Differential Effect of Hydroxen Peroxide οn Toxic Cyanobacteria of Hypertrophic Mediterranean Waterbodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Design of the Experiment
2.3. Phytoplankton Analysis
2.4. Pigment Analysis
2.4.1. Chlorophyll-a (Chl a) Analysis
2.4.2. Phycocyanin Analysis
2.5. MCYSTs Analysis
2.6. Statistical Analysis
3. Results
3.1. Water Physical—Chemical Parameters
Natural Phytoplankton Communities of the Waterbodies
3.2. H2O2 Degradation
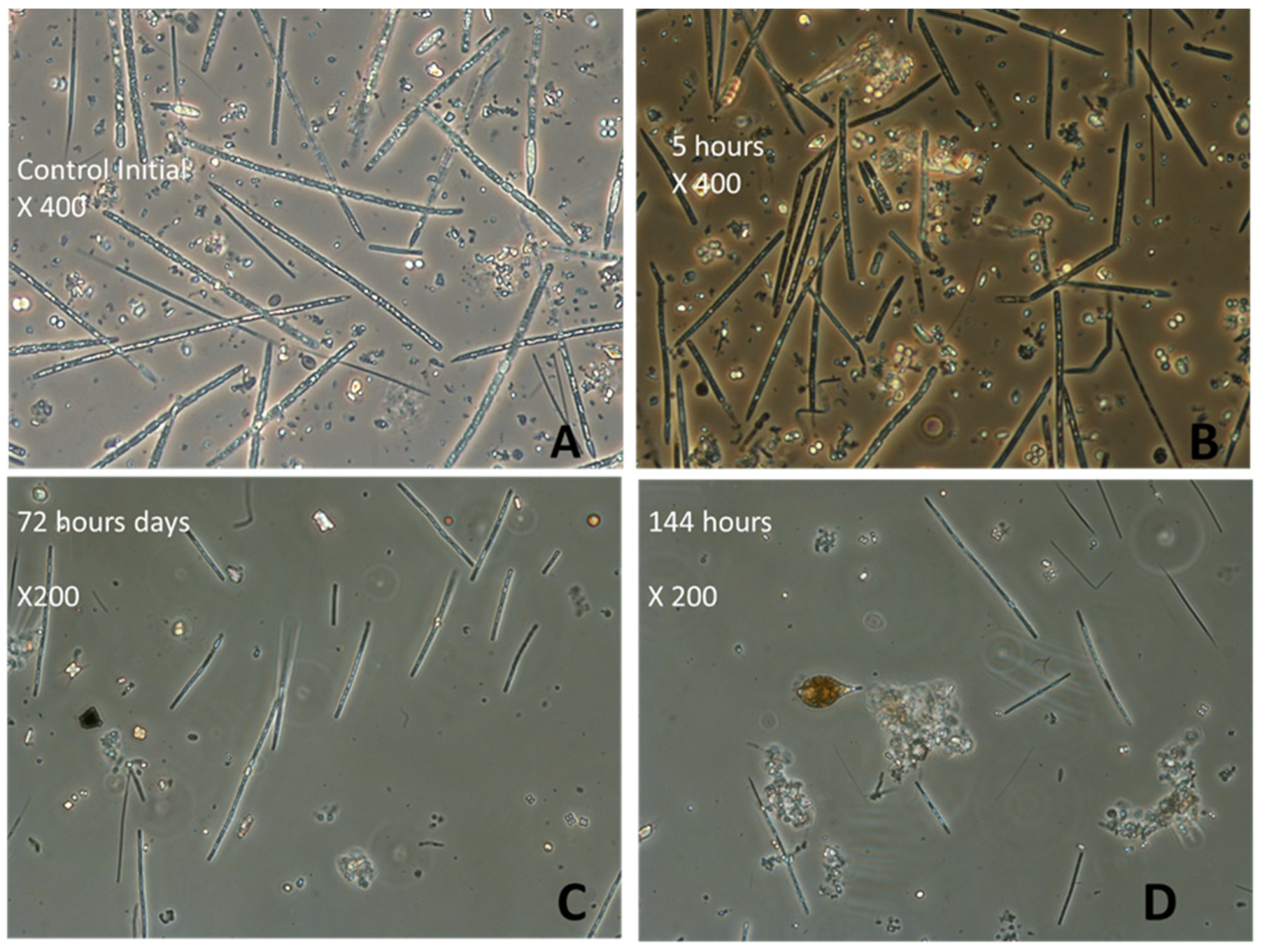
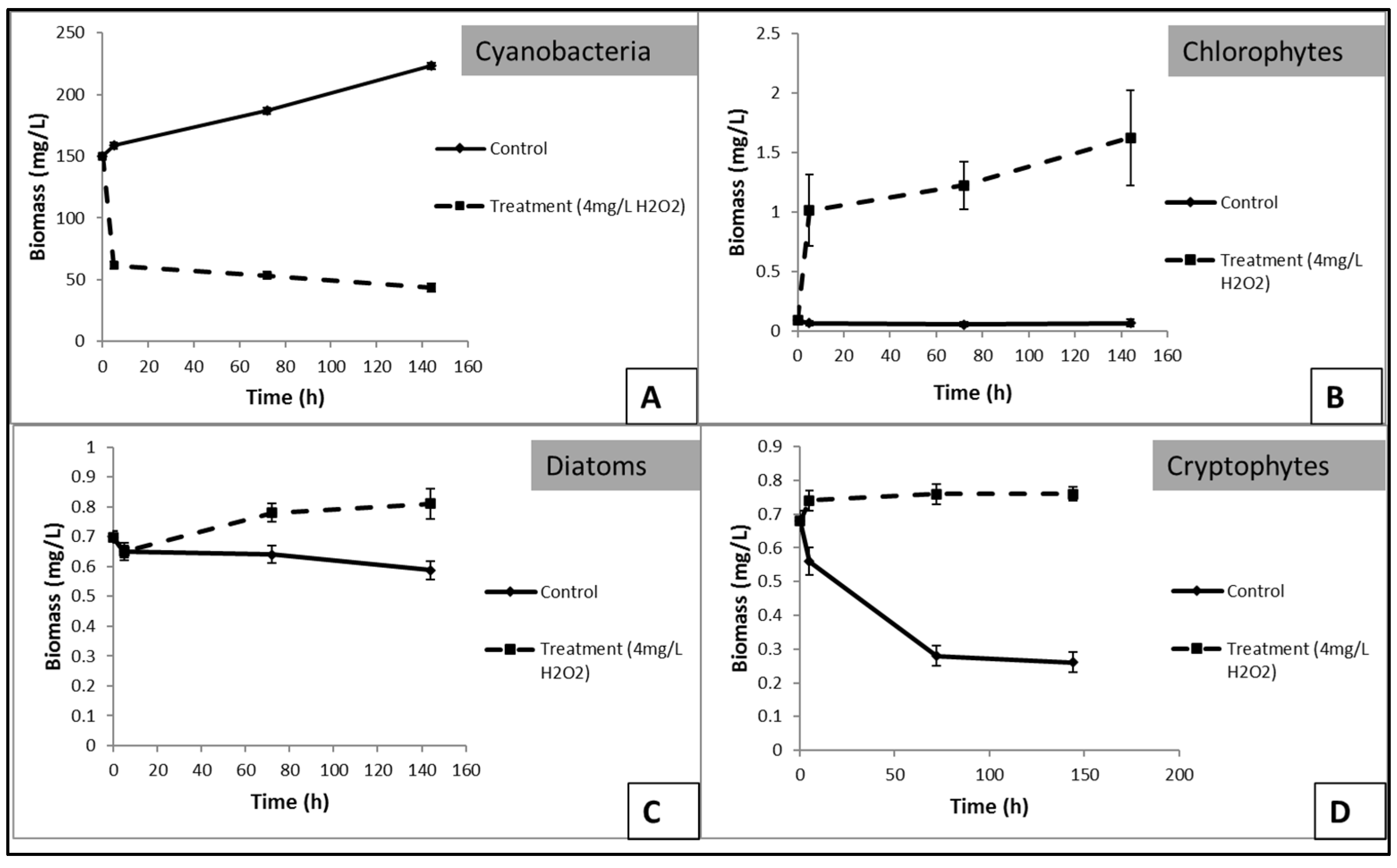
3.3. Effects of H2O2 on Karla Reservoirs’ Phytoplankton
3.4. Effects of H2O2 on Lake Pamvotis’ Phytoplankton
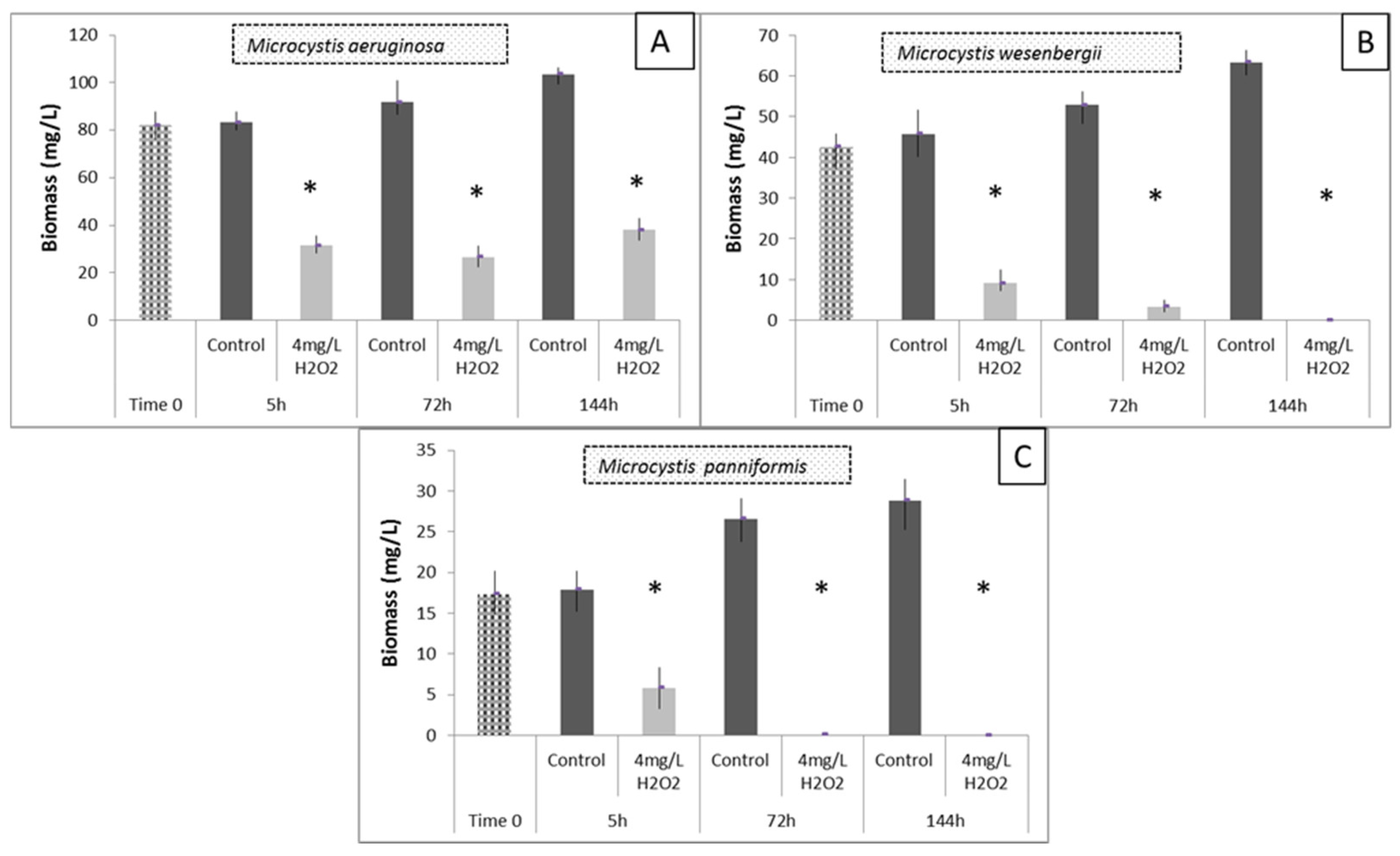
3.5. Effects of H2O2 on Different Species of Microcystis in Lake Pamvotis
3.6. Effects of H2O2 on Chl-a and Phycocyanin
3.7. Effects of H2O2 on MCYSTs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Francis, G. Poisonous Australian lake. Nature 1878, 18, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.-V.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Katsiapi, M.; Genitsaris, S.; Stefanidou, N.; Tsavdaridou, A.; Giannopoulou, I.; Stamou, G.; Michaloudi, E.; Mazaris, A.D.; Moustaka-Gouni, M. Ecological Connectivity in Two Ancient Lakes: Impact Upon Planktonic Cyanobacteria and Water Quality. Water 2020, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Benayache, Y.N.; Nguyen-Quang, T.; Hushchyna, K.; McLellan, K.; Afri-Mehennaoui, F.Z.; Noureddine Bouaï, N. An overview of cyanobacteria harmful algal bloom (CyanoHAB) issues in freshwater ecosystems. In Limnology-Some New Aspects of Inland Water Ecology; IntechOpen: London, UK, 2009. [Google Scholar]
- Moustaka-Gouni, M.; Sommer, U. Effects of Harmful Blooms of Large-Sized and Colonial Cyanobacteria on Aquatic Food Webs. Water 2020, 12, 1587. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffman, E.; Hamilton, D.P.; Brookes, J.D. Ecophysiological adaptations favour cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Moustaka-Gouni, M.; Sommer, U.; Katsiapi, M.; Vardaka, E. Monitoring of cyanobacteria for water quality: Doing the necessary right or wrong? Mar. Freshw. Res. 2019, 71, 717–724. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water; Chorus, I., Bartram, J., Eds.; World Health Organization: Geneva, Switzerland; E & FN Spon: London, UK, 1999; pp. 41–110. [Google Scholar]
- De Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotox. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology, and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [Green Version]
- MacKintosh, R.W.; Dalby, K.N.; Campbell, D.G.; Cohen, P.T.W.; Cohen, P.; MacKintosh, C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS Lett. 1995, 371, 236–240. [Google Scholar]
- Sotton, B.; Guillard, J.; Bony, S.; Devaux, A.; Domaizon, I.; Givaudan, N.; Crespeau, F.; Huet, H.; Anneville, O. Impact of Toxic Cyanobacterial Blooms on Eurasian Perch (Perca fluviatilis): Experimental Study and In Situ Observations in a Peri-Alpine Lake. PLoS ONE 2012, 7, e52243. [Google Scholar]
- Ma, J.; Feng, Y.; Jiang, S.; Li, X. Altered cellular metabolism of HepG2 cells caused by microcystin-LR. Environ. Pollut. 2017, 225, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.B.; de Silva, H.A. Cellular oxidative stress stimulated by microcystin: Review. Res. Soc. Dev. 2021, 10, e422101119765. [Google Scholar] [CrossRef]
- Zambrano, F.; Canelo, E. Effects of microcystin-LR on the partial reactions of the Naþ-Kþ pump of the gill of carp (Cyprinus carpio Linne). Toxicon 2004, 34, 451–458. [Google Scholar] [CrossRef]
- Jos, A.; Pichardo, S.; Prieto, A.I.; Repetto, G.; Vázquez, C.M.; Monero, I.; Cameán, A.M. Toxic cyanobacterial cells containing microcystins induce oxidative stress in exposed tilapia fish (Oreochromis sp.) under laboratory conditions. Aquat. Toxicol. 2005, 72, 261–271. [Google Scholar] [CrossRef]
- Karjalainen, M.; Engström-Öst, J.; Korpinen, S.; Peltonen, H.; Pääkkönen, J.-P.; Rönkkönen, S.; Suikkanen, S.; Viitasalo, M. Ecosystem Consequences of Cyanobacteria in the Northern Baltic Sea. AMBIO J. Hum. Environ. 2007, 36, 195–202. [Google Scholar] [CrossRef]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Huisman, J.J.A.E. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.R.; Quin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piehler, M. Watershed management strategies to prevent and control cyanobacterial harmful algal blooms. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 259–273. ISBN 978-0-387-75864-0. [Google Scholar]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Li, R.; Lepo, J.E.; Gu, J.-D. Potential for control of harmful cyanobacterial blooms using biologically derived substances: Problems and prospects. J. Environ. Manag. 2013, 125, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bauza, L.; Aguilera, A.; Echenique, R.; Andrinolo, D.; Giannuzzi, L. Application of hydrogen peroxide to the control of eutrophic lake systems in laboratory assays. Toxins 2014, 6, 2657–2675. [Google Scholar] [CrossRef]
- Samuilov, V.D.; Timofeev, K.N.; Sinitsyn, S.V.; Bezryadnov, D.V. H2O2-induced inhibition of photosynthetic O2 evolution by Anabaena variabilis cells. Biochemistry 2001, 69, 926–933. [Google Scholar]
- Drábková, M.; Admiraal, W.; Marsálek, B. Combined exposure to hydrogen peroxide and light: Selective effects on cyanobacteria, green algae, and diatoms. Environ. Sci. Technol. 2007, 41, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ho, L.; Hobson, P.; Daly, R.; Brookes, J. Application of various oxidants for cyanobacteria control and cyanotoxin removal in wastewater treatment. J. Environ. Eng. 2014, 140, 04014022. [Google Scholar] [CrossRef]
- Lürling, M.; Meng, D.; Faassen, E.J. Effects of hydrogen peroxide and ultrasound on biomass reduction and toxin release in the cyanobacterium, Microcystis aeruginosa. Toxins 2014, 6, 3260–3280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, X.; Chang, D.W.; Tseng, J.H.; Burch, M.D.; Lin, T.F. Exposure of Microcystis aeruginosa to hydrogen peroxide under light: Kinetic modelling of cell culture rupture and simultaneous microcystin degradation. Environ. Sci. Technol. 2015, 49, 5502–5510. [Google Scholar] [CrossRef]
- Barrington, D.J.; Reichwaldt, E.S.; Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 2013, 50, 86–94. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Yang, Z.; Buley, P.R.; Fernandez-Figueroa, E.G.; Barros, M.U.G.; Rajendran, S.; Wilson, A.E. Hydrogen peroxide treatment promotes chlorophytes over toxic cyanobacteria in a hyper-eutrophic aquaculture pond. Environ. Pollut. 2018, 240, 590–598. [Google Scholar] [CrossRef]
- Mikula, P.; Zezulka, S.; Jancula, D.; Marsalek, B. Metabolic activity and membrane integrity changes in Microcystis aeruginosa–new findings on hydrogen peroxide toxicity in cyanobacteria. Eur. J. Phycol. 2012, 47, 195–206. [Google Scholar] [CrossRef]
- Piel, T.; Sandrini, G.; White, E.; Xu, T.; Schuurmans, J.M.; Huisman, J.; Visser, P.M. Suppressing Cyanobacteria with Hydrogen Peroxide Is More Effective at High Light Intensities. Toxins 2020, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Song, Q.; Long, J.; Song, G.; Mi, W.; Bi, Y. Optimization method for Microcystis bloom mitigation by hydrogen peroxide and its stimulative effects on growth of chlorophytes. Chemosphere 2019, 228, 503–512. [Google Scholar] [CrossRef]
- Lusty, M.W.; Gobler, C.J. The Efficacy of Hydrogen Peroxide in Mitigating Cyanobacterial Blooms and Altering Microbial Communities across Four Lakes in NY, USA. Toxins 2020, 12, 428. [Google Scholar] [CrossRef]
- Piel, T.; Sandrini, G.; Muyzer, G.; Brussaard, C.P.D.; Slot, P.C.; van Herk, M.J.; Huisman, J.; Visser, P.M. Resilience of Microbial Communities after Hydrogen Peroxide Treatment of a Eutrophic Lake to Suppress Harmful Cyanobacterial Blooms. Microorganisms 2021, 9, 1495. [Google Scholar] [CrossRef]
- Oikonomou, A.; Katsiapi, M.; Karayanni, H.; Moustaka-Gouni, M.; Kormas, K. Plankton microorganisms coinciding with two consecutive mass fish kills in a newly reconstructed lake. Sci. World J. 2012, 2012, 504135. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, T.; Katsiapi, M.; Kormas, K.A.; Moustaka-Gouni, M.; Kagalou, I. Artificially born “killer” lake: Phytoplankton based water quality and microcystin affected fish in a reconstructed lake. Sci. Total Environ. 2013, 452–453, 116–124. [Google Scholar] [CrossRef]
- Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanus crispus) deaths in a Mediterranean reconstructed reservoir. Environ. Pollut. 2018, 234, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Levizou, E.; Statiris, G.; Papadimitriou, T.; Laspidou, C.S.; Kormas, K.A. Lettuce facing microcystins-rich irrigation water at different developmental stages: Effects on plant performance and microcystins bioaccumulation. Ecotoxicol. Environ. Saf. 2017, 143, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Levizou, E.; Papadimitriou, T.; Papavasileiou, E.; Papadimitriou, N.; Kormas, A.K. Root vegetables bioaccumulate microcystins-LR in a developmental stage dependent manner under realistic exposure scenario: The case of carrot and radish. Agric. Water Manag. 2020, 240, 106274. [Google Scholar] [CrossRef]
- Kagalou, I.; Tsimarakis, G.; Patsias, A. Phytoplankton dynamics and physicochemical features in Lake Pamvotis. Fresen. Environ. Bull. 2001, 10, 845–849. [Google Scholar]
- Stalikas, C.; Pilidis, G.; Karayannis, M. Heavy metal contents in sediments of the lake Ioannina and Kalamas river in northwestern Greece. Fresen. Environ. Bull. 1994, 3, 575–579. [Google Scholar]
- Papadimitriou, T.; Kagalou, I.; Stalikas, C.; Pilidis, G.; Leonardos, I.D. Assessment of microcystin distribution and biomagnification in tissues of aquatic food web compartments from a shallow lake and evaluation of potential risks to public health. Ecotoxicology 2012, 21, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- APHA. Handbook, Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VI, USA, 2005. [Google Scholar]
- Papadimitriou, T.; Kormas, K.; Dionysiou, D.D.; Laspidou, C. Using H2O2 treatments for the degradation of cyanobacteria and microcystins in a shallow hypertrophic reservoir. Environ. Sci. Pollut. Res. Int. 2016, 23, 21523–21535. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.O.; Hochanadel, C.J.; Ghormley, J.A.; Davis, R.W. Decomposition of water and aqueous solutions under mixed fast neutron and gamma radiation. J. Phys. Chem. 1952, 56, 575–586. [Google Scholar] [CrossRef]
- Zapomělova, E.; Jezberová, J.; Hrouzek, P.; Hisem, D.; Reháková, K.; Komárková, J. Polyphasic characterization of three strains of Anabaena reniformis and Aphanizomenon aphanizomenoides (cyanobacteria) and their reclassification to Sphaerospermum gen. nov. (incl. Anabaena kisseleviana). J. Phycol. 2010, 46, 415. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Katsiapi, M.; Moustaka, M.; Michaloudi, E.; Kormas, A.K. Phytoplankton and water quality in a Mediterranean drinking-water reservoir (Marathonas Reservoir, Greece). Environ. Monit. Assess 2011, 185, 563–575. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp.: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extractionmethods and stability studies on phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Mellios, N.; Kofinas, D.; Laspidou, C.; Papadimitriou, T. Mathematical modeling of trophic state and nutrient flows of Lake Karla using the PCLake model. Environ. Process 2015, 2, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Kotak, B.G.; Lam, A.K.Y.; Prepas, E.E. Variability of the hepatotoxin microcystin-LR in hypereutrophic drinking water lakes. J. Phycol. 1995, 31, 248–263. [Google Scholar] [CrossRef]
- Jouni, S. Current Trophic Status of Lake Karla, Greece, and Proposals for Remediation. Master’s Thesis, University of Edinburgh, Edinburgh, UK, 2011; p. 67. [Google Scholar]
- Chamoglou, M.; Papadimitriou, T.; Kagalou, I. Keys-Descriptors for the Functioning of a Mediterranean Reservoir: The Case of a New Lake Karla-Greece. Environ. Process 2014, 1, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Weenink, F.J.; Luimstra, V.M.; Schuurmans, J.M.; van Herk, M.J.; Visser, P.M.; Matthijs, H.C.P.; Magalhaes, V.F.; Pestana, C.J.; Edwards, C.; Lawton, L.A.; et al. Combatting cyanobacteria with hydrogen peroxide: A laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.A.; Guedes, D.O.; Barros, M.U.; Oliveira, S.; Pacheco, A.B.; Azevedo, S.M. Effect of hydrogen peroxide on natural phytoplankton and bacterioplankton in a drinking water reservoir: Mesocosm-scale study. Water Res. 2021, 197, 117069. [Google Scholar] [CrossRef] [PubMed]
- Arvin, E.; Pedersen, L.-F. Hydrogen peroxide decomposition kinetics in aquaculture water. Aquac. Eng. 2015, 64, 1–7. [Google Scholar] [CrossRef]
- Pflaumer, L.A. Hydrogen Peroxide in Eutrophic Lake Taihu, China: Addition Effects on Phytoplankton and Diel Variability in Natural Concentrations. Ph.D. Thesis, The University of North Carolina, Chapel Hill, NC, USA, 2006. [Google Scholar]
- Sinhá, A.K.; Eggleton, M.A.; Lochmann, R.T. An environmentally friendly approach for mitigating cyanobacterial bloom and their toxins in hypereutrophic ponds: Potentiality of a newly developed granular hydrogen peroxide-based compound. Sci. Total Environ. 2018, 637–638, 524–537. [Google Scholar] [CrossRef]
- Barroin, G.; Feuillade, M. Hydrogen-peroxide as a potential algicide for Oscillatoria-Rubescens Dc. Water Res. 1986, 20, 619–623. [Google Scholar] [CrossRef]
- Drabkova, M.; Matthijs, H.C.P.; Admiraal, W.; Marsalek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 2007, 45, 363–369. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [Green Version]
- Mustila, H.; Ermakova, M.; Bersanini, L.; Richaud, P.; Ajlani, G.; Battchikova, N.; Cournac, L.; Aro, E.-M. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. USA 2013, 110, 4111–4116. [Google Scholar]
- Passardi, F.; Zamocky, M.; Favet, J.; Jakopitsch, C.; Penel, C.; Obinger, C.; Dunand, C. Phylogenetic distribution of catalase-peroxidases: Are there patches of order in chaos? Gene 2007, 397, 101–113. [Google Scholar] [CrossRef]
- Wu, Z.; Gan, N.; Huang, Q.; Song, L. Response of Microcystis to copper stress—Do phenotypes of Microcystis make a difference in stress tolerance? Environ. Pollut. 2007, 147, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Golubić, S. Zwei wichtige Merkmale zur Abgrenzung der Blaualgengattungen. Schweiz. Z. Hydrol. 1967, 29, 176–184. [Google Scholar] [CrossRef]
- Hadjoudja, S.; Vignoles, C.; Deluchat, V.; Lenain, J.F.; Jeune, A.H.; Baudu, M. Short term copper toxicity on Microcystis aeruginosa and Chlorella vulgaris using flow cytometry. Aquat. Toxicol. 2009, 94, 255–264. [Google Scholar] [CrossRef]
- Gao, L.; Pan, X.; Zhang, D.; Mu, S.; Lee, D.J.; Halik, U. Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom-forming cyanobacterium Microcystis aeruginosa. Water Res. 2015, 69, 51–58. [Google Scholar] [CrossRef]
- Schuurmans, J.M.; Brinkmann, B.W.; Makower, A.K.; Dittmann, E.; Huisman, J.; Matthijs, H.C.P. Microcystin interferes with defense against high oxidativestress in harmful cyanobacteria. Harmful Algae 2018, 78, 47–55. [Google Scholar] [CrossRef]
- Bernroitner, M.; Zamocky, P.; Furtmüller, G.; Günter, A.; Peschek, C.; Obinger, C. Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria. J. Exp. Bot. 2009, 60, 423–440. [Google Scholar] [CrossRef] [Green Version]
- Spoof, L.; Jaakkola, S.; Važić, T.; Häggqvist, K.; Kirkkala, T.; Ventelä, A.-M.; Kirkkala, T.; Svirčev, Z.; Meriluoto, J. Elimination of cyanobacteria and microcystins in irrigation water—Effects of hydrogen peroxide treatment. Environ. Sci. Pollut. Res. 2020, 27, 8638–8652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellios, N.; Papadimitriou, T.; Laspidou, C. Predictive modeling of microcystin concentrations in a hypertrophic lake by means of Adaptive Neuro Fuzzy Inference System (ANFIS). Eur. Water 2016, 55, 91–103. [Google Scholar]
- Watanabe, M. Isolation, cultivation, and classification of bloom forming Microcystis in Japan. In Toxic Microcystis; Watanabe, M.F., Harada, K., Carmichael, W.W., Fujiki, H.P., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 13–34. [Google Scholar]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Biotech. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Bradt, S.; Villena, M.J. Detection of microcystins in the coastal lagoon La Albufera de Valencia, Spain by an enzyme-linked immunosorbent assay (E.L.I.S.A.). Limnetica 2002, 20, 187–196. [Google Scholar]
- Cooper, W.J.; Shao, C.W.; Lean, D.R.S.; Gordon, A.S.; Scully, F.E. Factors affecting the distribution of H2O2 in surface waters. In Environmental Chemistry of Lakes and Reservoirs; Baker, L.A., Ed.; American Chemical Society: Washington, DC, USA, 1994; Volume 237, pp. 391–422. [Google Scholar]
- Dziga, D.; Wasylewski, M.; Wladyka, B.; Nybom, S.; Meriluoto, J. Microbiological degradation of microcystins. Chem. Res. Toxicol. 2013, 26, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.I.; Tsuji, K. Persistence, and decomposition of hepatotoxic microcystins produced by cyanobacteria in natural environment. J. Toxicol. Toxin Rev. 1998, 17, 385–403. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Greseth, S.L.; Gaikowski, M.P.; Schmidt, L.J. Chronic toxicity of hydrogen peroxide to Daphnia magna in a continuous exposure, fow-through test system. Sci. Total Environ. 2008, 392, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Romano, N.; Shrivastava, J.; Monico, J.; Bishop, W.M. Oxidative stress, histopathological alterations, and antioxidant capacity in different tissues of largemouth bass (Micropterus salmoides) exposed to a newly developed sodium carbonate peroxyhydrate granular algaecide formulated with hydrogen peroxide. Aquat. Toxicol. 2020, 218, 105348. [Google Scholar] [CrossRef]
- Sandrini, G.; Piel, T.; Xu, T.; White, E.; Qin, H.; Slot, P.C.; Huisman, J.; Visser, P.M. Sensitivity to hydrogen peroxide of the bloom-forming cyanobacterium Microcystis PCC 7806 depends on nutrient availability. Harmful Algae 2020, 99, 101916. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Karla Reservoir | Lake Pamvotis |
|---|---|---|
| Temperature (°C) | 23.5 | 22.4 |
| Dissolved Oxygen (mg/L) | 8.00 | 8.10 |
| pH | 9.04 | 8.85 |
| Conductivity (m S/cm) | 3.21 | 1.97 |
| Nitrate (mg/L) | 0.57 | 0.68 |
| Nitrite (mg/L) | 0.02 | 0.03 |
| Ammonium (mg/L) | 1.56 | 1.28 |
| SRP (mg/L) | 0.32 | 0.25 |
| Pigments | ||
| Chl a (μg/L) | 250 | 570 |
| Phycocyanin (μg/L) | 456 | 1256 |
| MCYSTs | ||
| Intra-cellular (μg/L) | 5.45 | 8.10 |
| Extra-cellular (μg/L) | 3.65 | 5.21 |
| Lake Pamvotis (mg/L) | Karla Reservoir (mg/L) | |
|---|---|---|
| Cyanobacteria | ||
| Microcystis spp. (single cells) | 8.17 | |
| Microcystis aeruginosa (colonies) | 81.88 | |
| Microcystis wesenbergii (colonies) | 42.60 | |
| Microcystis panniformis (colonies) | 17.35 | |
| Raphidiopsis (Cylindrospermopsis) raciborskii | 21.72 | |
| Aphanizomenon favaloroi | 23.88 | |
| Planktolyngbya limnetica | 1.55 | |
| Chroococcus sp. | 1.91 | |
| Chlorophytes | ||
| Scenedesmus spp. | 0.07 | <0.01 |
| Monoraphidium contortum Monoraphidium minutum Monoraphidium spp. | 0.01 | |
| 0.006 | ||
| 0.01 | ||
| Diatoms | ||
| Small centric diatoms | 0.08 | <0.01 |
| Aulacoseira granulata Nitzschia spp. | 0.20 | |
| 0.37 | <0.01 | |
| Cryptophytes | ||
| Rhodomonas minuta | 0.68 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadimitriou, T.; Katsiapi, M.; Stefanidou, N.; Paxinou, A.; Poulimenakou, V.; Laspidou, C.S.; Moustaka-Gouni, M.; Kormas, K.A. Differential Effect of Hydroxen Peroxide οn Toxic Cyanobacteria of Hypertrophic Mediterranean Waterbodies. Sustainability 2022, 14, 123. https://doi.org/10.3390/su14010123
Papadimitriou T, Katsiapi M, Stefanidou N, Paxinou A, Poulimenakou V, Laspidou CS, Moustaka-Gouni M, Kormas KA. Differential Effect of Hydroxen Peroxide οn Toxic Cyanobacteria of Hypertrophic Mediterranean Waterbodies. Sustainability. 2022; 14(1):123. https://doi.org/10.3390/su14010123
Chicago/Turabian StylePapadimitriou, Theodoti, Matina Katsiapi, Natassa Stefanidou, Aikaterini Paxinou, Vasiliki Poulimenakou, Chrysi S. Laspidou, Maria Moustaka-Gouni, and Konstantinos A. Kormas. 2022. "Differential Effect of Hydroxen Peroxide οn Toxic Cyanobacteria of Hypertrophic Mediterranean Waterbodies" Sustainability 14, no. 1: 123. https://doi.org/10.3390/su14010123
APA StylePapadimitriou, T., Katsiapi, M., Stefanidou, N., Paxinou, A., Poulimenakou, V., Laspidou, C. S., Moustaka-Gouni, M., & Kormas, K. A. (2022). Differential Effect of Hydroxen Peroxide οn Toxic Cyanobacteria of Hypertrophic Mediterranean Waterbodies. Sustainability, 14(1), 123. https://doi.org/10.3390/su14010123










