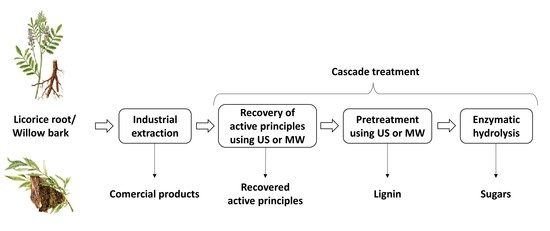
Ultrasonic or Microwave Cascade Treatment of Medicinal Plant Waste
Abstract
1. Introduction
2. Materials and Methods
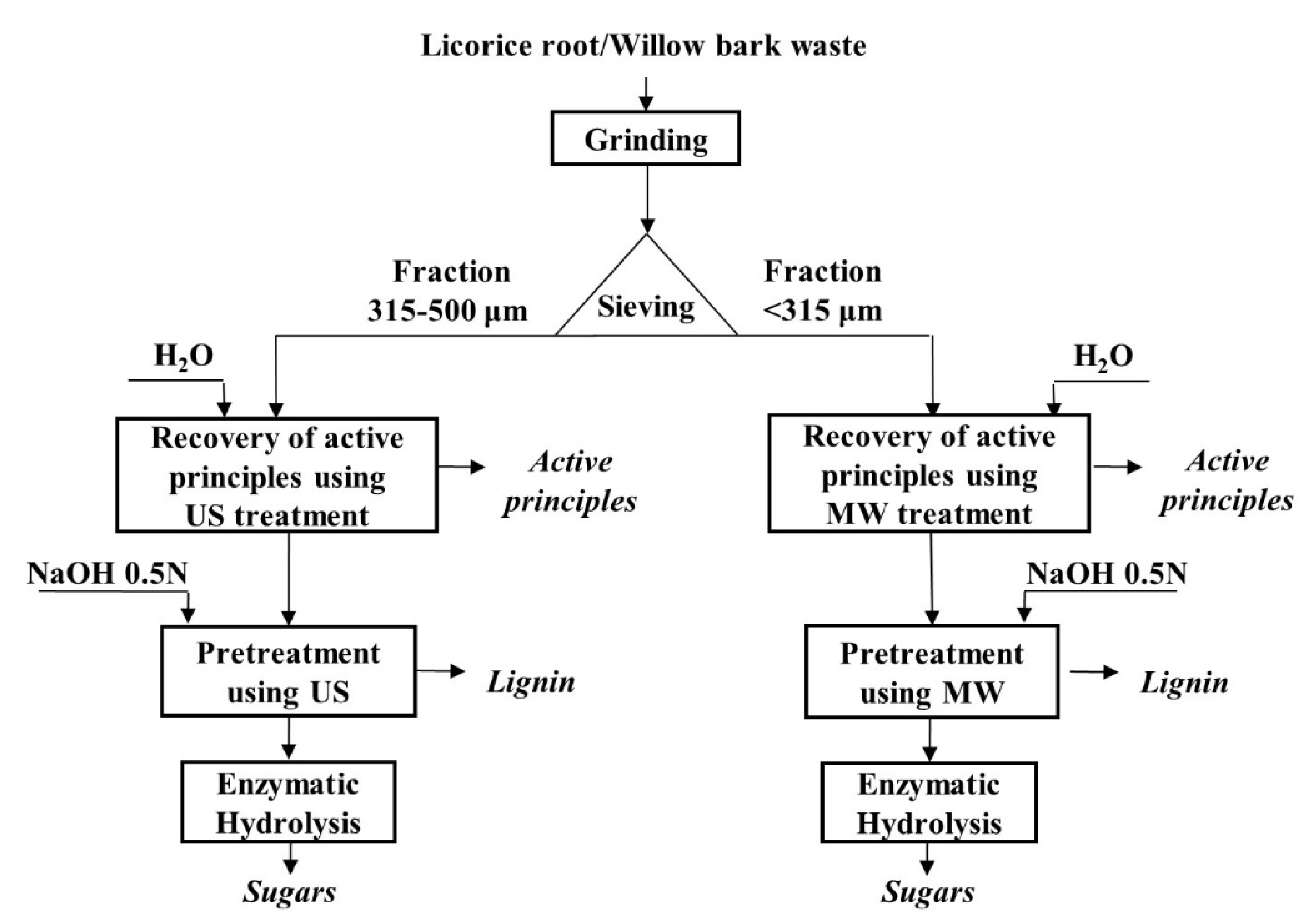
2.1. Materials
2.2. US and MW Treatments
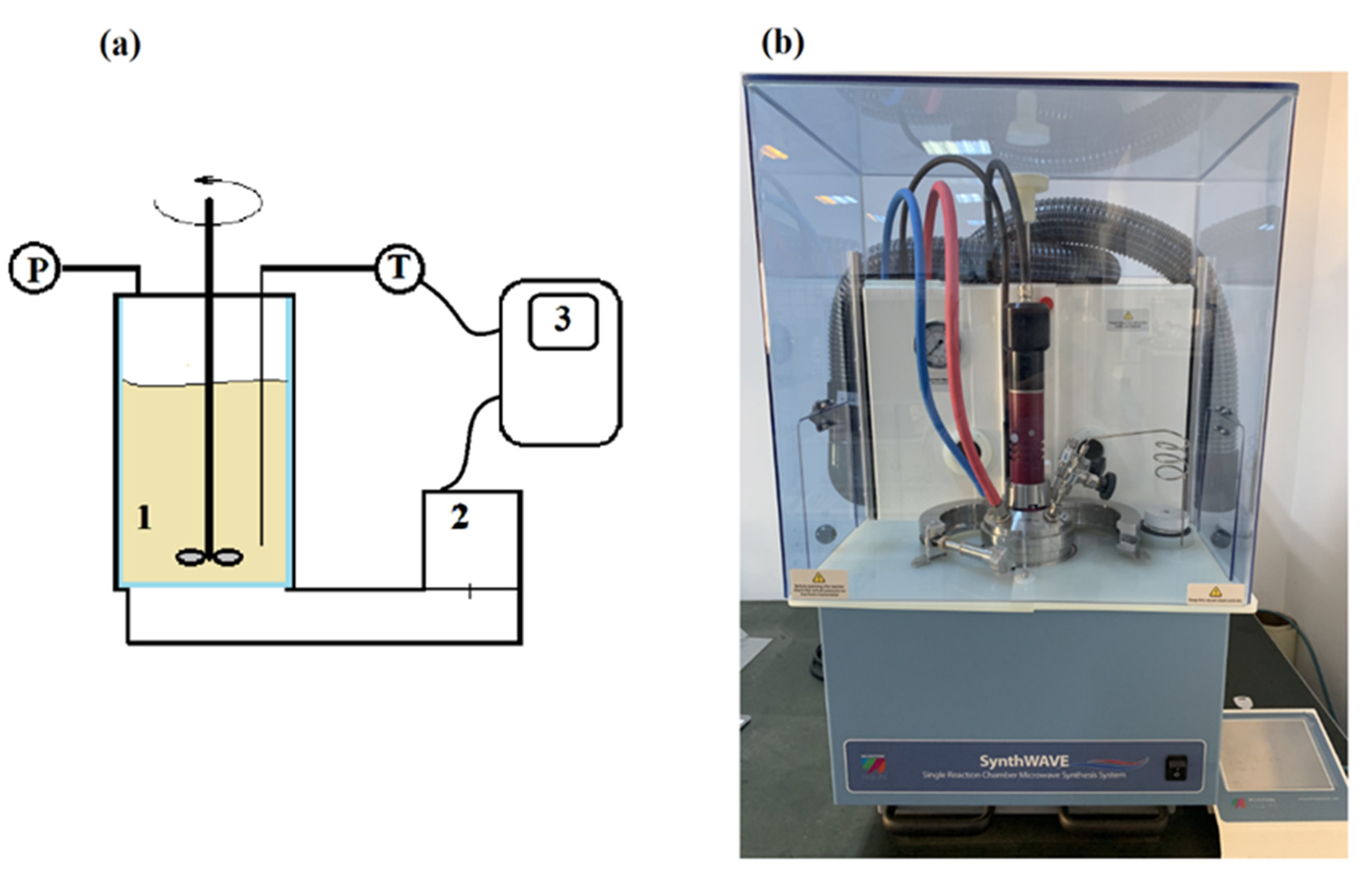
2.2.1. US Treatment Equipment and Procedure
2.2.2. MW Treatment Equipment and Procedure
2.2.3. Stability Studies of the Targeted Compounds
2.2.4. Delignification of the Waste Materials
2.3. Enzymatic Hydrolysis Procedure
2.4. Analyses
2.4.1. Determination of the Soluble Lignin Content
2.4.2. Determination of Saccharides Concentration
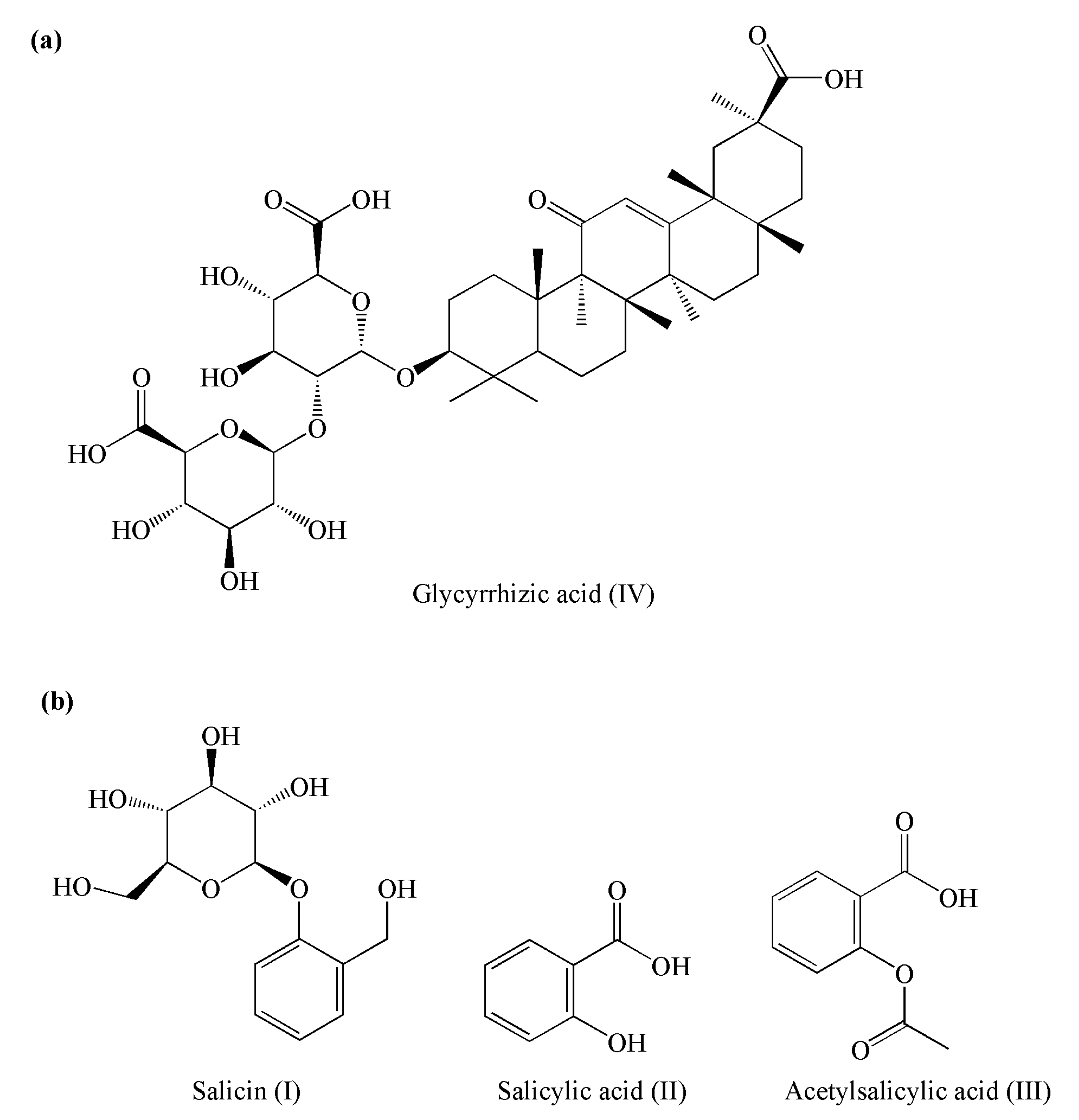
2.4.3. Determination of Glycyrrhizic Acid Content
2.4.4. Determination of the Salicin Derivatives Content
3. Results and Discussion
3.1. US and MW Extractions of Active Principles from the Lignocellulosic Materials
3.2. Stability of Active Principles during the US and MW Treatments
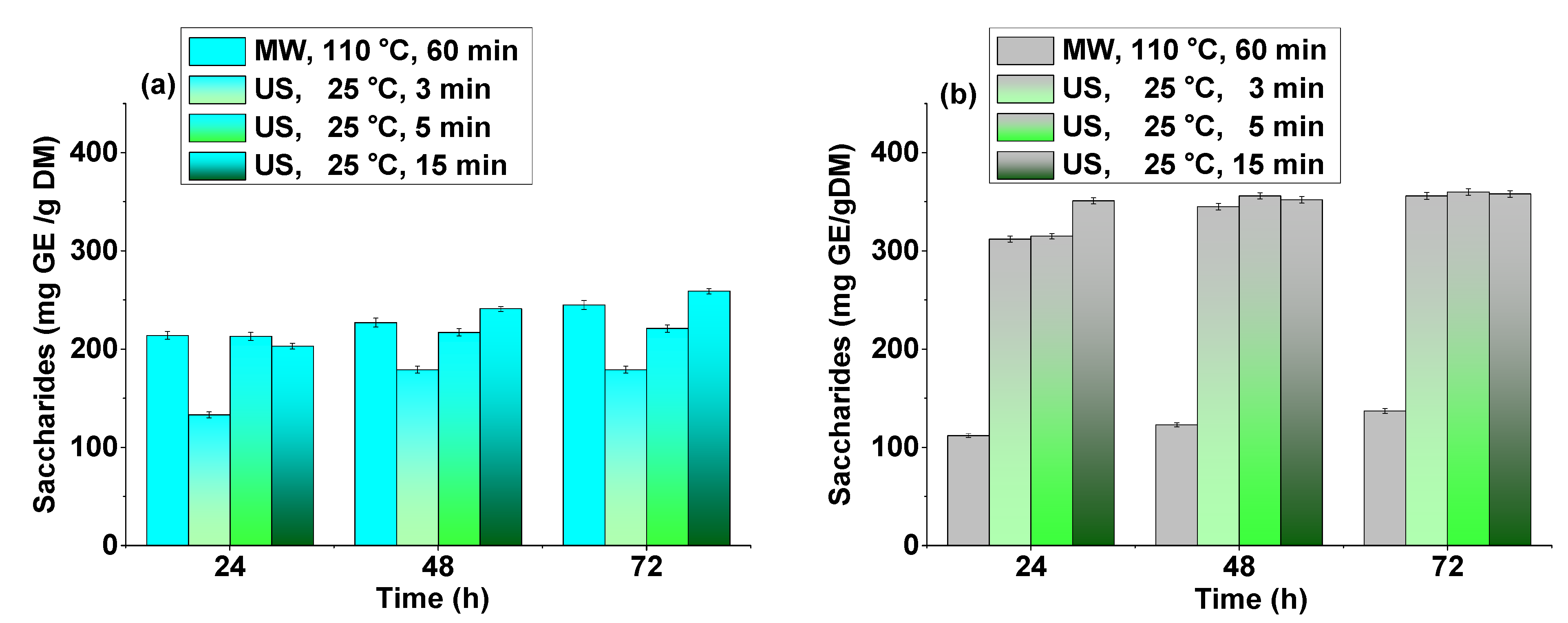
3.3. Delignification and Enzymatic Hydrolysis of Licorice Root and Willow Bark
3.4. Energy Consideration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mathews, S.L.; Pawlak, J.; Grunden, A.M. Bacterial biodegradation and bioconversion of industrial lignocellulosic streams. Appl. Microbiol. Biotechnol. 2015, 99, 2939–2954. [Google Scholar] [CrossRef]
- European Commission Biomass–Green Energy for Europe; European Commission: Luxembourg, 2005.
- Fardad, K.; Najafi, B.; Ardabili, S.F.; Mosavi, A.; Shamshirband, S.; Rabczuk, T. Biodegradation of Medicinal Plants Waste in an Anaerobic Digestion Reactor for Biogas Production. Comput. Mater. Contin. 2018, 55, 381–392. [Google Scholar] [CrossRef]
- Kulikowska, D.; Bernat, K. Waste Willow-Bark from Salicylate Extraction Successfully Reused as an Amendment for Sewage Sludge Composting. Sustainability 2021, 13, 6771. [Google Scholar] [CrossRef]
- Torres, E.; Rodriguez-Ortiz, L.A.; Zalazar, D.; Echegaray, M.; Rodriguez, R.; Zhang, H.; Mazza, G. 4-E (environmental, economic, energetic and exergetic) analysis of slow pyrolysis of lignocellulosic waste. Renew. Energy 2020, 162, 296–307. [Google Scholar] [CrossRef]
- Fernandez, A.; Rodriguez-Ortiz, L.; Asensio, D.; Rodriguez, R.; Mazza, G. Kinetic analysis and thermodynamics properties of air/steam gasification of agricultural waste. J. Environ. Chem. Eng. 2020, 8, 103829. [Google Scholar] [CrossRef]
- Zalazar-Garcia, D.; Torres, E.; Rodriguez-Ortiz, L.; Deng, Y.; Soria, J.; Bucala, V.; Rodriguez, R.; Mazza, G. Cleaner and sustainable processes for extracting phenolic compounds from bio-waste. J. Environ. Manag. 2020, 273, 111154. [Google Scholar] [CrossRef] [PubMed]
- Sette, P.; Fernandez, A.; Soria, J.; Rodriguez, R.; Salvatori, D.; Mazza, G. Integral valorization of fruit waste from wine and cider industries. J. Clean. Prod. 2020, 242, 118486. [Google Scholar] [CrossRef]
- De Sousa, C.B.D.C.; dos Anjos, G.L.; Nóbrega, R.S.; Magaton, A.D.S.; de Miranda, F.M.; Dias, F.D.S. Greener ultrasound-assisted extraction of bioactive phenolic compounds in Croton heliotropiifolius Kunth leaves. Microchem. J. 2020, 159, 105525. [Google Scholar] [CrossRef]
- Zou, S.; Wang, C.; Li, J.; He, J.; Gao, X.; Chang, Y. Microwave assisted solid phase microextraction for extraction and selective enrichment of four alkaloids in lotus leaf. Sustain. Chem. Pharm. 2020, 18, 100345. [Google Scholar] [CrossRef]
- Saleh, I.A.; Vinatoru, M.; Mason, T.J.; Abdel-Azim, N.S.; Aboutabl, E.A.; Hammouda, F.M. A possible general mechanism for ultrasound-assisted extraction (UAE) suggested from the results of UAE of chlorogenic acid from Cynara scolymus L. (artichoke) leaves. Ultrason. Sonochemistry 2016, 31, 330–336. [Google Scholar] [CrossRef]
- Lee, C.S.; Binner, E.; Winkworth-Smith, C.; John, R.; Gomes, R.; Robinson, J. Enhancing natural product extraction and mass transfer using selective microwave heating. Chem. Eng. Sci. 2016, 149, 97–103. [Google Scholar] [CrossRef]
- WHO Monographs. WHO Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS); World Health Organization: Geneva, Switzerland, 2010; p. 441. [Google Scholar]
- Roshan, A.; Verma, K.N.; Kumar, S.C.; Chandra, V.; Singh, P.D.; Panday, K.M. Phytochemical Constituent, Pharmacological Activities and Medicinal Uses Through the Millenia of Glycyrrhiza glabra Linn: A Review. Int. Res. J. Pharm. 2012, 3, 45–55. [Google Scholar] [CrossRef]
- Xiang, C.; Qiao, X.; Ye, M.; Guo, D.-a. Classification and distribution analysis of components in Glycyrrhiza using licorice compounds database. Acta Pharm. Sin. 2012, 47, 1023–1030. [Google Scholar]
- Alfauomy, G.A.; Seleem, H.A.; Ali, M.M.A. Evaluation of Pies Containing Licorice Roots (Glycyrrhiza glabra L.) Extracts. Middle East J. Agric. Res. 2020, 9, 545–557. [Google Scholar] [CrossRef]
- Shirazi, M.H.; Ranjbar, R.; Eshraghi, S.; Sadeghi, G.; Jonaidi, N.; Bazzaz, N.; Izadi, M.; Sadeghifard, N. An Evaluation of Antibacterial Activity of Glycyrrhiza glabra Extract on the Growth of Salmonella, Shigella and ETEC E. coli. J. Biol. Sci. 2007, 7, 827–829. [Google Scholar] [CrossRef]
- Yilmaz, F. Application of Glycyrrhiza glabra L. Root as a Natural Antibacterial Agent in Finishing of Textile. Ind. Crop. Prod. 2020, 157, 112899. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Soni, B.; Dalwadi, N. Evaluation of antioxidant and anti-atherogenic properties of Glycyrrhiza glabra root using in vitro models. Int. J. Food Sci. Nutr. 2009, 60, 135–149. [Google Scholar] [CrossRef]
- Yang, F.; Chu, T.; Zhang, Y.; Liu, X.; Sun, G.; Chen, Z. Quality assessment of licorice (Glycyrrhiza glabra L.) from different sources by multiple fingerprint profiles combined with quantitative analysis, antioxidant activity and chemometric methods. Food Chem. 2020, 324, 126854. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; Abdel-Wadood, Y.A. The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomedicine Plus 2021, 1, 100043. [Google Scholar] [CrossRef]
- Aboutalebi, S.; Shahsavandi, S.; Nemati, F.; Ebrahimi, N. The Potential Effect of Glycyrrhiza glabra on Early Step of Influenza Virus Replication. Iran. J. Virol. 2020, 14, 28–35. [Google Scholar]
- Srivastava, V.; Yadav, A.; Sarkar, P. Molecular docking and ADMET study of bioactive compounds of Glycyrrhiza glabra against main protease of SARS-CoV2. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, M.P.; Purushotham, K.G.; Sathish, M.; Vimal Raj, D.; Venkatesh, M. In-vitro Anti-inflammatory activity of Liquorice (Glycyrrhiza glabra) using Aqueous Extract. Int. J. Res. Pharm. Sci. 2020, 11, 657–662. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, A.; Hisaka, S.; Hayashi, H.; Nose, M. Effect of hot water extract of a glycyrrhizin-deficient strain of Glycyrrhiza uralensis on contact hypersensitivity in mice. J. Nat. Med. 2020, 74, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Weir, D.; Busse, P.; Yang, N.; Zhou, Z.; Emala, C.; Li, X.M. The Flavonoid 7,4’-Dihydroxyflavone Inhibits MUC5AC Gene Expression, Production, and Secretion via Regulation of NF-kappaB, STAT6, and HDAC2. Phytother. Res. 2015, 29, 925–932. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, Y.; Hu, X.; Wang, Q.; Lei, W.; Zhou, L.; Huang, J. Regulation of Th1/Th2 balance through OX40/OX40L signalling by glycyrrhizic acid in a murine model of asthma. Respirology 2016, 21, 102–111. [Google Scholar] [CrossRef]
- Gioti, K.; Papachristodoulou, A.; Benaki, D.; Beloukas, A.; Vontzalidou, A.; Aligiannis, N.; Skaltsounis, A.L.; Mikros, E.; Tenta, R. Glycyrrhiza glabra-Enhanced Extract and Adriamycin Antiproliferative Effect on PC-3 Prostate Cancer Cells. Nutr. Cancer 2020, 72, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhang, W.J.; Fan, Q.X.; Wang, L.X. Licochalcone A inhibits PI3K/Akt/mTOR signaling pathway activation and promotes autophagy in breast cancer cells. Oncol. Lett. 2018, 15, 1869–1873. [Google Scholar] [CrossRef]
- Isebrands, J.G.; Richardson, J. Poplars and Willows. Trees for Society and the Environment; The Food and Agriculture Organization of the United Nations and CABI: London, UK, 2014; p. 699. [Google Scholar]
- ESCOP Monographs. Salicis Cortex. Willow Bark; Mills, S., Hutchins, R., Eds.; European Scientific Cooperative on Phytotherapy: Exter, UK, 2017. [Google Scholar]
- Brereton, N.J.B.; Berthod, N.; Lafleur, B.; Pedneault, K.; Pitre, F.E.; Labrecque, M. Extractable phenolic yield variation in five cultivars of mature short rotation coppice willow from four plantations in Quebec. Ind. Crop. Prod. 2017, 97, 525–535. [Google Scholar] [CrossRef]
- Gligorić, E.I.; Igić, R.; Suvajdžić, L.Đ.; Teofilović, B.D.; Grujić-Letić, N.N. Salix eleagnos Scop.—A novel source of antioxidant and anti-inflammatory compounds: Biochemical screening and in silico approaches. S. Afr. J. Bot. 2020, 128, 339–348. [Google Scholar] [CrossRef]
- Seiwerth, J.; Tasiopoulou, G.; Hoffmann, J.; Wolfle, U.; Schwabe, K.; Quirin, K.W.; Schempp, C.M. Anti-Inflammatory Effect of a Novel Topical Herbal Composition (VEL-091604) Consisting of Gentian Root, Licorice Root and Willow Bark Extract. Planta Med. 2019, 85, 608–614. [Google Scholar] [CrossRef]
- Drummond, E.M.; Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D.; Gibney, E.R. An in vivo study examining the antiinflammatory effects of chamomile, meadowsweet, and willow bark in a novel functional beverage. J. Diet. Suppl. 2013, 10, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, N.A.; Mahmoudabady, M.; Soukhtanloo, M.; Hayatdavoudi, P.; Beheshti, F.; Niazmand, S. Salix alba attenuated oxidative stress in the heart and kidney of hypercholesterolemic rabbits. Avicenna J. Phytomedicine 2018, 8, 63–72. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.-C.; Baudelaire, É.; Scher, J.; Dicko, A. Antioxidant and antiacetylcholinesterase activities of different granulometric classes of Salix alba (L.) bark powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- Fayaz, M.; Sivakumaar, P.K. Phytochemical Analysis and Antimicrobial Activity of Salix alba against Dental Biofilm Forming Bacteria. Int. J. Pharm. Biol. Arch. 2014, 5, 137–140. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic Content, Antioxidant, Antimicrobial and Cytotoxic Activities of Ethanolic Extract of Salix alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef]
- Saracila, M.; Tabuc, C.; Panaite, T.D.; Papuc, C.P.; Olteanu, M.; Criste, R.D. Effect of the Dietary Willow Bark Extract (Salix alba) on the Caecal Microbial Population of Broilers (14–28 Days) Reared at 32 °C. Agric. Life Life Agric. Conf. Proc. 2018, 1, 155–161. [Google Scholar] [CrossRef][Green Version]
- Özbek, H.N.; Koçak Yanık, D.; Fadıloğlu, S.; Göğüş, F. Effect of microwave-assisted alkali pre-treatment on fractionation of pistachio shell and enzymatic hydrolysis of cellulose-rich residues. J. Chem. Technol. Biotechnol. 2020, 96, 521–531. [Google Scholar] [CrossRef]
- Sombatpraiwan, S.; Junyusen, T.; Treeamnak, T.; Junyusen, P. Optimization of microwave-assisted alkali pretreatment of cassava rhizome for enhanced enzymatic hydrolysis glucose yield. Food Energy Secur. 2019, 8, 1–15. [Google Scholar] [CrossRef]
- Wu, H.; Dai, X.; Zhou, S.L.; Gan, Y.Y.; Xiong, Z.Y.; Qin, Y.H.; Ma, J.; Yang, L.; Wu, Z.K.; Wang, T.L.; et al. Ultrasound-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Bioresour. Technol. 2017, 241, 70–74. [Google Scholar] [CrossRef]
- ULTRA-MINT Technologies. Available online: http://ultramint.chimie.upb.ro/en/index.php (accessed on 10 November 2021).
- Advanced Sonic Processing Systems. Available online: www.advancedsonicprocessingsystems.com/Dual-Frequency-Reactors/ (accessed on 10 November 2021).
- Milestone srl. Available online: https://www.milestonesrl.com/products/microwave-assisted-synthesis/synthwave/ (accessed on 10 November 2021).
- BioSolutions Novozymes. Available online: https://biosolutions.novozymes.com/en/juice-fruit-vegetables/products/vegetables/celluclast-1.5-l/ (accessed on 10 November 2021).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chim. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- TAPPI. Official Test Methods and Provisional Test Methods; TAPPI: Peachtree Corners, GA, USA, 1979. [Google Scholar]
- European Pharmacopoeia Commission. European Pharmacopoeia; European Pharmacopoeia Commission: Strasbourg, France, 2017. [Google Scholar]
- European Medicines Agency. Assessment Report on Salicis Cortex (Willow Bark) and Herbal Preparation(s) thereof with Well-Established Use and Traditional Use; European Medicines Agency: London, UK, 2009; pp. 1–27. [Google Scholar]
- Romanian Pharmacopoeia Commission. Romanian Pharmacopoeia; Romanian Pharmacopoeia Commission: Bucharest, Romania, 1993. [Google Scholar]
- Lavric, V.; Calinescu, I. Modeling the Temperature Field Dynamics During the Microwaves Assisted Extraction of Active Principles from Vegetables. In Proceedings of the 16th International Conference on Microwave and High Frequency Heating (AMPERE), Delft, The Netherlands, 18–21 September 2017; Eli Jerby; AMPERE Newsletter. Volume 95, pp. 14–20. [Google Scholar]




| Equipment | Grid Power (kW) * | Time (min) | Energy Consumption (kWh) |
|---|---|---|---|
| US | 1.2 | 5 | 0.1 |
| 15 | 0.3 | ||
| MW | 0.35 | 30 | 0.175 |
| 60 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staicu, V.; Luntraru, C.; Calinescu, I.; Chisega-Negrila, C.G.; Vinatoru, M.; Neagu, M.; Gavrila, A.I.; Popa, I. Ultrasonic or Microwave Cascade Treatment of Medicinal Plant Waste. Sustainability 2021, 13, 12849. https://doi.org/10.3390/su132212849
Staicu V, Luntraru C, Calinescu I, Chisega-Negrila CG, Vinatoru M, Neagu M, Gavrila AI, Popa I. Ultrasonic or Microwave Cascade Treatment of Medicinal Plant Waste. Sustainability. 2021; 13(22):12849. https://doi.org/10.3390/su132212849
Chicago/Turabian StyleStaicu, Vasile, Cristina Luntraru, Ioan Calinescu, Ciprian Gabriel Chisega-Negrila, Mircea Vinatoru, Miruna Neagu, Adina Ionuta Gavrila, and Ioana Popa. 2021. "Ultrasonic or Microwave Cascade Treatment of Medicinal Plant Waste" Sustainability 13, no. 22: 12849. https://doi.org/10.3390/su132212849
APA StyleStaicu, V., Luntraru, C., Calinescu, I., Chisega-Negrila, C. G., Vinatoru, M., Neagu, M., Gavrila, A. I., & Popa, I. (2021). Ultrasonic or Microwave Cascade Treatment of Medicinal Plant Waste. Sustainability, 13(22), 12849. https://doi.org/10.3390/su132212849