From Seaweeds to Cosmeceutics: A Multidisciplinar Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals & Algal Biomass
2.2. Microwave-Assisted Extraction (MAE)
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Total Poliphenolic Content (TPC)—Folin-Ciocalteau
2.5. Antioxidant Activity—DPPH
2.6. Metal Content Evaluation
2.7. Formulation
2.8. Formulation Efficacy—Tape Stripping
3. Results and Discussion
3.1. Non-Conventional Macroalgae Extraction
3.2. Total Poliphenolic Content (TPC)—Folin–Ciocalteau Essay
3.3. Antioxidant Activity—DPPH·
3.4. Metal Content Evaluation
3.5. Formulation
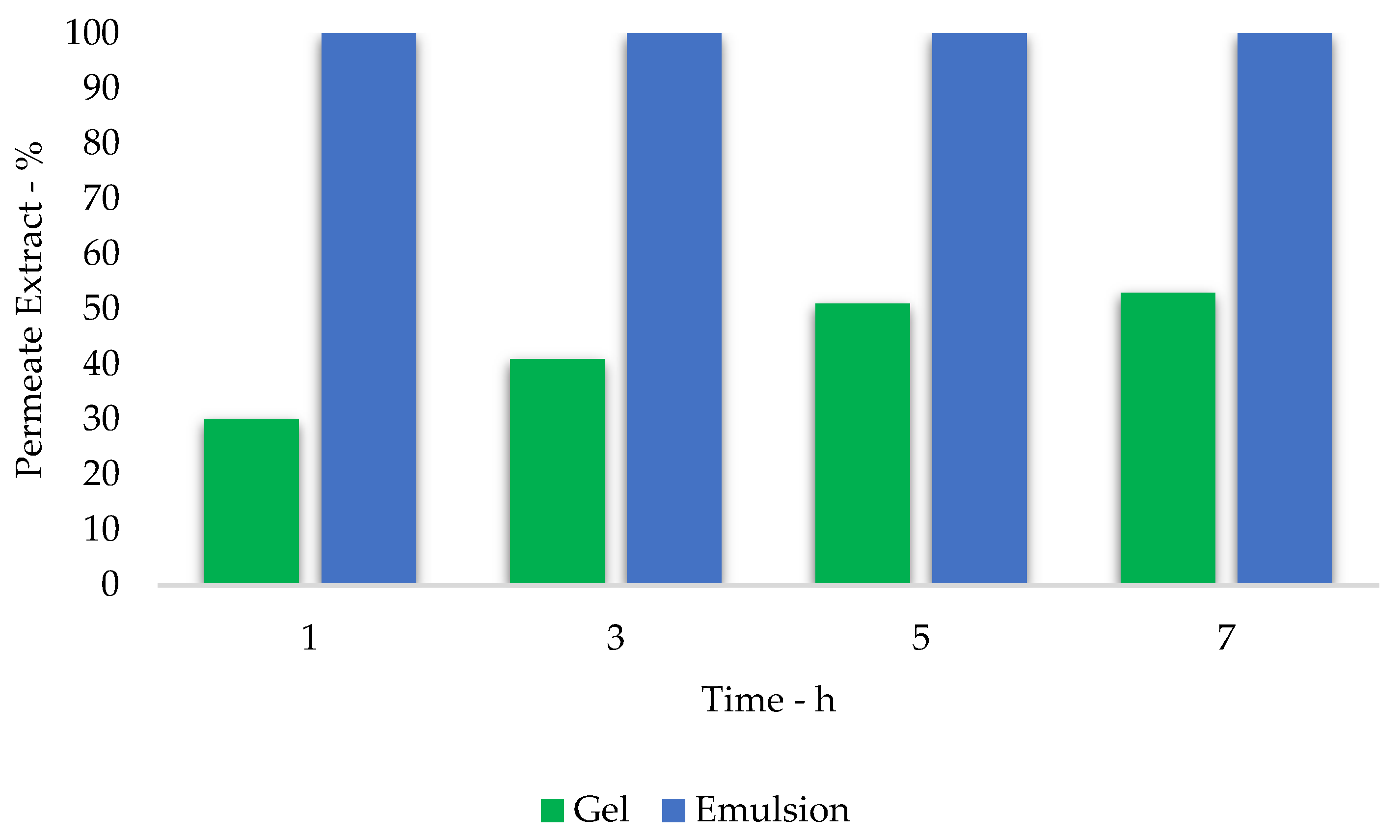
3.6. Formulate Efficacy—Tape Stripping
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milledge, J.J.; Maneein, S.; López, E.A.; Bartlett, D. Sargassum inundations in Turks and Caicos: Methane potential and proximate, ultimate, lipid, amino acid, metal and metalloid analyses. Energies 2020, 13, 1523. [Google Scholar] [CrossRef] [Green Version]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.-H. Seaweed-Based Molecules and Their Potential Biological Activities: An Eco-Sustainable Cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Jormalainen, V.; Honkanen, T. Variation in natural selection for growth and phlorotannins in the brown alga Fucus vesiculosus. J. Evol. Biol. 2004, 17, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Siahaan, E.A.; Pangestuti, R.; Munandar, H.; Kim, S.-K. Cosmeceuticals Properties of Sea Cucumbers: Prospects and Trends. Cosmetics 2017, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring bioactive fraction of Sargassum wightii: In vitro elucidation of angiotensin-I-converting enzyme inhibition and antioxidant potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Chen, X.; Wang, L.; Zhou, S.; Feng, H.; Chen, W.N.; Lau, R. Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour. Technol. 2013, 128, 337–344. [Google Scholar] [CrossRef]
- Wong, Y.C.; Shahirah, R. Effect of Different Solvent and Ratio Towards Microalgae Oil Production by Ul-trasonic Assisted Soxhlet Extraction Techniques. Orient. J. Chem. 2019, 35, 1377–1383. [Google Scholar] [CrossRef] [Green Version]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Chen, Y.; Liu, H.; Yuan, G.; Fan, Y.; Chen, K. Optimization of the microwave-assisted extraction of phlorotannins from Saccharina japonica Aresch and evaluation of the inhibitory effects of phlorotannin-containing extracts on HepG2 cancer cells. Chin. J. Oceanol. Limnol. 2013, 31, 1045–1054. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Q.; Qu, Y.; Xu, H.; Li, G. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemosa. J. Appl. Phycol. 2012, 24, 1527–1536. [Google Scholar] [CrossRef]
- Safari, P.; Rezaei, M.; Shaviklo, A.T. The optimum conditions for the extraction of antioxidant compounds from the Persian Gulf green algae (Chaetomorpha sp.) using response surface methodology. J. Food Sci. Technol. 2015, 52, 2974–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on -amylase, -glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Tuhy, Ł.; Chojnacka, K. Seaweed extract by microwave assisted extraction as plant growth biostimulant. Open Chem. 2015, 13, 1183–1195. [Google Scholar] [CrossRef]
- Pérez, L.; Conde, E.; Domínguez, H. Microwave hydrodiffusion and gravity processing of Sargassum muticum. Process Biochem. 2014, 49, 981–988. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Tabasso, S.; Grillo, G.; Cravotto, G.; Dreyer, T.; Schories, G.; Altenberg, S.; Jashina, L.; Telysheva, G. Wheat straw lignin extraction with bio-based solvents using enabling technologies. Comptes Rendus Chim. 2018, 21, 563–571. [Google Scholar] [CrossRef]
- Luque-García, J.; de Castro, M.L. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling phlorotannins from Fucus spp. of the Northern Portuguese coastline: Chemical approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Cho, H.; Doan, T.; Ha, T.; Kim, H.; Lee, B.; Pham, H.; Cho, T.; Oh, W.; Cho, H.M.; Doan, T.P.; et al. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cravotto, G.; Cintas, P. Forcing and Controlling Chemical Reactions with Ultrasound. Angew. Chem. Int. Ed. 2007, 46, 5476–5478. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Talarico, S.; Solarino, R.; Binello, A.; Cavaglià, G.; Bensaid, S.; Telysheva, G.; Cravotto, G. Batch and Flow Ultrasound-Assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-Kilo Scale. Antioxidants 2020, 9, 730. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Samorì, C.; Mazzei, L.; Ciurli, S.; Cravotto, G.; Grillo, G.; Guidi, E.; Pasteris, A.; Tabasso, S.; Galletti, P. Urease inhibitory potential and soil ecotoxicity of novel “polyphenols-deep eutectic solvents” formulations. ACS Sustain. Chem. Eng. 2019, 7, 15558–15567. [Google Scholar] [CrossRef]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.D.; Rinaldi, M.; Arlorio, M. Study of the DPPH•-scavenging activity: Development of a free software for the correct interpretation of data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Lerche, D.; Sobisch, T. Direct and Accelerated Characterization of Formulation Stability. J. Dispers. Sci. Technol. 2011, 32, 1799–1811. [Google Scholar] [CrossRef]
- EMA–European Medicines Agency. ICH Topic Q 1 A (R2) Stability Testing of new Drug Substances and Products Step. In Definitions 1–20. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products (accessed on 20 October 2021).
- Ansari, M.; Kazemipour, M.; Aklamli, M. The study of drug permeation through natural membranes. Int. J. Pharm. 2006, 327, 6–11. [Google Scholar] [CrossRef]
- Campos, P.M.; Praça, F.S.G.; Bentley, M.V.L.B. Quantification of lipoic acid from skin samples by HPLC using ultraviolet, electrochemical and evaporative light scattering detectors. J. Chromatogr. B 2016, 1019, 66–71. [Google Scholar] [CrossRef]
- Tommasi, E.; Cravotto, G.; Galletti, P.; Grillo, G.; Mazzotti, M.; Sacchetti, G.; Samorì, C.; Tabasso, S.; Tacchini, M.; Tagliavini, E. Enhanced and Selective Lipid Extraction from the Microalga P. tricornutum by Dimethyl Carbonate and Supercritical CO2 Using Deep Eutectic Solvents and Microwaves as Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 8316–8322. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging Processing Technologies for the Recovery of Valuable Bioactive Compounds from Potato Peels. Foods 2020, 9, 1598. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rajauria, G.; O’Donnell, C.; Tiwari, B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci. Technol. 2021, 110, 90–106. [Google Scholar] [CrossRef]
- Gouda, M.; Chen, K.; Li, X.; Liu, Y.; He, Y. Detection of microalgae single-cell antioxidant and electro-chemical potentials by gold microelectrode and Raman micro-spectroscopy combined with chemometrics. Sens. Actuators B Chem. 2021, 329, 129229–129239. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef] [Green Version]
- Marzocchi, M.; Badocco, D.; Piovan, A.; Pastore, P.; Di Marco, V.; Filippini, R.; Caniato, R. Metals in Undaria pinnatifida (Harvey) Suringar and Sargassum muticum (Yendo) Fensholt edible seaweeds growing around Venice (Italy). J. Appl. Phycol. 2016, 28, 2605–2613. [Google Scholar] [CrossRef]
- Davis, T.; Volesky, B.; Vieira, R.H.S. Sargassum seaweed as biosorbent for heavy metals. Water Res. 2000, 34, 4270–4278. [Google Scholar] [CrossRef] [Green Version]
- Regolamento (CE) n. 1223/2009 del Parlamento Europeo e del Consiglio Europeo sui prodotti cosmetici. Gazz. Uff. Dell’Unione Eur. (L342/59). 2009; pp. 52–59. Available online: https://www.salute.gov.it/imgs/C_17_pagineAree_145_listaFile_itemName_0_file.pdf (accessed on 20 October 2021).
- Signoretto, M.; Ghedini, E.; Menegazzo, F. Formulato Cosmetico a Rilascio Controllato di Principi Attivi. International Patent No. PCT/IB2019/053710, 7 May 2019. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Sachan, N.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, L.; De Cindio, B. Rheological investigation of pectin-based emulsion gels for pharmaceutical and cosmetic uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.-J.; Fluhr, J.W. The tape stripping procedure–evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef] [PubMed]

| Cosmetic Base a | A | Ch | Pe | Gly | G | O | W | Si |
|---|---|---|---|---|---|---|---|---|
| 0.2 | 1.5 | 0.3 | 2 | 0.5 | 7 | 85 | 4 |
| Sample | Water (%) | SD (%) | Organic Content (%) | SD (%) | Ashes (%) | SD (%) | Organic Content a (%) | Ashes a (%) |
|---|---|---|---|---|---|---|---|---|
| Sargassum muticum | 23.93 | ± 0.43 | 63.94 | ± 0.98 | 12.14 | ± 0.44 | 84.05 | 15.95 |
| Ulva lactuca | 20.41 | ± 0.51 | 65.40 | ± 1.02 | 14.19 | ± 0.69 | 82.17 | 17.83 |
| Solieria filiformis | 24.67 | ± 0.39 | 57.53 | ± 1.03 | 17.80 | ± 0.97 | 76.37 | 23.63 |
| Extraction Technology | Algae | Extraction Yield a | ||||
|---|---|---|---|---|---|---|
| Name | Type | mg/g | SD | % | SD | |
| MAE b | Sargassum muticum | Brown | 334.21 | ±12.10 | 33.42 | ±1.21 |
| Ulva lactuca | Green | 221.38 | ±8.43 | 22.14 | ±0.84 | |
| Solieria filiformis | Red | 92.053 | ±5.99 | 9.21 | ±0.60 | |
| UAE c | Sargassum muticum | Brown | 260.13 | ±8.02 | 26.01 | ±0.80 |
| Ulva lactuca | Green | 228.75 | ±10.19 | 22.88 | ±1.02 | |
| Solieria filiformis | Red | 78.15 | ±4.20 | 7.81 | ±0.42 | |
| Algal Sample | TPC Yield on DM * | TPC Yield on Organic Fraction | Ashes | |||||
|---|---|---|---|---|---|---|---|---|
| Name | Type | mgGAE/gExtr | SD | mgGAE/gMatrix | SD | mgGAE/gExtr | mgGAE/gMatrix | (%) |
| Sargassum muticum | Brown | 19.77 | ± 0.52 | 6.61 | ± 0.17 | 25.88 | 7.86 | 23.60 |
| Ulva lactuca | Green | 22.02 | ± 0.84 | 4.87 | ± 0.19 | 41.14 | 5.93 | 46.48 |
| Solieria filiformis | Red | 16.94 | ± 0.90 | 1.56 | ± 0.08 | 17.97 | 2.04 | 5.71 |
| Algal Sample | EC50 | Trolox® Eq. | ||
|---|---|---|---|---|
| Name | Type | mg/mL | Confidence Limit | mmolTrolox eq./gExtr |
| Sargassum muticum | Brown | 0.32 | 0.09–3.23 | 49.19 |
| Ulva lactuca | Green | 0.60 | 0.51–0.72 | 26.24 |
| Solieria filiformis | Red | 5.22 | 3.04–10.36 | 3.02 |
| Metal (ppm) | |||||||
|---|---|---|---|---|---|---|---|
| Seaweed | Type | Ni | Pb | Cr | Cd | Co | As |
| Sargassum muticum | Brown | 4630 | - | 9590 | 2.5 | 74.2 | - |
| Ulva lactuca | Green | 15 | 14.1 | 2.5 | - | - | - |
| mg Metal /kg Cosmetic (ppm) | |||||||
|---|---|---|---|---|---|---|---|
| Name | Type | Ni | Pb | Cr | Cd | Co | As |
| Sargassum muticum | Brown | 46.3 | - | 95.9 | 0.025 | 0.74 | - |
| Ulva lactuca | Green | 0.15 | 0.141 | 0.025 | - | - | - |
| Accepted Limits | 10 | 20 | 1 | 5 | 5 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillo, G.; Tabasso, S.; Solarino, R.; Cravotto, G.; Toson, C.; Ghedini, E.; Menegazzo, F.; Signoretto, M. From Seaweeds to Cosmeceutics: A Multidisciplinar Approach. Sustainability 2021, 13, 13443. https://doi.org/10.3390/su132313443
Grillo G, Tabasso S, Solarino R, Cravotto G, Toson C, Ghedini E, Menegazzo F, Signoretto M. From Seaweeds to Cosmeceutics: A Multidisciplinar Approach. Sustainability. 2021; 13(23):13443. https://doi.org/10.3390/su132313443
Chicago/Turabian StyleGrillo, Giorgio, Silvia Tabasso, Roberto Solarino, Giancarlo Cravotto, Clarissa Toson, Elena Ghedini, Federica Menegazzo, and Michela Signoretto. 2021. "From Seaweeds to Cosmeceutics: A Multidisciplinar Approach" Sustainability 13, no. 23: 13443. https://doi.org/10.3390/su132313443
APA StyleGrillo, G., Tabasso, S., Solarino, R., Cravotto, G., Toson, C., Ghedini, E., Menegazzo, F., & Signoretto, M. (2021). From Seaweeds to Cosmeceutics: A Multidisciplinar Approach. Sustainability, 13(23), 13443. https://doi.org/10.3390/su132313443