Exploring Temporal Trends of Plant Invasion in Mediterranean Coastal Dunes
Abstract
:1. Introduction
- detect changes affecting the occurrence and cover of alien species in invaded coastal dune habitats over the last 10–15 years (from 2005–2008 to 2017–2020);
- analyze different invasion trends through vegetation plots that experienced colonization, loss, or persistence of alien species, with particular emphasis on the impacts alien species may exert on native and diagnostic species.
2. Materials and Methods
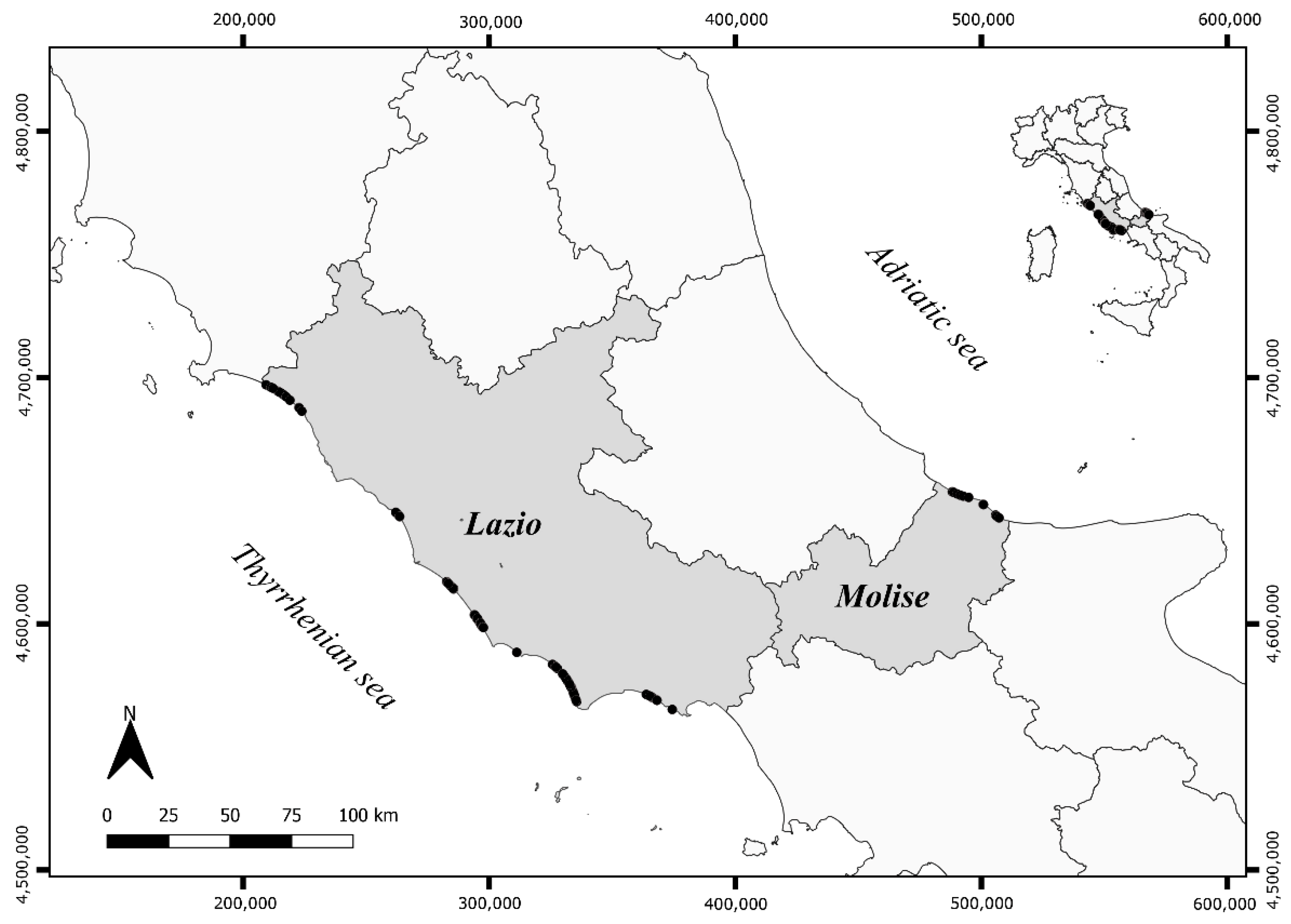
2.1. Data Selection
2.2. Overall Trends in Alien Species
2.3. Colonization, Loss, or Persistence of Alien Species
2.4. Changes in Diagnostic Species Cover
3. Results
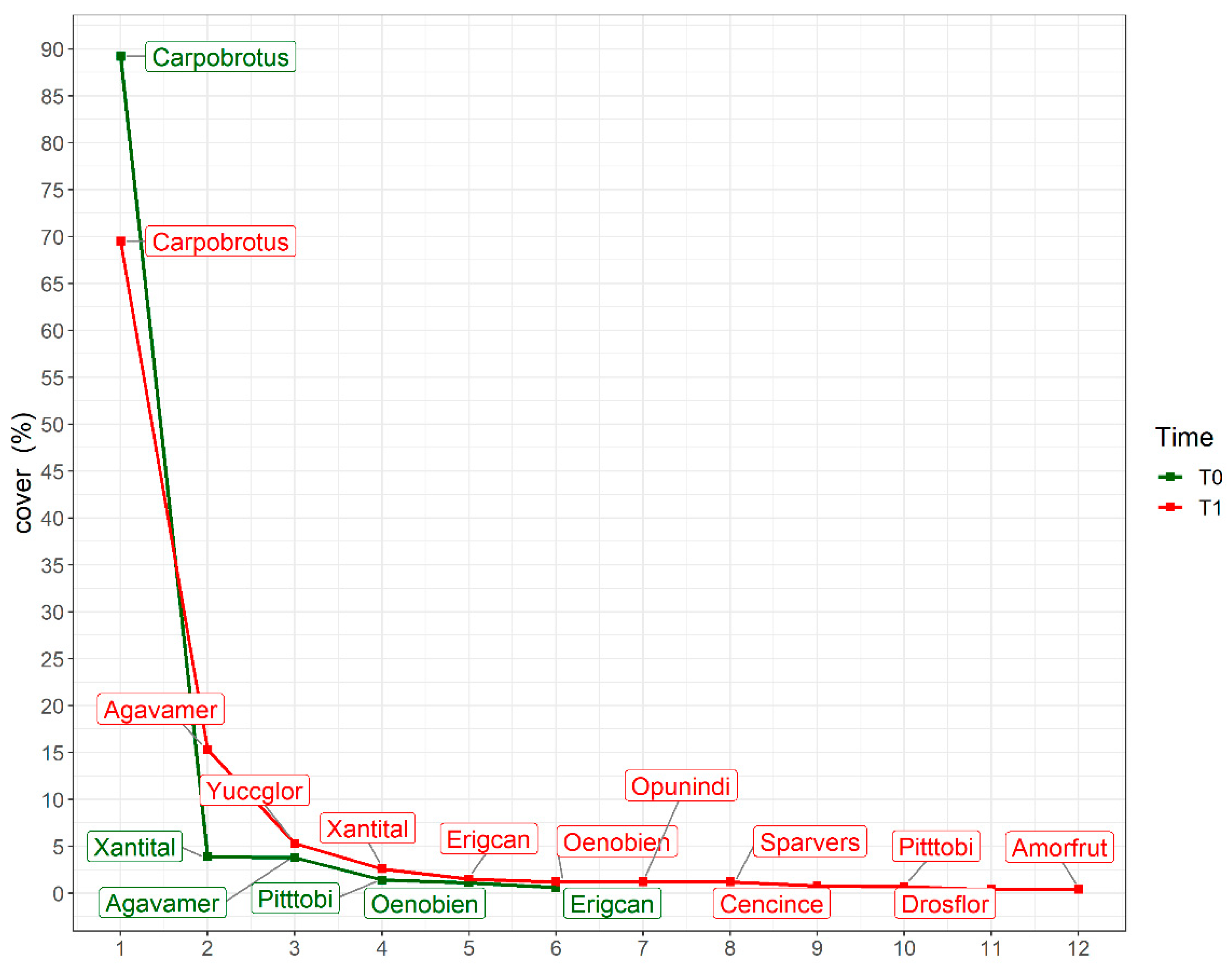
3.1. Overall Trends in Alien Species
3.2. Colonization, Loss, or Persistence of Alien Species
3.3. Changes in Diagnostic Species Cover
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien Species as a Driver of Recent Extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Sala, O.E. Global Biodiversity Scenarios for the Year 2100&nbs. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Bazzichetto, M.; Massol, F.; Carboni, M.; Lenoir, J.; Lembrechts, J.J.; Joly, R.; Renault, D. Once upon a time in the far south: Influence of local drivers and functional traits on plant invasion in the harsh sub-Antarctic islands. J. Veg. Sci. 2021, 32, e13057. [Google Scholar] [CrossRef]
- Kueffer, C. Plant Invasions in the Anthropocene. Science 2017, 358, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No Saturation in the Accumulation of Alien Species Worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.M.; Bellard, C.; Ricciardi, A. Alien versus Native Species as Drivers of Recent Extinctions. Front. Ecol. Environ. 2019, 17, 203–207. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological Impacts of Invasive Alien Plants: A Meta-Analysis of Their Effects on Species, Communities and Ecosystems: Ecological Impacts of Invasive Alien Plants. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A Global Assessment of Invasive Plant Impacts on Resident Species, Communities and Ecosystems: The Interaction of Impact Measures, Invading Species’ Traits and Environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Bacher, S.; Blackburn, T.M.; Essl, F.; Genovesi, P.; Heikkilä, J.; Jeschke, J.M.; Jones, G.; Keller, R.; Kenis, M.; Kueffer, C.; et al. Socio-economic Impact Classification of Alien Taxa (SEICAT). Methods Ecol. Evol. 2018, 9, 159–168. [Google Scholar] [CrossRef]
- Stricker, K.B.; Hagan, D.; Flory, S.L. Improving Methods to Evaluate the Impacts of Plant Invasions: Lessons from 40 Years of Research. AoB Plants 2015, 7, plv028. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the Long-Term Effects of Species Invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Branquart, E.; Brundu, G.; Buholzer, S.; Chapman, D.; Ehret, P.; Fried, G.; Starfinger, U.; van Valkenburg, J.; Tanner, R. A Prioritization Process for Invasive Alien Plant Species Incorporating the Requirements of EU Regulation No. 1143/2014. EPPO Bull. 2016, 46, 603–617. [Google Scholar] [CrossRef] [Green Version]
- D’Antonio, C.; Flory, S.L. Long-Term Dynamics and Impacts of Plant Invasions. J. Ecol. 2017, 105, 1459–1461. [Google Scholar] [CrossRef] [Green Version]
- Cleland, E.E.; Smith, M.D.; Andelman, S.J.; Bowles, C.; Carney, K.M.; Claire Horner-Devine, M.; Drake, J.M.; Emery, S.M.; Gramling, J.M.; Vandermast, D.B. Invasion in Space and Time: Non-Native Species Richness and Relative Abundance Respond to Interannual Variation in Productivity and Diversity: Productivity, Diversity and Invasion. Ecol. Lett. 2004, 7, 947–957. [Google Scholar] [CrossRef]
- Flory, S.L.; Bauer, J.; Phillips, R.P.; Clay, K. Effects of a Non-Native Grass Invasion Decline over Time. J. Ecol. 2017, 105, 1475–1484. [Google Scholar] [CrossRef] [Green Version]
- Marchante, H.; Marchante, E.; Freitas, H.; Hoffmann, J.H. Temporal Changes in the Impacts on Plant Communities of an Invasive Alien Tree, Acacia longifolia. Plant Ecol. 2015, 216, 1481–1498. [Google Scholar] [CrossRef]
- Mitchell, M.E.; Lishawa, S.C.; Geddes, P.; Larkin, D.J.; Treering, D.; Tuchman, N.C. Time-Dependent Impacts of Cattail Invasion in a Great Lakes Coastal Wetland Complex. Wetlands 2011, 31, 1143–1149. [Google Scholar] [CrossRef]
- Dostál, P.; Müllerová, J.; Pyšek, P.; Pergl, J.; Klinerová, T. The Impact of an Invasive Plant Changes over Time. Ecol. Lett. 2013, 16, 1277–1284. [Google Scholar] [CrossRef]
- Guido, A.; Pillar, V.D. Invasive Plant Removal: Assessing Community Impact and Recovery from Invasion. J. Appl. Ecol. 2017, 54, 1230–1237. [Google Scholar] [CrossRef] [Green Version]
- Yelenik, S.G.; D’Antonio, C.M. Self-Reinforcing Impacts of Plant Invasions Change over Time. Nature 2013, 503, 517–520. [Google Scholar] [CrossRef]
- Chytrý, M.; Maskell, L.C.; Pino, J.; Pyšek, P.; Vilà, M.; Font, X.; Smart, S.M. Habitat Invasions by Alien Plants: A Quantitative Comparison among Mediterranean, Subcontinental and Oceanic Regions of Europe. J. Appl. Ecol. 2008, 45, 448–458. [Google Scholar] [CrossRef]
- Chytrý, M.; Pyšek, P.; Wild, J.; Pino, J.; Maskell, L.C.; Vilà, M. European Map of Alien Plant Invasions Based on the Quantitative Assessment across Habitats. Divers. Distrib. 2009, 15, 98–107. [Google Scholar] [CrossRef]
- European Commission, Directorate General for the Environment. European Red List of Habitats. Part 2, Terrestrial and Freshwater Habitats; Publications Office: Luxembourg, 2016. [Google Scholar]
- Genovesi, P. Specie e Habitat di Interesse Comunitario in Italia: Distribuzione, Stato di Conservazione e Trend; ISPRA: Roma, Italy, 2014; ISBN 978-88-448-0644-6.
- Sarmati, S.; Bonari, G.; Angiolini, C. Conservation Status of Mediterranean Coastal Dune Habitats: Anthropogenic Disturbance May Hamper Habitat Assignment. Rend. Lincei Sci. Fis. Nat. 2019, 30, 623–636. [Google Scholar] [CrossRef]
- Ciccarelli, D. Mediterranean Coastal Sand Dune Vegetation: Influence of Natural and Anthropogenic Factors. Environ. Manag. 2014, 54, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Drius, M.; Jones, L.; Marzialetti, F.; de Francesco, M.C.; Stanisci, A.; Carranza, M.L. Not Just a Sandy Beach. The Multi-Service Value of Mediterranean Coastal Dunes. Sci. Total Environ. 2019, 668, 1139–1155. [Google Scholar] [CrossRef] [Green Version]
- Lithgow, D.; Martínez, M.L.; Silva, R.; Geneletti, D.; Gallego-Fernández, J.B.; Cerdán, C.R.; Mendoza, E.; Jermain, A. Ecosystem Services to Enhance Coastal Resilience in Mexico: The Gap between the Perceptions of Decision-Makers and Academics. J. Coast. Res. 2017, 77, 116–126. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The Value of Estuarine and Coastal Ecosystem Services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Basnou, C.; Iguzquiza, J.; Pino, J. Examining the Role of Landscape Structure and Dynamics in Alien Plant Invasion from Urban Mediterranean Coastal Habitats. Landsc. Urban Plan. 2015, 136, 156–164. [Google Scholar] [CrossRef]
- Cao Pinna, L.; Axmanová, I.; Chytrý, M.; Malavasi, M.; Acosta, A.T.R.; Giulio, S.; Attorre, F.; Bergmeier, E.; Biurrun, I.; Campos, J.A.; et al. The Biogeography of Alien Plant Invasions in the Mediterranean Basin. J. Veg. Sci. 2021, 32, e12980. [Google Scholar] [CrossRef]
- Lozano, V.; Marzialetti, F.; Carranza, M.L.; Chapman, D.; Branquart, E.; Dološ, K.; Große-Stoltenberg, A.; Fiori, M.; Capece, P.; Brundu, G. Modelling Acacia saligna Invasion in a Large Mediterranean Island Using PAB Factors: A Tool for Implementing the European Legislation on Invasive Species. Ecol. Indic. 2020, 116, 106516. [Google Scholar] [CrossRef]
- Jørgensen, R.H.; Kollmann, J. Invasion of Coastal Dunes by the Alien Shrub Rosa Rugosa Is Associated with Roads, Tracks and Houses. Flora-Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 289–297. [Google Scholar] [CrossRef]
- Malavasi, M.; Carboni, M.; Cutini, M.; Carranza, M.L.; Acosta, A.T.R. Landscape Fragmentation, Land-Use Legacy and Propagule Pressure Promote Plant Invasion on Coastal Dunes: A Patch-Based Approach. Landsc. Ecol. 2014, 29, 1541–1550. [Google Scholar] [CrossRef]
- Santoro, R.; Carboni, M.; Carranza, M.L.; Acosta, A.T.R. Focal Species Diversity Patterns Can Provide Diagnostic Information on Plant Invasions. J. Nat. Conserv. 2012, 20, 85–91. [Google Scholar] [CrossRef]
- Vila, M.; Tessier, M.; Suehs, C.M.; Brundu, G.; Galanidis, A.; Lambdon, P.; Manca, M.; Moragues, E.; Traveset, A.; Troumbis, A.Y.; et al. Local and Regional Assessments of the Impacts of Plant Invaders on Vegetation Structure and Soil Properties of Mediterranean Islands. J. Biogeogr. 2006, 9, 853–861. [Google Scholar] [CrossRef]
- Conser, C.; Connor, E.F. Assessing the Residual Effects of Carpobrotus edulis Invasion, Implications for Restoration. Biol. Invasions 2009, 11, 349–358. [Google Scholar] [CrossRef]
- Marchante, E.; Kjøller, A.; Struwe, S.; Freitas, H. Short- and Long-Term Impacts of Acacia longifolia Invasion on the Belowground Processes of a Mediterranean Coastal Dune Ecosystem. Appl. Soil Ecol. 2008, 40, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Santoro, R.; Jucker, T.; Carranza, M.; Acosta, A. Assessing the Effects of Carpobrotus Invasion on Coastal Dune Soils. Does the Nature of the Invaded Habitat Matter? Community Ecol. 2011, 12, 234–240. [Google Scholar] [CrossRef]
- Marcantonio, M.; Rocchini, D.; Ottaviani, G. Impact of Alien Species on Dune Systems: A Multifaceted Approach. Biodivers. Conserv. 2014, 23, 2645–2668. [Google Scholar] [CrossRef]
- Riva, E.G.; Godoy, O.; Castro-Díez, P.; Gutiérrez-Cánovas, C.; Vilà, M. Functional and Phylogenetic Consequences of Plant Invasion for Coastal Native Communities. J. Veg. Sci. 2019, 30, 510–520. [Google Scholar] [CrossRef]
- Tordoni, E.; Petruzzellis, F.; Nardini, A.; Savi, T.; Bacaro, G. Make It Simpler: Alien Species Decrease Functional Diversity of Coastal Plant Communities. J. Veg. Sci. 2019, 30, 498–509. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Pauchard, A.; Traveset, A.; Vilà, M. Linking the Impacts of Plant Invasion on Community Functional Structure and Ecosystem Properties. J. Veg. Sci. 2016, 27, 1233–1242. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Pizzo, L.; Buffa, G. The Response of Plant Community Diversity to Alien Invasion: Evidence from a Sand Dune Time Series. Biodivers. Conserv. 2015, 24, 371–392. [Google Scholar] [CrossRef] [Green Version]
- Sperandii, M.G.; Prisco, I.; Acosta, A.T.R. Hard Times for Italian Coastal Dunes: Insights from a Diachronic Analysis Based on Random Plots. Biodivers. Conserv. 2018, 27, 633–646. [Google Scholar] [CrossRef]
- Hédl, R.; Bernhardt-Römermann, M.; Grytnes, J.-A.; Jurasinski, G.; Ewald, J. Resurvey of Historical Vegetation Plots: A Tool for Understanding Long-Term Dynamics of Plant Communities. Appl. Veg. Sci. 2017, 20, 161–163. [Google Scholar] [CrossRef] [Green Version]
- Kapfer, J.; Grytnes, J.-A. Large Climate Change, Large Effect? Vegetation Changes over the Past Century in the European High Arctic. Appl. Veg. Sci. 2017, 20, 204–214. [Google Scholar] [CrossRef]
- Carranza, M.L.; Acosta, A.T.R.; Stanisci, A.; Pirone, G.; Ciaschetti, G. Ecosystem Classification for EU Habitat Distribution Assessment in Sandy Coastal Environments: An Application in Central Italy. Environ. Monit. Assess. 2008, 140, 99–107. [Google Scholar] [CrossRef]
- Sperandii, M.G.; Bazzichetto, M.; Gatti, F.; Acosta, A.T.R. Back into the Past: Resurveying Random Plots to Track Community Changes in Italian Coastal Dunes. Ecol. Indic. 2019, 96, 572–578. [Google Scholar] [CrossRef]
- Acosta, A.T.R. Coastal Dune Vegetation Zonation in Italy: Squeezed Between Environmental Drivers and Threats. In Tools for Landscape-Scale Geobotany and Conservation; Pedrotti, F., Box, E.O., Eds.; Geobotany Studies; Springer International Publishing: Cham, Switzerland, 2021; pp. 315–326. ISBN 978-3-030-74949-1. [Google Scholar]
- Sperandii, M.G.; Prisco, I.; Stanisci, A.; Acosta, A.T.R. RanVegDunes—A Random Plot Database of Italian Coastal Dunes. Phytocoenologia 2017, 47, 231–232. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An Updated Checklist of the Vascular Flora Alien to Italy. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Hennekens, S.M.; Knollová, I.; Janssen, J.A.M.; Rodwell, J.S.; Peterka, T.; Marcenò, C.; Landucci, F.; Danihelka, J.; et al. EUNIS Habitat Classification: Expert System, Characteristic Species Combinations and Distribution Maps of European Habitats. Appl. Veg. Sci. 2020, 23, 648–675. [Google Scholar] [CrossRef]
- Davies, C.E.; Moss, D.; Hill, M.O. EUNIS Habitat Classification Revised 2004; European Environment Agency (EEA): Brussels, Belgium, 2014; p. 310. [Google Scholar]
- Kindt, R.; Coe, R. Tree Diversity Analysis: A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agrofirestry Centre: Nairobi, Kenya, 2005; ISBN 978-92-9059-179-5. [Google Scholar]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Legendre, P. A Temporal Beta-diversity Index to Identify Sites That Have Changed in Exceptional Ways in Space–Time Surveys. Ecol. Evol. 2019, 9, 3500–3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dray, S.; Pélissier, R.; Couteron, P.; Fortin, M.-J.; Legendre, P.; Peres-Neto, P.R.; Bellier, E.; Bivand, R.; Blanchet, F.G.; De Cáceres, M.; et al. Community Ecology in the Age of Multivariate Multiscale Spatial Analysis. Ecol. Monogr. 2012, 82, 257–275. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; van de Wiel, M.A.; Zeileis, A. A Lego System for Conditional Inference. Am. Stat. 2006, 60, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Campoy, J.G.; Roiloa, S.R.; Santiso, X.; Retuerto, R. Ecophysiological Differentiation between Two Invasive Species of Carpobrotus Competing under Different Nutrient Conditions. Am. J. Bot. 2019, 106, 1454–1465. [Google Scholar] [CrossRef]
- Campoy, J.G.; Acosta, A.T.R.; Affre, L.; Barreiro, R.; Brundu, G.; Buisson, E.; González, L.; Lema, M.; Novoa, A.; Retuerto, R.; et al. Monographs of Invasive Plants in Europe: Carpobrotus. Bot. Lett. 2018, 165, 440–475. [Google Scholar] [CrossRef]
- Acosta, A.T.R.; Carranza, M.L.; Martino, L.D.; Frattaroli, A.; Izzi, C.F.; Stanisci, A. Patterns of Native and Alien Plant Species Occurrence on Coastal Dunes in Central Italy. In Plant Invasions: Human Perception, Ecological Impacts and Management; Tokarska-Guzik, B., Brock, J.H., Brundu, G., Child, L., Daehler, C.C., Pyšek, P., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2008; pp. 235–248. [Google Scholar]
- Carboni, M.; Thuiller, W.; Izzi, F.; Acosta, A. Disentangling the Relative Effects of Environmental versus Human Factors on the Abundance of Native and Alien Plant Species in Mediterranean Sandy Shores: Native-Alien Patterns on Coastal Dunes. Divers. Distrib. 2010, 16, 537–546. [Google Scholar] [CrossRef]
- Giulio, S.; Acosta, A.T.R.; Carboni, M.; Campos, J.A.; Chytrý, M.; Loidi, J.; Pergl, J.; Pyšek, P.; Isermann, M.; Janssen, J.A.M.; et al. Alien Flora across European Coastal Dunes. Appl. Veg. Sci. 2020, 23, 317–327. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Di Bugno, C.; Peruzzi, L. Checklist della flora vascolare psammofila della toscana. Atti Della Soc. Toscana Sci. Nat. Resid. Pisa Mem. Ser. B 2014, 121, 37–88. [Google Scholar] [CrossRef]


| T0 | T1 | |
|---|---|---|
| Invaded plots | 87 | 91 |
| Number of alien species | 6 | 12 |
| Total alien occurrences | 98 | 110 |
| Mean alien cover (%) | 25.06 | 23.46 |
| Category | Mean Relative Cover (T0) | Mean Relative Cover (T1) | Δ Cover | Trend | |
|---|---|---|---|---|---|
| Alien | Colonization | - | 0.19 | 0.19 | ↑ |
| Loss | 0.22 | - | −0.22 | ↓ | |
| Persistence | 0.36 | 0.24 | −0.12 | ↓ * | |
| Diagnostic | Colonization | 0.812 | 0.644 | −0.168 | ↓ * |
| Loss | 0.631 | 0.803 | 0.172 | ↑ * | |
| Persistence | 0.532 | 0.581 | 0.049 | ↑ |
| Category | Species | p-Value | Δ Cover |
|---|---|---|---|
| Colonization | Ammophila arenaria subsp. australis | 0.031 | −7.1% |
| Colonization | Cutandia maritima | 0.000 | +4.3% |
| Colonization | Salsola kali | 0.044 | −5.8% |
| Colonization | Sporobolus virginicus | 0.011 | −4.9% |
| Loss | Silene canescens | 0.011 | +3.2% |
| Persistence | Lotus cytisoides | 0.013 | +4.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascone, S.; Sperandii, M.G.; Cao Pinna, L.; Marzialetti, F.; Carranza, M.L.; Acosta, A.T.R. Exploring Temporal Trends of Plant Invasion in Mediterranean Coastal Dunes. Sustainability 2021, 13, 13946. https://doi.org/10.3390/su132413946
Cascone S, Sperandii MG, Cao Pinna L, Marzialetti F, Carranza ML, Acosta ATR. Exploring Temporal Trends of Plant Invasion in Mediterranean Coastal Dunes. Sustainability. 2021; 13(24):13946. https://doi.org/10.3390/su132413946
Chicago/Turabian StyleCascone, Silvia, Marta Gaia Sperandii, Luigi Cao Pinna, Flavio Marzialetti, Maria Laura Carranza, and Alicia Teresa Rosario Acosta. 2021. "Exploring Temporal Trends of Plant Invasion in Mediterranean Coastal Dunes" Sustainability 13, no. 24: 13946. https://doi.org/10.3390/su132413946
APA StyleCascone, S., Sperandii, M. G., Cao Pinna, L., Marzialetti, F., Carranza, M. L., & Acosta, A. T. R. (2021). Exploring Temporal Trends of Plant Invasion in Mediterranean Coastal Dunes. Sustainability, 13(24), 13946. https://doi.org/10.3390/su132413946








