An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid and Liquid Urine Derivatives Preparation
2.1.1. Hydrolyzed Urine
2.1.2. Stabilized Urine-Low pH
2.1.3. Stabilized Urine-High pH
2.1.4. Electrodialysis Concentrate
2.1.5. Aurin
2.1.6. K-Struvite
2.1.7. Urine Precipitate-NaOH
2.1.8. Urine Precipitate-CaO
2.2. Plant Material, Growth Conditions, and Experimental Design
2.3. Plants Manual Fertigation and Nutrient Solutions Electrical Conductivity
2.4. Soil Plant Analysis Development (SPAD) Index, Biomass Determination, and Growth Analysis
2.5. Total Nitrogen, Mineral, and Organic Acids Content Analysis
2.6. Statistical Analysis
3. Results
3.1. Agronomic Performance and SPAD Index
3.2. Mineral Composition and Nitrate Concentration
3.3. Organic Acids Content
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franklin-Johnson, E.; Figge, F.; Canning, L. Resource duration as a managerial indicator for Circular Economy performance. J. Clean. Prod. 2016, 133, 589–598. [Google Scholar] [CrossRef]
- Jurgilevich, A.; Birge, T.; Kentala-Lehtonen, J.; Korhonen-Kurki, K.; Pietikäinen, J.; Saikku, L.; Schösler, H. Transition towards circular economy in the food system. Sustainability 2016, 8, 69. [Google Scholar] [CrossRef]
- Koohafkan, P.; Altieri, M.A.; Holt Gimenez, E. Green Agriculture: Foundations for biodiverse, resilient and productive agricultural systems. Int. J. Agric. Sustain. 2012, 10, 61–75. [Google Scholar] [CrossRef]
- Toop, T.A.; Ward, S.; Oldfield, T.; Hull, M.; Kirby, M.E.; Theodorou, M.K. AgroCycle—Developing a circular economy in agriculture. Energy Procedia 2017, 123, 76–80. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Mayer, B.; Westerhoff, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84, 846–853. [Google Scholar] [CrossRef]
- Ciceri, D.; Manning, D.A.C.; Allanore, A. Historical and technical developments of potassium resources. Sci. Total Environ. 2015, 502, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Ganesapillai, M.; Simha, P.; Gupta, K.; Jayan, M. Nutrient Recovery and Recycling from Human Urine: A Circular Perspective on Sanitation and Food Security. Procedia Eng. 2016, 148, 346–353. [Google Scholar] [CrossRef]
- De Paepe, J.; Lindeboom, R.E.F.; Vanoppen, M.; De Paepe, K.; Demey, D.; Coessens, W.; Lamaze, B.; Verliefde, A.R.D.; Clauwaert, P.; Vlaeminck, S.E. Refinery and concentration of nutrients from urine with electrodialysis enabled by upstream precipitation and nitrification. Water Res. 2018, 144, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; Logrieco, M.E.; Monetti, J.; Ledezma, P.; Virdis, B.; Tsujimura, S. Self-powered bioelectrochemical nutrient recovery for fertilizer generation from human urine. Sustainability 2019, 11, 5490. [Google Scholar] [CrossRef]
- Tao, W.; Bayrakdar, A.; Wang, Y.; Agyeman, F. Three-stage treatment for nitrogen and phosphorus recovery from human urine: Hydrolysis, precipitation and vacuum stripping. J. Environ. Manag. 2019, 249, 109435. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.R.; Schott, C.; van der Weijden, R.D.; Leal, L.H.; Zeeman, G.; Buisman, C. Calcium phosphate granules recovered from black water treatment: A sustainable substitute for mined phosphorus in soil fertilization. Resour. Conserv. Recycl. 2020, 158, 104791. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, M.; Wang, C.; Liang, C.; Shen, Z.; Xu, K. A green method for the simultaneous recovery of phosphate and potassium from hydrolyzed urine as value-added fertilizer using wood waste. Resour. Conserv. Recycl. 2020, 157, 104793. [Google Scholar] [CrossRef]
- Harder, R.; Wielemaker, R.; Larsen, T.A.; Zeeman, G.; Öberg, G. Recycling nutrients contained in human excreta to agriculture: Pathways, processes, and products. Crit. Rev. Environ. Sci. Technol. 2019, 49, 695–743. [Google Scholar] [CrossRef]
- Mcheik, M.; Toufaily, J.; Haj Hassan, B.; Hamieh, T.; Abi Saab, M.T.; Rouphael, Y.; Ferracin, E.; da shio, B.; Bashabshah, I.; Al Hadidi, L. Reuse of treated municipal wastewater in irrigation: A case study from Lebanon and Jordan. Water Environ. J. 2017, 31, 552–558. [Google Scholar] [CrossRef]
- Grunbaum, M. Gee Whiz: Human Urine Is Shown to Be an effective Agricultural Fertilizer. Sci. Am. 2010. Available online: http://www.scientificamerican.com/article/human-urine-is-an-effective-fertilizer (accessed on 6 January 2021).
- Zhang, J.; Xie, M.; Tong, X.; Liu, S.; Qu, D.; Xiao, S. Recovery of ammonium nitrogen from human urine by an open-loop hollow fiber membrane contactor. Sep. Purif. Technol. 2020, 239, 116579. [Google Scholar] [CrossRef]
- Ganrot, Z.; Slivka, A.; Dave, G. Nutrient recovery from human urine using pretreated zeolite and struvite precipitation in combination with freezing-thawing and plant availability tests on common wheat. Clean-Soil Air Water 2008, 36, 45–52. [Google Scholar] [CrossRef]
- Beler-Baykal, B.; Allar, A.D.; Bayram, S. Nitrogen recovery from source-separated human urine using clinoptilolite and preliminary results of its use as fertilizer. Water Sci. Technol. 2011, 63, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Udert, K.M.; Larsen, T.A.; Biebow, M.; Gujer, W. Urea hydrolysis and precipitation dynamics in a urine-collecting system. Water Res. 2003, 37, 2571–2582. [Google Scholar] [CrossRef]
- Udert, K.M.; Larsen, T.A.; Gujer, W. Biologically induced precipitation in urine-collecting systems. Water Sci. Technol. Water Supply 2003, 3, 71–78. [Google Scholar] [CrossRef]
- Udert, K.M.; Larsen, T.A.; Gujer, W. Fate of major compounds in source-separated urine. Water Sci. Technol. 2006, 54, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Saetta, D.; Boyer, T.H. Mimicking and Inhibiting Urea Hydrolysis in Nonwater Urinals. Environ. Sci. Technol. 2017, 51, 13850–13858. [Google Scholar] [CrossRef]
- Ray, H.; Saetta, D.; Boyer, T.H. Characterization of urea hydrolysis in fresh human urine and inhibition by chemical addition. Environ. Sci. Water Res. Technol. 2018, 4, 87–98. [Google Scholar] [CrossRef]
- Randall, D.G.; Krähenbühl, M.; Köpping, I.; Larsen, T.A.; Udert, K.M. A novel approach for stabilizing fresh urine by calcium hydroxide addition. Water Res. 2016, 95, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Fumasoli, A.; Etter, B.; Sterkele, B.; Morgenroth, E.; Udert, K.M. Operating a pilot-scale nitrification/distillation plant for complete nutrient recovery from urine. Water Sci. Technol. 2016, 73, 215–222. [Google Scholar] [CrossRef]
- Udert, K.M.; Wächter, M. Complete nutrient recovery from source-separated urine by nitrification and distillation. Water Res. 2012, 46, 453–464. [Google Scholar] [CrossRef]
- Xu, K.; Wang, C.; Wang, X.; Qian, Y. Laboratory experiments on simultaneous removal of K and P from synthetic and real urine for nutrient recycle by crystallization of magnesium-potassium-phosphate-hexahydrate in a draft tube and baffle reactor. Chemosphere 2012, 88, 219–223. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic constitution, phytochemical and macronutrient content in three species of microgreens as modulated by natural fiber and synthetic substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Peiro, E.; Pannico, A.; Colleoni, S.G.; Bucchieri, L.; Rouphael, Y.; De Pascale, S.; Paradiso, R.; Gòdia, F. Air Distribution in a Fully-Closed Higher Plant Growth Chamber Impacts Crop Performance of Hydroponically-Grown Lettuce. Front. Plant Sci. 2020, 11, 537. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Petropoulos, S.A.; Giordano, M.; Colla, G.; Troise, A.D.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. Dataset on the organic acids, sulphate, total nitrogen and total chlorophyll contents of two lettuce cultivars grown hydroponically using nutrient solutions of variable macrocation ratios. Data Br. 2020, 29, 105135. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis: Part 2 CHemical and Microbiological Properties Agronomy Monograph 9; Black, C.A., Evans, D., White, J.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. (Amsterdam) 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Alemayehu, Y.A.; Asfaw, S.L.; Terfie, T.A. Nutrient recovery options from human urine: A choice for large scale application. Sustain. Prod. Consum. 2020, 24, 219–231. [Google Scholar] [CrossRef]
- Volpin, F.; Chekli, L.; Phuntsho, S.; Cho, J.; Ghaffour, N.; Vrouwenvelder, J.S.; Kyong Shon, H. Simultaneous phosphorous and nitrogen recovery from source-separated urine: A novel application for fertiliser drawn forward osmosis. Chemosphere 2018, 203, 482–489. [Google Scholar] [CrossRef]
- Ushakova, S.; Tikhomirov, A.; Shikhov, V.; Kudenko, Y.; Anischenko, O.; Gros, J.B.; Lasseur, C. Increased BLSS closure using mineralized human waste in plant cultivation on a neutral substrate. Adv. Sp. Res. 2009, 44, 971–978. [Google Scholar] [CrossRef]
- Ryu, H.D.; Lee, S.I. Struvite recovery from swine wastewater and its assessment as a fertilizer. Environ. Eng. Res. 2016, 21, 29–35. [Google Scholar] [CrossRef]
- Cerrillo, M.; Palatsi, J.; Comas, J.; Vicens, J.; Bonmatí, A. Struvite precipitation as a technology to be integrated in a manure anaerobic digestion treatment plant-removal efficiency, crystal characterization and agricultural assessment. J. Chem. Technol. Biotechnol. 2015, 90, 1135–1143. [Google Scholar] [CrossRef]
- Ricardo, G.P.; López-de-Sá, E.G.; Plaza, C. Lettuce response to phosphorus fertilization with struvite recovered from municipal wastewater. HortScience 2009, 44, 426–430. [Google Scholar] [CrossRef]
- Savvas, D.; Passam, H.C.; Olympios, C.; Nasi, E.; Moustaka, E.; Mantzos, N.; Barouchas, P. Effects of ammonium nitrogen on lettuce grown on pumice in a closed hydroponic system. HortScience 2006, 41, 1667–1673. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Lea, P.J. Do plants need nitrate? the mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Paradiso, R.; Buonomo, R.; Dixon, M.A.; Barbieri, G.; De Pascale, S. Effect of bacterial root symbiosis and urea as source of nitrogen on performance of soybean plants grown hydroponically for Bioregenerative Life Support Systems (BLSSs). Front. Plant Sci. 2015, 6, 888. [Google Scholar] [CrossRef]
- EI-Kazzaz, A. Soilless Agriculture a New and Advanced Method for Agriculture Development: An Introduction. Agric. Res. Technol. Access J. 2017, 3, 63–72. [Google Scholar] [CrossRef]
- Andriolo, J.L.; da Luz, G.L.; Witter, M.H.; Godoi, R.d.S.; Barros, G.T.; Bortolotto, O.C. Growth and yield of lettuce plants under salinity. Hortic. Bras. 2005, 23, 931–934. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. (Amsterdam) 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Samarakoon, U.C.; Weerasinghe, P.A.; Weerakkody, W.A. Effect of Electrical Conductivity [EC] of the Nutrient Solution on Nutrient Uptake, Growth and Yield of Leaf Lettuce (Lactuca sativa L.) in Stationary Culture. Trop. Agric. Res. 2006, 18, 13–21. [Google Scholar]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. (Amsterdam) 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Gunes, A.; Post, W.N.K.; Kirkby, E.A.; Aktas, M. Influence of partial replacement of nitrate by amino acid nitrogen or urea in the nutrient medium on nitrate accumulation in nft grown winter lettuce. J. Plant Nutr. 1994, 17, 1929–1938. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. (Amsterdam) 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Corrado, G.; Lucini, L.; Miras-Moreno, B.; Chiaiese, P.; Colla, G.; De Pascale, S.; Rouphael, Y. Metabolic insights into the anion-anion antagonism in sweet basil: Effects of different nitrate/chloride ratios in the nutrient solution. Int. J. Mol. Sci. 2020, 21, 2482. [Google Scholar] [CrossRef]
- Duri, L.G.; El-Nakhel, C.; Caporale, A.G.; Ciriello, M.; Graziani, G.; Pannico, A.; Palladino, M.; Ritieni, A.; De Pascale, S.; Vingiani, S.; et al. Mars regolith simulant ameliorated by compost as in situ cultivation substrate improves lettuce growth and nutritional aspects. Plants 2020, 9, 628. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in macronutrient content, phytochemical constitution and in vitro antioxidant capacity of green and red butterhead lettuce dictated by different developmental stages of harvest maturity. Antioxidants 2020, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; Bloszies, S.; Li, X.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stresses on respiratory metabolism, antioxidant system, nutritional status, and organic acid metabolism in tomato roots. Acta Physiol. Plant. 2014, 36, 2167–2181. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Dung, V.V.; Kuchaeva, L. The role of organic acids in heavy metal tolerance in plants. Biol. Commun. 2018, 63, 9–16. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Loera, R.D.C.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Marqués, C.D.; Pardo-de-Santayana, M.; Tardío, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
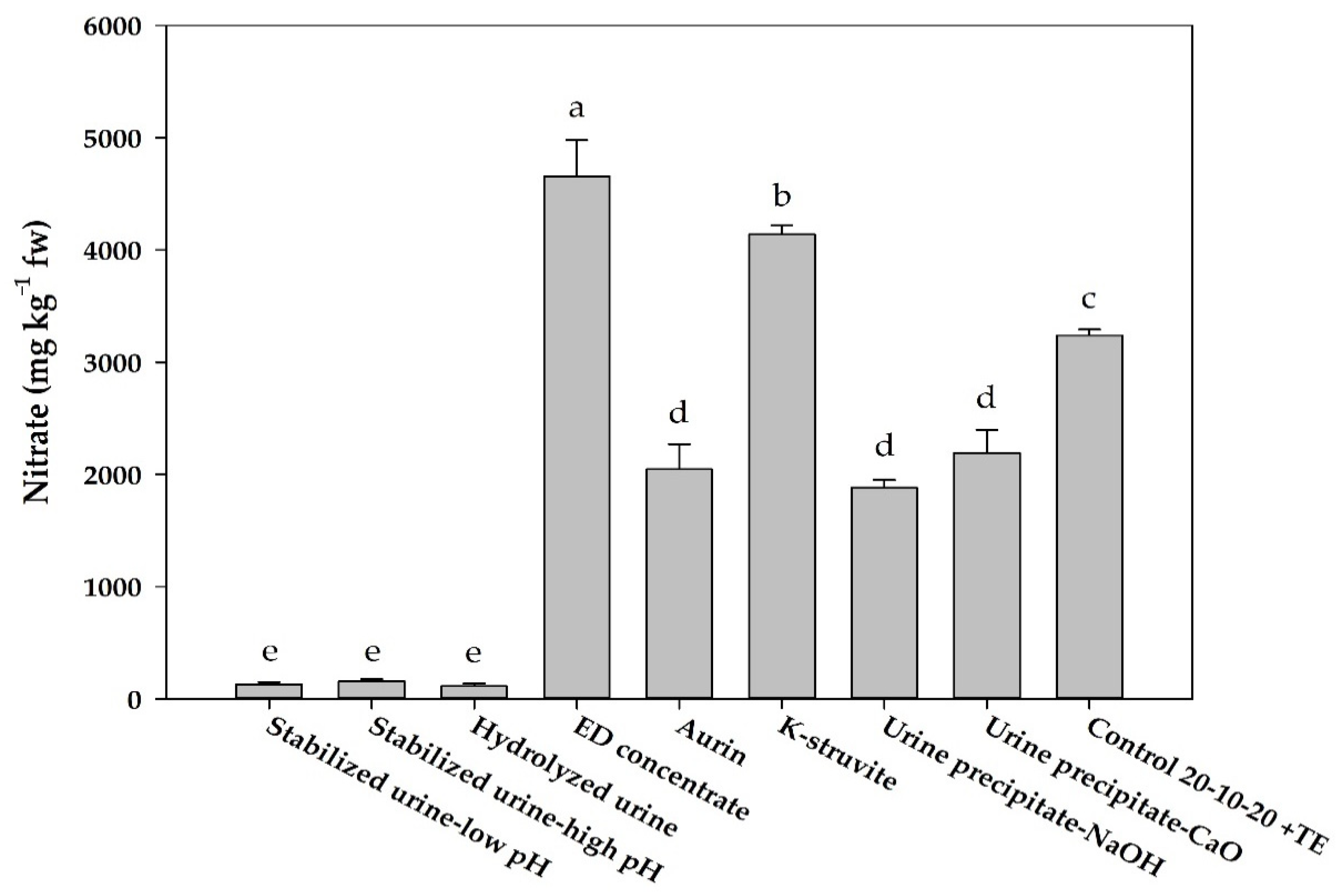
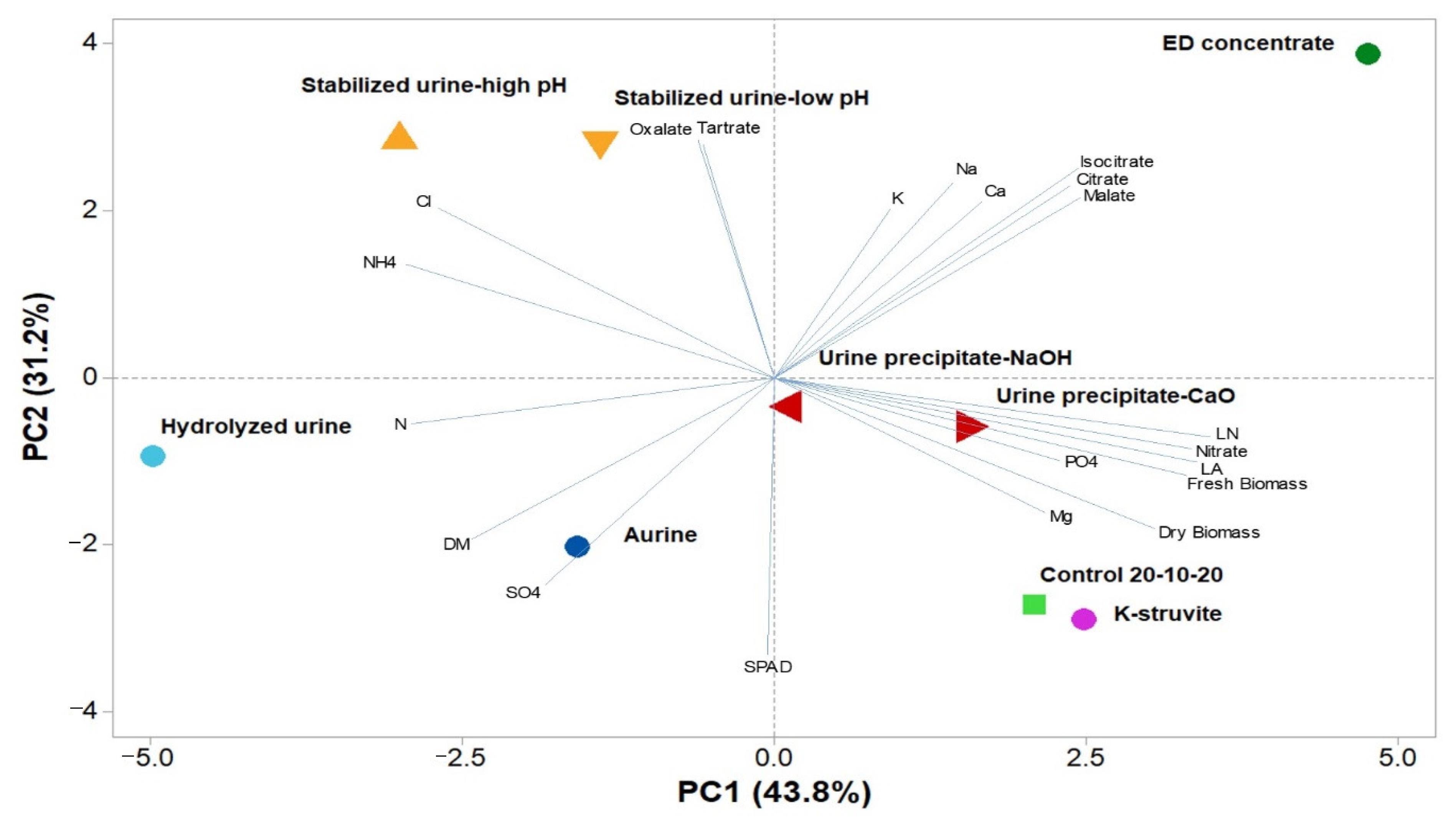
| Treatments | Total N | NH4+-N | NO2−-N | NO3−-N | PO43−-P | K+ | SO42− | Ca2+ | Mg2+ | Na+ | Cl− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stabilized urine-low pH | 6396.0 | 502.1 | 1.4 | 12.4 | 258.7 | 2648.4 | 912.6 | - | - | 2719.6 | 6826.8 |
| Stabilized urine-high pH | 5610.0 | 403.3 | 4.6 | 9.0 | 3.0 | 1620.0 | 791.4 | 10.2 | 110.0 | 2506.7 | 3431.2 |
| Hydrolyzed urine | 3376.0 | 3300.7 | 0.0 | 0.2 | 87.2 | 1354.0 | 531.1 | - | - | 1360.6 | 2227.7 |
| ED concentrate | 4500.0 | 12.0 | 122.8 | 3885.5 | 74.8 | 1853.5 | 225.0 | - | - | 8776.2 | 3919.4 |
| Aurin | 42,000.0 | 20,346.0 | 0.0 | 21,654.0 | 1746.0 | 14,943.0 | - | - | - | 17,000.0 | 31,000.0 |
| K-Struvite | 5.2 | 4.2 | 0.0 | 0.0 | 61.0 | 42.4 | - | 17.7 | 15.3 | 52.9 | 6.5 |
| Urine precipitate-NaOH | 147.1 | 22.1 | 0.0 | 0.0 | 20.6 | 52.3 | - | 2.4 | 0.54 | 115.5 | 100.4 |
| Urine precipitate-CaO | 81.2 | 7.5 | 0.1 | 0.1 | 15.5 | 35.3 | 0.0 | 22.6 | 0.1 | 29.4 | 66.9 |
| NPK 20-10-20 + TE | 200.0 | 80.0 | 0.0 | 120.0 | 100.0 | 200.0 | - | - | 1.5 | - | - |
| Treatments | Leaf Number | Leaf Area | Fresh Biomass | Dry Biomass | Dry Matter | SPAD Index |
|---|---|---|---|---|---|---|
| (no. plant−1) | (cm2 plant−1) | (g plant−1) | (g plant−1) | (%) | ||
| Stabilized urine-low pH | 13 ± 0.22 bcd | 565 ± 111 bc | 17.38 ± 3.62 b | 0.88 ± 0.27 de | 4.85 ± 0.52 ef | 14.94 ± 0.26 bc |
| Stabilized urine-high pH | 12 ± 0.80 de | 387 ± 76 cd | 10.33 ± 2.54 c | 0.59 ± 0.14 e | 5.74 ± 0.26 cde | 13.96 ± 0.86 cd |
| Hydrolyzed urine | 10 ± 0.48 e | 324 ± 31 d | 7.88 ± 0.82 c | 0.60 ± 0.06 e | 7.79 ± 0.14 a | 15.89 ± 0.60 ab |
| ED concentrate | 16 ± 1.73 ab | 1283 ± 85 a | 38.18 ± 2.05 a | 1.81 ± 0.05 b | 4.76 ± 0.13 f | 13.13 ± 0.60 d |
| Aurin | 13 ± 0.19 cd | 620 ± 20 b | 18.78 ± 0.82 b | 1.30 ± 0.11 cd | 6.92 ± 0.51 b | 17.24 ± 0.64 a |
| K-struvite | 17 ± 0.33 a | 1379 ± 127 a | 44.37 ± 3.38 a | 2.51 ± 0.30 a | 5.64 ± 0.25 def | 17.50 ± 0.73 a |
| Urine precipitate-NaOH | 14 ± 0.68 bcd | 776 ± 36 b | 23.14 ± 0.88 b | 1.52 ± 0.07 bc | 6.59 ± 0.10 bc | 16.98 ± 0.28 a |
| Urine precipitate-CaO | 15 ± 1.47 abc | 1308 ± 70 a | 42.78 ± 1.69 a | 2.33 ± 0.07 a | 5.46 ± 0.07 def | 16.89 ± 0.50 a |
| Control 20-10-20 + TE | 16 ± 0.48 ab | 1290 ± 18 a | 42.78 ± 0.96 a | 2.49 ± 0.09 a | 5.86 ± 0.18 cd | 16.77 ± 0.25 a |
| Significance | *** | *** | *** | *** | *** | *** |
| Treatments | Total N | PO4 | K | Ca | Mg | Na | Cl | SO4 |
|---|---|---|---|---|---|---|---|---|
| (g kg−1 dw) | (g kg−1 dw) | (g kg−1 dw) | (g kg−1 dw) | (g kg−1 dw) | (g kg−1 dw) | (g kg−1 dw) | (g kg−1 dw) | |
| Stabilized urine-low pH | 51.1 ± 0.92 ab | 17.13 ± 1.56 bcd | 75.63 ± 3.81 a | 3.65 ± 0.97 a | 1.63 ± 0.17 c | 15.11 ± 1.01 c | 53.32 ± 5.33 a | 6.31 ± 0.34 ab |
| Stabilized urine-high pH | 52.9 ± 2.5 a | 14.25 ± 1.55 de | 67.70 ± 1.26 b | 1.88 ± 0.33 b | 1.24 ± 0.03 cde | 11.84 ± 0.18 de | 41.16 ± 1.46 b | 5.45 ± 0.63 b |
| Hydrolyzed urine | 53.3 ± 0.30 a | 14.76 ± 0.59 de | 63.94 ± 2.78 b | 1.36 ± 0.14 b | 1.02 ± 0.07 de | 7.70 ± 0.67 f | 44.21 ± 2.48 b | 6.99 ± 0.48 ab |
| ED concentrate | 42.4 ± 0.42 f | 18.38 ± 0.58 abc | 78.66 ± 2.44 a | 3.64 ± 0.89 a | 1.54 ± 0.24 cd | 32.06 ± 2.06 a | 21.22 ± 1.34 d | 3.37 ± 0.62 c |
| Aurin | 47.6 ± 0.92 cd | 15.62 ± 0.63 cde | 64.04 ± 1.03 b | 1.18 ± 0.16 b | 0.93 ± 0.09 e | 10.01 ± 0.10 ef | 28.35 ± 1.18 cd | 6.51 ± 0.24 ab |
| K-struvite | 50.7 ± 0.10 abc | 20.48 ± 0.31 a | 65.96 ± 0.96 b | 1.47 ± 0.14 b | 3.52 ± 0.28 a | 9.03 ± 0.42 f | 7.63 ± 0.68 e | 6.81 ± 0.17 ab |
| Urine precipitate-NaOH | 47.7 ± 0.60 cd | 17.99 ± 1.10 abc | 51.08 ± 2.82 c | 2.03 ± 0.13 b | 2.53 ± 0.12 b | 25.56 ± 1.14 b | 29.22 ± 2.74 c | 7.26 ± 0.44 a |
| Urine precipitate-CaO | 46.5 ± 0.82 e | 13.68 ± 0.56 e | 62.02 ± 0.94 b | 3.99 ± 0.47 a | 2.17 ± 0.14 b | 13.49 ± 0.11 cd | 27.59 ± 0.59 cd | 6.11 ± 0.34 ab |
| Control 20-10-20 + TE | 4.82 ± 0.03 bcd | 20.07 ± 0.66 ab | 67.11 ± 0.96 b | 1.99 ± 0.24 b | 2.43 ± 0.25 b | 1.08 ± 0.04 g | 5.35 ± 0.60 e | 6.25 ± 0.68 ab |
| Significance | *** | *** | *** | ** | *** | *** | *** | *** |
| Treatments | Malate | Tartrate | Oxalate | Citrate | Isocitrate |
|---|---|---|---|---|---|
| (g kg−1 dw) | (g kg−1 dw) | (g kg−1 dw) | (g kg−1 dw) | (mg kg−1 dw) | |
| Stabilized urine-low pH | 21.32 ± 2.03 cd | 3.58 ± 0.65 a | 2.92 ± 0.27 b | 4.38 ± 0.30 c | 113.9 ± 9.79 b |
| Stabilized urine-high pH | 22.60 ± 1.38 cd | 3.80 ± 0.36 a | 3.59 ± 0.24 a | 3.51 ± 0.07 d | 90.17 ± 12.8 bc |
| Hydrolyzed urine | 4.95 ± 0.40 g | 2.55 ± 0.80 abc | 2.04 ± 0.05 c | 1.32 ± 0.06 g | 39.00 ± 12.0 d |
| ED concentrate | 46.54 ± 3.09 a | 2.94 ± 0.15 ab | 2.71 ± 0.29 b | 6.24 ± 0.26 a | 206.9 ± 30.6 a |
| Aurin | 10.45 ± 0.38 f | 1.45 ± 0.04 c | 2.06 ± 0.16 c | 2.07 ± 0.09 f | 64.60 ± 1.51 cd |
| K-struvite | 17.94 ± 1.20 de | 2.46 ± 0.28 abc | 2.14 ± 0.02 c | 3.37 ± 0.17 de | 81.47 ± 9.20 bc |
| Urine precipitate-NaOH | 34.81 ± 1.22 b | 2.19 ± 0.11 bc | 2.61 ± 0.09 bc | 4.99 ± 0.05 b | 112.1 ± 1.94 b |
| Urine precipitate-CaO | 25.31 ± 0.19 c | 2.51 ± 0.27 abc | 2.09 ± 0.13 c | 3.43 ± 0.21 de | 94.33 ± 8.67 bc |
| Control 20-10-20 + TE | 16.31 ± 1.41 e | 2.15 ± 0.38 bc | 2.13 ± 0.02 c | 2.91 ± 0.14 e | 72.43 ± 8.14 bcd |
| Significance | *** | * | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Nakhel, C.; Geelen, D.; De Paepe, J.; Clauwaert, P.; De Pascale, S.; Rouphael, Y. An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System. Sustainability 2021, 13, 4218. https://doi.org/10.3390/su13084218
El-Nakhel C, Geelen D, De Paepe J, Clauwaert P, De Pascale S, Rouphael Y. An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System. Sustainability. 2021; 13(8):4218. https://doi.org/10.3390/su13084218
Chicago/Turabian StyleEl-Nakhel, Christophe, Danny Geelen, Jolien De Paepe, Peter Clauwaert, Stefania De Pascale, and Youssef Rouphael. 2021. "An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System" Sustainability 13, no. 8: 4218. https://doi.org/10.3390/su13084218
APA StyleEl-Nakhel, C., Geelen, D., De Paepe, J., Clauwaert, P., De Pascale, S., & Rouphael, Y. (2021). An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System. Sustainability, 13(8), 4218. https://doi.org/10.3390/su13084218









