Effect of Natural and Chemical Colorant Supplementation on Performance, Egg-Quality Characteristics, Yolk Fatty-Acid Profile, and Blood Constituents in Laying Hens
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Birds and Experimental Diets
2.3. Laying Performance
2.4. Egg Quality
2.5. Egg Yolk Fatty-Acid Profiles
2.6. Blood Constituents
2.7. Data Analysis
3. Results
3.1. Laying Growth Performance
3.2. Egg Quality
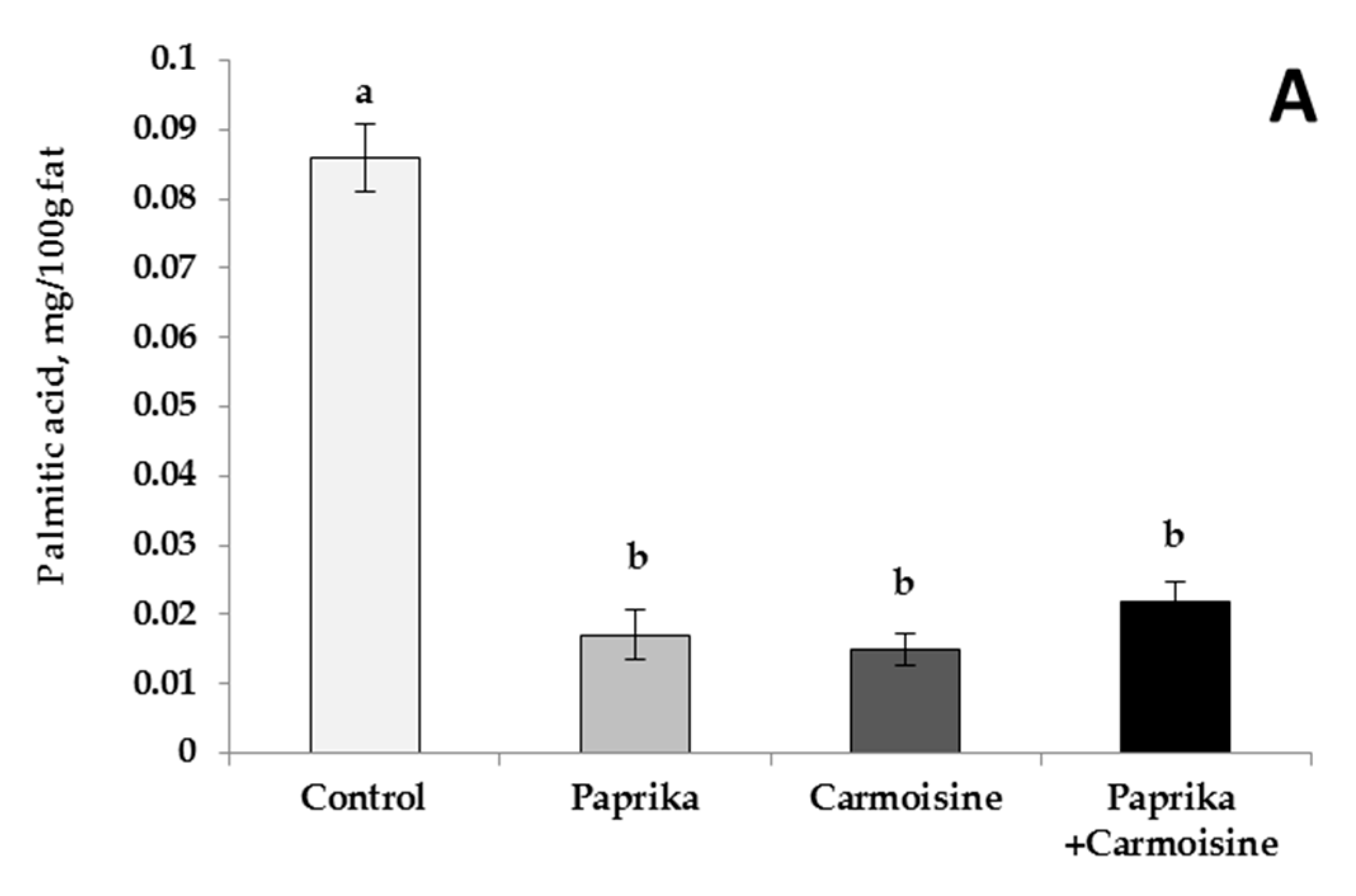
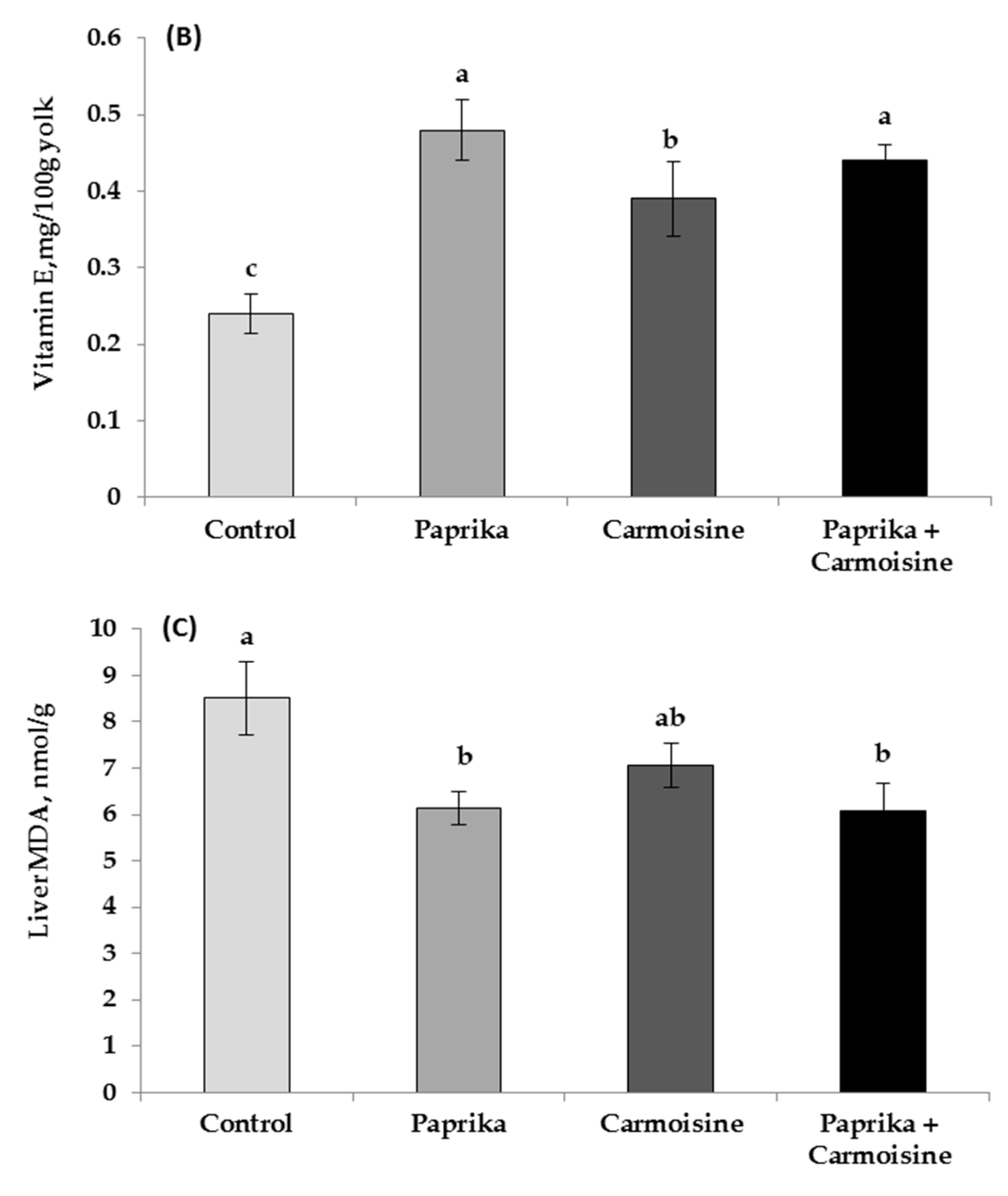
3.3. Egg Yolk Fatty-Acid Profiles
3.4. Blood Constituents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miranda, J.M.; Anton, X.; Redondo-Valbuena, C.; Roca-Saavedra, P.; Rodriguez, J.A.; Lamas, A.; Franco, C.M.; Cepeda, A. Egg and egg-derived foods: Effects on human health and use as functional foods. Nutrients 2015, 7, 706–729. [Google Scholar] [CrossRef]
- Williams, W.D. Origin and impact of color on consumer preference for food. Poult. Sci. 1992, 71, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Hencken, H. Chemical and physiological behavior of feed carotenoids and their effects on pigmentation. Poult. Sci. 1992, 71, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-La Roche, F. Egg Yolk Pigmentation with Carophyll, 3rd ed.; Co. Ltd.: Basel, Switzerland, 1988. [Google Scholar]
- Lesnierowski, G.; Stangierski, J. What’s new in chicken egg research and technology for human health promotion?—A review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Barbosa, V.C.; Gaspar, A.; Calixto, L.F.L.; Agostinho, T.S.P. Stability of the pigmentation of egg yolks enriched with omega-3 and carophyll stored at room temperature and under refrigeration. Rev. Bras. Zootec. 2011, 40, 1540–1544. [Google Scholar] [CrossRef]
- Li, I.-C.; Wu, S.-Y.; Liou, J.-F.; Liu, H.-H.; Chen, J.-H.; Chen, C.-C. Effects of Deinococcus spp. supplement on egg quality traits in laying hens. Poult. Sci. 2018, 97, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Conradie, T.A.; Pieterse, E.; Jacobs, K. Application of Paracoccus marcusii as a potential feed additive for laying hens. Poult. Sci. 2018, 97, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Nys, Y. Dietary carotenoids and egg yolk coloration-a review. Arch. Für Geflugelkd. 2000, 64, 45–54. [Google Scholar]
- Hudon, J. Biotechnological applications of research on animal pigmentation. Biotechnol. Adv. 1994, 12, 49–69. [Google Scholar] [CrossRef]
- Surai, P.; Speake, B.; Sparks, N. Carotenoids in avian nutrition and embryonic development. 1. Absorption, availability and levels in plasma and egg yolk. J. Poult. Sci. 2001, 38, 1–27. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Negro, J.J.; Surai, P.F.; Prieto, P. Carotenoids in eggs and plasma of red-legged partridges: Effects of diet and reproductive output. Physiol. Biochem. Zool. 2003, 76, 367–374. [Google Scholar] [CrossRef]
- An, S.; Kim, D.; An, B.K. Effects of dietary calcium levels on productive performance, eggshell quality and overall calcium status in aged laying hens. Asian Australas. J. Anim. Sci. 2016, 29, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D. Modern application of xanthophylls in animal feeding—A review. Trends Food Sci. Technol. 2007, 18, 501–506. [Google Scholar] [CrossRef]
- Lokaewmanee, K.; Yamauchi, K.-E.; Komori, T.; Saito, K. Effects on egg yolk colour of paprika or paprika combined with marigold flower extracts. Ital. J. Anim. Sci. 2010, 9, e67. [Google Scholar] [CrossRef]
- Beardsworth, P.; Hernandez, J. Yolk colour—An important egg quality attribute. Int. Poult. Prod. 2004, 12, 17–18. [Google Scholar]
- Skřivan, M.; Englmaierová, M. The deposition of carotenoids and α-tocopherol in hen eggs produced under a combination of sequential feeding and grazing. Anim. Feed Sci. Technol. 2014, 190, 79–86. [Google Scholar] [CrossRef]
- Skřivan, M.; Marounek, M.; Englmaierová, M.; Skřivanová, E. Effect of increasing doses of marigold (Tagetes erecta) flower extract on eggs carotenoids content, colour and oxidative stability. J. Anim. Feed Sci. 2016, 25, 58–64. [Google Scholar] [CrossRef]
- Sirri, F.; Iaffaldano, N.; Minelli, G.; Meluzzi, A.; Rosato, M.; Franchini, A. Comparative pigmentation efficiency of high dietary levels of apo-ester and marigold extract on quality traits of whole liquid egg of two strains of laying hens. J. Appl. Poult. Res. 2007, 16, 429–437. [Google Scholar] [CrossRef]
- Niu, Z.; Fu, J.; Gao, Y.; Liu, F. Influence of paprika extract supplement on egg quality of laying hens fed wheat-based diet. Int. J. Poult. Sci. 2008, 7, 887–889. [Google Scholar] [CrossRef]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Calislar, S.; Uygur, G. Effects of dry tomato pulp on egg yolk pigmentation and some egg yield characteristics of laying hens. J. Anim. Vet. Adv. 2010, 9, 96–98. [Google Scholar]
- Tuormaa, T.E. The adverse effects of food additives on health: A review of the literature with a special emphasis on childhood hyperactivity. J. Orthomol. Med. 1994, 9, 225–243. [Google Scholar]
- Reza, M.S.A.; Hasan, M.M.; Kamruzzaman, M.; Hossain, M.I.; Zubair, M.A.; Bari, L.; Abedin, M.Z.; Reza, M.A.; Khalid-Bin-Ferdaus, K.M.; Haque, K.M.F. Study of a common azo food dye in mice model: Toxicity reports and its relation to carcinogenicity. Food Sci. Nutr. 2019, 7, 667–677. [Google Scholar] [CrossRef]
- Saleh, A.A.; Zaki, A.; El-Awady, A.; Amber, K.; Badwi, N.; Eid, Y.; Ebeid, T. Effect of substituting wheat bran with cumin seed meal on laying performance, egg quality characteristics and fatty acid profile in laying hens. Vet. Arh. 2020, 90, 47–56. [Google Scholar] [CrossRef]
- Rossi, P.; Nunes, J.; Rutz, F.; Anciuti, M.; Moraes, P.; Takahashi, S.; Bottega, A.; Dorneles, J. Effect of sweet green pepper on yolk color and performance of laying hens. J. Appl. Poult. Res. 2015, 24, 10–14. [Google Scholar] [CrossRef]
- NRC. Nutrition Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Radwan Nadia, L.; Hassan, R.; Qota, E.; Fayek, H. Effect of natural antioxidant on oxidative stability of eggs and productive and reproductive performance of laying hens. Int. J. Poult. Sci. 2008, 7, 134–150. [Google Scholar] [CrossRef]
- Saleh, A.A.; Ahmed, E.A.; Ebeid, T.A. The impact of phytoestrogen source supplementation on reproductive performance, plasma profile, yolk fatty acids and antioxidative status in aged laying hens. Reprod. Domest. Anim. 2019, 54, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Surai, P. Vitamin E and egg quality. In VI European Symposium on the Quality of Egg and Egg Products, Proceedings of the XII European Symposium on the Quality of Poultry Meat and the VI European Symposium on the Quality of Eggs and Egg Products, Zaragoza, Spain, 25–29 September, 1995; Zaragoza University: Zaragoza, Spain, 1995; pp. 387–394. [Google Scholar]
- Ebeid, T.; Saleh, A.A.; Kirrella, A.A.; Dawood, M.A. Effect of dietary inclusion of cumin seed oil on the performance, egg quality, immune response and ovarian development in laying hens under high ambient temperature. Anim. Physiol. Anim. Nutr. 2019, 103, 1810–1817. [Google Scholar]
- Saleh, A.A.; Eltantawy, M.S.; Gawish, E.M.; Younis, H.H.; Amber, K.A.; Abd El-Moneim, A.E.-M.E.; Ebeid, T.A. Impact of Dietary Organic Mineral Supplementation on Reproductive Performance, Egg Quality Characteristics, Lipid Oxidation, Ovarian Follicular Development, and Immune Response in Laying Hens Under High Ambient Temperature. Biol. Trace Elem. Res. 2019, 195, 506–514. [Google Scholar] [CrossRef]
- Abiodun, B.S.; Adedeji, A.S.; Abiodun, E. Lesser known indigenous vegetables as potential natural egg colourant in laying chickens. J. Anim. Sci. Technol. 2014, 56, 18. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkhair, R.; Selim, S.; Hussein, E. Effect of supplementing layer hen diet with phytogenic feed additives on laying performance, egg quality, egg lipid peroxidation and blood biochemical constituents. Anim. Nutr. 2018, 4, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Spasevski, N.; Tasić, T.; Vukmirović, Ð.; Banjac, V.; Rakita, S.; Lević, J.; Ðuragić, O. Effect of different levels of marigold and paprika on egg production and yolk colour. Arch. Zootech. 2017, 20, 51–57. [Google Scholar]
- Lokaewmanee, K.; Yamauchi, K.; Okuda, N. Effects of dietary red pepper on egg yolk colour and histological intestinal morphology in laying hens. J. Anim. Physiol. Anim. Nutr. 2013, 97, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Lokaewmanee, K.; Yamauchi, K.; Komori, T.; Saito, K. Enhancement of egg yolk color by paprika combined with a probiotic. J. Appl. Poult. Res. 2011, 20, 90–94. [Google Scholar] [CrossRef]
- Deli, J.; Molnár, P.; Matus, Z.; Tóth, G. Carotenoid composition in the fruits of red paprika (Capsicumannuum var. lycopersiciforme rubrum) during ripening; bi-osynthesis of carotenoids in red paprika. J. Agric. Food Chem. 2001, 49, 1517–1523. [Google Scholar]
- Lin, H.; Wang, L.; Song, J.; Xie, Y.; Yang, Q. Effect of dietary supplemental levels of vitamin A on the egg production and immune responses of heat-stressed laying hens. Poult. Sci. 2002, 81, 458–465. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; da Silva, W.D.; Castro, H.; de Queiroz Barros Moreira, E.; de Oliveira Ferreira, L.; de Sousa Gomes, Y.; de Souza Junior, M.A.P. Paprika and/or marigold extracts in diets for laying hens. Rev. Bras. Saúde Produção Anim. 2017, 18, 293–302. [Google Scholar] [CrossRef]
- Saleh, A.A.; Ebeid, T.A.; Abudabos, A.M. Effect of dietary phytogenics (herbal mixture) supplementation on growth performance, nutrient utilization, antioxidative properties and immune response in broilers. Environ. Sci. Pollut. Res. 2018, 25, 14606–14613. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.A.; Abd El-Moneim, A.E.; Arif, M.; Khafaga, A.; Shaheen, H.; Samak, D.; Swelum, A.A. Putative impacts of phytogenic additives to ameliorate lead toxicity in animal feed. Environ. Sci. Pollut. Res. 2019, 26, 23209–23218. [Google Scholar] [CrossRef]
- Surai, P.F.; Bortolotti, G.R.; Fidgett, A.L.; Blount, J.D.; Speake, B.K. Effects of piscivory on the fatty acid profiles and antioxidants of avian yolk: Studies on eggs of the gannet, skua, pelican and cormorant. J. Zool. 2001, 255, 305–312. [Google Scholar] [CrossRef]
- Rowghani, E.; Maddahian, A.; Abousadi, M.A. Effects of addition of marigold flower, safflower petals, red pepper on egg-yolk color and egg production in laying hens. Pak. J. Biol. Sci. 2006, 9, 1333–1337. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A. Human requirement for N-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed; Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of saponified paprika extract, containing capsanthin as main carotenoid source, for poultry for fattening and laying (except turkeys). EFSA J. 2020, 18, e06023. [Google Scholar] [PubMed]
- Hamilton, P.B.; Tirado, F.J.; Garcia-Hernandez, F. Deposition in egg yolks of the carotenoids from saponified and unsaponified oleoresin of red pepper (Capsicum annuum) fed to laying hens. Poult. Sci. 1990, 69, 462–470. [Google Scholar] [CrossRef]
- Saleh, A.A.; Ijiri, D.; Ohtsuka, A. Effects of summer shield supplementation on the growth performance, nutrient utilization, and plasma lipid profiles in broiler chickens. J. Vet. Med. 2014, 59, 536–542. [Google Scholar]
- Ebeid, T.; Eid, Y.; Saleh, A.; Abd El-Hamid, H. Ovarian Follicular Development, Lipid Peroxidation, Antioxidative Status and Immune Response in Laying Hens Fed Fish Oil Supplemented Diets to Produce Omega-3 Enriched Eggs. Animal 2008, 2, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Moneim, A.E.; Sabic, E.M. Beneficial effect of feeding olive pulp and Aspergillus awamori on productive performance, egg quality, serum/yolk cholesterol and oxidative status in laying Japanese quails. J. Anim. Feed Sci. 2019, 28, 52–61. [Google Scholar] [CrossRef]
- Abd El-Moneim, A.E.; Sabic, E.M.; Abu-Taleb, A.M. Influence of dietary supplementation of irradiated or non-irradiated olive pulp on biochemical profile, antioxidant status and immune response of Japanese quails. Biol. Rhythm Res. 2019. [Google Scholar] [CrossRef]
- Omri, B.; Chalghoumi, R.; Abdouli, H. Study of the effects of dietary supplementation of linseeds, fenugreek seeds and tomatopepper mix on laying hen’s performances, egg yolk lipids and antioxidants profiles and lipid oxidation status. J. Anim. Sci. Livest. Prod. 2017, 1, 2–6. [Google Scholar]
- Shivaji, S.; Munegowda, D.; Arpitha, T. Recent advancements in designer egg production. Life Sci. Int. Res. J. 2017, 4, 155–158. [Google Scholar]
- Lee, B.D.; Kim, D.J.; Lee, S.J. Nutritive and economic values of high oil corn in layer diet. Poult. Sci. 2001, 80, 1527–1534. [Google Scholar] [CrossRef]
- Krinsky, N. Carotenoids as antioxidants. Nutrition 2001, 17, 815–817. [Google Scholar] [CrossRef]
- Luqman, S.; Rizvi, S. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006, 20, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Kentaro, K.; Satoru, G.; Miki, N.; Mina, Y.; Kazutoyo, A.; Chie, O.; Hironori, S.; Takenori, K.; Hiroshi, T. Mechanism of potent antiperoxidative effect of capsaicin. Biochim. Biophys. Acta 2002, 1573, 84–92. [Google Scholar]
- da Costa, L.; de Moura, N.; Marangoni, C.; Mendes, C.; Teixeira, A. Antioxidant activities of peppers of the genus Capsicum. Food Sci. Technol. 2010, 30, 51–59. [Google Scholar]


| Ingredient | g/kg |
|---|---|
| Corn | 605 |
| Soybean meal, 46% | 238 |
| Gluten meal, 62% | 32 |
| Soybean oil | 18 |
| Dicalcium phosphate | 20 |
| DL-Methionine, 99% | 2.1 |
| Threonine, 99% | 0.5 |
| Limestone | 72 |
| NaCl | 3 |
| Vitamin mineral premix 1 | 4 |
| Sodium bicarbonate | 2.4 |
| Potassium carbonate | 3 |
| Total | 1000 |
| Calculated nutrient levels 2 | |
| Crude protein, % | 17.49 |
| ME, Kcal/kg diet | 2851 |
| Calcium, % | 3.26 |
| Total phosphorus, % | 0.71 |
| Available phosphorus, % | 0.46 |
| Ether extract, % | 4.46 |
| Fiber, % | 2.80 |
| Lysine, % | 0.88 |
| Methionine, % | 0.49 |
| Item | Diets | p-Value | |||
|---|---|---|---|---|---|
| Control | Paprika | Carmoisine | Paprika + Carmoisine | ||
| Initial body weight (42 wks), g | 1908 ± 28 | 1945 ± 42 | 1927 ± 33 | 1940 ± 25 | 0.205 |
| Final body weight (54 wks), g | 1974 ± 22 | 1987 ± 36 | 1996 ± 34 | 1974 ± 22 | 0.320 |
| Body weight gain, g/84 days | 66 ± 15 | 42 ± 21 | 68 ± 20 | 34 ± 13 | 0.133 |
| Feed intake, g/day | 117.2 ± 0.65 | 114.2 ± 0.18 | 114.3 ± 0.70 | 115.1 ± 0.23 | 0.180 |
| Egg weight, g | 59.2 ± 2 | 56.9 ± 2 | 57.8 ± 1 | 56.8 ± 1 | 0.453 |
| Egg production, % | 79.8 ± 1.6 b | 83.7 ± 1.6 a | 81.8 ± 1.6 ab | 81.2 ± 1.6 ab | 0.048 |
| Egg mass, g/hen/day | 47.2 ± 3.2 | 47.6 ± 2.6 | 47.3 ± 2.4 | 46.1 ± 1.9 | 0.142 |
| Feed conversion ratio, g feed/g egg | 2.48 ± 0.39 a | 2.39 ± 0.21 b | 2.42 ± 0.14 ab | 2.49 ± 0.08 a | 0.048 |
| Item | Diets | p-Value | |||
|---|---|---|---|---|---|
| Control | Paprika | Carmoisine | Paprika + Carmoisine | ||
| At the beginning, 42 wks of age | |||||
| Egg weight, g | 60.32 ± 2.72 | 59.21 ± 1.62 | 59.32 ± 2.59 | 60.44 ± 3.68 | 0.136 |
| Egg length, cm | 5.54 ± 0.05 | 5.52 ± 0.11 | 5.47 ± 0.21 | 5.49 ± 0.15 | 0.989 |
| Egg width, cm | 4.49 ± 0.06 | 4.34 ± 0.06 | 4.25 ± 0.07 | 4.37 ± 0.07 | 0.127 |
| Eggshell thickness, µm | 470 ± 8.37 | 472 ± 12 | 468 ± 16.85 | 456 ± 13.27 | 0.820 |
| Yolk width, cm | 3.80 ± 0.09 | 3.89 ± 0.09 | 3.96 ± 0.19 | 4.02 ± 0.08 | 0.639 |
| Albumen width, cm | 8.41 ± 0.35 | 11.39 ± 2.23 | 9.36 ± 0.49 | 9.54 ± 1.24 | 0.462 |
| Yolk height, mm | 17.95 ± 0.08 | 18.32 ± 0.43 | 17.58 ± 0.40 | 18.90 ± 0.52 | 0.148 |
| Albumen height, mm | 9.14 ± 0.57 | 7.32 ± 1.08 | 6.17 ± 0.61 | 7.70 ± 0.90 | 0.122 |
| Yolk weight, g | 15.25 ± 0.58 | 15.21 ± 0.37 | 14.98 ± 0.48 | 15.11 ± 0.84 | 0.957 |
| Albumen weight, g | 33.84 ± 1.02 | 32.82 ± 0.86 | 33.33 ± 1.94 | 34.17 ± 3.41 | 0.451 |
| Yolk color, Roche fan | 6.22 ± 0.37 | 6.23 ± 0.37 | 6.41 ± 0.4 | 6.61 ± 0.24 | 0.830 |
| Shell weight, g | 11.21 ± 1.58 | 11.17 ± 0.8 | 10.99 ± 1.33 | 11.12 ± 0.71 | 0.104 |
| At the end, 54 wks. of age | |||||
| Egg weight, g | 64.23 ± 0.32 | 64.22 ± 0.97 | 64.43 ± 1.86 | 63.90 ± 0.68 | 0.437 |
| Egg length, cm | 5.71 ± 0.09 | 5.82 ± 0.037 | 5.62 ± 0.07 | 5.62 ± 0.09 | 0.249 |
| Egg width, cm | 4.56 ± 0.05 | 4.40 ± 0.03 | 4.45 ± 0.06 | 4.48 ± 0.05 | 0.163 |
| Eggshell thickness, µm | 478 ± 8.14 b | 503 ± 0.66 a | 502 ± 0.87a | 497 ± 4.49 a | 0.005 |
| Yolk width, cm | 3.99 ± 0.05 b | 4.13 ± 0.04 a | 4.14 ± 0.04 a | 3.96 ± 0.05 b | 0.021 |
| Albumen width, cm | 7.46 ± 1.62 b | 10.90 ± 0.44 a | 10.52 ± 0.57 a | 9.30 ± 0.33 a | 0.044 |
| Yolk height, mm | 17.69 ± 0.39 | 17.07 ± 0.37 | 17.19 ± 0.64 | 16.55 ± 0.33 | 0.377 |
| Albumen height, mm | 6.34 ± 0.51 a | 5.01 ± 0.25 b | 4.68 ± 0.47 b | 6.69 ± 0.25 a | 0.005 |
| Yolk weight, g | 16.12 ± 0.32 | 17.80 ± 0.49 | 17.62 ± 0.6 | 17.41 ± 0.87 | 0.065 |
| Albumen weight, g | 37.01 ± 0.71 a | 34.71 ± 1.44 b | 35.02 ± 1.22 b | 34.91 ± 0.51 b | 0.042 |
| Yolk color, Roche fan | 7.81 ± 0 b | 9.40 ± 0.24 a | 8.82 ± 0.2 a | 9.21 ± 0.37 a | 0.005 |
| Shell weight, g | 11.12 ± 0.63 | 11.70 ± 0.71 | 11.81 ± 2.49 | 11.62 ± 0.6 | 0.872 |
| Item | Diets | p-Value | |||
|---|---|---|---|---|---|
| Control | Paprika | Carmoisine | Paprika + Carmoisine | ||
| Total lipid, mg/dL | 4.51 ± 0.25 | 4.56 ± 0.31 | 4.43 ± 0.29 | 4.22 ± 0.40 | 0.446 |
| Total cholesterol, mg/dL | 160 ± 14.62 a | 148 ± 12.60 b | 157 ± 5.04 ab | 159 ± 8.39 ab | 0.048 |
| Triglyceride, mg/dL | 1.57 ± 0.14 a | 0.99 ± 0.13 c | 1.41 ± 0.15 ab | 1.26 ± 0.20 b | 0.036 |
| HDL, mg/dL | 14.5 ± 1.71b c | 21.5 ± 5.45 a | 16.0 ± 1.47 b | 19.2 ± 4.16 ab | 0.034 |
| LDL, mg/dL | 146 ± 6.49 | 126 ± 9.83 | 141 ± 4.5 | 139 ± 5.5 | 0.081 |
| Total protein, mg/dL | 2.83 ± 0.13 | 2.72 ± 0.20 | 2.83 ± 0.16 | 2.85 ± 0.21 | 0.935 |
| Albumin, mg/dL | 1.575 ± 0.19 | 1.375 ± 0.19 | 1.812 ± 0.19 | 1.823 ± 0.25 | 0.435 |
| Globulin, mg/dL | 1.25 ± 0.13 | 1.33 ± 0.14 | 1.01 ± 0.26 | 1.05 ± 0.13 | 0.514 |
| Glucose, mg/dL | 204 ± 12.87 | 209 ± 3.22 | 209 ± 6.92 | 218 ± 5.36 | 0.688 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, A.A.; Gawish, E.; Mahmoud, S.F.; Amber, K.; Awad, W.; Alzawqari, M.H.; Shukry, M.; Abdel-Moneim, A.-M.E. Effect of Natural and Chemical Colorant Supplementation on Performance, Egg-Quality Characteristics, Yolk Fatty-Acid Profile, and Blood Constituents in Laying Hens. Sustainability 2021, 13, 4503. https://doi.org/10.3390/su13084503
Saleh AA, Gawish E, Mahmoud SF, Amber K, Awad W, Alzawqari MH, Shukry M, Abdel-Moneim A-ME. Effect of Natural and Chemical Colorant Supplementation on Performance, Egg-Quality Characteristics, Yolk Fatty-Acid Profile, and Blood Constituents in Laying Hens. Sustainability. 2021; 13(8):4503. https://doi.org/10.3390/su13084503
Chicago/Turabian StyleSaleh, Ahmed A., Esraa Gawish, Samy F. Mahmoud, Khairy Amber, Wael Awad, Mohammed H. Alzawqari, Mustafa Shukry, and Abdel-Moneim Eid Abdel-Moneim. 2021. "Effect of Natural and Chemical Colorant Supplementation on Performance, Egg-Quality Characteristics, Yolk Fatty-Acid Profile, and Blood Constituents in Laying Hens" Sustainability 13, no. 8: 4503. https://doi.org/10.3390/su13084503
APA StyleSaleh, A. A., Gawish, E., Mahmoud, S. F., Amber, K., Awad, W., Alzawqari, M. H., Shukry, M., & Abdel-Moneim, A.-M. E. (2021). Effect of Natural and Chemical Colorant Supplementation on Performance, Egg-Quality Characteristics, Yolk Fatty-Acid Profile, and Blood Constituents in Laying Hens. Sustainability, 13(8), 4503. https://doi.org/10.3390/su13084503










