Effects of Combined Application of Potassium Silicate and Salicylic Acid on the Defense Response of Hydroponically Grown Tomato Plants to Ralstonia solanacearum Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
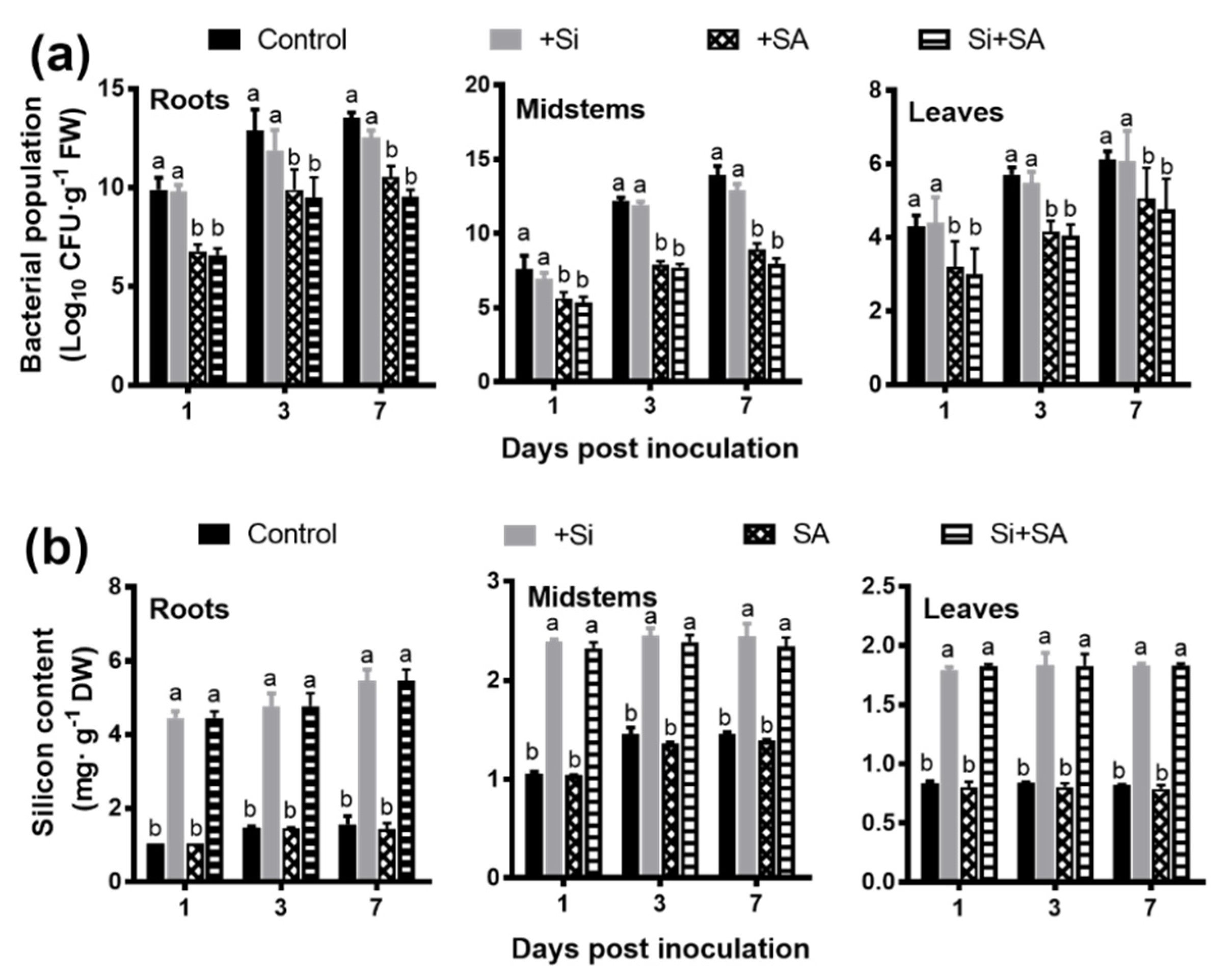
2.2. Bacteria Quantification, Symptom Evaluation, Measurement of Root Morphological Traits and Determination of Si Content
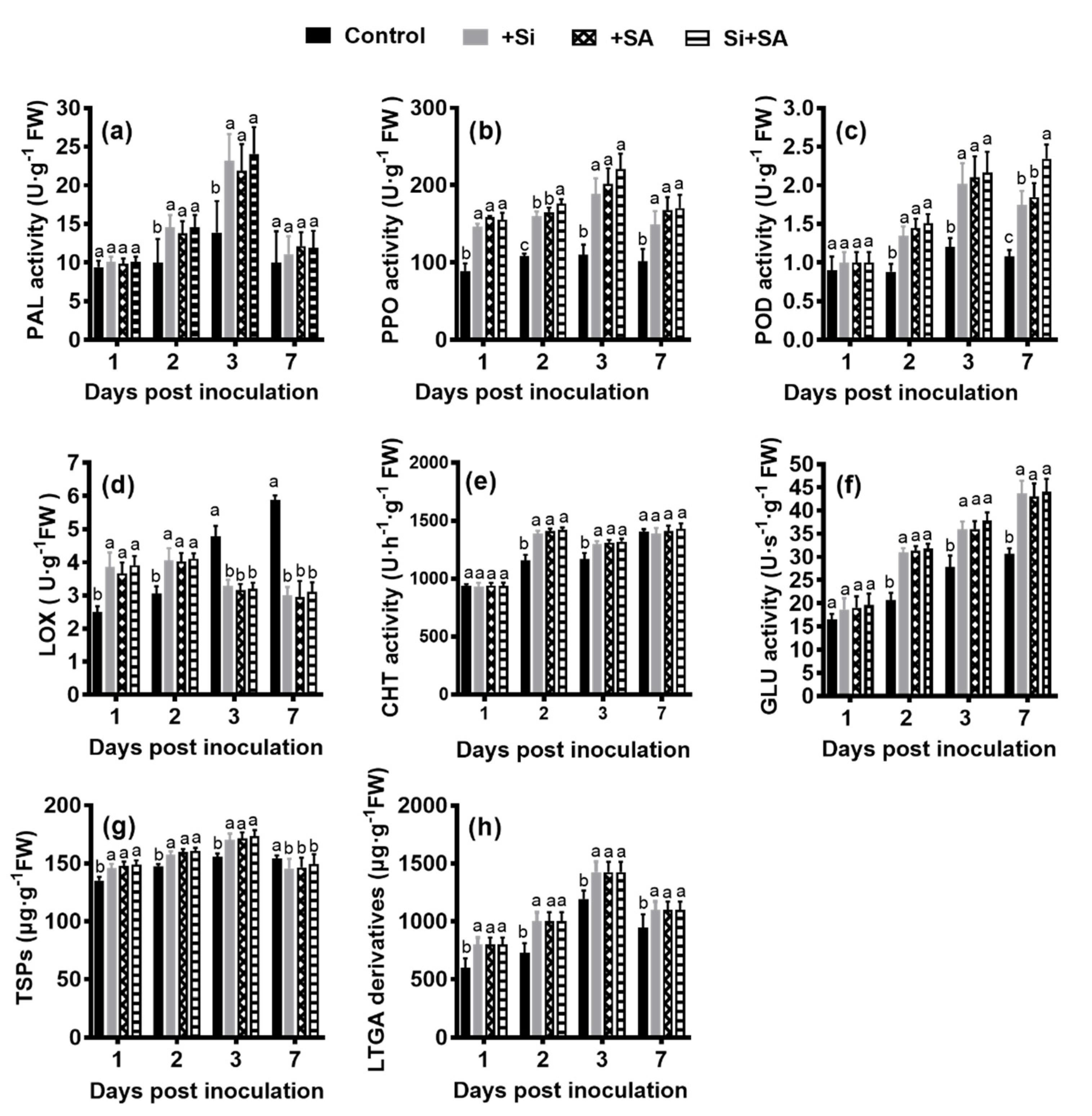
2.3. Enzyme Activity
2.4. Determination of Total Soluble Phenolics, Lignin, Lignin-Like Phenolic Polymers and Sucrose
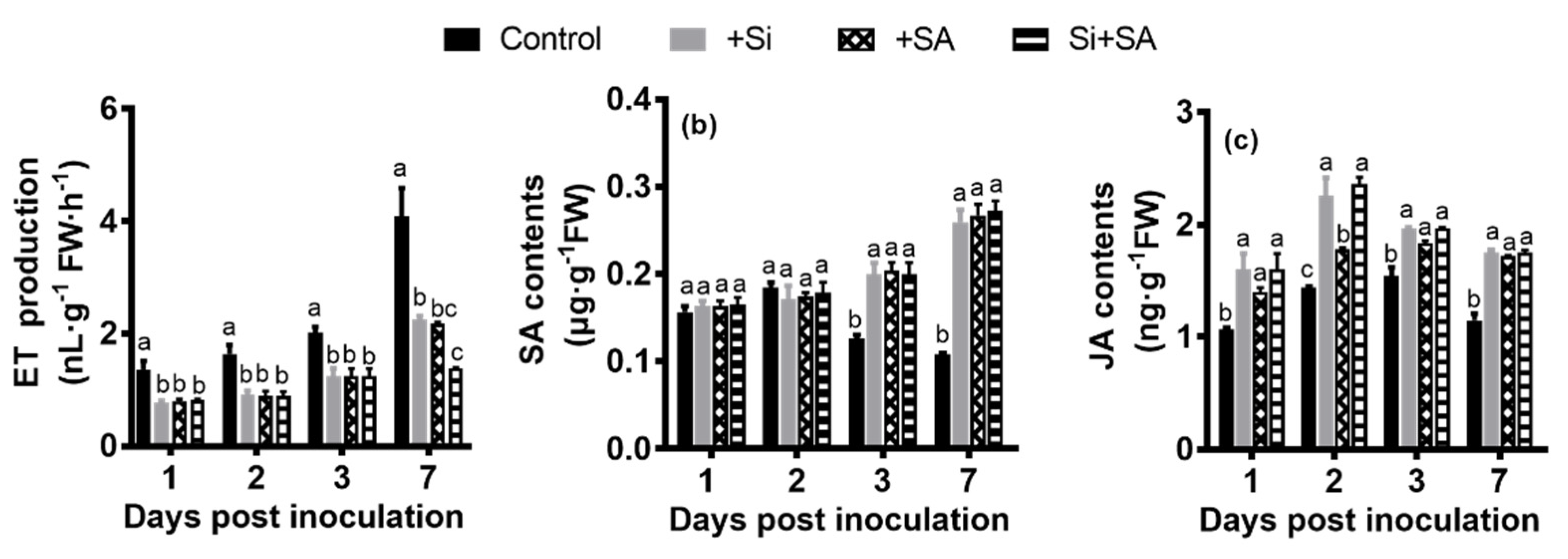
2.5. Measurements of Hormone Contents
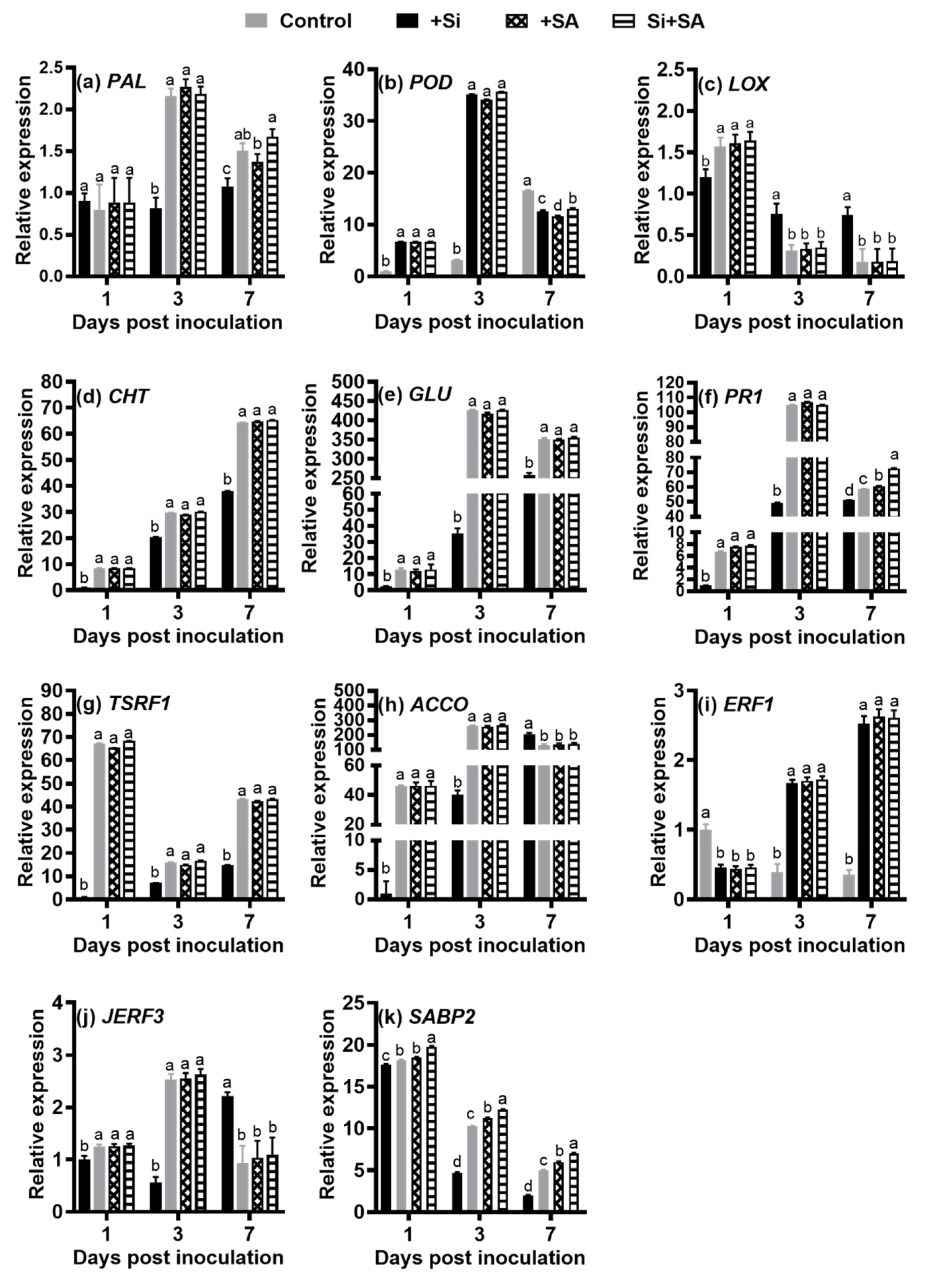
2.6. qRT-PCR Analysis of Gene Expression of Defense-Related Genes
2.7. Statistical Analysis
3. Results
3.1. Combined Application of Si and SA Can Enhance Disease Resistance to R. solanacearum in Hydroponically-Grown Tomato
3.2. Effects of Combined Si and SA Treatment on Sucrose Metabolism in Hydroponically Grown Tomato Plants Inoculated with R. solanacearum
3.3. Effects of Combined Si and SA Treatment on Biochemical Parameters in Hydroponically Grown Tomato Roots Inoculated with R. solanacearum
3.4. Effects of Combined Si and SA Treatment on the Changes of ET, JA and SA Contents in Hydroponically Grown Tomato Roots Inoculated with R. solanacearum
3.5. Effects of Combined Si and SA Treatment on the Expression Levels of Defense-Related Genes in Hydroponically Grown Tomato Roots Inoculated with R. solanacearum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Genin, S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 2010, 187, 920–928. [Google Scholar] [CrossRef]
- Dannon, E.A.; Wydra, K. Interaction between silicon amendment, bacterial wilt development and phenotype of Ralstonia solanacearum in tomato genotypes. Physiol. Mol. Plant Pathol. 2004, 64, 233–243. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Wang, L.; Lin, W.; Fan, X.; Cai, K. Proteomic characterization of silicon-mediated resistance against Ralstonia solanacearum in tomato. Plant Soil 2015, 387, 425–440. [Google Scholar] [CrossRef]
- Singh, S.; Gautam, R.K.; Singh, D.R.; Sharma, T.V.R.S.; Sakthivel, K.; Roy, S.D. Genetic approaches for mitigating losses caused by bacterial wilt of tomato in tropical islands. Eur. J. Plant Pathol. 2015, 143, 205–221. [Google Scholar] [CrossRef]
- Pradhanang, P.M.; Momol, M.T.; Olson, S.M.; Jones, J.B. Effects of Plant Essential Oils on Ralstonia solanacearum Population Density and Bacterial Wilt Incidence in Tomato. Plant Dis. 2003, 87, 423–427. [Google Scholar] [CrossRef]
- Messiha, N.A.S.; Van Diepeningen, A.D.; Wenneker, M.; Van Beuningen, A.R.; Janse, J.D.; Coenen, T.G.C.; Termorshuizen, A.J.; Van Bruggen, A.H.C.; Blok, W.J. Biological Soil Disinfestation (BSD), a new control method for potato brown rot, caused by Ralstonia solanacearum race 3 biovar 2. Eur. J. Plant Pathol. 2007, 117, 403–415. [Google Scholar] [CrossRef]
- Ghareeb, H.; Bozsó, Z.; Ott, P.G.; Repenning, C.; Stahl, F.; Wydra, K. Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 2011, 75, 83–89. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, X.; Lin, W.; Wang, G.; Cai, K. Transcriptome Analysis Reveals New Insights into the Bacterial Wilt Resistance Mechanism Mediated by Silicon in Tomato. Int. J. Mol. Sci. 2019, 20, 761. [Google Scholar] [CrossRef]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef]
- Sheng, H.; Chen, S. Plant silicon-cell wall complexes: Identification, model of covalent bond formation and biofunction. Plant Physiol. Biochem. 2020, 155, 13–19. [Google Scholar] [CrossRef]
- Jonas, V.B.; David, D.V.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar]
- Vivancos, J.; Labbé, C.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol. Plant Pathol. 2014, 16, 572–582. [Google Scholar] [CrossRef]
- Chain, F.; Côté-Beaulieu, C.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. A Comprehensive Transcriptomic Analysis of the Effect of Silicon on Wheat Plants Under Control and Pathogen Stress Conditions. Mol. Plant-Microbe Interact. 2009, 22, 1323–1330. [Google Scholar] [CrossRef]
- Lux, A.; Lukačová, Z.; Vaculík, M.; Švubová, R.; Kohanová, J.; Soukup, M.; Martinka, M.; Bokor, B. Silicification of Root Tissues. Plants 2020, 9, 111. [Google Scholar] [CrossRef]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.G.; Bélanger, R.R. Silicon-Mediated Accumulation of Flavonoid Phytoalexins in Cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kiirika, L.M.; Stahl, F.; Wydra, K. Phenotypic and molecular characterization of resistance induction by single and combined application of chitosan and silicon in tomato against Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2013, 81, 1–12. [Google Scholar] [CrossRef]
- Shetty, R.; Fretté, X.; Jensen, B.; Shetty, N.P.; Jensen, J.D.; Jørgensen, H.J.L.; Newman, M.-A.; Christensen, L.P. Silicon-Induced Changes in Antifungal Phenolic Acids, Flavonoids, and Key Phenylpropanoid Pathway Genes during the Interaction between Miniature Roses and the Biotrophic Pathogen Podosphaera pannosa. Plant Physiol. 2011, 157, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Bockhaven, J.V.; Spíchal, L.; Novák, O.; Strnad, M.; Asano, T.; Kikuchi, S.; Höfte, M.; Vleesschauwer, D.D. Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytol. 2015, 206, 761–773. [Google Scholar] [CrossRef]
- Kurabachew, H.; Stahl, F.; Wydra, K. Global gene expression of rhizobacteria-silicon mediated induced systemic resistance in tomato (Solanum lycopersicum) against Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2013, 84, 44–52. [Google Scholar] [CrossRef]
- Rasoolizadeh, A.; Labbé, C.; Sonah, H.; Deshmukh, R.K.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. Silicon protects soybean plants against Phytophthora sojae by interfering with effector-receptor expression. BMC Plant Biol. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Kurabachew, H.; Wydra, K. Induction of systemic resistance and defense-related enzymes after elicitation of resistance by rhizobacteria and silicon application against Ralstonia solanacearum in tomato (Solanum lycopersicum). Crop. Prot. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Lin, W.-P.; Jiang, N.-H.; Peng, L.; Fan, X.-Y.; Gao, Y.; Wang, G.-P.; Cai, K.-Z. Silicon impacts on soil microflora under Ralstonia Solanacearum inoculation. J. Integr. Agric. 2020, 19, 251–264. [Google Scholar] [CrossRef]
- Wang, L.; Cai, K.; Chen, Y.; Wang, G. Silicon-Mediated Tomato Resistance Against Ralstonia solanacearum is Associated with Modification of Soil Microbial Community Structure and Activity. Biol. Trace Elem. Res. 2013, 152, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of Silicon on Plant–Pathogen Interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- French, E.; Kim, B.-S.; Rivera-Zuluaga, K.; Iyer-Pascuzzi, A.S. Whole Root Transcriptomic Analysis Suggests a Role for Auxin Pathways in Resistance to Ralstonia solanacearum in Tomato. Mol. Plant-Microbe Interact. 2018, 31, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Y.; Lin, W.-P.; Liu, R.; Jiang, N.-H.; Cai, K.-Z. Physiological response and phenolic metabolism in tomato (Solanum lycopersicum) mediated by silicon under Ralstonia solanacearum infection. J. Integr. Agric. 2018, 17, 2160–2171. [Google Scholar] [CrossRef]
- Vlot, A.C.; Liu, P.-P.; Cameron, R.K.; Park, S.-W.; Yang, Y.; Kumar, D.; Zhou, F.; Padukkavidana, T.; Gustafsson, C.; Pichersky, E.; et al. Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance inArabidopsis thaliana. Plant J. 2008, 56, 445–456. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Jacobs, J.M.; Ailloud, F.; Fochs, B.; Prior, P.; Allen, C. Degradation of the plant defense signal salicylic acid protects Ralstonia solanacearum from toxicity and enhances virulence on tobacco. MBio 2016, 7, e00656-16. [Google Scholar] [CrossRef]
- Chamnongpol, S.; Willekens, H.; Moeder, W.; Langebartels, C.; Sandermann, H., Jr.; Van Montagu, M.; Inzé, D.; Van Camp, W. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc. Natl. Acad. Sci. USA 1998, 95, 5818–5823. [Google Scholar] [CrossRef]
- Mandal, S.; Mallick, N.; Mitra, A. Salicylic acid-induced resistance to Fusarium oxysporum f. sp. lycopersici in tomato. Plant Physiol. Biochem. 2009, 47, 642–649. [Google Scholar] [CrossRef]
- Afroz, A.; Khan, M.R.; Ahsan, N.; Komatsu, S. Comparative proteomic analysis of bacterial wilt susceptible and resistant tomato cultivars. Peptides 2009, 30, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Narasimhamurthy, K.; Soumya, K.; Udayashankar, A.; Srinivas, C.; Niranjana, S. Elicitation of innate immunity in tomato by salicylic acid and Amomum nilgiricum against Ralstonia solanacearum. Biocatal. Agric. Biotechnol. 2019, 22, 101414. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lin, Y.M.; Chao, T.C.; Wang, J.F.; Liu, A.C.; Ho, F.I.; Cheng, C.P. Virus-induced gene silencing reveals the involvement of ethylene-, salicylic acid- and mitogen-activated protein kinase-related defense pathways in the resistance of tomato to bacterial wilt. Physiol. Plant. 2009, 136, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.M.; Babujee, L.; Meng, F.; Milling, A.; Allen, C. The In Planta Transcriptome of Ralstonia solanacearum: Conserved Physiological and Virulence Strategies during Bacterial Wilt of Tomato. mBio 2012, 3, e00114-12. [Google Scholar] [CrossRef]
- Yu, J.Q.; Lee, K.S.; Matsui, Y. Effect of the addition of activated charcoal to the nutrient solution on the growth of tomato in hydroponic culture. Soil Sci. Plant Nutr. 1993, 39, 13–22. [Google Scholar] [CrossRef]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Moon, K.-B.; Park, J.-S.; Park, Y.-I.; Song, I.-J.; Lee, H.-J.; Cho, H.S.; Jeon, J.-H.; Kim, H.-S. Park Development of Systems for the Production of Plant-Derived Biopharmaceuticals. Plants 2019, 9, 30. [Google Scholar] [CrossRef]
- Shimizu, K.; Matsuda, Y.; Nonomura, T.; Ikeda, H.; Tamura, N.; Kusakari, S.; Kimbara, J.; Toyoda, H. Dual protection of hydroponic tomatoes from rhizosphere pathogens Ralstonia solanacearum and Fusarium oxysporum f.sp. radicis-lycopersici and airborne conidia of Oidium neolycopersici with an ozone-generative electrostatic spore precipitator. Plant Pathol. 2007, 56, 987–997. [Google Scholar] [CrossRef]
- Picot, A.; Cobo-Díaz, J.F.; Pawtowski, A.; Donot, C.; Legrand, F.; Le Floch, G.; Déniel, F. Water Microbiota in Greenhouses With Soilless Cultures of Tomato by Metabarcoding and Culture-Dependent Approaches. Front. Microbiol. 2020, 11, 1354. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Kastelein, P.; De Vries, P.M.; Van Overbeek, L.S. Effects of ecological factors on the survival and physiology of Ralstonia solanacearum bv. 2 in irrigation water. Can. J. Microbiol. 2001, 47, 842–854. [Google Scholar] [CrossRef]
- Sui, X.C. The Endemic Factors of Tomato Bacterial Wilt in Soilless Culture System and Prevention Approache by Micro-Ecological Environment. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2007. [Google Scholar]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. The response of barley to salinity stress differs between hydroponic and soil systems. Funct. Plant Biol. 2010, 37, 621–633. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Liu, Y.; Mo, M.; Guan, X.; Huang, L.; Sun, C.; Yang, S.T.; Chang, X.L. Toxicity of graphene oxide to naked oats (Avena satival.) in hydroponic and soil cultures. RSC Adv. 2018, 8, 15336–15343. [Google Scholar] [CrossRef]
- Ahsan, M.; Wright, D. Wheat Varietal Behavior in Hydroponic and Soil Culture Under Saline Conditions. Pak. J. Biol. Sci. 1998, 1, 318–320. [Google Scholar] [CrossRef]
- Holubík, O.; Vaněk, A.; Mihaljevič, M.; Vejvodová, K. Higher Tl bioaccessibility in white mustard (hyper-accumulator) grown under the soil than hydroponic conditions: A key factor for the phytoextraction use. J. Environ. Manag. 2020, 255, 109880. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Gao, D.; Luo, S.; Zeng, R.; Yang, J.; Zhu, X. Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol. Plant. 2008, 134, 324–333. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of Postharvest Decay of Apple Fruit with Candida saitoana and Induction of Defense Responses. Phytopathology 2003, 93, 344–348. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Dell’Amico, J.; Bolarín, M.C.; Pérez-Alfocea, F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol. Plant. 2000, 110, 503–511. [Google Scholar] [CrossRef]
- DallAgnol, L.J.; Rodrigues, F.A.; Pascholati, S.F.; Fortunato, A.A.; Camargo, L.E.A. Comparison of root and foliar applications of potassium silicate in potentiating post-infection defences of melon against powdery mildew. Plant Pathol. 2015, 64, 1085–1093. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△Ct method. Methods 2012, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Saile, E.; McGarvey, J.A.; Schell, M.A.; Denny, T.P. Role of Extracellular Polysaccharide and Endoglucanase in Root Invasion and Colonization of Tomato Plants by Ralstonia solanacearum. Phytopathology 1997, 87, 1264–1271. [Google Scholar] [CrossRef]
- Ailloud, F.; Lowe, T.M.; Robène, I.; Cruveiller, S.; Allen, C.; Prior, P. In planta comparative transcriptomics of host-adapted strains of Ralstonia solanacearum. PeerJ 2016, 4, e1549. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Nikolic, N.; Liang, Y.; Kirkby, E.A.; Römheld, V. Germanium-68 as an Adequate Tracer for Silicon Transport in Plants. Characterization of Silicon Uptake in Different Crop Species. Plant Physiol. 2007, 143, 495–503. [Google Scholar] [CrossRef]
- Sofo, A.; Vitti, A.; Nuzzaci, M.; Tataranni, G.; Scopa, A.; Vangronsveld, J.; Remans, T.; Falasca, G.; Altamura, M.M.; Degola, F.; et al. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiol. Plant. 2013, 149, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Vitti, A.; Nuzzaci, M.; Scopa, A.; Tataranni, G.; Remans, T.; Vangronsveld, J.; Sofo, A. Auxin and Cytokinin Metabolism and Root Morphological Modifications in Arabidopsis thaliana Seedlings Infected with Cucumber mosaic virus (CMV) or Exposed to Cadmium. Int. J. Mol. Sci. 2013, 14, 6889–6902. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Lee, I.-J. Silicon: A duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit. Rev. Biotechnol. 2016, 36, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Abozeid, A.; Ying, Z.; Lin, Y.; Liu, J.; Zhang, Z.; Tang, Z. Ethylene improves root system development under cadmium stress by modulating superoxide anion concentration in Aarabidopsis thaliana. Front. Plant Sci. 2017, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, V.; de la Peña, M.; Azcárate, L.; Aranjuelo, I.; Gonzalez, E.M. Functional analysis of the taproot and fibrous roots of Medicago truncatula: Sucrose and proline catabolism primary response to water deficit. Agric. Water Manag. 2019, 216, 473–483. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Asthir, B.; Rai, P.K.; Bains, N.S.; Sohu, V.S. Genotypic Variation for High Temperature Tolerance in Relation to Carbon Partitioning and Grain Sink Activity in Wheat. Am. J. Plant Sci. 2012, 3, 381–390. [Google Scholar] [CrossRef]
- Kou, J.; Wei, Y.; He, X.; Xu, J.; Xu, F.; Shao, X. Infection of post-harvest peaches by Monilinia fructicola accelerates sucrose decomposition and stimulates the Embden–Meyerhof–Parnas pathway. Hortic. Res. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, W.; Bian, J.; Xie, H.; Li, Y.; Xu, C.; Ma, J.; Guo, S.; Chen, J.; Cai, X.; et al. Proteomics and Phosphoproteomics of Heat Stress-Responsive Mechanisms in Spinach. Front. Plant Sci. 2018, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.H.; Xui, Z.J.; Qi, Y.J.; Wang, X.J.; Sun, D.L.; Bian, N.F.; Wang, X. Effect of exogenous α-naphthaleneacetic acid on carbon metabolism of soybean under drought stress at flowering stage. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2018, 29, 1215–1224. [Google Scholar]
- Su, Y.; Li, T.-L.; Li, N.; Yang, F.-J.; Lu, S.-W. Effects of salicylic acid on sucrose metabolism of tomato seedlings under NaCl stress. J. Appl. Ecol. 2009, 20, 1525. [Google Scholar]
- Lu, S.W.; Li, T.L.; Jiang, J. Tomato key sucrose metabolizing enzyme activities and gene expression under NaCI and PEG Iso-osmotic stresses. Agric. Sci. China 2009, 8, 1046–1052. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Mazrou, Y.S.; Hafez, Y.M. Silicon Foliar Application Mitigates Salt Stress in Sweet Pepper Plants by Enhancing Water Status, Photosynthesis, Antioxidant Enzyme Activity and Fruit Yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef]
- Jun, Y.; Jun, Z.; Tao, W.; Zhao, M.L.; Rong, L.; Pim, G.; Huang, Q.W.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar]
- Saldajeno, M.G.B.; Naznin, H.A.; Elsharkawy, M.M.; Shimizu, M.; Hyakumachi, M. Enhanced resistance of plants to disease using Trichoderma spp. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2014; pp. 477–494. [Google Scholar]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Kim, J.F. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1117. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, X.; Yang, T.; Friman, V.; Geisen, S.; Wei, Z.; Xu, Y.; Jousset, A.; Shen, Q. Chemical structure predicts the effect of plant-derived low-molecular weight compounds on soil microbiome structure and pathogen suppression. Funct. Ecol. 2020, 34, 2158–2169. [Google Scholar] [CrossRef]
- Atkinson, M.M.; Baker, C.J. Alteration of plasmalemma sucrose transport in Phaseolus vulgaris by Pseudomonas syringae pv. syringae and its association with K+/H+ exchange. Phytopathology 1987, 77, 1573–1578. [Google Scholar] [CrossRef]
- Qamar, A.; Mysore, K.S.; Senthil-Kumar, M. Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 2015, 6, 503. [Google Scholar] [CrossRef]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.; Campo, S.; Rufat, M.; Estopà, M.; Messeguer, J.; Segundo, B.S.; Coca, M. Sucrose-Mediated Priming of Plant Defense Responses and Broad-Spectrum Disease Resistance by Overexpression of the Maize Pathogenesis-Related PRms Protein in Rice Plants. Mol. Plant-Microbe Interact. 2007, 20, 832–842. [Google Scholar] [CrossRef]
- Duan, L.; Liu, H.; Li, X.; Xiao, J.; Wang, S. Multiple phytohormones and phytoalexins are involved in disease resistance to Magnaporthe oryzae invaded from roots in rice. Physiol. Plant. 2014, 152, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Nikraftar, F.; Taheri, P.; Rastegar, M.F.; Tarighi, S. Tomato partial resistance to Rhizoctonia solani involves antioxidative defense mechanisms. Physiol. Mol. Plant Pathol. 2013, 81, 74–83. [Google Scholar] [CrossRef]
- Ma, Q.-H.; Zhu, H.-H.; Qiao, M.-Y. Contribution of both lignin content and sinapyl monomer to disease resistance in tobacco. Plant Pathol. 2017, 67, 642–650. [Google Scholar] [CrossRef]
- Chezem, W.R.; Memon, A.; Li, F.-S.; Weng, J.-K.; Clay, N.K. SG2-Type R2R3-MYB Transcription Factor MYB15 Controls Defense-Induced Lignification and Basal Immunity in Arabidopsis. Plant Cell 2017, 29, 1907–1926. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y. Salicylic acid induces the accumulation of defense-related enzymes in Whangkeumbae pear and protects from pear black spot. Front. Agric. China 2010, 4, 215–219. [Google Scholar] [CrossRef]
- Jendoubi, W.; Harbaoui, K.; Hamada, W. Salicylic acid-induced resistance against Fusarium oxysporumf.s.pradicis lycopercisi in hydroponic grown tomato plants. J. New Sci. 2015, 21, 985–995. [Google Scholar]
- Mandal, S.; Kar, I.; Mukherjee, A.K.; Acharya, P. Elicitor-induced defense responses in Solanum lycopersicum against Ralstonia solanacearum. Sci. World J. 2013, 2013, 561056. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Klessig, D.F. Salicylic Acid Is a Modulator of Tobacco and Mammalian Catalases. J. Biol. Chem. 1996, 271, 28492–28501. [Google Scholar] [CrossRef]
- Liu, Q.; Li, K.; Guo, X.; Ma, L.; Guo, Y.; Liu, Z. Developmental characteristics of grapevine seedlings root border cells and their response to ρ-hydroxybenzoic acid. Plant Soil 2019, 443, 199–218. [Google Scholar] [CrossRef]
- Heil, M.; Bostock, R.M. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann. Bot. 2002, 89, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Dana, M.D.L.M.; Pintor-Toro, J.A.; Cubero, B. Transgenic Tobacco Plants Overexpressing Chitinases of Fungal Origin Show Enhanced Resistance to Biotic and Abiotic Stress Agents. Plant Physiol. 2006, 142, 722–730. [Google Scholar] [CrossRef]
- Baichoo, Z.; Jaufeerally-Fakim, Y. Ralstonia solanacearum upregulates marker genes of the salicylic acid and ethylene signaling pathways but not those of the jasmonic acid pathway in leaflets of Solanum lines during early stage of infection. Eur. J. Plant Pathol. 2016, 147, 615–625. [Google Scholar] [CrossRef]
- Ishihara, T.; Mitsuhara, I.; Takahashi, H.; Nakaho, K. Transcriptome Analysis of Quantitative Resistance-Specific Response upon Ralstonia solanacearum Infection in Tomato. PLoS ONE 2012, 7, e46763. [Google Scholar] [CrossRef]
- Chen, S.-C.; Liu, A.-R.; Zou, Z.-R. Overexpression of glucanase gene and defensin gene in transgenic tomato enhances resistance to Ralstonia solanacearum. Russ. J. Plant Physiol. 2006, 53, 671–677. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van Der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Mengiste, T. Plant Immunity to Necrotrophs. Annu. Rev. Phytopathol. 2012, 50, 267–294. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Chang, Y.; Jiang, C.Z. Comparative transcriptomic analysis reveals that ethylene/H2O2-mediated hypersensitive response and programmed cell death determine the compatible interaction of sand pear Andalternaria alternate. Front. Plant Sci. 2017, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van Der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Narancio, R.; Zorrilla, P.; Robello, C.; Gonzalez, M.; Vilaró, F.; Pritsch, C.; Dalla Rizza, M. Insights on gene expression response of a caracterized resistant genotype of Solanum commersonii dun. against Ralstonia olanacearum. Eur. J. Plant Pathol. 2013, 136, 823–835. [Google Scholar] [CrossRef]
- Groen, S.C.; Whiteman, N.K. The Evolution of Ethylene Signaling in Plant Chemical Ecology. J. Chem. Ecol. 2014, 40, 700–716. [Google Scholar] [CrossRef] [PubMed]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of Antioxidant Systems and Storability of Two Plum Cultivars by Preharvest Treatments with Salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Fauteux, F.; Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Conrath, U. Systemic acquired resistance. Plant Signal. Behav. 2006, 1, 179–184. [Google Scholar] [CrossRef]
- Smith-Becker, J.; Marois, E.; Huguet, E.J.; Midland, S.L.; Sims, J.J.; Keen, N.T. Accumulation of Salicylic Acid and 4-Hydroxybenzoic Acid in Phloem Fluids of Cucumber during Systemic Acquired Resistance Is Preceded by a Transient Increase in Phenylalanine Ammonia-Lyase Activity in Petioles and Stems. Plant Physiol. 1998, 116, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Delaney, T.P. Over-expression ofTGA5, which encodes a bZIP transcription factor that interacts with NIM1/NPR1, confers SAR-independent resistance inArabidopsis thalianatoPeronospora parasitica. Plant J. 2002, 32, 151–163. [Google Scholar] [CrossRef]
- Hosseini, S.; Elfstrand, M.; Heyman, F.; Jensen, D.F.; Karlsson, M. Deciphering common and specific transcriptional immune responses in pea towards the oomycete pathogens Aphanomyces euteiches and Phytophthora pisi. BMC Genom. 2015, 16, 627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Nie, Y.F.; He, L.; Li, Y.F.; Wang, Z.Z. Changes of resistance-related defense enzymes activities and endogenous salicylic acid in rice induced by exogenous salicylic acid. J. Huazhong Agric. Univ. 2010, 29, 541–545. [Google Scholar]
- Smith, H.B. Signal transduction in systemic acquired resistance. Plant Cell 2000, 12, 179–181. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection With Potato Virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Zhang, L.-Q.; Song, L.-L.; Duan, K.; Li, N.; Wang, Y.-X.; Gao, Q.-H. The different interactions of Colletotrichum gloeosporioides with two strawberry varieties and the involvement of salicylic acid. Hortic. Res. 2016, 3, 16007. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, X.; Zhou, X.; Huang, L.; Yan, L.; Lei, Y.; Liao, B.; Huang, J.; Huang, S.; Wei, W.; et al. Dynamics in the resistant and susceptible peanut (Arachis hypogaea L.) root transcriptome on infection with the Ralstonia solanacearum. BMC Genom. 2014, 15, 1078. [Google Scholar] [CrossRef]


| Treatment | Root Length (cm) | Root Surface Area (cm2) | Average Root Diameter (mm) | Root Volume (cm3) |
|---|---|---|---|---|
| Control | 306.4 ± 28.2 b | 25.2 ± 3.6 c | 0.285 ± 0.013 c | 0.252 ± 0.023 b |
| +Si | 321.5 ± 44.85 b | 36.4 ± 2.7 b | 0.335 ± 0.018 ab | 0.315 ± 0.048 a |
| +SA | 311.6 ± 27.2 b | 35.6 ± 2.5 b | 0.321 ± 0.004 b | 0.322 ± 0.011 a |
| Si+SA | 452.5 ± 50.1 a | 56.8 ± 7.2 a | 0.342 ± 0.012 a | 0.347 ± 0.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.-H.; Zhang, S.-H. Effects of Combined Application of Potassium Silicate and Salicylic Acid on the Defense Response of Hydroponically Grown Tomato Plants to Ralstonia solanacearum Infection. Sustainability 2021, 13, 3750. https://doi.org/10.3390/su13073750
Jiang N-H, Zhang S-H. Effects of Combined Application of Potassium Silicate and Salicylic Acid on the Defense Response of Hydroponically Grown Tomato Plants to Ralstonia solanacearum Infection. Sustainability. 2021; 13(7):3750. https://doi.org/10.3390/su13073750
Chicago/Turabian StyleJiang, Ni-Hao, and Shi-Han Zhang. 2021. "Effects of Combined Application of Potassium Silicate and Salicylic Acid on the Defense Response of Hydroponically Grown Tomato Plants to Ralstonia solanacearum Infection" Sustainability 13, no. 7: 3750. https://doi.org/10.3390/su13073750
APA StyleJiang, N.-H., & Zhang, S.-H. (2021). Effects of Combined Application of Potassium Silicate and Salicylic Acid on the Defense Response of Hydroponically Grown Tomato Plants to Ralstonia solanacearum Infection. Sustainability, 13(7), 3750. https://doi.org/10.3390/su13073750





