Phytosanitary and Technical Quality Challenges in Export Fresh Vegetables and Strategies to Compliance with Market Requirements: Case of Smallholder Snap Beans in Kenya
Abstract
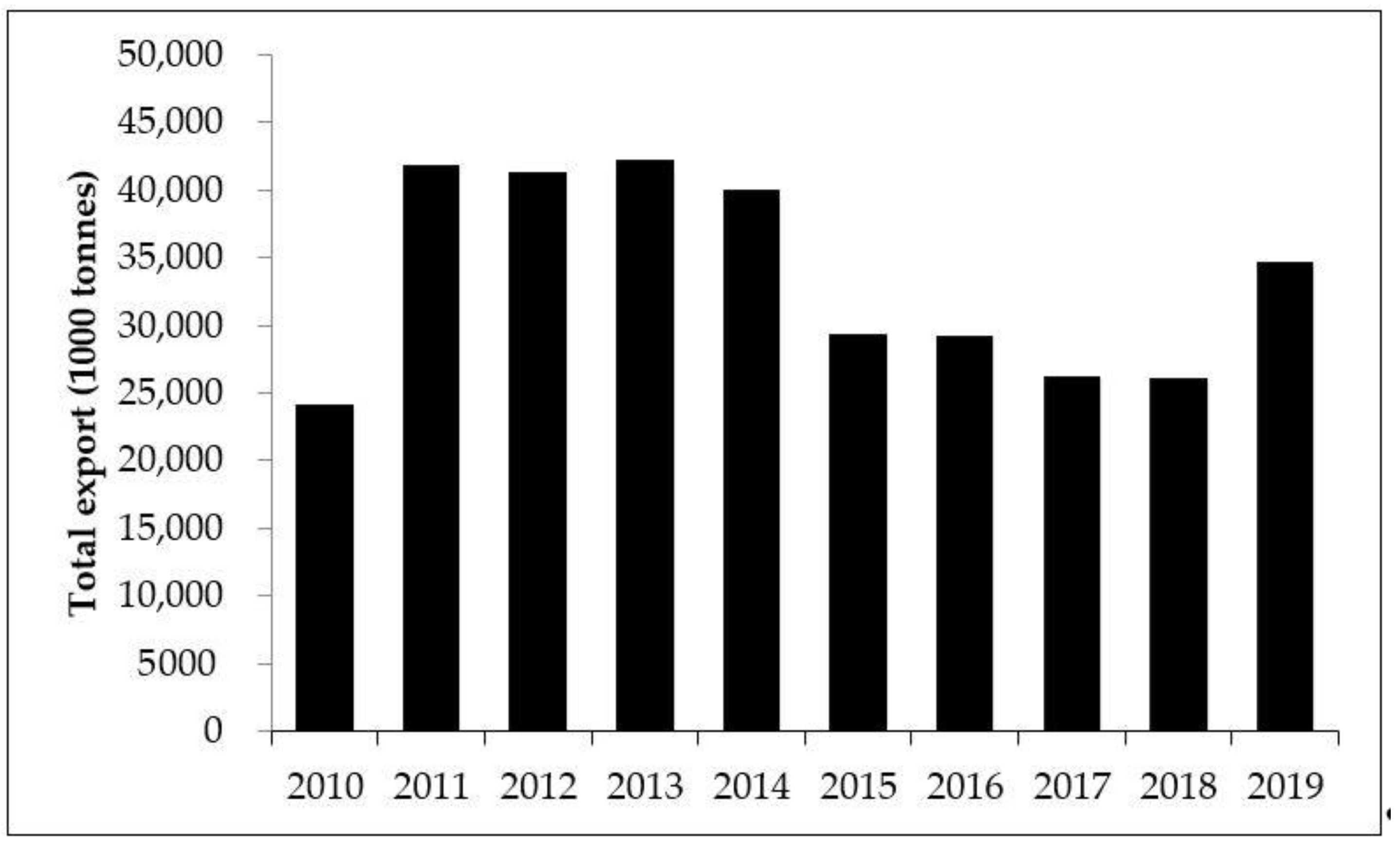
1. Introduction
2. Agronomic Practices in Smallholder Snap Bean Production
3. Phytosanitary and Technical Quality Issues in Snap Bean Production and Marketing
3.1. Phytosanitary Requirements for Export Snap Beans
3.2. Phytosanitary Treatment for Fresh Fruits and Vegetables
| Type of Treatment | Type of Fruits and Vegetables | Target Pests | Pros and Cons | Reference | Comments |
|---|---|---|---|---|---|
| Radiation treatment 50–400 Gy to prevent egg hatching, development and emergence of adult pests | All fruits and vegetables | Fruit flies (Anastrepha sp., Bactrocera sp., Ceratitis sp.) Codling moth (Cydia sp. Grapholita sp.) Apple maggot fly (Rhagoletis sp.) Weevil (Conotrachelus sp., Cylas sp., Euscepes sp.) Mealy bugs (Dysmicoccus sp., Planococcus sp.), Corn borer (Ostrinia sp.) | High investment in irradiation facility Cost-effective over the long term Adequate throughput is required for profitability Reduces efficacy of Modified atmosphere packaging (MAP) and controlled atmosphere (CA) storage | [31,32,35,36] | Internationally approved Dosage depends on the target pest |
| Vapour Heat (TPT-VH) Temperature raised to 40–50 °C in a forced hot air chamber for 20–70 min Kills insect eggs and larvae | Papaya (Carica papaya) Caricaceae: Brassicales Melon (Cucumis melo var. reticulatus), Mango (Mangifera indica) | Melon fly (Bactocera cucurbitae) Fruit flies (Bactrocera sp.) | May cause internal and external damage to sensitive produce May accelerate color development | [31] | Internationally approved |
| Cold treatment temperature between 0 to 3 °C for 15–20 or more continuous days | Citrus (Citrus limon) sweet orange (Citrus sinensis) Tangerine (Citrus reticulata) Pomelo (Citrus maxima) Clementine (Citrus clementina) | Fruit flies (Bactrocera sp., Ceratitis sp.) | Environmentally friendly Safe for employers and consumers. Increase fresh plant product shelf life and quality Easy to apply | [31,36,37,38] | Internationally approved |
| Fumigation Ethylene dibromide (EDB) Methyl bromide Ethanol and vinegar vapour Hydrogen cyanide Fungicides (imazalil, thiabendazole, pyrimethanil, and fludioxonil) | Oranges, Grapefruit Tangerines Plums Mangoes Vegetables | Fruit flies (Anastrepha sp., Ceratatitis sp., Dacus sp., Rhaqoletis sp.) Collectotrichum gloeosporioides Penicillium sp. | Methyl bromide is stratospheric ozone-depleting and its uses are regulated and reduced Chemicals have adverse effects on humans | [39,40] | Chemical fumigation is usually avoided due to strict requirements by importing countries on pesticide residues |
| Hot water treatment Dip in water at 52–62 °C for 2–60 min Used for insect and disease control | Citrus Mango (Mangifera indica) | Penicillium spp. Fruit fly (Ceratitis capitate, Bactrocera invadens) Anthracnose (Colletotrichum sp.) | Loss of firmness and weight Uniform fruit-color and positively modify the pH Enhances fruit ripening | [40,41] | High temperatures may cause fruit damage May affect fruit color, appearance, and eating quality |
3.3. Technical Quality Requirements for Export Snap Beans
| Active Ingredient | Maximum Residue Levels (mg/Kg) | Post-Harvest Interval (PHI) (Days) |
|---|---|---|
| Abamectin | 0.01 | 7 |
| Acetamiprid | 0.06 | 7 |
| Alpha-cypermethrin | 0.01; 0.5 | 3, 7 |
| Azoxystrobin + difenoconazole | 1 | 7 |
| Permethrin | 1 | 7 |
| Carbendazim | 0.2 | 9 |
| Chlorantraniliprole | 0.1 | 3 |
| Bifenthrin | 0.5 | 7 |
| Tebucanozole | 0.14 | 14 |
| Imadacloprid | 2 | 8 |
| Emamectin | 0.01 | 7 |
| Year | Type of Notification | Chemical | Concentration (mg/kg-ppm) | Fruits and Vegetables |
|---|---|---|---|---|
| 2019 | Border rejection | methamidophos u, acephate u | 0.6, 0.14, respectively | white beans |
| 2018 | Information for attention | carbofuran u | 0.015 | organic avocado |
| 2018 | Border rejection | carbofuran u | 0.14 | mangetout peas |
| 2016 | Border rejection | acephate u | 0.03 | snow peas |
| 2016 | Border rejection | carbofuran u | 0.14 | fresh snow peas |
| 2015 | Border rejection | carbendazim u | 1.5 | peas |
| 2015 | Border rejection | propamocarb, fluopicolide | 0.22, 0.034, respectively | peas |
| 2015 | Alert | carbofuran u | 0.018 | aubergines |
| 2015 | Border rejection | dimethoate | 0.18 | pea pods |
| 2015 | Border rejection | carbendazim u | 0.45 | fresh beans with pods |
| 2015 | Border rejection | methamidophos u | 0.3 | green beans |
| 2015 | Border rejection | methamidophos u | 0.067 | green beans with pods |
| 2015 | Border rejection | mandipropamid | 0.052 | fresh pea pods |
| 2015 | Border rejection | hexaconazole u | 0.021 | green beans |
| 2015 | Border rejection | carbendazim u | 1.2 | peas |
| 2015 | Border rejection | oxydemetun-methyl | 0.14 | fresh beans |
| 2015 | Border rejection | methomyl | 0.2 | green beans |
| 2015 | Border rejection | lufenuron | 0.089 | green beans |
| 2015 | border rejection | dimethoate, profenofos u | 0.049, 0.02, respectively | mangetout |
| 2015 | Border rejection | mataxyl | 0.29 | peas |
| Quality | Attributes | Quality Tolerance | |
|---|---|---|---|
| Quality classes | |||
| Extra class | Superior quality | Turgid, quickly snapped, very tender, practically straight and stringless | 5% of class I |
| Class I | Good quality | Turgid, young and tender, practically stringless and slight defects in shape and color accepted | 10% of class II No bean spot |
| Class II | Neither superior or good quality | Reasonably tender and meets minimum requirements | 10% of either class and or minimum requirements No bean spot |
| Size classes | |||
| Extra fine | 6 mm | 10% not satisfying given size | |
| Fine | 9 mm | ||
| Bobby/medium | 12 mm | ||
4. Challenges Faced by Smallholder Farmers in Meeting Phytosanitary and Quality Requirements
| County | Pesticide | Finding | Reference |
|---|---|---|---|
| Meru | Azoxystrobin | MRLswere below that set by EU | [57] |
| Carbendazim and metalaxyl | MRLs exceeded that set by EU | ||
| Nairobi | Dimethoate | MRLswere below that set by EU | [58] |
| Chlorpyrifos and dimethoate | MRLs exceeded that set by EU | ||
| Murang’a and Kiambu | Imidacloprid, chlorantraniliprole, spirotetramat, indoxacarb and metalaxyl | MRLswere below that set by EU | [59] |
| Meru | Cabendazim, acetamiprid imidacloprid chlorpyrifos | MRLswere below that set by EU | [60] |
5. Enforcement and Facilitation of Phytosanitary and Quality Regulations
| Institutions/Standards | Roles | Reference |
|---|---|---|
| National regulatory institutions | ||
| Agriculture Food and Fisheries Authority (AFFA) | Promoting and regulating best practices from in production to marketing of produce. | [79] |
| Horticulture Competent Authority Coordinating Committee | Streamlining sanitary and phytosanitary measures to stop rejection of produce in the international market | [79] |
| KEPHIS | Phytosanitary certification, monitoring, and analyzing of pesticide residue | [79] |
| HCD | Regulating pack-houses and players in the vegetable value chain, including enforcement of export standards, registration exporters, produce traceability, and quarterly reporting by processors and exporters | [80,81] |
| PCPB | Registration, formulation analysis, and control of protection products. | [49] |
| Kenya Agricultural and Livestock Research Organization (KALRO) | Facilitating the use of up to date production technology and establishing feedback systems to improve export capacities in horticultural products. | [79] |
| National private institutions and standards | ||
| Fresh Produce Exporters Association of Kenya (FPEAK); Kenya GAP | Advocating for a favorable trading environment for fresh vegetable growers/exporters, supporting and enhancing members’ ability to comply with international standards; promoting Kenyan products at international markets and providing members with market and technical information. | [82] |
| Inter-governmental regulatory agencies | ||
| International Plant Protection Convention (IPPC) | Setting the International Standards for Phytosanitary Measures (ISPMs); Ensuring that exported horticultural produce are compliant and allowed entry into markets | [80,83] |
| European and Mediterranean Plant Protection Organization (EPPO) | It is the regional plant protection organization for Europe responsible for cooperation in plant protection in the European and Mediterranean region. It develops regional standards for phytosanitary measures. | [4] |
| Codex Alimentarius Commission (CODEX) | Developing and encouraging the implementation of standards, codes of practice, guidelines, and recommendations on food safety | [84] |
| European Commission | Setting legislation on plant health to prevent the introduction and spread of organisms harmful to plants and plant products in the EU | [4] |
| Organization of Economic Cooperation and Development (OECD) | Providing a complete and internationally harmonized export quality inspection system for member countries | [85] |
| Hazard Analysis Critical Control Points (HACCP) | HACCP entails programs about the handling of snap beans and documenting | [86] |
| International private standards | ||
| Global Gap | Developing good agricultural practices for adoption across the value chain of fruits and vegetables | [54,72] |
| British Retail Consortium (BRC) | Specification of technical requirements that entails production, packaging, and distribution of produce | [87] |
| EU organic | Stipulating that the areas of cultivation of organic vegetables are reasonably synthetic agrochemicals free. | [88] |
6. Alternative Pest Management Approaches to Overcome Phytosanitary and Quality Challenges
| Active Substance | Target Pest | Source |
|---|---|---|
| Microbial Pesticides | ||
| Verticillium lecanii | Bemisia tabaci and Trialeurodes vaporariorum | [49] |
| Bacillus thuringiensis | Helicoverpa armigera, Aphis craccivora, Tetranychus spp. | [49,95] |
| Paecilomyces lilacinus, 1 × 109 cfu/mL | Bemisia tabaci and Trialeurodes Root-knot nematodes | [49] |
| Beauveria bassiana, 1.0 × 108 cfu/mL | Megalurothrips sjostedti, Aphis craccivora, Tetranychus spp. | [49,95] |
| Trichoderma harzianum, 8 × 109 spores/g | Frankliniella schultzei, and F. occidentalis, Soil-borne pathogens | [49] |
| Trichoderma asperullum | Soil-borne pathogens | [49] |
| Botanical Pesticides | ||
| Pyrthrins, 25% w/w | Bemisia tabaci and Trialeurodes vaporariorum | [49] |
| Azadirachtin | Megalurothrips sjostedti, Frankliniella schultzei, and F. occidentalis | [49] |
| Pyrethrin | Megalurothrips sjostedti, Frankliniella schultzei and F. occidentalis | [49] |
| Neem oil | Bemisia tabaci and Trialeurodes vaporariorum | [49] |
| Predators | ||
| Chrysoperla carnae | Aphis craccivora, Tetranychus spp. | [95] |
| Coccinella sp. | [96] | |
| Parasitoids | ||
| Trichogramma sp., Trichogrammatoidea sp., Encarsia sp., Telenomus sp. | Trialeurodes vaporariorum, Ostrinia nubilalis, Aspidiotus destructor, Helopeltis antonii, Opisina arenosella, Helicoverpa armigera | [97] |
| Pheromones | ||
| Kairomone capsule, | Frankliniella occidentalis | [98] |
| Methyl-isonicotinate | Frankliniella spp. | [99] |
| Pheromone traps (4-methyl-3,5-heptanedione) | Aphis craccivora, Tetranychus spp. | [95] |
7. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Farmers Information Service (NAFIS). French Beans Value Chain Development Murang’a County. Available online: http://www.nafis.go.ke/vegetables/ (accessed on 20 June 2016).
- Wahome, S.W.; Kimani, P.M.; Muthomi, J.W.; Narla, R.D. Quality and yield of snap bean lines locally develop din Kenya. Int. J. Agron. Agric. Res. 2013, 3, 1–10. [Google Scholar] [CrossRef]
- United States Agency for International Development-Kenya Horticulture Competitiveness Project (USAID-KHCP). Global Competitiveness Study: Benchmarking Kenya’s Horticulture Sector for Enhanced Export Competitiveness; Fintrac. Inc.: Nairobi, Kenya, 2015. [Google Scholar]
- Kok, M.G.; Osena, E.; Snel, H. Food Loss in the French Bean Supply Chain of VEGPRO-Group Kenya; Analysis of the French bean pilot; Report WCDI-19-085/WFBR-1999; Wageningen University & Research: Wageningen, The Netherlands, 2019. [Google Scholar]
- Masiga, R.; Kasina, M.; Mbugi, J.; Odhiambo, C.; Kinuthia, W.; Gemmill-Herren, B.; Vaissièr, B.E. Do French beans (Phaseolus vulgaris) grown inproximity to Mt. Kenya forestin Kenya experience pollination deficit? J. Pollinat. Ecol. 2014, 14, 255–260. [Google Scholar] [CrossRef]
- Trad Map. Trade Statistics for International Business Development; Monthly, Quarterly and Yearly TradeData. Importand Export Values, Volumes, Growth Rates, Market Shares, etc. Available online: https://www.trademap.org/cbi/Country_SelProduct_TS.aspx?nvpm=1%7c%7c%7c%7c%7c070820%7c%7c%7c6%7c1%7c1%7c2%7c2%7c1%7c2%7c2%7c1 (accessed on 12 April 2020).
- Okello, J.J.; Swinton, S.M. International food safety standards and the use of pesticides infresh export vegetable productionindeveloping countries: Implications for farmer health and the environment. In Pesticides—Formulations, Effects, Fate; Stoytcheva, M., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Horticultural Crop Development Authority (HCDA). Vegetables, French Beans. Available online: http://www.hcda.or.ke/?page_id=1054 (accessed on 22 December 2014).
- Kenya National Bureauof Statistics (KNBS). Statistical Abstract; Kenya National Bureau of Statistics: Nairobi, Kenya, 2019; ISBN 978-9966-102-10-2.
- Hortnews. United States Opens Market for Kenyan French-Beans. Available online: http://www.hortinews.co.ke/2016/01/28/united-states-opens-market-for-kenyan-french-beans/ (accessed on 18 February 2018).
- Kuehn, J. Glossary of Phytosanitary Terms. National Standard for Phytosanitor Measures. 2015. Available online: https://pflanzengesundheit.julius-kuehn.de/dokumente/upload/e3aff_np3-glossary_phytosanitary_terms2015_en.pdf (accessed on 18 October 2016).
- Infonet-Biovision. Crops. Available online: http://www.infonet-biovision.org/default/ct/118/crops (accessed on 27 June 2016).
- Jong-Woo, K.; Dorothea, R. Impact of Sanitary and Phytosanitary Measures and TechnicalBarriersonInternationalTrade.MunichPersonalRePEcArchive. MPRA Paper No. 82352. 2017. Available online: https://mpra.ub.uni-muenchen.de/82352/ (accessed on 30 December 2019).
- Centre for the Promotion of Imports from Developing Countries (CBI). What Requirements Should Fresh Fruitsor Vegetables Comply with to be Allowed on the European Market. Available online: https://www.cbi.eu/market-information/fresh-fruit-vegetables/buyer-requirements/ (accessed on 10 February 2018).
- Value Chain Analysis for Development (VCAD). Green Beans Value Chain Analysis in Kenya; Directorate General International Cooperation and Development—Europe Aid, Directorate Sustainable Growth and Development: Nairobi, Kenya, 2018. [Google Scholar]
- Shingal, A.; Malte, E. Trade Effect of MRL Harmonization in the EU: Improved Access for Non-EU Partners; European University Institute: Florence, Italy, 2018. [Google Scholar]
- European Union (EU). New EU Plant Health Rules: Regulation (EU) 2016/2031 on Protective Measures against Plant Pests (“Plant Health Law”). Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/legislation/new_eu_rules_en (accessed on 15 July 2020).
- MFarm. Growing French Beansin Kenya. 2014. Available online: https://www.mfarm.co.ke/blog/post/growing-french-beans-in-kenya (accessed on 18 October 2016).
- Wamucii, S. Why French Beans Have Become a Top Horticultural Choice in New Regions of Kenya. Available online: https://www.selinawamucii.com/why-french-beans-have-become-a-top-horticultural-choice-in-new-regions-of-kenya/ (accessed on 7 October 2020).
- Fanelli, R.M. The (un)sustainability of the land use practice sand agricultural production in EU countries. Int. J. Environ. Stud. 2019, 1–22. [Google Scholar] [CrossRef]
- Fanelli, R.M. The spatial land temporal variability of the effects of agricultural practices on the environment. Environments 2020, 7, 33. [Google Scholar] [CrossRef]
- Green life Crop Protection Africa. French Beans. Available online: https://www.greenlife.co.ke/french-beans/ (accessed on 25 October 2018).
- Nyasetia, D.M.S. Antifungal Activity of Selected Crude Plant Extracts on Bean Rust (Uromycesappendiculatus) and Their Effectson Physiological Activities of French Beans. Master’s Thesis, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2011. [Google Scholar]
- Okello, J.J. Exit, voice and loyalty in Kenya’s French bean industry: What lessons can we learn from small holder farmers’ past response to international food safety standards? Afr. J. Food Agric. Nutr. Dev. 2011, 11, 4460–4475. [Google Scholar] [CrossRef]
- Pesticide Residues in Food (PRF). The Expert Committee on Pesticide Residues in Food. Annual Report; 2016. Available online: https://www.gov.uk/government/publications/pesticide-residues-in-food-quarterly-monitoring-results-for-2016 (accessed on 1 October 2017).
- Pest Control Products Board (PCPB). Pest Control Products Registered for Use in Kenya. Fully Registered Pest Control Products, 10th ed.; Pest Control Products Board: Nairobi, Kenya, 2015. [Google Scholar]
- IPPC. Adopted International Standards for Phytosanitary Measures (ISPMs). International Plant Protection Convention. 2017. Available online: https://www.ippc.int/en/core-activities/standards-setting/ispms/#publications (accessed on 2 June 2018).
- European and Mediterranean Plant Protection Organization (EPPO). Quarantine List A1. 2017. Available online: https://www.eppo.int/QUARANTINE/listA1.htm (accessed on 2 March 2018).
- Gathura, G. Fresh Produce Processing Factories Risking Kenya’s Export Market through Unhygienic Practices. 2017. Available online: www.rocketscience.co.ke (accessed on 23 July 2018.).
- Webgate, E.C. Rapid Alert System for Food and Feed (RASFF); Notifications. Available online: https://ec.europa.eu/food/safety/rasff_en (accessed on 13 April 2020).
- Hennessey, M.K.; Jeffers, L.; Nendick, D.; Ken, G.; Floyd, L.; Hansen, J.D.; Bailey, W.D.; Winborne, I.; Bartels, D.; Ramsey, C.; et al. Phytosanitary Treatments. In The Handbook of Plant Biosecurity; Gordh, G., McKirdy, S., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Hallman, G.J. Process control in phytosanitary irradiation of fresh fruits and vegetables as a model for other phytosanitary treatment processes. Food Control 2017, 72, 372–377. [Google Scholar] [CrossRef]
- Saeed, N.; Tonina, L.; Battisti, A.; Mori, N. Postharvestshortcoldtemperaturetreatmenttopreservefruitqualityafter Drosophila suzukii damage. Int. J. Pest Manag. 2020, 66, 23–30. [Google Scholar] [CrossRef]
- Nicholas, A.; Lidbetter, F.; Eagleton, N.; Spohr, L.; Harris, A.; Barchia, I. Phytosanitary irradiation of three species of spider mites (Trombidiformes: Tetranychidae). Austral Entomol. 2018. [Google Scholar] [CrossRef]
- Follett, P.A.; Neven, L.G. Phytosanitary irradiation: Does modified atmosphere packaging or controlled atmosphere storage creating allow oxygen environment threaten treatment efficacy? Radiat. Phys. Chem. 2020, 108874. [Google Scholar] [CrossRef]
- Bustos-Griffin, E.; Hallman, G.J.; Griffin, R.L. Phytosanitary irradiation in ports of entry: A practical solution for developing countries. Int. J. Food Sci. Technol. 2015, 50, 249–255. [Google Scholar] [CrossRef]
- Dias, V.S.; Hallman, G.J.; Cardoso, A.S.; Hurtado, N.V.; CMaxwell, F.R.; Cáceres-Barrios, C.E.; Vreysen, M.J.B.; Myers, S.W. Relative Tolerance of three Morphotypes of the Anastrepha fraterculus complex (Diptera:Tephritidae) to Cold Phytosanitary Treatment. J. Econ. Entomol. 2020, 113, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Dohino, T.; Suzuki, N.; Miyazaki, I.; Tanno, M.; Yoshinaga, M.; Shukuya, T. Comparison of Cold Tolerance of egg sand larvae of Bactrocera dorsalis (Diptera: Tephritidae) among citrus fruits. Res. Bull. Pl. Prot. Jpn. 2019, 55, 1–10. [Google Scholar]
- Suwapanich, R.; Krusong, W.; Thompson, A.K. Postharvest control of anthracnose in mangoes by fumigation with vinegar and ethanol vapours. Int. J. Postharv. Technol. Innov. 2019, 6, 179–191. [Google Scholar] [CrossRef]
- Papoutsisa, K.; Mathioudakisc, M.M.; Hasperuéd, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum. Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Hernández, E.; Rivera, P.; Bravo, B.; Toledo, J.; Caro-Corrales, J.; Montoya, P. Hot-water phytosanitary treatment against Ceratitis capitate (Diptera: Tephritidae) in “Ataulfo” mangoes. J. Econ. Entomol. 2012, 105, 1940–1953. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Ameer, K.; Song, B.-S.; Kim, J.-K.; Park, H.-Y.; Lee, K.-C.; Eun, J.-B.; Park, J.-H. Effects of X-ray irradiation on the postharvest quality characteristics of ‘Maehyang’ strawberry (Fragaria × ananassa). Food Chem. 2020. [Google Scholar] [CrossRef]
- Serapian, T.; Prakash, A. Comparative evaluation of the effect of methyl bromide fumigation and phytosanitary irradiation on the quality of fresh strawberries. Sci. Hortic. 2016, 201, 109–117. [Google Scholar] [CrossRef]
- Centre for the Promotion of Imports from Developing Countries (CBI). Buyer Requirements: Fresh Fruit and Vegetables. CBI Market Intelligence. Available online: www.cbi.eu/market-information (accessed on 23 June 2016).
- Centre for the Promotion of Imports from Developing Countries (CBI). Product Factsheet Fresh Beans, Peas, and Other Leguminous Vegetables in Europe; CBI Ministry of Foreign Affairs: The Haque, The Netherlands, 2015. [Google Scholar]
- European Commission. The European Commission Directive 2009/128/EC on Maximum Residue Levels. 2009. Available online: https://ec.europa.eu/food/plant/pesticides/max_residue_levels_en (accessed on 15 April 2017).
- Pesticide Residues in Food (PRF): The Expert Committee on Pesticide Residues in Food (PRF) Report on the Pesticide Residues Monitoring Programme: Quarter 3 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/879216/prif-monitoring-2019-quarter3.pdf (accessed on 30 September 2020).
- CUTS. Standards and Market Access: Opportunities and Challenges for East African Exports into European Union. 2009. Available online: http://www.cuts-international.org/ARC/Nairobi/BIEAC/PDF/BIEAC-Standards_and_Market_Access.pdf (accessed on 30 July 2017).
- Pest Control Products Board (PCPB). Pest Control Products Registered for Use in Kenya. Fully Registered Pest Control Products, Version 1_2018; Pest Control Products Board: Nairobi, Kenya, 2019. [Google Scholar]
- USAID-Kenya Agricultural Value Chain Enterprises (USAID-KAVES) Project. Initial Environmental Examination, Amendment Pesticide Evaluation Report and Safer Use Action Plan (PERSUAP) for USAID/Kenya’s Kenya Agricultural Value Chain Enterprises (KAVES) Project; Fintrac. Inc.: Nairobi, Kenya, 2014. [Google Scholar]
- Shingal, A.; Ehrich, M. Trade Effects of Standards Harmonization in the EU: Improved Access for Non-EU Partners. ©Indian Council for Research on International Economic Relations. 2019. Available online: http://hdl.handle.net/11540/9984 (accessed on 13 May 2020).
- European Union (EU). EU Legislation on MRLs. Available online: https://ec.europa.eu/food/plant/pesticides/max_residue_levels/eu_rules_en (accessed on 14 April 2020).
- CBI. Practical Market Insights for Your Product: Fresh Beans and Pulses in Germany; The Haque, The Netherlands, 2015; Available online: https://www.importpromotiondesk.de/fileadmin/user_upload/Publikationen/factsheet/obst_gemuese/Beans___Pulses_160331_eng.pdf (accessed on 14 May 2020).
- United States Agency for International Development-Kenya Horticulture Competitiveness Project (USAID-KHCP). The EU Market for Green Beans. 2011. Available online: www.GrowKenya.org|khcp@fintrac.com|www.fintrac.com|MarketSurvey#03 (accessed on 14 December 2018).
- United Nations Economic Commission for Europe (UNECE). ENECE Standard FFV-06 Concerning the Marketing and Commercial Quality Control of Beans; United Nations: Geneva, Switzerland, 2017. [Google Scholar]
- Kenya Plant Health Inspectorate Service (KEPHIS). EU Requirements. Available online: www.kephis.org (accessed on 21 June 2017).
- Marete, G.M.; Shikuku, V.O.; Lalah Mputhia, J.O.; Wekesa, V.W. Occurrence of pesticides residues in French beans, tomatoes, and kale in Kenya, and their human health risk in dicators. Environ. Monit. Assess. 2020, 192, 692. [Google Scholar] [CrossRef]
- Inonda, R.; Njage, E.; Ngeranwa, J.; Mutai, C. Determination of pesticide residues in locally consumed vegetables in Kenya. Afr. J. Pharm. 2015, 4, 1–6. [Google Scholar]
- Kipkemoi, E.; Andayi, W.A.; Njagi, E.C.; Ptoton, B. Analysis of Pesticide Residues in Tomatoes and French Beans from Murang’a and Kiambu Counties, Kenya. Eur. J. Nutrit. Food Saf. 2020, 12, 121–132. [Google Scholar] [CrossRef]
- Marete, M.; Lalah, J.O.; Mputhia, J.; Wekesa, W.V. Determination of level so for organophosphorus pesticide residue sin kales, tomatoes and French beans from Meru County, Kenya by GC-MS. Afric. J. Emerg. Issues 2019, 1, 20–29. [Google Scholar]
- Otim, M.; Kasina, M.; Nderitu, J.; Katafiire, M.; Mcharo, M.; Kaburu, M.; GBwire, J.B.; Cheminingw’a, G.; Olubayo, F.; Ugen, M. Effectiveness and profitability of insecticide formulations used for managing snap bean pests. Ugan. J. Agric. Sci. 2016, 17, 111–124. [Google Scholar] [CrossRef]
- Nyakundi, W.O.; Magma, G.; Ochoa, J.; Nene, A.B. A survey of pesticide use and application patterns among farmers: A case study from selected horticultural farms in Rift Valley and Central Provinces, Kenya. OJS Sci. Conf. Proc. 2012, 686, 618–630. [Google Scholar]
- Pretty, J.; Pervez, B.Z. Integrated pest management for sustainable intensification of agriculture in Asia and Africa. Insects 2015, 6, 152–182. [Google Scholar] [CrossRef]
- Macharia, I. Pesticides and Health in Vegetable Production in Kenya. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- European Commission. Final Report of an Audit Carried out in Kenya from 12 to 19 November 2013 in order to Evaluate Controls of Pesticides in Food of Plant Origin Intended for Export to the European Union; DG (SANCO) 2013-6692-MR FINAL; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Fening, K.O.; Adama, I.; Tegbe, R.E. On-farm evaluation of homemade pepper extract in the management of pests of cabbage, Brassica oleraceae L., and French beans, Phaseolus vulgaris L., in two agro-ecological zones in Ghana. Afr. Entomol. 2014, 22, 552–560. [Google Scholar] [CrossRef]
- Koigi, B. Digital traceability: Reigniting Kenya’s International Trade. The Technical Centre for Agricultural and Rural Cooperation (CTA). 2018. Available online: http://spore.cta.int/en/research/reigniting-kenyas-international-trade.html (accessed on 13 October 2018).
- Business Daily Africa. EU Forces Pesticide Chemical Banning. 2012. Available online: https://www.freshplaza.com/article/2092322/kenya-eu-forces-pesticide-chemical-banning/ (accessed on 22 January 2015).
- Andae, G. Fresh Exports Get Boost as KEPHIS to Monitor EU Sales. Business Daily. Available online: http://www.businessdailyafrica.com/Fresh-exports-get-boost-as-Kephis-to-monitor-EU-sales/-/539552/3048684/-/13dhxpsz/-/index.html (accessed on 24 June 2016).
- Hortfresh. EU Lifts Ban on Kenya’s Beans. Hortfresh J. Available online: http://hortfreshjournal.com/eu-lifts-ban-on-kenyas-beans/ (accessed on 23 June 2016).
- Infonet-Biovision. Export Requirements for Fruits and Vegetables in Kenya. Available online: https://www.infonet-biovision.org/EnvironmentalHealth/Basic-Export-Requirements-FruitVegetable-Kenya (accessed on 21 July 2020).
- Global-GAP. What We Do. Available online: https://www.globalgap.org/uk_en/what-we-do/ (accessed on 20 October 2017).
- Wahome, S.W.; Kimani, P.M.; Muthomi, J.W.; Narla, R.D.; Buruchara, R. Multiple disease resistance in snap bean genotypes in Kenya. Afr. Crop Sci. J. 2011, 4, 289–302. [Google Scholar]
- Odhiambo, O. D.Competitiveness of Small holder Snap Bean Production in Kirinyaga County, Kenya. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2012. [Google Scholar]
- Research Solutions Africa (RSA) Ltd. Report of a Study on Fresh Vegetables Market in Kenya. Desk Review; Research Solutions Africa (RSA) Ltd.: Nairobi, Kenya, 2015. [Google Scholar]
- Kenya Plant Health Inspectorate Service (KEPHIS). Phytosanitary Services. Available online: https://www.kephis.org/index.php/import-requirements/2014-09-19-08-11-21 (accessed on 24 January 2020).
- Nassilah, S. Kenya Plant Health Inspectorate Service (KEPHIS) 2016 Annual Report. Available online: https://issuu.com/nassilah/docs/2017_annual_report_24.04.2017_12.37 (accessed on 5 October 2018).
- Kleih, U.; Allen, C.; Basset-Mens, C.; Edewa, A. Green Beans Value Chain Analysis in Kenya; Report for the European Commission; Value Chain Analysis for Development Project (VCA4D CTR 2016/375-804), + annexes; DG-DEVCO: Brussels, Belgium, 2017; 171p. [Google Scholar]
- United States Agency for International Development-Kenya Agricultural Value Chain Enterprises (USAID-KAVES). French Bean Value Chain Analysis; Fintrac. Inc.: Nairobi, Kenya, 2015. [Google Scholar]
- Otieno, G.A. Standards and Development: Perspectives from Kenya’s Horticultural Export Industry. Ph.D. Thesis, Erasmus University Rotterdam, Rotterdam, The Netherlands, 2016. [Google Scholar]
- Agriculture and Food Authority (AFA). Horticultural Crops Directorate (HCD). 2018. Available online: https://horticulture.agricultureauthority.go.ke/?page_id=20 (accessed on 4 June 2018).
- Fresh Produce Exporters Association of Kenya (FPEAK). Making Kenyan Horticulture the Global Choice. 2018. Available online: http://fpeak.org/ (accessed on 4 June 2018).
- Food and Agriculture Organization of the United Nations (FAO). International Plant Protection Convention. Available online: https://www.ippc.int/en/ (accessed on 18 March 2018).
- Food and Agriculture Organization of the United Nations (FAO). Codex Alimentarius International Food Standards. Available online: http://www.fao.org/fao-who-codexalimentarius/en/ (accessed on 18 March 2018).
- Organisation for Economic Co-operation and Development (OECD). About Fruit and Vegetables. Available online: http://www.oecd.org/tad/code/aboutfruitandvegetables.htm (accessed on 5 October 2018).
- Export Development Authority and Green Trade Initiative. Green Beans Market Overview in France. 2018. Available online: https://eda-gti.org/wp-content/uploads/2018/08/green-beans-market-overview-in-France.pdf (accessed on 18 January 2021).
- BRC Global Standards. British Retail Consortium (BRC) Global Standards. Available online: https://www.brcglobalstandards.com/ (accessed on 12 March 2018).
- European Commission. What is Organic Farming and Organic Certification? Available online: https://ec.europa.eu/agriculture/organic/organic-farming/what-is-organic-farming/organic-certification (accessed on 13 February 2018).
- Sharmah, D.; Rahman, S. Management of the major pests of French bean through development and validation of certain IPM modules, Assam. India J. Appl. Nat. Sci. 2017, 9, 674–679. [Google Scholar] [CrossRef]
- Murali-Baskaran, R.K.; Sharma, K.C.; Kaushal, P.; Kumar, J.; Parthiban, P.; Senthil-Nathan, S.; Mankin, R.W. Role of kairomones in biological control of crop pests-Review. Physiol. Mol. Plant Pathol. 2017, 1–13. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Krishan, J. Global Trends in the Development and Use of Biopesticides. Regional Experts Workshop on Development, Regulation and Use of Biopesticides in East Africa; Nairobi, Kenya, 2014; Available online: https://www.slideshare.net/ILRI/bioinnovate-jindal-may2014 (accessed on 21 December 2017).
- Croplife. Integrated Pest Management. Available online: https://croplife.org/wp-content/uploads/pdf_files/Integrated-pest-management.pdf (accessed on 12 February 2018).
- Kimani, V. Biopesticides Development, Use and Regulation in Kenya. Regional Experts Workshop on Development, Regulation and Use of Biopesticide in East Africa Slide Share 2014. Available online: https://www.slideshare.net/ILRI/bioinnovate-kimani-may2014 (accessed on 21 December 2017).
- Mondal, A.; Shankar, U.; Abrol, D.P.; Singh, I.; Norboo, T. Evaluation of pest management strategies against sucking insect-pests for the safety of beneficial insects in vegetable French bean (Phaseolus vulgaris). Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1441–1448. [Google Scholar] [CrossRef]
- Sushil, S.N.; Singh, J.P.; Ghosh, P.K.; Akhtar, M. Integrated Pest Management (IPM) in French Beans (Phaseolus Vulgaris) for Export Purpose; Plant Protection Advisor, Directorate of Plant Protection, Quarantine and Storage: Faridabad, India, 2018. [Google Scholar]
- Fathipour, Y.; Maleknia, B. Ecofriendly Pest Management for Food Security; Omkar, Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 329–366. [Google Scholar] [CrossRef]
- Harbi, A.; Elimem, M.; Chermiti, B. Use of synthetic kairomone to control Frankliniella occidentalis. Pergande (thysanoptera: Thripidae) in protected pepper in Tunisia. Afr. J. Plant Sci. Biotechnol. 2013, 7, 42–47. [Google Scholar]
- Muvea, A.M.; Kutima, H.L.; Lagat, S.O.; Waiganjo, M.; Subramanian, S. Evaluation of coloured traps with kairomones attractant for monitoring thrips population dynamics on tomato crops in East Africa. Int. J. Trop. Insect Sci. 2017, 37, 89–97. [Google Scholar] [CrossRef]
- Niassy, S.; Maniania, N.K.; Subramanian, S.; Gitonga, M.L.; Maranga, R.; Obonyo, A.B.; Ekesi, S. Compatibility of Metarhizium anisopliae isolate ICIPE69 with agrochemical used French bean production. Int. J. Pest Manag. 2012, 58, 131–137. [Google Scholar] [CrossRef]
- Muthomi, J.W.; Fulano, A.M.; Wagacha, J.M.; Mwang’ombe, A.W. Management of snap bean insect pests and diseases by use of antagonist stick fungi and plant extracts. Sustain. Agric. Res. 2017, 6, 52–63. [Google Scholar] [CrossRef]
- Zyton, M.A.; Ahmed, G.A. Management of bean rust by some bioagents and essential plant oils. Egypt. J. Phytopathol. 2019, 44, 167–186. [Google Scholar] [CrossRef]
- Niassy, S.; Maniania, N.K.; Subramanian, S.; Gitonga, L.M.; Ekesi, S. Performance of a semiochemical-baited auto inoculation device treated with Metarhizium anisopliae for control of Frankliniella occidentalis on French bean in field cages. Entomol. Exp. Appl. 2012, 142, 97–103. [Google Scholar] [CrossRef]
- Kuboka, M.N. Effect of Temperature on the Efficacy of Metarhizium Anisopliae in Management of Western Flower Thrips in French Beans. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2013. [Google Scholar]
- Kasina, J.M.; Nderitu, J.H.; Nyamasyo, G.H.N.; Olubayo, F.; Waturu, C.N.; Obudho, E. Evaluation of companioncrops for thrips (Thysanoptera: Thripidae) management in French beans (Phaseolus vulgaris) L. Int. J. Trop. Insect Sci. 2006, 26, 121–125. [Google Scholar] [CrossRef]
- Zilahi-balogh, G.M.G.; Shipp, J.L.; Cloutier, C.; Brodeur, J. Predation by Neoseiulus cucumeris on western flower thrips, and its oviposition on greenhouse cucumber under winter vs. summer conditions in atemperate climate. Biol. Control 2007, 40, 160–167. [Google Scholar] [CrossRef]
- Nyasani, J.O.; Subramanian, S.; Poehling, H.M.; Maniania, N.K.; Ekesi, S.; Meyhöfer, R. Optimizing Western Flower Thrips Management on French Beans by Combined Use of Beneficials and Imidacloprid. Insects 2015, 6, 279–296. [Google Scholar] [CrossRef]
- Mohammed, A. An Overview of Distribution, Biology and the Management of Common Bean Anthracnose. J. Plant Pathol. Microb. 2013, 4, 193. [Google Scholar] [CrossRef]
- Dheeraj, J.; Susan, V.P.; Nisha, S. Fatty Acid Metallic Salts and Pyrethroids-Environmental friendly Pesticides. Int. J. Sci. Res. Rev. 2013, 2, 43–51. [Google Scholar]
- Wafula, G.O. Potential of Potassium Salts and Integrated Pest Management Strategies in Management of Snap Bean Pests. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2014. [Google Scholar]
- Wafula, G.O.; Muthomi, J.W.; Nderitu, J.H.; Chemining’wa, G.N. Efficacy of potassium salts of fatty acids in the management of thrips and whitefly on snap beans. Sustain. Agric. Res. 2017, 6, 45–54. [Google Scholar] [CrossRef]
- Monda, E.O.; Munene, S.; Ndegwa, A. Snap bean production constraints in Kenya. Afr. Crop Sci. Conf. Proc. 2003, 6, 683–687. [Google Scholar]
- Muthomi, J.W.; Kinyungu, T.N.; Nderitu, J.H.; Olubayo, F.M. Spatial arrangement of maize as border crop to manage aphids and aphid-transmitted viruses in potato. East Afr. Agric. For. J. 2010, 76, 21–29. [Google Scholar]
- Muthomi, J.W.; Kinyungu, T.N.; Nderitu, J.H.; Olubayo, F.M. Incidence of aphid-transmitted viruses in farmer-produced seed potato tubers in Kenya. Afr. J. Hort. Sci. 2011, 5, 18–25. [Google Scholar] [CrossRef]
- Nderitu, J.; Mwangi, F.; Nyamasyo, G.; Kasina, A.M. Evaluation of cropping systems as a strategy for managing snap beanflower thrips in Kenya. Int. J. Sustain. Crop Prod. 2009, 4, 22–25. [Google Scholar]
- Waiganjo, M.M.; Muriuki, J.; Mbugua, G.W. Potential of indigenous leafy vegetables as companion crops for pest management of high-value legumes: Acase study of Gynandropsis gynandra in Kenya. Acta Hort (ISHS) 2007, 752, 319–321. [Google Scholar] [CrossRef]
- Muthomi, J.W.; Wafula, G.O.; Nderitu, J.H.; Chemining’wa, G.N. Integration of seed dressing, bio-pesticides and intercropping to reduce pesticide use in snap bean production. Int. J. Agric. Sci. Nat. Resour. 2018, 5, 12–20. [Google Scholar]


| Pesticide | Status | MRLs (mg/Kg) |
|---|---|---|
| Acephate | Unauthorized | 0.01 |
| Carbendazim | Unauthorized | 0.2 |
| Chlorpyrifos | Not for foliar use | 0.05 |
| Dimethoate | Not for foliar use | 0.02 |
| Hezaconazole | Unauthorized | 0.01 |
| Lufenuron | Unauthorized | 0.02 |
| Methamidophos | Unauthorized | 0.01 |
| Omethoate | Not for foliar use | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulano, A.M.; Lengai, G.M.W.; Muthomi, J.W. Phytosanitary and Technical Quality Challenges in Export Fresh Vegetables and Strategies to Compliance with Market Requirements: Case of Smallholder Snap Beans in Kenya. Sustainability 2021, 13, 1546. https://doi.org/10.3390/su13031546
Fulano AM, Lengai GMW, Muthomi JW. Phytosanitary and Technical Quality Challenges in Export Fresh Vegetables and Strategies to Compliance with Market Requirements: Case of Smallholder Snap Beans in Kenya. Sustainability. 2021; 13(3):1546. https://doi.org/10.3390/su13031546
Chicago/Turabian StyleFulano, Alex M., Geraldin M. W. Lengai, and James W. Muthomi. 2021. "Phytosanitary and Technical Quality Challenges in Export Fresh Vegetables and Strategies to Compliance with Market Requirements: Case of Smallholder Snap Beans in Kenya" Sustainability 13, no. 3: 1546. https://doi.org/10.3390/su13031546
APA StyleFulano, A. M., Lengai, G. M. W., & Muthomi, J. W. (2021). Phytosanitary and Technical Quality Challenges in Export Fresh Vegetables and Strategies to Compliance with Market Requirements: Case of Smallholder Snap Beans in Kenya. Sustainability, 13(3), 1546. https://doi.org/10.3390/su13031546







