Abstract
Prunus padus L., is not very popular plant, it is commonly found due to low soil requirements and easy to settle in various places. As for now, concerning food technology, there is no wide application for P. padus. Therefore, the aim of this study is to evaluate the possibility of using bird cherry bark as an ingredient in herbal functional teas. In the first step, the conditions for extraction of the bark were electrochemically optimized. It was proven that the highest content of polyphenols could be found in the sample consisting of chamomile, linden flower, and calendula (7939.8 ± 106.6 mg/100 g dm). In the beta-carotene bleaching test, the highest activity could be spotted for calendula tea (16.7 ± 1.1c%) and chamomile tea (15.0 ± 2.0c%) and concerning the test for linden flower tea without added bark (134.4 ± 15.1b μg ascorbic acid /mL). The property of the tested teas to inhibit cholinesterases was proven. What is more, P. padus bark infusion showed the highest activity of 15.8 ± 1.1d μg neostigmine/mL, for acetylcholinesterases (AChE) inhibition and 21.2 ± 1.0c μg neostigmine/mL for butyrylcholinesterases (BChE). The same tea also showed the highest activity to reduce ions of iron (Fe(III)): 25.3 ± 0.9c μg Trolox /mL and glutathione reductase and glutathione peroxidase inhibition, 87.0 ± 1.1e% and 64.9 ± 2.0d% respectively. The use of P. padus bark may be vital in the preventive care concerning modern-age diseases and allow for the production of a new range of products with distinctive sensory characteristics and functional properties and, at the same time, in combating the spread of P. padus in the farm and forest ecosystem.
Keywords:
Prunus padus; bird cherry; antioxidants; SWV; cholinesterase; COX-2; SOD; catalase; glutathione reductase; inhibition; bioactive food 1. Introduction
1.1. Soil Conditions and Physiology of Bird Cherry as Invasive Plant
Prunus padus L. (bird cherry) belongs to the Rosaceae family, Padus subgenus of Prunus genus L. Taking into consideration all plants from Prunus species, bird cherry grows farthest to the north in Europe, spreading to the coasts of the Arctic Ocean. What is more, in the Alps it grows at a higher altitude than any other deciduous tree [1]. This species is highly invasive, tolerant of low and high temperatures, as well as tolerant of many soil types. It owes its ecological invasion to the high efficiency of its vegetative and generative propagation, thanks to characteristics such as the high capacity and speed of seed germination, its overproduction, its longevity, its spread, the rapid growth and development of individuals, its low requirements in relation to habitat factors, and its high tolerance to climatic conditions. In many European countries, the bird cherry is called a “forest weed” due to its dense undergrowth, which prevents the regeneration and growth of many tree species. Dense bird cherry bushes cause displacement of native species and completely prevent their natural regeneration, leading to the complete degradation of forest phytocenoses. Therefore, the negative impact of P. padus on the natural environment involves the displacement of native species. The intensity of this displacement depends on the original condition of the existing forest community. Despite determining many methods of removal of bird cherry, the most effective one has not yet been discovered.
Therefore, the expansion of this species is a threat to biodiversity conservation, and the development of effective strategies to combat invasive species is one of the most challenging tasks facing nature conservation, both concerning the preservation of natural vegetation and native plants. The literature on the topic contains statements that show that depriving bird cherry of their bark can limit and weaken their growth [2].
1.2. Nutritional and Functional Potential Related to the Phytocomponents Present in Prunus padus L.
Bird cherry contains numerous compounds that might be healthy. Its bark has a pleasant, distinctive pungent scent, similar to that of black currant. As a herbaceous plant, it is harvested in summer. It contains, among others: tocopherols, vitamins, tannins, terpenes, and polyphenols [3].
P. padus bark is known for its anti-inflammatory, antimicrobial, and antioxidant properties [4]. Bird cherry bark has the property of iron ions chelation and reduction [5]. It is used to brew infusions with medical properties [6]. The compounds present in bird cherry may prove vital for herbal medicine or in the production of extracts, as they have health-promoting properties, e.g., lowering the risk of cancer, cardiovascular disease, and diabetes. In addition, they reduce inflammation [7]. This is due to the polyphenols which improve the oxidant/antioxidant balance in the organism. Polyphenols can support the effects of health-promoting substances [8].
Modern medicine uses synthetic acetylcholinesterases (AChE) and butyrylcholinesterases (BChE) inhibitors for the symptomatic treatment of dementia, but these drugs often cause side effects that could be minimized by supporting therapy using phytochemicals [9]. The literature confirms the possible effect of polyphenols as preventive and supportive agents for the treatment of neurodegenerative diseases. Polyphenols may also be useful in inhibiting other enzymes that are crucial in eliminat-ing the effects of oxidative stress. This is due to the multidirectional action of polyphenols as antioxidants [10].
In recent years, the nutritional use of P. padus is increasing. Due to the presence of active compounds, its health-promoting effects may be expected in terms of chelating and reducing metal ions, and it may also be a source of substances helpful in inhibiting cholinesterase activity and inhibiting glutathione reductase and glutathione peroxidase. The aim of this study was to evaluate the possibility of using it as an ingredient in functional teas with a broad spectrum of health-promoting properties, while also inhibiting the spread of the species.
2. Materials and Methods
2.1. Preparation of Tea Blends and Infusions with P. padus Bark
The test sample was bird cherry (P. padus) bark from an orchard in Ozierany Małe in Podlasie region, Poland (53° 13′ 14.865″ N 23° 51′ 9.327″ E) and was prepared as described above [5]. The ingredients for the preparation of infusions (Prunus padus L. bark), linden flower (Tilia europaea L.), chamomile flower (Matricarnia Chamomilla L.) and calendula (Calendula officinalis L.) were ground in a Retsch (Haan, Germany) Grindomix GM 200 for 15 s at 500 rpm at 21 °C to obtain a particle size of 0.5–0.9 mm. The teas were prepared by mixing the dry ingredients and weighing 2-gram portions, which were packed in disposable tissue paper teabags. Eleven samples were prepared, four of which contained only one component: P (100% P. padus), Ch (100% Matricarnia Chamomilla L.), C (100% Calendula officinalis L.) and T (100% Tilia europaea L.), the first sample was a base labeled B (33.33% Ch + 33.33% C + 33.33% T), and six samples contained P. padus (P) bark at different concentrations: BP5 (5% P + 95%B), BP10 (10% P + 90%B), BP15 (15% P + 85%B), BP20 (20% P + 80%B), BP25 (25% P + 75%B), BP30 (30% P + 70%B). The infusion was obtained by extracting the bag, containing dry product, with 200 mL of water at 80 °C in 15 min.
2.2. An Electrochemical Method for Optimizing Bird Cherry Bark Extraction Conditions
The content of redox compounds in bark extracts was determined using square wave voltammetry (SWV) and the procedure described previously [11]. Measurements were performed using a potentiostat PGSTAT12 with the GPES 4.9 software (EcoChemie, The Netherlands) and a three-electrode system consisting of a reference (Ag/AgCl, 3 M KCl) (Mineral, Poland), auxiliary (platinum) (Mineral, Poland), and working (carbon paste electrode: CPE). The CPE was made by itself according to a described procedure [12]. Carbon paste was made by mixing graphite powder (Sigma) with mineral oil (Sigma) in the ratio of 70:30 (w/w). The surface of the CPE was reestablished before use by removing its outer layer on a filter paper, application of fresh paste, and polishing it to a smooth finish on a frosted glass slide. Before carrying out measurement, the surface of CPE was conditioned in 0.05 M phosphate buffer with 0.01 M KCl (pH 7.0) at +1.7 V for 60 s. Next, the electrodes were immersed for 120 s in the extract dissolved in phosphate buffer (1:1, v/v) and the three measurements of SWV in the range from −0.3 V to + 1.4 V were taken. SWV parameters were: step potential of 5 mV, frequency of 50 Hz, and amplitude of 40 mV. Baselines with moving average were subtracted from SWV voltammograms, and the peak potential, peak height (current), peak area for each signal, and the total peak areas were determined. Additionally, the electrochemical index (EI) was calculated, describing the electrochemical activity of extracts, expressed as the total area of all redox signals, in relation to 1 g dry matter of plant material [11]. One ml of the tested extract contained 0.03125 g dry plant material.
2.3. Phenolic Acids, Flanovols, and Low Molecular Weight Organic Acid (LMWOA)
Phenolic compounds in the samples were analyzed after alkaline and acid hydrolysis. The analysis was performed using a Waters Acquity Class H UPLC system equipped with a Waters Acquity Photodiode Array (PDA) detector (Waters, USA). Chromatographic separation was performed on an Acquity UPLC® BEH C18 column (100 mm × 2.1 mm, particle size 1.7 μm) (Waters, Ireland). Gradient elution was conducted using the following mobile phase composition: A: acetonitrile with 0.1% formic acid, B: 1% aqueous formic acid mixture (pH = 2). The concentrations of phenolic compounds were determined using an internal standard at wavelengths λ = 320 nm and 280 nm and expressed in mg/100 g dry weight. The detection level was 1 μg/g.
Low molecular weight organic acid (LMWOA) analysis was performed using a Waters Acquity Class H UPLC system. The separation was performed on an Acquity UPLC BEH C18 column (150 mm × 2.1 mm, 1.7 um, Waters) thermostatically heated to 35 °C. Gradient elution was performed with water and acetonitrile (both containing 0.1% formic acid, pH = 2) at a flow rate of 0.4 mL/min. Detection was performed using a Waters Photodiode Array (PDA) Detector (Waters Corporation, Milford, MA, USA) at λ = 280 nm. Results were expressed as milligrams per 100 g dry weight of tea.
2.4. Linoleic Acid Oxidation, Beta-Carotene Bleaching Test and FRAP Method
Linoleic acid oxidation. The study used the method published by Kozarski et al. (2012) with some modifications [13]. Linoleic acid (800 mg) was dissolved in 20 mL of absolute MeOH and 200 mL of 0.2 M sodium phosphate buffer (pH 6.5) and Tween 20 (6.5 mM conc. of Tween 20 was obtained). The emulsion was sonicated for 10 min. 1.8 mL linoleic acid emulsion was mixed with 0.2 mL of the sample and incubated at 37 °C. Samples (0.1 mL) were taken out after 6 h of incubation and mixed with 1.2 mL of absolute MeOH. The absorbance against the blank sample was measured at 234 nm, without the studied solution. Ten ascorbic acid solutions (concentration in the range 124.5–1824.3 μg ascorbic acid/mL) were used for the preparation of the calibration curve in the same way as the tested samples. In addition, the content was measured at 234 nm (a mixture containing the studied sample and buffer only [13]. Each sample was analyzed at least four times.
Beta-carotene bleaching test. The study used the method published by Öztürk et al. (2011) with some modifications [14]. Seven mg of β-carotene in 5 mL of chloroform was mixed with 350 μL of linoleic acid and 2.8 g of Tween 80. Vacuum evaporation of chloroform was performed (40 °C) and 100 mL of DDI (doubly distilled water) saturated with oxygen was added followed by vigorous shaking. The sample (200 μL) was mixed with β-carotene/linoleic acid emulsion (200 μL). Zero time absorbance and the change in absorbance were measured at 463 nm after 4 h at 50 °C. A positive sample was prepared using ascorbic acid. A total of 0.94 mg/mL of stock solution, used instead of samples, was prepared followed by a serial dilution (9.4–94 μg/mL), as described above. In addition, the content of samples was measured (a mixture containing the studied sample and DDI water only). The percentage of activity of the samples was calculated according to the blank samples containing emulsion and DDI water only [14]. Each sample was analyzed at least four times.
The FRAP method of Hanafy et al. (2017) with slight modifications was used [15]. The amount of 20 μL of the tested solution was mixed with 1900 μL of FRAP (ferric ion reducing antioxidant power) solution, shaken for 30 min at room temperature and the absorbance was read at 593 nm, after shaking for 30 s. The FRAP solution was prepared by mixing 2.5 mL 5 mM TPTZ solution (prepared in 40 mM HCl solution), 2.5 mL 5 mM FeCl3 solution, and 25 mL acetate buffer (0.3M pH 3.6), and warming in water at 37 °C for 20 min. A blank (reagent) sample was prepared with buffer instead of the sample and the content of the sample was measured (a mixture containing the studied sample and buffer only). All samples were measured at 593 nm. The calibration curve was prepared using Trolox solution: 0.51 mg of Trolox was dissolved in 1 mL of DDI water. An amount of 20 μL of 20 standard solutions (containing 0.0243–0.524 mg Trolox/mL) was studied, as described above [15]. Each sample was analyzed at least four times.
2.5. Cholinesterase (AChE and BChE), Catalase and SOD Inhibition
Inhibition of AChE and BChE tests were performed as described in a work by Baranowska-Wójcik et al. (2020) [16]. Neostigmine standard solutions (neostigmine bromide, Sigma Aldrich N2001, 2.57–22.85 µg/mL in ethanol: DDI (doubly distilled water) 1:1) were prepared and analyzed using the same method as tested samples. The calibration curve was developed and the inhibition of enzymes was expressed in the equivalent concentration of neostigmine (µg/mL). Each sample was analyzed at least four times.
Inhibition of catalase test. The method of Watanabe et al. (2007), with slight modifications, was used [17]. The reaction mixture was composed of 50 samples, 945 µL EDTA (ethylenediaminetetraacetic acid) solution (6.2 mg EDTA in 1 M Tri-HCl, pH 7.0, previously filtered through 0.45 μm membrane), and 12 µL of 30% H2O2 solution (final conc. 0.035 M/L). The reaction was started by adding 2.5 μL catalase solution (Sigma C3515, previously diluted 100-fold using 1M Tris-HCl, pH 7.0). Absorbances were measured at 240 nm at the start and after 2 min. In controls, the tested sample was replaced by Tris buffer. The calculations were performed as follows: Inhibition [%] = 100 − 100 × (Abs. of the sample 0 min − Abs. of the sample 2 min)/(Abs. of the control 0 min − Abs. of the control 2 min) [17]. Each sample was analyzed at least four times.
This study adopted methods for inhibition of SOD from Parschat et ale. (2001) implementing their own modifications [18]. The test mixture was composed of 50 μL of the sample, 10 μL SOD (0.24 U), 160 μL of nitro blue tetrazolium (NBT, 0.0025 M), 140 μL phosphate buffer (0.2 M, pH 7.5), 30 μL xanthine (150 mM in 1 M NaOH), and 10 μL xanthine oxidase (0.065 U, Sigma Aldrich X4875). The change in the absorbance in the control sample (without test sample) at 550 nm was 0.02/min. The change in the absorbance was measured after 0 min and 20 min. The calculations were performed as follows: Inhibition [%] = 100 − 100 × (Abs. of the sample 0 min. − Abs. of the sample 2 min)/(Abs. of the control 0 min − Abs. of the control 2 min) [18]. Each sample was analyzed at least four times.
2.6. Inhibition of Glutathione Reductase, Inhibition of Glutathione Peroxidase
Inhibition of glutathione reductase. The method of Moreira et al. (2014) was used [19]. The studied sample (150 μL) was mixed with 660 μL of 0.1 mM sodium phosphate buffer. Then, 25 μL of EDTA solution and 30 μL of GSSG (glutathione disuflide) solution were added, samples were incubated for 5 mins at 25 °C, and 40 μL of NADPH (nicotinamide adenine dinucleotide phosphate) solution was added (all reagents were dissolved in 0.1 mM sodium phosphate buffer, pH 7.6) (concentrations of reagents in the final mixture (805 μL) were as follows: 0.5 mM EDTA, 10 mM GSSG, and 10 mM NADPH). The initial absorbance (340 nm) was recorded, and the reaction was initiated by adding 2 U enzyme (2 μL, Sigma Aldrich G3664). The absorbance (340 nm) was recorded after 2.5 min of incubation at 25 °C. A blank sample was prepared with buffer instead of the sample and the content of the sample was measured (a mixture containing the studied sample and buffer only). One unit of enzyme activity was defined as nmol of NADPH consumed/min − mL of the sample, in comparison with nmol of NADPH consumed/min in the blank (reagent) sample [19]. Each sample was analyzed at least four times.
Inhibition of glutathione peroxidase. In the study, the method of Singh et al. (2000) was used with some modifications [20]. The sample (150 μL) was a mix of 20 μL EDTA solution, 3 μL of glutathione reductase (0.2 U Sigma G3664), 10 μL of GSH solution, 3 μL of glutathione peroxidase (0.04 U Sigma G6137), 55 μL of H2O2, and 663 μL of 50 mM sodium phosphate, pH 7.0). To start the reaction, 10 μL of NADPH solution (N5130) was added and, after 2 min of incubation at 25 °C, the decrease in the absorbance (340 nm) was read. All solutions were prepared in a 50 mM buffer solution and the concentration of the reagents in the final mixture was as follows: 1 mM EDTA, 0.2 U glutathione reductase, 2 mM GSH, 0.04 U glutathione peroxidase, 1.5 mMH2O2, and 0.8 mM NADPH. A blank sample was prepared with buffer instead of the sample and the content of the sample was measured (a mixture containing the studied sample and buffer only). One unit of enzyme activity has been defined as nmol of NADPH consumed/min − mL of the sample, in comparison with nmol of NADPH consumed/min in the blank (reagent) sample [20]. Each sample was analyzed at least four times.
2.7. Sensory Analysis by Sensory Profiling
For a detailed sensory description of these samples the method of quantitative descriptive analysis, i.e., sensory profiling, was applied, which was performed by a 20-person team specially trained for this purpose. Individual quality descriptors selected in preliminary studies were evaluated: color (clear, straw, brown, green), taste (sweet, sour, bitter, fruity, astringent, grassy, foreign), scent (fruity, earthy, sweet, sour, unfamiliar, intense) and overall desirability of the teas. The intensity of each quality descriptor was determined using a 10-cm unstructured linear scale with appropriate boundary markings. The results obtained were described as numerical values expressed in conventional units.
2.8. Statistical Analysis
All tests were conducted three times and the results are expressed as mean ± SD. The routine statistical tests (average values and standard deviation) were performed. Statistical differences were calculated with significant differences identified at p < 0.05 (Statistica Software ver. 13.1 StatSoft, Cracow, Poland). Additionally, the analysis of the principal components was performed (PCA). The electrochemical results were treated as an additional factor to the model based on standard analytical techniques. The p values for Levene’s test of independent variables were calculated.
3. Results
3.1. Electrochemical Analysis
Optimization of the process conditions is aimed at determining the best solutions concerning the criterion of the quality of the final product. The stage that determines the content of phenolic compounds, as well as the antioxidant activity of infusions, is the extraction process. What is more, a vital element also includes the choice of time and temperature. Electrochemical activity (antioxidant potential) of the bark of P. padus was tested using different extraction temperatures (80 °C and 100 °C ) and extraction duration (15, 30, or 45 min). The electrochemical (SWV: square wave voltammetry) measurement procedure was identical to the one adopted in the previous studies [5,21].
The total peak current, total peak area and electrochemical activity of the extracts from the bark of P. padus, prepared using different temperature and extraction time, was tested.
Below (Table 1), there is a summary of average results (number of measurements n = 3).

Table 1.
Electrochemical parameters determined for Prunus padus L. (P. padus) bark extracts.
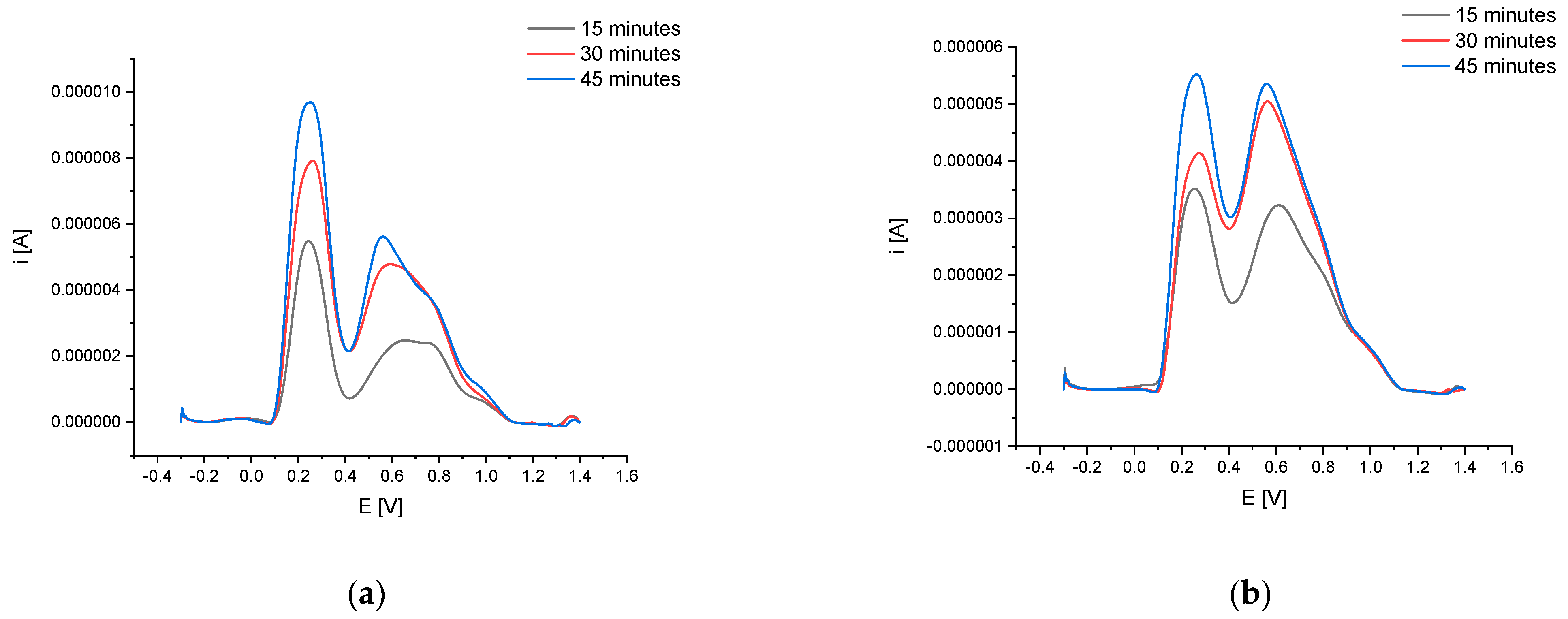
Concerning the electrochemical test, one could see that the highest antioxidant activity was characteristic for an infusion prepared at 45 min at 80 °C (total peak area 3.933 V × µA) (Figure 1).
Figure 1.
Square wave voltammetry (SWV) voltammograms for P. padus bark extracts (80 °C (a) and 100 °C (b)).
Extending the extraction process time from 15 to 45 min increased the tested electrochemical activity by 170%, however, due to the very long brewing time, the most practical brewing time, from the consumer’s point of view, of 15 min and a temperature of 80 °C were chosen for further analysis. The infusions obtained in this way were also characterized by high electrochemical activity.
3.2. Content of Phytochemicals Compounds in Infusions with P. padus Bark Added
Of the samples tested, sample B (Table 2) was characterized by the highest polyphenol content, in which the total content of polyphenolic compounds was 7939.8 ± 106.6 mg/100 g dw. It had the highest content of isorhamnetins (1951.3 ± 3.6 mg/100 g dw), as well as catechins (1251.6 ± 3.2 mg/100 g dw) and apigenins (900 ± 1.5 mg/100 g dw). Nonetheless, oxalic acid (1.2 ± 0.1 mg/100 g dw) and maleic acid (7.1 ± 0.1 mg/100 g dw) were compounds with the lowest content in the sample. Among the infusions with added P. padus bark, the total polyphenols content ranged from 7509.2 ± 189.6 mg/100 g in sample BP5 to 6546.4 ± 66.5 mg/100 g in sample BP30. One could see that the content of isorhamnetin (between 1841.6 ± 20.7 and 1362.2 ± 3.2 mg/100 g dm) and catechins (between 1206.4 ± 4.8 and 1140.9 ± 2.5 mg/100 g dm) was the highest. On the contrary, the infusions with bird cherry bark added were characterized by low content of oxalic acid (between 0.9 ± 0.1 and 1.1 ± 0.1 mg/100 g dm) and vanillic acid (between 4.8 ± 0.3 and 6.2 ± 0.1 mg/100 g dm). Concerning low molecular weight organic acids (LMWOA), their lowest value was determined in sample Ch, while the highest value was obtained for the infusion prepared from bark alone (B) and was 1300% higher than in the tea with chamomile. All teas with P. padus bark contained a very high amount of acids.

Table 2.
Content of phytochemicals compounds in dietary infusions with the bark of P. padus.
3.3. Linoleic Acid Oxidation, Beta-Carotene Bleaching Test and FRAP Method
It was decided to broaden the scope of the study by incorporating the linoleic acid oxidation test, in which the highest antioxidant activity against this acid was determined in tea without added bird cherry bark (B) (134.4 ± 15.1b μg ascorbic acid /mL), and the lowest in tea with its 20% content (BP20) (107.3 ± 7.2a μg ascorbic acid /mL). Concerning the beta-carotene bleaching test, a common method used to assess antioxidant activity, the highest results were obtained for samples: C (16.7 ± 1.1c%) and Ch (15.0 ± 2.0c%), and the lowest was shown by the BP20 sample (9.2 ± 0.4a%). The infusions with the addition of bark were characterized in terms of their ability to reduce Fe (III) to Fe (II). This ability represents antioxidant activity as expressed by its mechanism of action as the ability to donate an electron. This reaction is associated with an increase in the negative charge and, as a result, a decrease in the degree of oxidation. In the assay for the ability to reduce Fe (III) iron ions, one could see that this property was most visible for infusions containing only bird cherry bark (P), respectively: 25.3 ± 0.9c μg Trolox /mL, and for an infusion with 20% addition of the tested material (BP20) 22.1 ± 0.3b (Table 3).

Table 3.
Linoleic acid oxidation and beta-carotene bleaching test.
3.4. Inhibition of AChE, BChE, Catalase and SOD
The ability to inhibit acetylcholinesterases (AChE) and butyrylcholinesterases (BChE) was demonstrated for all tested extracts. The highest ability to inhibit cholinesterase was determined for an infusion of bird cherry bark alone (P) 15.8 ± 1.1d μg neostigmine/mL for AChE and 21.2 ± 1.0c μg neostigmine/mL for BChE (Table 4). Concerning bark teas, the highest activity was determined for the infusion with 20% addition of the tested material (BP20) 12.1 ± 0.1c μg neostigmine/mL for AChE and 18.5 ± 1.5bc μg neostigmine/mL for BChE. Cholinesterases are enzymes that cleave choline esters. There are two enzymes belonging to the cholinesterase group, AChE, which is present in the nervous system and interrupts neurotransmission, and BChE found in the brain, serum, and nervous system.

Table 4.
Inhibition of acetylcholinesterases (AChE), butyrylcholinesterases (BChE), catalase and (SOD) superoxide dismutase in teas with P. padus bark added.
Catalase, on the other hand, is a protein enzyme, a type of haemoprotein that plays a vital role in cell protection and as an antioxidant. Superoxide dismutase enzymes catalyze the dismutation of superoxide radicals to hydrogen peroxide (H2O2) and molecular oxygen (O2), providing cellular defense against reactive oxygen species and consequently constitute an important defense mechanism against superoxide radicals’ toxicity. As indicated by the statistical analysis of the results, the mixture containing the addition of bird cherry bark in the range of 10–30% of the share was characterized by a statistically higher activity than the raw material base or the mixture with the addition of 5% bark. Catalasa and SOD inhibition for the BP20, BP25, and BP30 trials, although nominally different, did not differ statistically.
The results obtained for the inhibition of catalase and SOD enzymes were similar. Again, the highest inhibitory activity was determined for the infusion of bird cherry bark alone (P), 62.9 ± 2.8e % for catalase and 37.5 ± 2.3e% for SOD, and the lowest for the infusion consisting of the base alone (B), 34.5 ± 3.6a% and 23.9 ± 1.3b%, respectively. Concerning the bark teas, the highest activity was discovered for the infusion with 20% addition of the tested material, the same as for AChE and BChE.
3.5. Inhibition of Glutathione Reductase, Inhibition of Glutathione Peroxidase
Glutathione reductase is a homodimeric flavoprotein and key antioxidant enzyme in regard to maintaining an intracellular reducing environment, which is crucial for the cell in combating oxidative stress. Glutathione peroxidase-1 is an intracellular antioxidant enzyme that decreases hydrogen peroxide to water enzymatically in order to limit its damaging effects.
Testing the ability to inhibit glutathione reductase and glutathione peroxidase, one could spot that in both cases the highest values were obtained for the infusion in which the only ingredient was bird cherry bark—87.0 ± 1.1e% for glutathione reductase and 64.9 ± 2.0d% for peroxidase (Table 5). Among teas with added bark, the highest activity was determined for infusions with 25% addition of the tested material (BP25) (77.9 ± 1.6d% and 53.3 ± 3.2c%) as well as 20% addition of bark (BP20) (74.2 ± 2.9cd and 53.7 ± 2.7c) (Table 5).

Table 5.
Inhibition of glutathione reductase, inhibition of glutathione peroxidase.
3.6. Sensory Analysis of Teas with the Addition of the Bark of P. padus
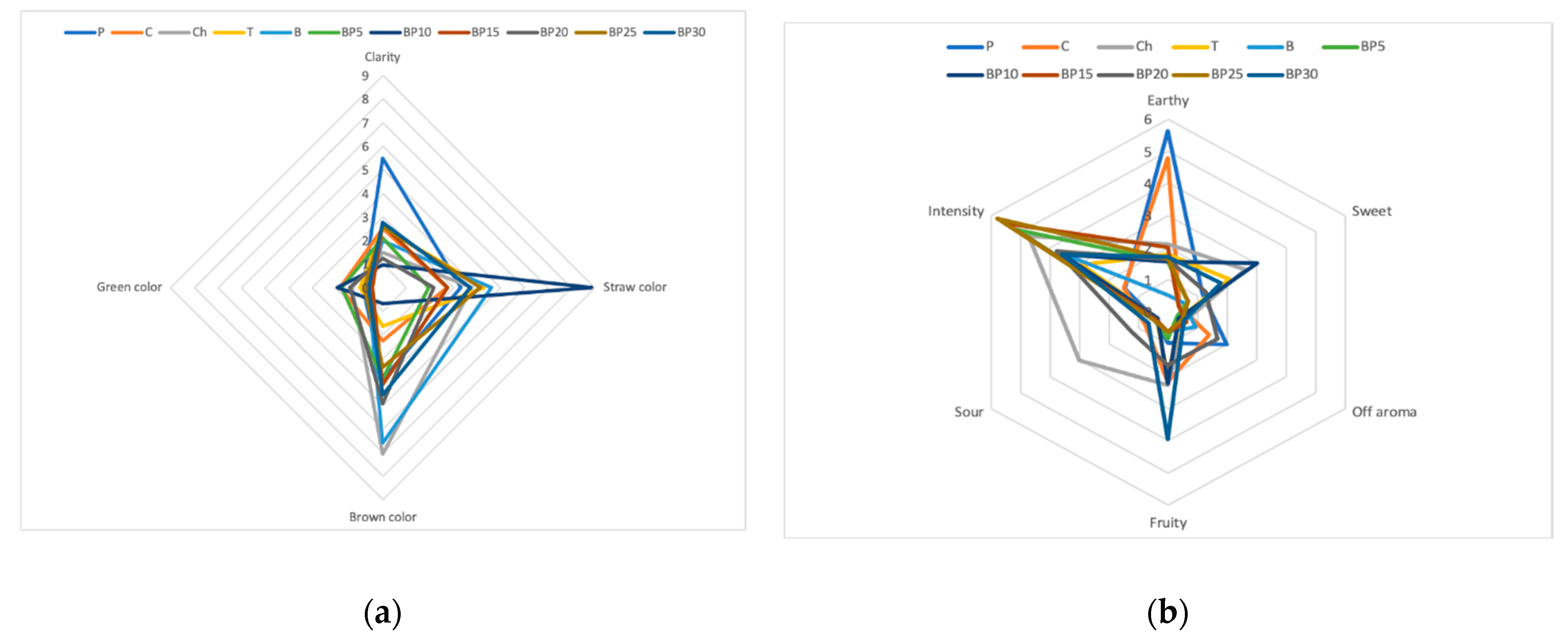
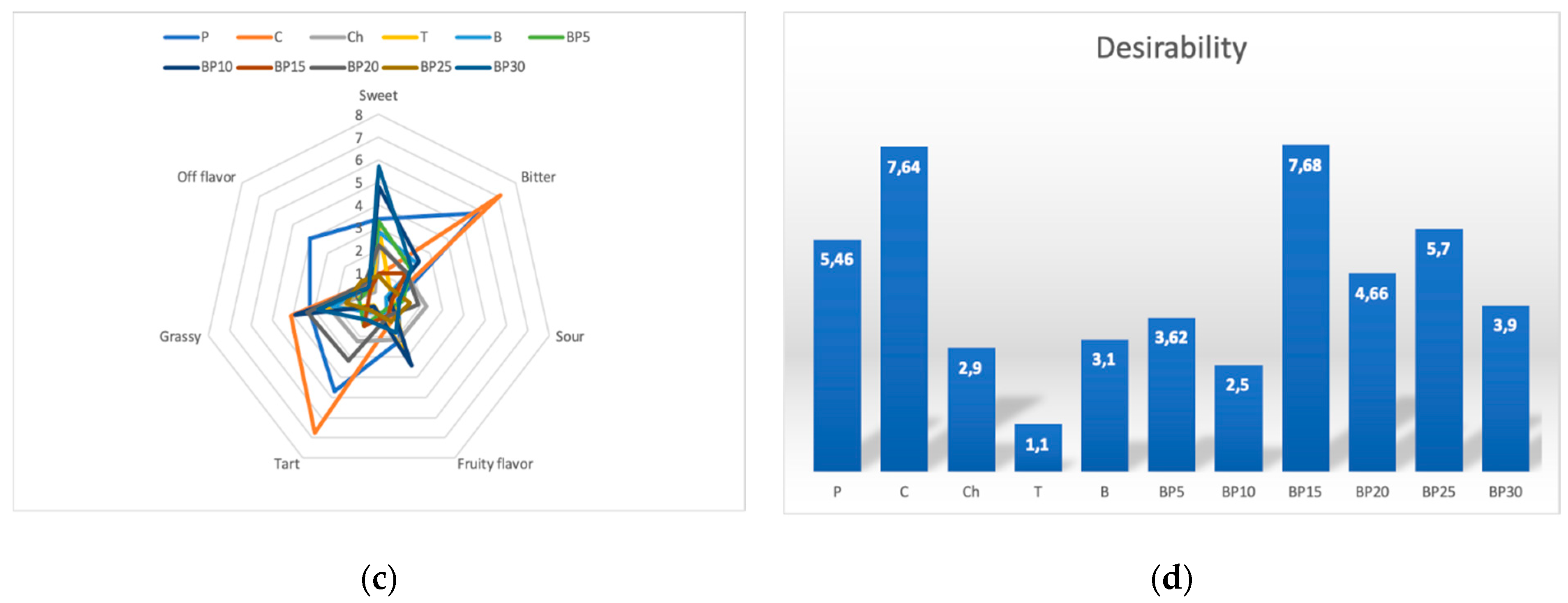
The dominant color for most samples of the tested teas containing the bark of bird cherry was brown (Figure 2). Among all samples, green was the least noticeable color and it ranged, in the terms of value, from 0.62 to 1.92. The samples were of heterogeneous clarity, with sample P having the highest mean score of 5.5 and sample BP10 with the lowest score (0.96). The teas containing bark of bird cherry were characterized by a variety of flavors, the distinguishing feature of a sour aftertaste was dominant and it was followed by grassy flavor, which was the second dominant flavor. It was demonstrated that sweet flavor was rated on a 10-point scale ranging from 0.9 for sample C to 5.7 in the case of sample BP30. It was also discovered that, among all studied types of teas, the fruity flavor was found to be poorly recognizable, with intensity scores ranging from 0.7 to 3.44. Almost all samples of the tea that contained the bark of bird cherry were scored very similarly in the terms of color distinguishing feature and it was closer to brown. The Ch sample was of a more intense brown color and was clearer.
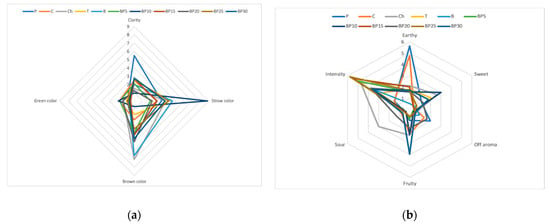
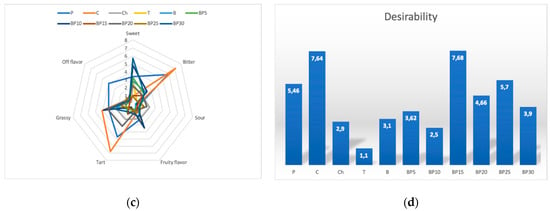
Figure 2.
Sensory profiles of teas with added bark of P. padus: (a) color, (b) taste, (c) flavor, (d) overall desirability.
4. Discussion and Conclusions
In agriculture as well as in forestry, bird cherry is an issue because it disrupts the ecosystem. Due to the abundant occurrence of bird cherry in natural ecosystems and its low requirement when it comes to the growth conditions, and thus its ability to spread easily and quickly, it seems unquestionable that finding an effective method of using its properties in a wide industrial or food spectrum should become one of the most important tasks for scientists and technologists nowadays. The use, and the elimination of bird cherry at the same time, can limit its growth and its bark can be a source of many bioactive compounds. Due to the high content of active compounds and the antioxidant and inhibitory activities of bird cherry, as determined in this study, a new method can be indicated for the elimination of the tree from the environment, with simultaneous efficient use of its bark for nutritional purposes.
Products such as herbal infusions rich in polyphenol can support the prevention of chronic diseases [5,10,22,23]. Bird cherry, due to the fact that it contains a lot of active compounds, can be an attractive raw material to use in teas, however, the high content of those substances can also make the taste of herbal infusions unattractive and specific. Therefore, to meet the expectations of consumers and to improve the sensory values, it can be used as an ingredient in mixtures that are used to prepare herbal teas. Such practices are common in food technology, for example, to enrich products with bioactive components. Similar research was conducted by Dziedzinski et al. (2020), and it was related to infusions with the addition of hops, bringing an intense aroma, and in that case, it was also proved that the developed recipe, which included hops as an ingredient, could be an interesting alternative and a proposal in supporting the treatment and preventing the development of neurodegenerative diseases [24].
New raw materials, such as bird cherry bark, which have not been used in industry so far, may be a source of active compounds increasing the health-promoting value of food. Currently, there are visible trends for the search for this type of raw materials, as well as trends related to the management of waste materials or by-products of the food industry, such products are, for example, bran, spent grain, or pulp after juice production. Therefore, sustainable development that takes into account the acquisition of raw materials that do not require agrotechnical treatments (bark of invasive plants) as well as the use of by-products and additional processes increasing the stability of compounds in the processing process, e.g., through the process of microencapsulation, allows you to maintain balance in the environment and increase the nutritional value and functional properties of products [25].
In this study, it was confirmed that the addition of the bark of bird cherry can influence the functional properties, which is the result of the specific composition of the compounds present in the bark. According to earlier studies, a significant amount of polyphenols was identified in certain anatomical parts of P. padus [5]. This may be the future of the management of bird cherry and the use of its bark for the purposes of food production. The bark of bird cherry contains many bioactive compounds that are particularly rich in catechins and their derivatives which contribute to the beneficial effects of herbal or tea infusions, they effectively eliminate reactive oxygen species in vitro and may also act indirectly as antioxidants thanks to their effects on transcription factors and enzyme activity [26]. In 2015, scientists from Korea also studied the total content of polyphenol in methanolic extracts from the bark of P. padus [27]. The bark was also a research material for another group of scientists who determined the high content of phenolic in water extracts and it was suggested by them that this could be a way to eliminate it from the environment [28]. Those benefits may also be the result of the high antioxidant potential, e.g., the reduction activity [27].
The classic antioxidant activity of polyphenols, based on their reducing properties, includes the direct scavenging of reactive oxygen and nitrogen species (ROS and RFA) and the reduction of the amount of ROS and/or RFA formed. This action is indirect through the regeneration of other antioxidants such as α-tocopherol or β-carotene and by chelating or reducing transition metals. Polyphenols have antioxidant properties due to their chem-ical structure which determines the ability to easily donate hydrogen atoms and/or elec-trons. Moreover, the phenoxy radicals of the polyphenols may participate in subsequent stages in reactions characteristic for radicals, such as dimerization or reactions with other radicals, they may also undergo further oxidation to the ochinone form. Moreover, in ad-dition to their classic antioxidant activity, polyphenols can act as antioxidants, influenc-ing the intracellular redox balance, thanks to other mechanisms such as inhibition of “pro-oxidative” enzymes, such as eg lipoxygenase [5,27].
Such actions may indicate the personalized use of the bark of bird cherry in the prevention of neurodegenerative diseases. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) are two enzymes that are sensitive to different chemicals and have the ability to bind to key parts of enzymes. They are sensitive to a broad spectrum of molecules. The research on natural inhibitors which resulted in creating, e.g., physostigmine and galantamine, began years ago, and more recently, synthetic synthetic versions of these products were also introduced [29]. Our study presented a highly visible property of inhibiting cholinesterases in bird cherry bark. The search for this type of material was commenced by other researchers. Yang et al. (2015) analyzed Peganum harmala L. and stated that the 2-aldehyde tetrahydroharmine contained in it can inhibit both AChE and BChE [30]. Polyphenols may also have other properties concerning catalase inhibition. Catalase is produced by aerobic organisms. It takes part in cell protection by degrading reactive oxygen species. Thus, catalase may prove helpful in chronic inflammation [31], whereas SOD is one of the main antioxidant enzymes that protect against the harmful effects of reactive oxygen species on the cell [32]. Our results for inhibition of catalase and SOD enzyme indicate high inhibitory activity of bird cherry bark, proving that it can be a relevant ingredient for improving the functional properties of food products.
The research also evaluated the property of infusions to inhibit glutathione peroxidase, which is an intracellular antioxidant enzyme, and glutathione reductase, which catalyzes the reduction of glutathione disulfide to the sulfhydryl form, a molecule crucial for combating oxidative stress and maintaining the intracellular reducing environment [33,34].
The quality of food consists of its nutritional value, health safety, and, above all, sensory characteristics which should be accepted by consumers. This can be achieved by using different types of combinations, mixing together ingredients with a specific taste, aroma, and color to obtain a product with the best health-promoting property for the consumer and a pleasant and desirable taste. When carrying out this study, a product both tasty and having many health-promoting properties resulting from the antioxidant and inhibitory activity against key enzymes in neurodegenerative diseases was obtained.
Author Contributions
Conceptualization, A.T. and J.K.-C.; methodology, J.K.-C., M.L., K.S.-S., and D.S.; software, A.T. and H.B.; validation, A.T., P.S., and D.S.; formal analysis, H.B.; investigation, A.T., M.L.; resources, K.S.-S.; data curation, A.T. and J.K.-C.; writing—original draft preparation, A.T.; writing—review and editing, J.K.-C. and P.S.; visualization, M.L. and D.S.; supervision, J.K.-C.; project administration, P.S. and H.B.; funding acquisition, H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding from the Department of Gastronomy Sciences and Functional Foods of Poznan University of Life Sciences, grant number 506.751.03.00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caudullo, G.; Tinner, W.; de Rigo, D. Picea abies in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publications Office of the European Union: Luxembourg, 2016; pp. 114–116. [Google Scholar]
- Uusitalo, M. European Bird Cherry (Prunus padus L.)—A Biodiverse Wild Plant for Horticulture; MTT Agrifood Research Finland: Jokioinen, Finland, 2004; Volume 61. [Google Scholar]
- Olszewska, M.A.; Kwapisz, A. Metabolite profiling and antioxidant activity of Prunus padus L. flowers and leaves. Nat. Prod. Res. 2011, 25, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Cha, D.S.; Jeon, H. Anti-inflammatory and anti-nociceptive properties of Prunus padus. J. Ethnopharmacol. 2012, 144, 379–386. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Stuper-Szablewska, K.; Ligaj, M.; Tichoniuk, M.; Szymanowska, D.; Szulc, P. Exploring antimicrobial and antioxidant properties of phytocomponents from different anatomical parts of Prunus padus L. Int. J. Food Prop. 2020, 23, 2097–2109. [Google Scholar] [CrossRef]
- Schultes, R.E. Cornucopia: A source book of edible plants. J. Ethnopharmacol. 1991, 34, 291–292. [Google Scholar] [CrossRef]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 1–12. [Google Scholar] [CrossRef]
- De Farias, C.C.; Maes, M.; Bonifacio, K.L.; Matsumoto, A.K.; Bortolasci, C.C.; Nogueira, A.; Brinholi, F.F.; Morimoto, H.K.; de Melo, L.B.; Moreira, E.G.; et al. Parkinson’s Disease is Accompanied by Intertwined Alterations in Iron Metabolism and Activated Immune-inflammatory and Oxidative Stress Pathways. CNS Neurol. Disord. Drug Targets 2017, 16, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Iftikhar, M.; Saleem, M.; Hussain, S.; Rehmat, F.; Afzal, Z.; Khawar, S.; Ashraf, M.; Al-Rashida, M. New synthetic 1,2,4-triazole derivatives: Cholinesterase inhibition and molecular docking studies. Results Chem. 2020, 2, 100041. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szulc, P.; Szczepaniak, O.; Dziedziński, M.; Szymanowska, D.; Szymandera-Buszka, K.; Goryńska-Goldmann, E.; Gazdecki, M.; Telichowska, A.; Ligaj, M. Variability of Hordeum vulgare L. Cultivars in Yield, Antioxidant Potential, and Cholinesterase Inhibitory Activity. Sustainability 2020, 12, 1938. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Ligaj, M.; Kobus-Cisowska, J.; Maciejewska, P.; Tichoniuk, M.; Szulc, P. Application for novel electrochemical screening of antioxidant potential and phytochemicals in Cornus mas extracts. CYTA J. Food 2019, 17, 781–789. [Google Scholar] [CrossRef]
- Ligaj, M.; Tichoniuk, M.; Filipiak, M. Detection of bar gene encoding phosphinothricin herbicide resistance in plants by electrochemical biosensor. Bioelectrochemistry 2008, 74, 32–37. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J.L.D. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Öztürk, M.; Duru, E.; Kivrak, Ş.; Mercan-Doĝan, N.; Türkoglu, A.; Özler, M.A. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food Chem. Toxicol. 2011, 49, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, D.M.; Prenzler, P.D.; Burrows, G.E.; Ryan, D.; Nielsen, S.; El Sawi, S.A.; El Alfy, T.S.; Abdelrahman, E.H.; Obied, H.K. Biophenols of mints: Antioxidant, acetylcholinesterase, butyrylcholinesterase and histone deacetylase inhibition activities targeting Alzheimer’s disease treatment. J. Funct. Foods 2017, 33, 345–362. [Google Scholar] [CrossRef]
- Baranowska-wójcik, E.; Szwajgier, D.; Winiarska-mieczan, A. Regardless of the brewing conditions, various types of tea are a source of acetylcholinesterase inhibitors. Nutrients 2020, 12, 709. [Google Scholar] [CrossRef]
- Watanabe, M.; de Moura Neiva, L.B.; da Costa Santos, C.X.; Rafael Martins Laurindo, F.; de Fátima Fernandes Vattimo, M. Isoflavone and the heme oxygenase system in ischemic acute kidney injury in rats. Food Chem. Toxicol. 2007, 45, 2366–2371. [Google Scholar] [CrossRef]
- Parschat, K.; Canne, C.; Hüttermann, J.; Kappl, R.; Fetzner, S. Xanthine dehydrogenase from Pseudomonas putida 86: Specificity, oxidation-reduction potentials of its redox-active centers, and first EPR characterization. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2001, 1544, 151–165. [Google Scholar] [CrossRef]
- Moreira, P.R.; Maioli, M.A.; Medeiros, H.C.D.; Guelfi, M.; Pereira, F.T.V.; Mingatto, F.E. Protective effect of bixin on carbon tetrachloride-induced hepatotoxicity in rats. Biol. Res. 2014, 47, 49. [Google Scholar] [CrossRef]
- Singh, R.P.; Padmavathi, B.; Rao, A.R. Modulatory influence of Adhatoda vesica (Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism, antioxidant status and lipid peroxidation in mice. Mol. Cell. Biochem. 2000, 213, 99–109. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Ligaj, M.; Stuper-Szablewska, K.; Szymanowska, D.; Tichoniuk, M.; Szulc, P. Polyphenol content and antioxidant activities of Prunus padus L. And Prunus serotina L. Leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties. Open Chem. 2020, 18, 1125–1135. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Serrano, C.; Sapata, M.; Oliveira, M.C.; Gerardo, A.; Viegas, C. Encapsulation of oleoresins for salt reduction in food. Acta Sci. Pol. Technol. Aliment. 2020, 19, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Dziedziński, M.; Szczepaniak, O.; Telichowska, A.; Kobus-Cisowska, J. Antioxidant capacity and cholinesterase inhibiting properties of dietary infusions with humulus lupulus. J. Elem. 2020, 25, 657–673. [Google Scholar] [CrossRef]
- Carvalho, C.; Pagani, A.; Teles, A.; Santos, J.; Pacheco, T.; Junior, R.C.; Pozza, M. Jamelao capsules containing bioactive compounds and its aplication in yoghurt. Acta Sci. Pol. Technol. Aliment. 2020, 19, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.C.; Kim, J.S. In vitro screening for antioxidant, antimicrobial, and antidiabetic properties of some Korean native plants on Mt. Halla, Jeju Island. Indian J. Pharm. Sci. 2015, 77, 668–674. [Google Scholar]
- Hwang, D.; Kim, H.; Shin, H.; Jeong, H.; Kim, J.; Kim, D. Cosmetic effects of Prunus padus bark extract. Korean J. Chem. Eng. 2014, 31, 2280–2285. [Google Scholar] [CrossRef]
- Kostelnik, A.; Pohanka, M. Inhibition of acetylcholinesterase and butyrylcholinesterase by a plant secondary metabolite boldine. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, X.; Liu, W.; Chou, G.; Wang, Z.; Wang, C. Potent AChE and BChE inhibitors isolated from seeds of Peganum harmala Linn by a bioassay-guided fractionation. J. Ethnopharmacol. 2015, 168, 279–286. [Google Scholar] [CrossRef]
- Kaushal, J.; Mehandia, S.; Singh, G.; Raina, A.; Arya, S.K. Catalase enzyme: Application in bioremediation and food industry. Biocatal. Agric. Biotechnol. 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Assady, M.; Farahnak, A.; Golestani, A.; Esharghian, M.R. Superoxide dismutase (SOD) enzyme activity assay in fasciola spp. parasites and liver tissue extract. Iran. J. Parasitol. 2011, 6, 17–22. [Google Scholar]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Seefeldt, T.; Chen, W.; Wang, X.; Matthees, D.; Hu, Y.; Guan, X. Effects of glutathione reductase inhibition on cellular thiol redox state and related systems. Arch. Biochem. Biophys. 2009, 485, 56–62. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).