Abstract
The limitation of fossil fuel sources and negative environmental impact persuade scientists around the world to find a solution. One possible solution is by using renewable fuel to replace fossil fuel with an inexpensive, fast, and effective production process. The objective of this study is to investigate the biodiesel production from crude Reutealis trisperma oil using the conventional and the ultrasonic bath stirrer method through the esterification and transesterification process. The result shows that the most effective reaction time with an optimum condition for the esterification and transesterification of Reutealis trisperma oil is at 2 h 30 min by using the ultrasonic bath stirrer method. The optimum conditions at a temperature of 55 °C for the esterification and at 60 °C for transesterification with 2% (v/v) of sulphuric acid with catalyst concentration of 0.5 wt.% were a methanol-to-oil ratio of 60%, and agitation speed of 1000 rpm. This optimum condition gives the highest yield of 95.29% for the Reutealis trisperma biodiesel. The results showed that the ultrasonic bath stirrer method had more effect on the reaction time needed than using the conventional method and reduced half of the conventional method reaction time. Finally, the properties of Reutealis trisperma biodiesel fulfilled the ASTM D6751 and EN 14214 biodiesel standards with density, 892 kg/m3; pour point, −2 °C; cloud point, −1 °C; flash point, 206.5 °C; calorific value, 40.098 MJ/kg; and acid value, 0.26 mg KOH/g.
1. Introduction
The consumption of fossil fuels causes serious environmental concerns and global warming from excessive emissions of carbon dioxide by burning fossil fuels. These factors have prompted the research and development of biofuel and bioenergy that are environmentally friendly [1]. Biodiesel is one of the solutions for fossil fuel. Biodiesel is composed of mono-alkyl esters derived from long-chain fatty acids that can be made from renewable lipid feedstock such as animal fats and vegetable oils that are available in a large amounts in nature [2,3]. Biodiesel is considered as one of the candidates to replace petroleum-based fuels because its characteristics are almost similar but produce less emissions, sulphur-free, it has a higher cetane number and it is biodegradable [4,5,6]. The main purpose of biodiesel is to replace the petroleum diesel fuel or be mixed with petroleum diesel fuel in any certain type of use to reduce greenhouse gas emission.
Some studies focus on getting renewable sources that are ideal for the production of biodiesel without competing with food resources. Regarding this, there are some non-edible oil methyl ester (biodiesel) investigated and reported such as Ceiba pentandra [7], Sterculia foetida L [8], Calophyllum inophyllum L [9] and Jatropha curcas L [10]. Those works prove that those non-edible oils can replace the use of vegetable oils. For example, from vegetable oils, there are Jatropha curcas, Pongamiapinnata (Karanja), Madhucaindica (Mahua), Linseed, Cottonseed, Azadirachtaindica (Neem), Camelina, Eutealis Trisperma, Hevea Brasiliensis, Ricinus Communis, Schleichera Oleosa, Cerbera manghas and beauty leaf tree or polanga [11,12,13,14]. From wasted or recycled oils, there is cooking oil, frying oil, vegetable oil soap stocks, and pomace oil [15,16,17,18,19,20,21,22]. Also, from animal fats, there is beef tallow, pork lard, yellow grease, chicken fat, and products from fish oil [23,24,25,26,27,28]. Currently, there is a lot of research on algae as biodiesel production [29]. This is due to the potential of the algae in supplying enough oil for global consumption and utilization, and due to global warming caused by burning fossil fuel [30,31,32,33,34,35,36].
Reutealis trisperma, locally known as Philippine Tung, is one of the non-edible oils that belongs to the family Euphorbiaceae. It is a native plant of the Philippines and southeast Asia [37]. This plant can grow up to 10–15 m, and can produce 25–30 kg of dry beans per tree and per year, with a Reutealis trisperma oil content of 50–52% (w/w). Figure 1 shows Reutealis trisperma tree, fruit, seeds and kernels [38,39,40]. Figure 2 shows the crude oil and biodiesel of Reutealis trisperma; the fruits of Reutealis trisperma can be found around the countryside in Malaysia and Indonesia. In Indonesia, Reutealis trisperma is especially distributed in West Java. Recently, it has been cultivated in the Sumedang area. Figure 3 shows the distribution map of Reutealis trisperma around the world.
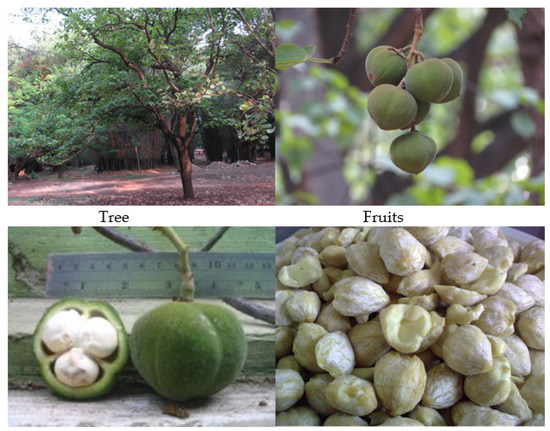
Figure 1.
Reutealis trisperma tree, fruit, seeds and kernels [39,40].

Figure 2.
Crude oil and biodiesel of Reutealis trisperma.
In the biodiesel production process, various methods can be used such as conventional, ultrasound-assisted, non-catalytic supercritical, ultrasonic, and microwave methods. Among these methods, ultrasonic and conventional are more preferable and widely studied by using varied raw materials. In many cases, the conventional method is preferred because it is easy to use and simple, while on the other hand, the ultrasonic method offers advantages due to its short processing time [41,42]. Ultrasonic waves that are propagated in the liquid will cause an effect referred to as the cavitation phenomenon. In an ultrasound-assisted reaction, fluid pressure would increase when the positive amplitude is propagated and subsequently decrease when the negative amplitude is distributed [43]. Simultaneous changes in pressure with the high frequency of the ultrasonic wave are reacted to slowly by the liquid, resulting in the creation of microbubbles. The bubble continues to grow with the application of the ultrasonic energy so that the diameter of the bubble grows larger until it collapses violently, resulting in the cavitation effect. This cavitation phenomenon will make the temperature and pressure increase by releasing the vapor contained in the bubble and will help to improve the transfer of mass and heat into the liquid [44]. Utilizing the ultrasonic waves for the esterification process has shown their ability to reduce the free fatty acid content and improve the transesterification process to produce high-yield biodiesel [45]. Andrade-Tacca et al. have conducted a study using ultrasonic irradiation to reduce the acid value of the Jatropha oil from 36.5 to 0.236 mg KOH/g [46]. There are many proven results of using ultrasound for the transesterification process that produces high biodiesel yield. Chen et al. have successfully made biodiesel with a fatty acid methyl ester (FAME) of 92.7% using heterogeneous catalyst [47]. From the numerous studies that have been conducted, it has been shown that ultrasonic irradiation is a very cost- and energy-efficient method to produce biodiesel. Georgogiani et al. [48] reported that using the ultrasonic method for processing sunflower seed oil and by using methanol as the chemical can produce biodiesel with ester yields as high as (98%) in 40 min of reaction time. Using the conventional method gave the lower yield that is (88%) even after 4 h of reaction time.
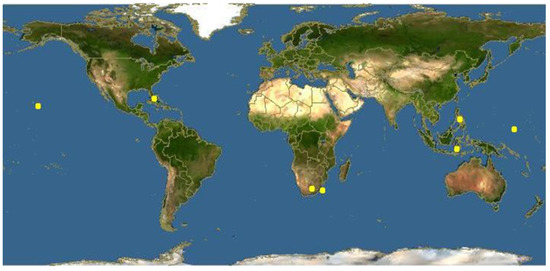
Figure 3.
Distribution map of Reutealis trisperma around the world [49].
Meanwhile, Holilah et al. [50] investigated the Reutealis trisperma oil by using conventional methods through two stages esterification and transesterification with an optimum result of biodiesel production yield of 95.15% at a temperature of 65 °C with 3 h of reaction time. However, the application of ultrasound for biodiesel production from the Reutealis trisperma oil still has not received practitioners’ and scientists’ attention and there is even very little information available in the literature about the production of biodiesel from crude Reutealis Trisperma oil. However, many have successfully proven the applicablility of biodiesel fuels in blending form to be applied to the unmodified diesel engine [51,52]. The objective of this study is to produce biodiesel with the shortest processing time from crude Reutealis trisperma oil and to compare the quality of the biodiesel produced based on several important characteristics such as the acid value, kinematic viscosity, and yield between conventional and ultrasonic bath stirrer method by using potassium hydroxide as a catalyst.
2. Materials and Methods
2.1. Materials and Apparatus
Crude Reutealis trisperma oil from Indonesia was produced by the Department of Chemical Engineering, University of Indonesia, Jakarta, Indonesia. All reagents used are methanol, sulphuric acid (H2SO4), phosphoric acid (H3PO4), potassium hydroxide (KOH), and Whatman filter papers size 150 mm (filter fioroni, France) were purchased from local suppliers. The equipment used for the experimental process of crude Reutealis trisperma oil esterification and transesterification process is presented in Figure 4. The ultrasonic bath stirrer (Model: Powersonic 410, 500 W–40 kHz) with bath size (mm) 300 × 240 × 150 is made by Copens Scientific (M) Sdn. Bhd (Malaysia) and the thermometer was settled down using the rubber stand in one neck of the flask.
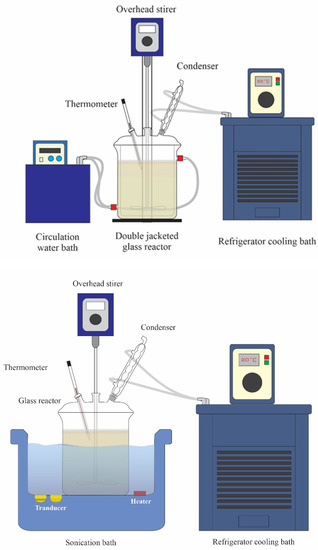
Figure 4.
The equipment of Ultra sonication and Double jacketed glass reactor for the experimental process of crude Reutealis trisperma oil.
2.2. Degumming Process
In this process, 5 vol.% of phosphoric acid (H3PO4 20%) was added into the crude Reutealis trisperma oil at 60 °C with a stirring speed of 1000 rpm for 30 min. This was followed by a simple filtration process for 4–5 h, and it could be seen at the bottom of the flask there was the formation of the gums (phosphatides) from the experiment. The gums were removed manually from the oil and washed several times with warm water at temperature 45–50 °C. After being washed and separating oil from water, the oil was evaporated using a vacuum pump at 60 °C for approximately 20–30 min to remove remaining water in the oil.
2.3. Esterification Process
In this research, the biodiesel production process for Reutealis trisperma oil went through two steps—(1) esterification, and (2) transesterification. The main objective of this esterification process is to reduce the amount of free fatty acids contained in crude oil. There are two methods used in the esterification process which are conventional and ultrasonic bath. For the conventional method, 2% (v/v) of sulphuric acid (H2SO4) and a methanol-to-oil molar ratio of 60% were added to 500 mL of degummed Reutealis trisperma oil then placed in a double jacketed glass reactor with a stirrer speed of 1000 rpm and the temperature was set to 55 °C. The reaction time parameter ranged from 60 to 180 min. For the second method of esterification, the same amount of catalyst, methanol, and oil were used and it was carried out using a glass reactor placed in an ultrasonic bath and the ultrasonic bath power supply was set to deliver 100% of total power (Powersonic 410, 500 W) of 40 kHz with stirrer speed at 1000 rpm at different time parameters (60, 90, 120, 150 and 180 min) and a temperature of 55 °C. Once the reaction based on the required parameter was completed, the product was poured into a separation funnel to separate H2SO4, methanol, and impurities. After leaving it for 6 h in the separating funnel, H2SO4, methanol and impurities were found at the top layer of the oil while and the esterified oil at lower layer was obtained. After that, the esterified Reutealis trisperma oil was collected and placed in a rotary evaporator at a temperature of 60 °C for 30 min under vacuum conditions to remove water and methanol residues in the esterified oil.
2.4. Transesterification Process
In this process, the esterified Reutealis trisperma oil was preheated at a temperature 60 °C and the methanol-to-oil ratio was 60%. Then, 0.5 wt.% of potassium hydroxide (KOH) was dissolved into methanol. This methanol and KOH mixed solution were added in the preheated oil at a temperature of 60 °C and the reaction continued for different time parameters of 60, 90 and 120 min. During the transesterification process by the conventional method, the oil was stirred constantly at a speed of 1000 rpm using an overhead stirrer for the conventional method. Meanwhile, for the second method, the ultrasonic bath was conducted at the maximum frequency of 40 kHz with a stirrer speed of 1000 rpm and the temperature was constant at 60 °C. After the reaction of each parameter was completed, methyl ester was poured into each separating funnel in order to separate the glycerol from methyl ester (biodiesel) for approximately 7 h. Two layers were observed from the separating funnel. The bottom layer consisted of excess methanol, where impurities and glycerol can be removed at this stage. Then, the methyl ester was put into the rotary evaporator and set the temperature to 60 °C for 15 min to evaporate extra methanol. It was subsequently washed with warm water at 45–50 °C several times. Then, the product was poured once again into the rotary evaporator with the temperature set at 60–65 °C for 30 min to remove water completely from biodiesel and then filtered with filter paper.
2.5. Characterization Fuel Properties
The types of equipment used to analyse the chemical properties and physical properties of crude oil and biodiesel are shown in Table 1. The properties were tested according to the ASTM 6751 and EN 14214 standard and every variable was tested three times [53].

Table 1.
List of equipment and standard method used for the properties test.
3. Results and Discussion
3.1. Properties of Crude Reutealis trisperma Oil
The physicochemical properties and fatty acid composition of crude Reutealis trisperma such as the acid value, density at 15 °C, viscosity at 40 °C and flash point were analysed and compared with other non-edible oils as presented in Table 2. The test results showed that the acid value and viscosity measured were the highest, which were 44.681 mg KOH/g and 76.927 mm2/s, respectively. The density and flash point obtained were 937 kg/m3 and 226.5 °C, respectively. The fatty acid composition of crude Reutealis trisperma oil (CRTO) showed that the primary components were palmitic acid, oleic acid, and linoleic acid which comprised 13.1%, 16.1%, and 18.7%, respectively. For Crude Ceiba petandra oil (CCPO), Crude Sterculia feotida oil (CSFO) and Crude Calophyllum inophyllum oil (CCIO), the dominant compositions were linoleic acid, palmitic acid, and oleic acid, which comprised 39.7%, 17.7%, and 46.1%, respectively. Crude Reutealis trisperma oil contains about 19.5% saturated fatty acids and 35.3% of unsaturated fatty acids.

Table 2.
The properties and fatty acid composition of crude Reutealis trisperma oil compared with other non-edible oils.
3.2. Fourier Transform Infrared Spectrum of the Reutealis trisperma Biodiesel
Fourier transform infrared spectrum (FTIR) is one technique to overcome the problem of the quantification and identification in some material substances such as fuel, chemistry, and the environmental chemical composition [55,56]. FTIR is one of the analytical techniques commonly used because of the cost, speed, and quality screening considerations. This method is the technique of molecular “fingerprinting”. The FTIR analysis of the Reutealis trisperma biodiesel is presented in Figure 5. As can bee seen in Table 3, the FTIR results of the Reutealis trisperma biodiesel such as Wavenumber, Group attribution, Absorption intensity, and Vibration type of the absorption peaks have been detected. The results showed that the biodiesel from Reutealis trisperma is comprised of long-chain fatty acid esters. The spectrum of the biodiesel product in transesterification is similar to chemical precursors (refined oil), C=O is stretching is 1741 cm−1 and the peak is located in the region 1800–1700 cm−1. This is a spectrum of typical ester, and it is usually encountered in FAME and oil-refined products [57,58]. In the range area of 1700–700 cm−1, biodiesel from Reutealis trisperma showed a peak at 1244 cm−1 corresponding to the bending vibration −CH3 which is known as a “fingerprint”, which is a major region of the spectrum [59].
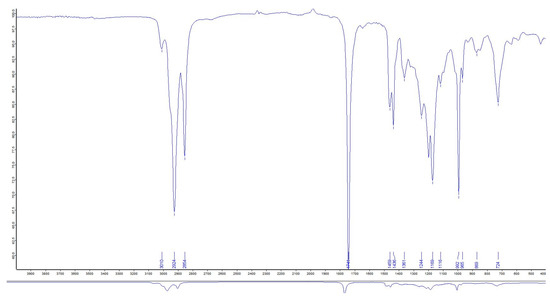
Figure 5.
Fourier transform infrared spectrum of the Reutealis trisperma biodiesel.

Table 3.
The Fourier transform infrared spectrum of the Reutealis trisperma biodiesel.
3.3. Physicochemical Properties of Reutealis trisperma Biodiesel Compare to Other Biodiesels
The physicochemical properties of Reutealis trisperma biodiesel with the optimum results in the shortest time for the esterification process and transesterification process by using the ultrasonic bath stirrer method are summarized in Table 4. The properties of Reutealis trisperma biodiesel produced from the esterification process of 60 min at a temperature of 55 °C using parameters (H2SO4 concentration: 2% (v/v), methanol-to-oil ratio: 60%, agitation speed: 1000 rpm) and transesterification process of 90 min at temperature 60 °C using parameters (KOH catalyst concentration: 0.5 wt.%, methanol-to-oil ratio: 60%, agitation speed: 1000 rpm) are shown.

Table 4.
Physicochemical properties of Reutealis trisperma biodiesel and others biodiesels.
The physicochemical properties of Reutealis trisperma methyl ester (RTME) are compared to other biodiesels, which are listed in Table 4. It is found that most of the properties of RTME biodiesel fulfilled the ASTM D6751 and EN 14214 standard except for kinematic viscosity. The kinematic viscosity of RTME was 6.48 mm2/s, which is relatively higher than other biodiesels, but slightly lower than previous work. According to Holilah et al. [50], the viscosity for RTME, Sterculia foetida methyl ester (SFME), Calophyllum inophyllum methyl ester (CIME), and Ceiba pentandra methyl ester (CPME) was obtained as 6.71, 3.96, 3.45, and 4.61 mm2/s, respectively.
Furthermore, the density limit was 880 kg/m3 at 15 °C for ASTM D6751 and 860–900 kg/m3 at 15 °C for EN 14214 biodiesel standards. The density results were 892, 879.1, 877.6, and 876.9 kg/m3 for RTME, SFME, CIME, and CPME, respectively. The obtained flash point results were higher compared to other biodiesels, which are 206.5, 160.5, 165.5, and 156.5 °C for RTME, SFME, CIME, and CPME, respectively. Furthermore, the lower heating values were 40.098, 40.427, 41.442, and 40.493 MJ/kg for RTME, SFME, CIME, and CPME, respectively, which fall within ASTM and EN biodiesel standards. The obtained acid values were 0.26, 0.14, 0.34, and 0.38 mg KOH/g for RTME, SFME, CIME, and CPME, respectively, which are in line with ASTM D6751 and EN 14214 biodiesel standards which should be lower than 0.5%.
3.4. Effect of Esterification Process to Acid Value vs. Time
The high percentages of FFA (free fatty acid) content and water in vegetable oil may affect the conversion process to biodiesel due to the saponification reaction that produces soap with the base catalysts. Holilah et al. [50] reported that the FFA content is about 2.4% in Reutealis trisperma oil. Nevertheless, this kind of oil can be esterified using conventional methods and an acid catalyst requires a longer reaction time, which is uneconomic. It has been shown that Holilah et al. used 3 wt.% acid catalyst (H2SO4) for 2 h in the esterification process [50].
The trend of acid value versus time from esterified Reutealis trisperma oil using the conventional and ultrasonic bath stirrer method is presented in Figure 6. As illustrated in Figure 6, the ultrasonic bath stirrer method had the lowest acid value after 1 h of the esterification process. The observed acid value was 0.476 mg KOH/g. Increasing the time of the esterification process for the ultrasonic bath stirrer method by more than 1 h will increase the acid value. Meanwhile, for the conventional method, the lowest acid value was found after 3 h of the esterification process. The obtained acid value was 0.367 mg KOH/g. From the experimental result, it can be seen that the lowest acid value with a shorter time is found using the ultrasonic bath stirrer method after a 1-hour process. However, the conventional method spends up to 3 h for the esterification process to produce the lowest acid value. Esterification by using an ultrasonic bath stirrer promotes more vibration and collision between molecules, reducing the time of reaction. As shown in Figure 6, the acid value increases after the esterification process is prolonged beyond the required time. A similar trend was observed from Devaraj Naik and Udayakumar’s research; prolonging the reaction time after the optimum value will decrease the acid conversion (which means increasing the acid value) due to the available longer duration favoring the reversible reaction [62]. Another study reported increasing reaction time has no significant increase in yield after the system reaches the optimum result [63].
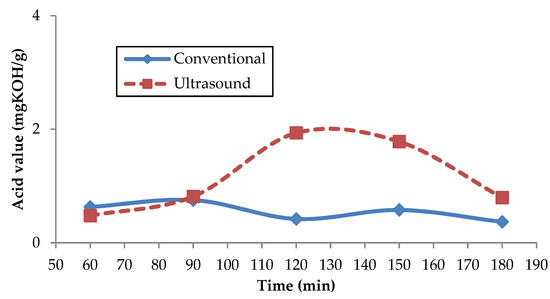
Figure 6.
Effect of esterification process on acid value.
3.5. Effect of Transesterification Process on Acid Value vs. Time
Maghami et al. [64] investigated the effect of acidity on the biodiesel yield using 1 wt.% KOH as the catalyst for waste fish oil (WFO) with a variation of temperatures (40, 50, 60 °C), and the result found that with reduction of oil acidity, the biodiesel yield of reaction increased [64]. In this study, the experiments have been conducted with 0.5 wt.% potassium hydroxide (KOH) as an alkaline catalyst at temperatures of 60 °C and the time varied as (60, 90, and 120 min) with a comparison of the conventional and ultrasonic methods. The trend of acid value versus time from Reutealis trisperma biodiesel is presented in Figure 7. As can be seen in Figure 7, the ultrasonic bath stirrer method has the shortest time and the lowest acid value after 90 min of the transesterification process, and the obtained acid value was 0.268 mg KOH/g. Meanwhile, for the ultrasonic method, the increase in time of the transesterification process up to 120 min will increase the acid value. Extending the reaction time beyond the optimum result will not promote more methyl ester. Simultaneously, it will deteriote the FAME quality that resulted in higher acid value due to the loss of methanol during the extension of reaction time [65]. However, in the conventional method, it needs up to 120 min to approach acid value in the ultrasonic method; the observed acid value was 0.273 mg KOH/g after 120 min of the transesterification process. Therefore, the ultrasound method fastens the transesterification process compared with the conventional way. Ultrasound promotes ultrasonic waves during the conversion process that create tiny drops of each liquid in the opposite phase that enhanced mass transfer between phases and accelerated the reaction [66].
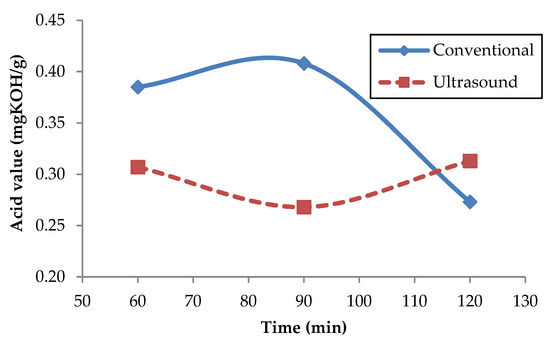
Figure 7.
Effect of transesterification process on acid value.
3.6. Effect of Transesterification Process to Viscosity vs. Time
Holilah et al. [50] investigated the Reutealis trisperma oil for biodiesel production using the conventional method through the esterification and transesterification process with a constant stirring speed. He found that the kinematic viscosity of Reutealis trisperma biodiesel was 6.71 mm2/s and believed the presence of triglycerides produced as a by-product from the unreacted Reutealis trisperma oil affects the kinematic viscosity of the biodiesel. However, in this work, the effects of the transesterification process using the conventional method and ultrasonic bath stirrer methods on the viscosity versus time for Reutealis trisperma biodiesel were not very significant. From Figure 8, it can be seen that after the transesterification process for 120 min with the conventional method, the lowest viscosity was 6.2863 mm2/s. Meanwhile, for the ultrasonic bath stirrer method, the lowest viscosity after 120 min of the transesterification process was 6.3716 mm2/s. The experiment found that the viscosity of Reutealis trisperma biodiesel was slightly above the limit of the ASTM D6751 and EN 14214 standard, however, it is still acceptable. However, the author expected that further researcher will find an economical experimental method to solve this problem. Mishra et al. claimed that biodiesel’s viscosity is based on its composition, molecular weight, number of carbon atoms, and double bonds [67]. Therefore, the differences in viscosity found in this study are depended on the FAME quality of the biodiesel produced.
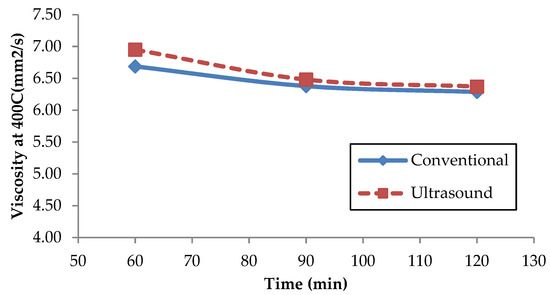
Figure 8.
Effect of the transesterification process to viscosity.
3.7. Effect of the Transesterification Process to Yield vs. Time
Ultrasonic methods reduced the processing time with the higher yield as compared to conventional methods, based on the fact that ultrasound will increase the interaction among the phases due to the ultrasonic jet which consequently increased the reaction [41]. Figure 9 shows the yield of the Reutealis trisperma oil transesterification process as a function of time. As shown in the figure, the ultrasonic bath stirrer method of 90 min was the optimum value, with the optimum yield of 95.29%. At the beginning of the transesterification process, the biodiesel yield is proportional to the reaction time due to the high mass transfer between the reactant and the oil that promotes the transesterification process. Further increasing the transesterification process for over 90 min could reduce biodiesel yield as the biodiesel conversion is saturated. For the conventional method, the biodiesel yield is rising after 90 min of the transesterification process. These results proved that ultrasound converts oil to biodiesel in a way faster than the conventional method. This result corresponds with the study done by Sundaramahalingam et al. that used ultrasound to produce Annona squamosa biodiesel. Their results have shown that their sonication time to make biodiesel is 113 min, and the maximum FAME result was 98.4% [68].
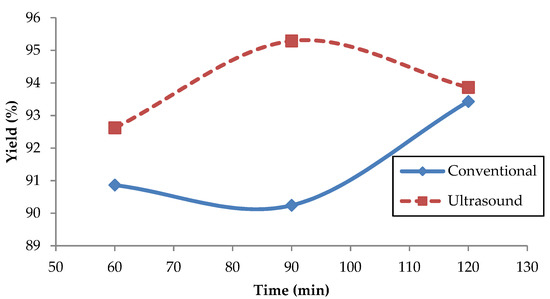
Figure 9.
Effect of transesterification process on biodiesel yield.
4. Conclusions
The results of this study show that crude Reutealis trisperma oil will be a potential feedstock to be used as a biodiesel source in the future. Investigation results on the effect acid value, kinematic viscosity, and yield versus time of reaction on biodiesel production using the conventional method and ultrasonic bath stirrer method through the esterification process and transesterification process have been presented and discussed deeply. The result shows that the optimum quality of biodiesel with the shortest time obtained the yield up to 95.29%, with acid value 0.268 mg KOH/g, and kinematic viscosity 6.48 mm2/s by using the ultrasonic bath stirrer method for a total time of esterification and transesterification process of 150 min. On the other hand, the conventional method takes nearly 5 h for the esterification and transesterification process to achieve similar results. The properties of Reutealis trisperma biodiesel were tested and most of the properties were in agreement with the ASTM D6751 and EN 14214 standard except for kinematic viscosity.
Author Contributions
T.M.I.R.: Writing—original draft, Formal analysis, Investigation, Visualization. A.S.S., J.M., A.H.S. (Abd. Halim Shamsuddin) and A.H.S. (Abdi Hanra Sebayang): Conceptualization, Methodology, Resources. R., J.S.: Data curation and Validation. R.T. and T.M.I.M.: Supervision and Writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to acknowledgement the research is supported by Institut Teknologi Sumatera under Research Grant for Hibah Publikasi GBU-45 and Pendidikan Tinggi Republik Indonesia and Politeknik Negeri Medan, Medan, Indonesia. This research is funded by the Centre for Advanced Modeling and Geospatial Information Systems (CAMGIS), UTS under Grants 321740.2232397.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chong, C.T.; Mong, G.R.; Ng, J.-H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.-M.; Ling, T.C.; Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Mahlia, T.; Syazmi, Z.; Mofijur, M.; Abas, A.P.; Bilad, M.; Ong, H.C.; Silitonga, A. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.; Mahlia, T.; Silitonga, A.; Chong, W.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Shamsuddin, A.H.; Wang, C.-T.; Mahlia, T.M.I.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Silitonga, A.; Shamsuddin, A.; Mahlia, T.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.; Masjuki, H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Marri, V.B.; Kotha, M.M.; Gaddale, A.P.R.; Babu, M.V.; Murthy, K.M.; Rao, G.A.P. Production process optimisation of Sterculia foetida methyl esters (biodiesel) using response surface methodology. Int. J. Ambient. Energy 2020, 1–10. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K.; Darla, S.; Chyuan, O.H.; Ramesh, A.; Jacob, A.; Sahil, G.; Thiyagarajan, S.; Geo, V.E. Comparative assessment of hexanol and decanol as oxygenated additives with calophyllum inophyllum biodiesel. Energy 2019, 173, 494–510. [Google Scholar] [CrossRef]
- Silitonga, A.; Masjuki, H.; Mahlia, T.; Ong, H.; Chong, W.; Boosroh, M. Overview properties of biodiesel diesel blends from edible and non-edible feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Mallah, T.A.; Sahito, A.R. Optimization of castor and neem biodiesel blends and development of empirical models to predicts its characteristics. Fuel 2020, 262, 116341. [Google Scholar] [CrossRef]
- Nain, P.; Jaiswal, S.K.; Prakash, N.T.; Prakash, R.; Gupta, S.K. Influence of acyl acceptor blends on the ester yield and fuel properties of biodiesel generated by whole-cell catalysis of cottonseed oil. Fuel 2020, 259, 116258. [Google Scholar] [CrossRef]
- Dwivedi, G.; Jain, S.; Sharma, M.P. Diesel engine performance and emission analysis using biodiesel from various oil sources—Review. J. Mater. Environ. Sci. 2013, 4, 434–447. [Google Scholar]
- Singh, S.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Leung, D.Y. Development of a Clean Biodiesel Fuel in Hong Kong Using Recycled Oil. Water Air Soil Pollut. 2001, 130, 277–282. [Google Scholar] [CrossRef]
- Mushrush, G.W.; Wynne, J.H.; Willauer, H.D.; Lloyd, C.T.; Hughes, J.M.; Beal, E.J. Recycled Soybean Cooking Oils As Blending Stocks for Diesel Fuels. Ind. Eng. Chem. Res. 2004, 43, 4944–4946. [Google Scholar] [CrossRef]
- Kumar, M.S.; Jaikumar, M. A comprehensive study on performance, emission and combustion behavior of a compression ignition engine fuelled with WCO (waste cooking oil) emulsion as fuel. J. Energy Inst. 2014, 87, 263–271. [Google Scholar] [CrossRef]
- López, I.; Pinzi, S.; Leiva-Candia, D.; Dorado, M. Multiple response optimization to reduce exhaust emissions and fuel consumption of a diesel engine fueled with olive pomace oil methyl ester/diesel fuel blends. Energy 2016, 117, 398–404. [Google Scholar] [CrossRef]
- de Almeida, V.F.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Biodiesel production from mixtures of waste fish oil, palm oil and waste frying oil: Optimization of fuel properties. Fuel Process. Technol. 2015, 133, 152–160. [Google Scholar] [CrossRef]
- Sanli, H.; Canakci, M.; Alptekin, E.; Turkcan, A.; Ozsezen, A. Effects of waste frying oil based methyl and ethyl ester biodiesel fuels on the performance, combustion and emission characteristics of a DI diesel engine. Fuel 2015, 159, 179–187. [Google Scholar] [CrossRef]
- Zaher, F.A.; Megahed, O.A.; El Kinawy, O.S. Utilization of used frying oil as diesel engine fuel. Energy Sources 2003, 25, 819–826. [Google Scholar] [CrossRef]
- Silitonga, A.; Masjuki, H.; Ong, H.C.; Sebayang, A.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Ashok, B.; Raj, R.T.K.; Nanthagopal, K.; Tapaswi, A.; Jindal, A.; Subbish, S.H. Animal fat methyl ester as a fuel substitute for DI compression ignition engine. Int. J. Thermodyn. 2016, 19, 206–212. [Google Scholar]
- Ramos, Á.; García-Contreras, R.; Armas, O. Performance, combustion timing and emissions from a light duty vehicle at different altitudes fueled with animal fat biodiesel, GTL and diesel fuels. Appl. Energy 2016, 182, 507–517. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Couto, M.B.; Souza, G.S.; Filho, M.A.; Assis, J.C.; Guimarães, P.R.; Pontes, L.A.; Almeida, S.Q.; Teixeira, J.S. Characterization of beef tallow biodiesel and their mixtures with soybean biodiesel and mineral diesel fuel. Biomass Bioenergy 2010, 34, 438–441. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Krishnamoorthy, V.; Rana, D.; Saravanan, S.; Nagendran, A.; Kumar, B.R. A sustainable and eco-friendly fueling approach for direct-injection diesel engines using restaurant yellow grease and n-pentanol in blends with diesel fuel. Fuel 2017, 193, 419–431. [Google Scholar] [CrossRef]
- Hanafi, S.A.; Elmelawy, M.S.; Shalaby, N.H.; El-Syed, H.A.; Eshaq, G.; Mostafa, M.S. Hydrocracking of waste chicken fat as a cost effective feedstock for renewable fuel production: A kinetic study. Egypt. J. Pet. 2016, 25, 531–537. [Google Scholar] [CrossRef]
- Yahyaee, R.; Ghobadian, B.; Najafi, G. Waste fish oil biodiesel as a source of renewable fuel in Iran. Renew. Sustain. Energy Rev. 2013, 17, 312–319. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Calixto, C.D.; Santana, J.K.D.S.; Tibúrcio, V.P.; Pontes, L.D.F.B.L.D.; Sassi, C.F.D.C.; da Conceição, M.M.; Sassi, R. Productivity and fuel quality parameters of lipids obtained from 12 species of microalgae from the northeastern region of Brazil. Renew. Energy 2018, 115, 1144–1152. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Prathima, A. Microalgae biofuel with CeO2 nano additives as an eco-friendly fuel for CI engine. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 1332–1338. [Google Scholar] [CrossRef]
- Makareviciene, V.; Gumbyte, M.; Skorupskaite, V.; Sendzikiene, E. Biodiesel fuel production by enzymatic microalgae oil transesterification with ethanol. J. Renew. Sustain. Energy 2017, 9, 23101. [Google Scholar] [CrossRef]
- Mason, I.; Page, S.; Williamson, A. A 100% renewable electricity generation system for New Zealand utilising hydro, wind, geothermal and biomass resources. Energy Policy 2010, 38, 3973–3984. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Silitonga, A.; Mahlia, T.; Kusumo, F.; Dharma, S.; Sebayang, A.; Sembiring, R.; Shamsuddin, A. Intensification of Reutealis trisperma biodiesel production using infrared radiation: Simulation, optimisation and validation. Renew. Energy 2019, 133, 520–527. [Google Scholar] [CrossRef]
- Kumar, K.R.; Channarayappa; Chandrika, K.; Prasanna, K.T.; Gowda, B. Biodiesel production and characterization from non-edible oil tree species Aleurites trisperma Blanco. Biomass Convers. Biorefinery 2015, 5, 287–294. [Google Scholar] [CrossRef]
- Wikipedia, Reutealis Trisperma. 2016. Available online: https://en.wikipedia.org/wiki/Reutealis. (accessed on 13 April 2016).
- Sunan-drajat.blogspot.my, Kemiri Sunan. 2016. Available online: http://sunan-drajat.blogspot.my/ (accessed on 13 April 2016).
- Takase, M.; Feng, W.; Wang, W.; Gu, X.; Zhu, Y.; Li, T.; Yang, L.; Wu, X. Silybum marianum oil as a new potential non-edible feedstock for biodiesel: A comparison of its production using conventional and ultrasonic assisted method. Fuel Process. Technol. 2014, 123, 19–26. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Amid, S.; Hosseinzadeh-Bandbafha, H.; Khoshnevisan, B.; Kianian, G. Life cycle assessment analysis of an ultrasound-assisted system converting waste cooking oil into biodiesel. Renew. Energy 2020, 151, 1352–1364. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Encinar, J.; González, J.F.G.; Pardal, A. Transesterification of castor oil under ultrasonic irradiation conditions. Preliminary results. Fuel Process. Technol. 2012, 103, 9–15. [Google Scholar] [CrossRef]
- Andrade-Tacca, C.A.; Chang, C.-C.; Chen, Y.-H.; Manh, D.-V.; Chang, C.-Y. Esterification of jatropha oil by sequential ultrasonic irradiation with auto-induced temperature rise and dosing of methanol and sulfuric acid catalyst. J. Taiwan Inst. Chem. Eng. 2014, 45, 1523–1531. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Shi, J.; Yan, B. Ultrasonic-assisted production of biodiesel from transesterification of palm oil over ostrich eggshell-derived CaO catalysts. Bioresour. Technol. 2014, 171, 428–432. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Kontominas, M.G.; Tegou, E.; Avlonitis, D.; Gergis, V. Biodiesel Production: Reaction and Process Parameters of Alkali-Catalyzed Transesterification of Waste Frying Oils. Energy Fuels 2007, 21, 3023–3027. [Google Scholar] [CrossRef]
- T.p. Corporation, Reutealis Trisperma 2016. Available online: http://www.discoverlife.org/mp/20m?map=Reutealis+trisperma (accessed on 23 February 2018).
- Holilah, H.; Prasetyoko, D.; Oetami, T.P.; Santosa, E.B.; Zein, Y.M.; Bahruji, H.; Fansuri, H.; Ediati, R.; Juwari, J. The potential of Reutealis trisperma seed as a new non-edible source for biodiesel production. Biomass Convers. Biorefinery 2015, 5, 347–353. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Optimization of palm oil biodiesel blends and engine operating parameters to improve performance and PM morphology in a common rail direct injection diesel engine. Fuel 2020, 260, 116326. [Google Scholar] [CrossRef]
- Silitonga, A.; Ong, H.; Mahlia, T.; Masjuki, H.; Chong, W. Characterization and production of Ceiba pentandra biodiesel and its blends. Fuel 2013, 108, 855–858. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, L. Process optimization for biodiesel production from Jatropha, Karanja and Polanga oils. Fuel 2009, 88, 1588–1594. [Google Scholar] [CrossRef]
- Sedman, J.; van de Voort, F.R.; Ismail, A.A. Simultaneous determination of lodine value and trans content of fats and oils by single-bounce horizontal attenuated total reflectance fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 399–403. [Google Scholar] [CrossRef]
- Pereira, R.C.C.; Skrobot, V.L.; Castro, E.V.R.; Fortes, I.C.P.; Pasa, V.M.D. Determination of Gasoline Adulteration by Principal Components Analysis-Linear Discriminant Analysis Applied to FTIR Spectra. Energy Fuels 2006, 20, 1097–1102. [Google Scholar] [CrossRef]
- Soares, I.P.; Rezende, T.F.; Silva, R.C.; Castro, E.V.R.; Fortes, I.C.P. Multivariate Calibration by Variable Selection for Blends of Raw Soybean Oil/Biodiesel from Different Sources Using Fourier Transform Infrared Spectroscopy (FTIR) Spectra Data. Energy Fuels 2008, 22, 2079–2083. [Google Scholar] [CrossRef]
- Dharma, S.; Masjuki, H.; Ong, H.C.; Sebayang, A.; Silitonga, A.; Kusumo, F.; Mahlia, T. Optimization of biodiesel production process for mixed Jatropha curcas–Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Rabelo, S.N.; Ferraz, V.P.; Oliveira, L.S.; Franca, A.S. FTIR Analysis for Quantification of Fatty Acid Methyl Esters in Biodiesel Produced by Microwave-Assisted Transesterification. Int. J. Environ. Sci. Dev. 2015, 6, 964–969. [Google Scholar] [CrossRef]
- Silitonga, A.; Ong, H.; Masjuki, H.; Mahlia, T.; Chong, W.; Yusaf, T.F. Production of biodiesel from Sterculia foetida and its process optimization. Fuel 2013, 111, 478–484. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.; Mahlia, T.; Silitonga, A.; Chong, W.; Leong, K. Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers. Manag. 2014, 81, 30–40. [Google Scholar] [CrossRef]
- Naik, B.D.; Udayakumar, M. Optimization studies on esterification of waste cooking oil using sulfated montmorillonite clay acidic catalyst. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Joshi, S.M.; Gogate, P.R.; Kumar, S.S. Intensification of esterification of karanja oil for production of biodiesel using ultrasound assisted approach with optimization using response surface methodology. Chem. Eng. Process. Process. Intensif. 2018, 124, 186–198. [Google Scholar] [CrossRef]
- Maghami, M.; Sadrameli, S.; Ghobadian, B. Production of biodiesel from fishmeal plant waste oil using ultrasonic and conventional methods. Appl. Therm. Eng. 2015, 75, 575–579. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Kusumo, F.; Dharma, S.; Sebayang, A.H.; Cheah, M.Y.; Wang, C.-T. Physicochemical property enhancement of biodiesel synthesis from hybrid feedstocks of waste cooking vegetable oil and Beauty leaf oil through optimized alkaline-catalysed transesterification. Waste Manag. 2018, 80, 435–449. [Google Scholar] [CrossRef]
- Paiva, E.J.M.; da Silva, M.L.C.P.; Barboza, J.C.S.; de Oliveira, P.C.; de Castro, H.F.; Giordani, D.S. Non-edible babassu oil as a new source for energy production–A feasibility transesterification survey assisted by ultrasound. Ultrason. Sonochem. 2013, 20, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bukkarapu, K.R.; Krishnasamy, A. A composition based approach to predict density, viscosity and surface tension of biodiesel fuels. Fuel 2021, 285, 119056. [Google Scholar] [CrossRef]
- Sundaramahalingam, M.A.; Karthikumar, S.; Kumar, R.S.; Samuel, K.J.; Shajahan, S.; Sivasubramanian, V.; Sivashanmugam, P.; Varalakshmi, P.; Syed, A.; Marraiki, N.; et al. An intensified approach for transesterification of biodiesel from Annona squamosa seed oil using ultrasound-assisted homogeneous catalysis reaction and its process optimization. Fuel 2021, 291, 120195. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).