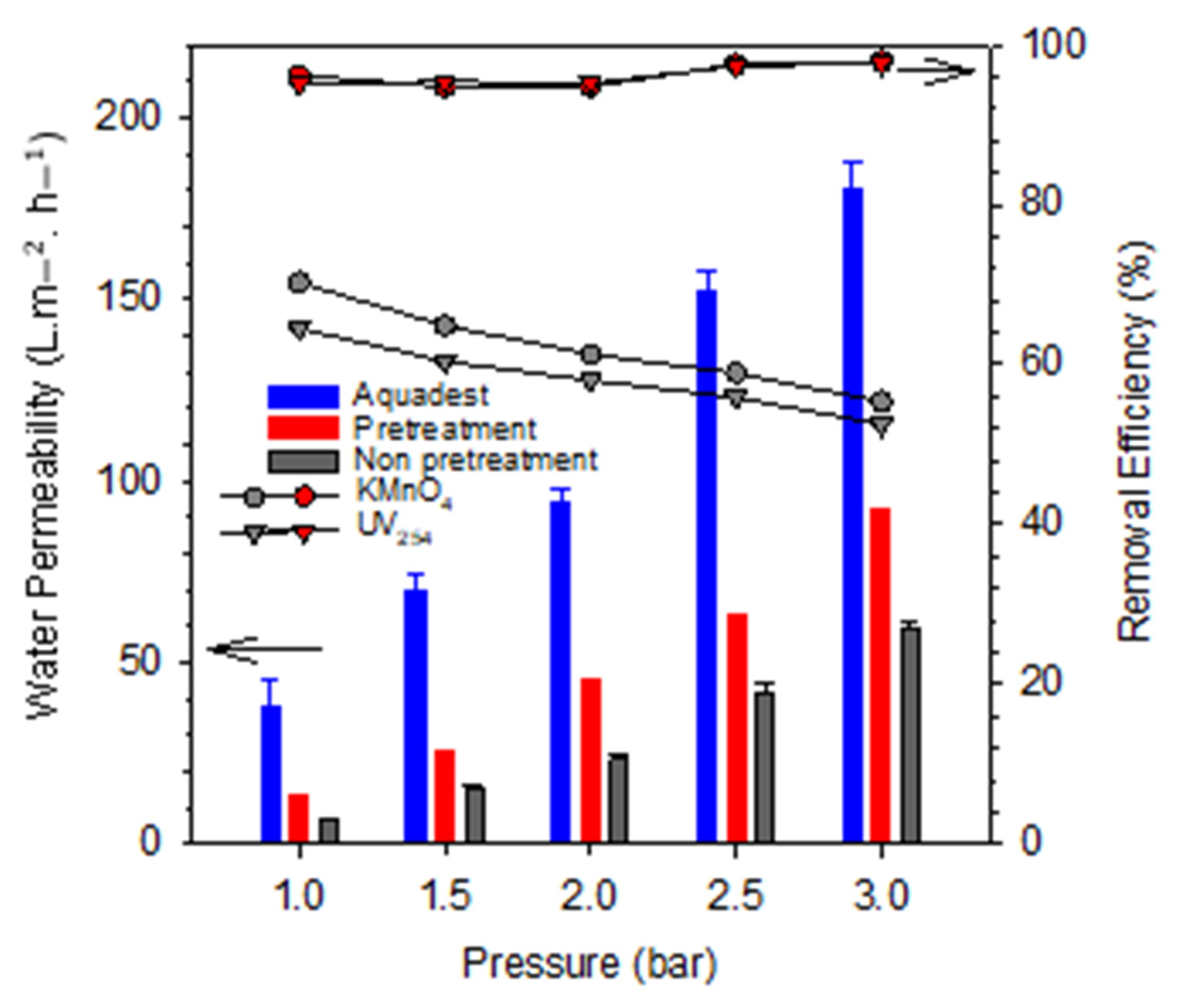
3.5. Coagulation-Adsorption-Membrane Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, C.; He, Z.; Zhao, F.; Liang, X.; Li, Z. Effects of Cations on The Formation of Ultrafiltration Membrane Fouling Layers When filtering Fulvic Acid. Desalination 2014, 352, 174–180. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Menya, E.; Olupot, P.; Storz, H.; Lubwama, M.; Kiros, Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chem. Eng. Res. Des. 2018, 129, 271–296. [Google Scholar] [CrossRef]
- McMeen, C.R.; Benjamin, M.M. NOM removal by slow sand filtration through iron oxide–coated olivine. J. Am. Water Work. Assoc. 1997, 89, 57–71. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Phuntsho, S.; Gao, B.Y.; Yang, Y.Z.; Kim, J.-H.; Shon, H.K. Comparison of A Novel Polytitanium Chloride Coagulant With Polyaluminium Chloride: Coagulation Performance And Floc Characteristics. J. Environ. Manag. 2015, 147, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.-Z.; Chen, Y.; Gao, N.-Y.; Fan, J.-C. Effect of Coagulation Pretreatment on The Fouling of Ultrafiltration Membrane. J. Environ. Sci. 2007, 19, 278–283. [Google Scholar] [CrossRef]
- Rahma, A.; Elma, M.; Mahmud, M.; Irawan, C.; Pratiwi, A.E.; Rampun, E.L.A. Removal of natural organic matter for wetland saline water desalination by coagulation-pervaporation. J. Kim. Sains Dan Apl. 2019, 22, 85–92. [Google Scholar] [CrossRef]
- Elma, M.; Rahma, A.; Pratiwi, A.E.; Rampun, E.L.A. Coagulation as pretreatment for membrane-based wetland saline water desalination. Asia Pac. J. Chem. Eng. 2020, 15, e2461. [Google Scholar] [CrossRef]
- Cui, X.; Choo, K.-H. Reviewer Paper: Natural Organic Matter Removal and Fouling Control in Low-Pressure Membrane Filtration for Water Treatment. Environ. Eng. 2014, 19, 1–8. [Google Scholar]
- Song, Y.; Dong, B.; Gao, N.; Ma, X. Powder Activated Carbon Pretreatment of A Microfiltration Membrane for The Treatment of Surface Water. Environ. Res. Public Health 2015, 12, 11269–11277. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Amal, R. TiO2-Coated Natural Zeolite: Rapid Humic Acid Adsorption And Effective Photocatalytic Regeneration. Chem. Eng. Technol. 2014, 105, 46–52. [Google Scholar] [CrossRef]
- Gamage, S.M.K.; Sathasivan, A. A Review: Potential And Challenges of Biologically Activated Carbon to Remove Natural Organic Matter in Drinking Water Purification Process. Chemosphere 2017, 167, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Rahma, A.; Elma, M.; Rampun, E.L.A.; Pratiwi, A.E.; Rakhman, A.; Fitriani. Rapid Thermal Processing and Long Term Stability of Interlayer-free Silica-P123 Membranes for Wetland Saline Water Desalination. Adv. Res. Fluid Mech. Therm. Sci. 2020, 71, 1–9. [Google Scholar] [CrossRef]
- Rahma, A.; Elma, M.; Pratiwi, A.E.; Rampun, E.L. Performance of interlayer-free pectin template silica membranes for brackish water desalination. Membr. Technol. 2020, 2020, 7–11. [Google Scholar] [CrossRef]
- Lestari, R.A.; Elma, M.; Rahma, A.; Suparsih, D.; Anadhliyah, S.; Sari, N.L.; Pratomo, D.A.; Sumardi, A.; Lestari, A.E.; Assyaifi, Z.L.; et al. Organo Silica Membranes for Wetland Saline Water Desalination: Effect of membranes calcination temperatures. E3S Web Conf. 2020, 148, 07006. [Google Scholar] [CrossRef]
- Elma, M.; Rampun, E.L.A.; Rahma, A.; Assyaifi, Z.L.; Sumardi, A.; Lestari, A.E.; Saputro, G.S.; Bilad, M.R.; Darmawan, A. Carbon templated strategies of mesoporous silica applied for water desalination: A review. J. Water Process Eng. 2020, 38, 101520. [Google Scholar] [CrossRef]
- Elma, M.; Mujiyanti, D.R.; Ismail, N.M.; Bilad, M.R.; Rahma, A.; Rahman, S.K.; Rakhman, A.; Rampun, E.L.A. Development of Hybrid and Templated Silica-P123 Membranes for Brackish Water Desalination. Polymers 2020, 12, 2644. [Google Scholar] [CrossRef]
- Elma, M.; Riskawati, N.; Marhamah. Silica Membranes for Wetland Saline Water Desalination: Performance and Long Term Stability. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012006. [Google Scholar] [CrossRef]
- Elma, M.; Hairullah; Assyaifi, Z.L. Desalination Process via Pervaporation of Wetland Saline Water. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012009. [Google Scholar] [CrossRef]
- Elma, M.; Setyawan, H.; Rahma, A.; Pratiwi, A.; Rampun, E.L.A. Fabrication of Interlayer-free P123 Caronised Template Silica Membranes for Water Desalination: Conventional Versus Rapid Thermal Processing (CTP vs RTP) Techniques. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012076. [Google Scholar] [CrossRef]
- Elma, M.; Setyawan, H. Synthesis of Silica Xerogels Obtained in Organic Catalyst via Sol Gel Route. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012008. [Google Scholar] [CrossRef]
- Rahman, S.K.; Maimunawaro; Rahma, A.; Syauqiah, I.; Elma, M. Functionalization of hybrid organosilica based membranes for water desalination—Preparation using Ethyl Silicate 40 and P123. Mater. Today: Proc. 2020, 31, 60–64. [Google Scholar] [CrossRef]
- Mawaddah, Y.; Wati, L.S.; Rampun, E.L.A.; Sumardi, A.; Lestari, A.E.; Elma, M. FTIR Studies of the TEOS/TEVS xerogel structure using rapid thermal processing method. Konversi 2020, 9, 73–78. [Google Scholar] [CrossRef]
- Maimunawaro; Rahman, S.K.; Rampun, E.L.A.; Rahma, A.; Elma, M. Deconvolution of carbon silica templated thin film using ES40 and P123 via rapid thermal processing method. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Lestari, A.E.; Elma, M.; Rabiah, S.; Rampun, E.L.A.; Rahma, A.; Pratiwi, A.E. Performance of Mesoporous Organo Silica Membrane for Desalination. In Proceedings of Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; pp. 285–292. [Google Scholar]
- Elma, M.; Rezki, M.R.; Mahmud, M.; Sunardi, S.; Pratiwi, E.N.; Oktaviana, E.N.; Fatimah, S.; Rahma, A. Membran karbon templated silika dari karbon nipah (Nypa fruticans) untuk aplikasi desalinasi air rawa asin [Carbon templated silica membranes from nypa carbon (Nypa fruticans) applied for wetland saline water desalination]. J. Ris. Ind. Has. Hutan 2020, 12, 83–92. [Google Scholar] [CrossRef]
- Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Handayani, N. The Performance of Membranes Interlayer-Free Silica-Pectin Templated for Seawater Desalination via Pervaporation Operated at High Temperature of Feed Solution. Mater. Sci. Forum 2020, 981, 349–355. [Google Scholar] [CrossRef]
- Elma, M.; Fitriani; Rakhman, A.; Hidayati, R. Silica P123 Membranes for Desalination of Wetland Saline Water in South Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012007. [Google Scholar] [CrossRef]
- Mahmud; Elma, M.; Rampun, E.L.A.; Rahma, A.; Pratiwi, A.E.; Abdi, C.; Rosadi, R. Effect of Two Stages Adsorption as Pre-Treatment of Natural Organic Matter Removal in Ultrafiltration Process for Peat Water Treatment. Mater. Sci. Forum 2020, 988, 114–121. [Google Scholar] [CrossRef]
- Herwati, N.; Mahmud; Abdi, C. Pengaruh pH Air Gambut Terhadap Fouling Membran Ultrafiltrasi. Jukung J. Tek. Lingkung 2015, 1, 59–73. [Google Scholar]
- Mahmud; Abdi, C.; Mu’min, B. Removal Natural Organic Matter (NOM) in Peat Water from Wetland Area by Coagulation-Ultrafiltration Hybrid Process with Pretreatment Two-Stage Coagulation. J. Wetl. Environ. Manag. 2013, 1, 42–49. [Google Scholar] [CrossRef]
- Lee, J.; Vigneswaran, S.; Zhang, Y.; Raj Reddy, R.S.; Liu, Z. Effective Natural Organic Matter Removal In Pond Water by Carbon Nanotube Membrane with Flocculation/Adsorption. Water Sci. Technol. Water Supply 2017, 17, 1080–1087. [Google Scholar] [CrossRef][Green Version]
- Aryanti, P.T.P.; Joscarita, S.R.; Wardani, A.K.; Subagjo; Ariono, D.; Wenten, I.G. The Influence of PEG 400 and Acetone on Polysulfone Membrane Morphology and Fouling Behaviour. J. Eng. Technol. Sci. 2016, 48, 135–149. [Google Scholar] [CrossRef]
- Wardani, A.K. Effect of Additives on Polysulfone Based Ultrafiltration Membrane For Peat Water Purification; Institut Teknologi Bandung: Bandung, Indonesia, 2013; pp. 1–37. [Google Scholar]
- Tutriyanti. Synthesis and Characterization of Polysulfone Membranes by Phase Inversion Technique: Effect of Concentration of Impregnant KCl on Coagulant Against Pore Structure of Membranes. Bachelor’s Thesis, Universitas Lambung Mangkurat, Banjarbaru, Indonesia, 2017. [Google Scholar]
- Ariono, D.; Aryanti, P.T.P.; Subagjo, S.; Wenten, I.G. The Effect of Polymer Concentration on Flux Stability of Polysulfone Membrane. In Proceedings of the International Conference on Engineering Science and Nanotechnology (ICESNANO 2016), Solo, Indonesia, 3–5 August 2016; American Institute of Physics: College Park, MD, USA, 2017; pp. 1–10. [Google Scholar]
- Saputra, A.A. Hybrid Coagulation-Ultrafiltration Process in Removing Natural Organic Matter (NOM) in Peat Water: The Effect of Variations in Coagulant Doses on Membrane Fouling. Bachelor’s Thesis, Universitas Lambung Mangkurat, Banjarbaru, Indonesia, 2014. [Google Scholar]
- Kurniawan, C.; Waluyo, T.B.; Sebayang, P. Particle Size Analysis Using Free Software Image-J. In Seminar Nasional Fisika; Pusat Penelitian Fisika-LIPI: Jakarta, Indonesia, 2011; pp. 1–8. [Google Scholar]
- Aisyahwalsiah, A. Optimization of Peat Water Treatment Using a Combined Process of Coagulation with Peaty Clay and Activated Carbon Adsorption. Bachelor’s Thesis, Universitas Lambung Mangkurat, Banjarbaru, Indonesia, 2013. [Google Scholar]
- Mahmud; Notodarmojo, S.; Padmi, T.; Soewondo, P. Adsorpsi Bahan Organik Alami (BOA) Air Gambut Pada Tanah Lempung Gambut Alami dan Teraktivasi: Studi Kesetimbangan Isoterm dan Kinetika Adsorpsi. INFO-TEKNIK 2012, 13, 28–37. [Google Scholar]
- Kang, S.K.; Choo, K.H. Why Does A Mineral Oxide Adsorbent Control Fouling Better than Powdered Activated Carbon in Hybrid Ultrafiltration Powder Treatment? J. Membr. Sci. 2010, 355, 69–77. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, D.G.; Ko, S.O. Adsorption Characteristics of Effluent Organic Matter and Natural Organic Matter by Carbon Based Nanomaterials. KSCE J. Civ. Eng. 2016, 21, 119–126. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, M.R.; Sakinah, M.; Matsuura, T. Application of Coagulation–Ultrafiltration Hybrid Process for Drinking Water Treatment: Optimization of Operating Conditions Using Experimental Design. Sep. Purification Technol. 2009, 65, 193–210. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherland, 1996. [Google Scholar]
- Chih, Y.C.; Chung, Y.W.; Ying, C.C. The Coagulation Characteristics of Humic Acid By Using Acid-Soluble Chitosan, Water-Soluble Chitosan, And Chitosan Coagulant Mixtures. Environ. Technol. 2014, 36, 1141–1146. [Google Scholar]
- Suslova, О.; Govorukha, V.; Brovarskaya, О.; Matveeva, N.; Tashyreva, H.; Tashyrev, O. Method for Determining Organic Compound Concentration in Biological Systems by Permanganate Redox Titration. Int. J. Bioautomation 2014, 18, 45–52. [Google Scholar]
- Rahman, R.A. Hybrid Coagulation-Ultrafiltration Process in Removal Natural Organic Matter (NOM) in Peat Water: Effect of Coagulants Type and Stirring Speed on Membrane Fouling. Bachelor’s Thesis, Universitas Lambung Mangkurat, Banjarbaru, Indonesia, 2014. [Google Scholar]
- Fachrozi, M. Ultrafiltration Coagulation Hybrid Process in Removal Natural Organic Matter (NOM) in Peat Water: Effect of Coagulants Type on Membrane Fouling. Bachelor’s Thesis, Universitas Lambung Mangkurat, Banjarbaru, Indonesia, 2013. [Google Scholar]
- Tomaszewska, M.; Mozia, S.Y.; Morawski, A.W. Removal of Organic Matter by Coagulation Enhanced with Adsorption on PAC. Desalination 2004, 161, 79–87. [Google Scholar] [CrossRef]
- Abdillah, A.I.; Darjito; Khunur, M.M. Pengaruh pH dan Waktu Kontak pada Adsorpsi Ion Logam Cd2+ Menggunakan Adsorben Kitin Terikat Silang Glutaraldehid. Kim. Stud. J. 2015, 1, 826–832. [Google Scholar]
- Oxidation of Organic Molecules by KMnO4; National Science Foundation University of California, LibreTexts Library: Sacramento, CA, USA, 2015.
- Adams, F.V.; Nxumalo, E.N.; Krause, R.W.M.; Hoek, E.M.V.; Mamba, B.B. Application of polysulfone/cyclodextrin mixed-matrix membranes in the removal of natural organic matter from water. Phys. Chem. Earth Parts A/B/C 2014, 67–69, 71–78. [Google Scholar] [CrossRef]
- Notodarmojo, S.; Deniva, A. Penurunan Zat Organik dan Kekeruhan Menggunakan Teknologi Membran Ultrafiltrasi dengan Sistem Aliran Dead-End (Studi Kasus: Waduk Saguling, Padalarang). Proc. ITB Sains Technol. A 2004, 36, 63–82. [Google Scholar] [CrossRef]
- Aryanti, P.T.P.; Khoiruddin; Wenten, I.G. Influence of Additives on Polysulfone-Based Ultrafiltration Membrane Performance during Peat Water Filtration. J. Water Sustain. 2013, 3, 85–96. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Benjamin, M.M.; Chang, Y. Fouling and Natural Organic Matter Removal in Adsorbent/Membrane Systems for Drinking Water Treatment. Environ. Sci. Technol. 2003, 37, 1663–1669. [Google Scholar] [CrossRef] [PubMed]


| No | Parameter | Units | Week | Average | STDEV | |||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||
| 1 | pH | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 0 | |
| 2 | DOC (dissolved organic carbon) | mg/L | 36.40 | - | - | 36.40 | 36.4 | - |
| 3 | UV254 absorbance | 1/cm | 0.968 | 1.005 | 0.977 | 0.955 | 0.976 | 0.02 |
| 4 | KMnO4 organic substances | mg KMnO4/L | 120.08 | 126.4 | 120.08 | 113.76 | 120.08 | 5.16 |
| 5 | SUVA254 | L/mg.m | 2.659 | 2.761 | 2.684 | 2.624 | 2.682 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Mahmud, M.; Abdi, C.; Rosadi, R.; Yanto, D.H.Y.; Bilad, M.R. Combination of Coagulation, Adsorption, and Ultrafiltration Processes for Organic Matter Removal from Peat Water. Sustainability 2022, 14, 370. https://doi.org/10.3390/su14010370
Elma M, Pratiwi AE, Rahma A, Rampun ELA, Mahmud M, Abdi C, Rosadi R, Yanto DHY, Bilad MR. Combination of Coagulation, Adsorption, and Ultrafiltration Processes for Organic Matter Removal from Peat Water. Sustainability. 2022; 14(1):370. https://doi.org/10.3390/su14010370
Chicago/Turabian StyleElma, Muthia, Amalia Enggar Pratiwi, Aulia Rahma, Erdina Lulu Atika Rampun, Mahmud Mahmud, Chairul Abdi, Raissa Rosadi, Dede Heri Yuli Yanto, and Muhammad Roil Bilad. 2022. "Combination of Coagulation, Adsorption, and Ultrafiltration Processes for Organic Matter Removal from Peat Water" Sustainability 14, no. 1: 370. https://doi.org/10.3390/su14010370
APA StyleElma, M., Pratiwi, A. E., Rahma, A., Rampun, E. L. A., Mahmud, M., Abdi, C., Rosadi, R., Yanto, D. H. Y., & Bilad, M. R. (2022). Combination of Coagulation, Adsorption, and Ultrafiltration Processes for Organic Matter Removal from Peat Water. Sustainability, 14(1), 370. https://doi.org/10.3390/su14010370








