Abstract
The production of volatile fatty acids (VFAs) from waste stream has been recently getting attention as a cost-effective and environmentally friendly approach in mechanical–biological treatment plants. This is the first study to explore the use of a functional bacterium, AM5 isolated from forest soil, which is capable of enhancing the production of VFAs in the presence of soil bacteria as a co-digester in non-strict anaerobic fermentation processes of food waste leachates. Batch laboratory-scale trials were conducted under thermophilic conditions at 55 °C and different pH values ranging from approximately 5 to 11, as well as under uncontrolled pH for 15 days. Total solid content (TS) and volatile solid content (VS) were observed with 58.42% and 65.17% removal, respectively. An effluent with a VFA concentration of up to 33,849 mg/L (2365.57 mg/g VS; 2244.45 mg/g chemical oxygen demand (COD)-VFA VS; 1249 mg/g VSremoved) was obtained at pH 10.5 on the second day of the batch culture. The pH resulted in a significant effect on VFA concentration and composition at various values. Additionally, all types of VFAs were produced under pH no-adjustment (approximately 5) and at pH 10.5. This research might lead to interesting questions and ideas for further studies on the complex metabolic pathways of microbial communities in the mixture of a soil solution and food waste leachate.
1. Introduction
Food waste (FW) generation has sharply increased each year and accounted for 32% of all the food produced worldwide, including food waste and loss in the supply chain. The notable environmental effects of FW include the contribution to global warming due to the emission of greenhouse gases through transportation, storage, and other disposal operations [1]. Due to its properties, such as high organic and moisture content, FW is important not only because of waste treatment but also because of the commercial applications using products generated during the treatment process, such as volatile fatty acids (VFAs). Thus, FW exploitation for VFA production is a sustainable alternative in the “biorefinery” concept that represents an innovative approach in environmental management [2].
VFAs are line short-chain mono-carboxylate compounds produced mainly by microbial fermentation, including acetic, propionic, butyric, caproic, valeric, and heptanoic acid. Due to their renewability, degradability, and sustainability, VFAs are attractive carbon sources for biological nutrient removal, the production of biodiesel and biogas, bioplastic production, and electricity generation via microbial fuel cells [3,4].
Numerous studies have investigated various treatment methods to improve the hydrolysis and solubilization of complex organic compounds in order to enhance VFA production [5,6,7]. Hydrolysis is considered the stage-limiting step for anaerobic digestion because macromolecules are reduced and transformed into other soluble compounds, such as amino acids, sugar, and long-chain fatty acids, in this step [1]. Moreover, the hydrolysis and acidification stages are extremely influenced by environmental factors such as inocula, substrate, pH, temperature, and hydraulic retention time (HRT) [8,9,10,11]. Some studies have investigated numerous pretreatment methods, such as physical, chemical, and biological approaches, separately or in combination, to enhance the hydrolysis step [12,13,14,15,16]. Biological pretreatment and bioaugmentation have been recognized as promising approaches to increase VFA yield since these methods are eco-friendly and affordable. Thus, the co-digestion of multiple substrates and waste instead of mono-digestion has been used during anaerobic fermentation (AF) via the addition of 2.5% w/w of mature compost to food waste as a co-digester to increase hydrolysis, with VFA concentration observed to increase up to 51.2% ± 12.3% compared to the concentration with no compost addition [17,18]. Additionally, the investigation of each functional bacterial group that is responsible for VFA production is of interest to many researchers via microbial routes. The main bacterial groups involved in VFA production are the acid-forming bacteria, including acetogenic and homoacetogenic bacteria [19,20].
The use of pure cultures of microorganisms that can metabolize organic substrates as energy and carbon sources has been previously explored. In the fermentative pathway, various microbes, such as Acetobacter, Acetomicrobium, Clostridium, Gluconobacter, and Thermoanaerobacter, may be useful for acetic acid production, especially, Acetobacter and Gluconobacter, which have been reported as having promising commercial potential [21]. Clostridium lentocellum can produce 17 g/L and 30.98 g/L of acetic acid using various sources of biomass, such as lignocelluloses and cellulose, respectively [22]. Members of the genus Propionibacterium have been investigated as propionic acid-producing bacteria that are able to produce up to 34.93 g/L in the case of the engineered P. jensenii [23]. Clostridium tyrobutyricum is a representative of the genus Clostridium that produces 34.2 g/L of butyric acid [24]. Moreover, in fed-batch fermentations using a fibrous bed bioreactor with immobilized C. tyrobutyricum, a production of 86.9 g/L of butyric acid was achieved [25]. Isobutyric acid production was also investigated using Propionibacterium freudenreichii, Escherichia coli, and Pseudomonas sp. strains [26,27,28]. Regarding isovaleric acid production, Thierry et al. (2004) found that P. freudenreichii is an isovaleric acid-producing strain from Swiss cheese [29]. Hou and co-workers demonstrated the usefulness of the application of food waste fermentation to prepare soil conditioners [30]. Despite the abundance of bacterial species in the soil, more than 99% of these species cannot be cultured using traditional techniques. Thus, their functions are still unknown and potentially attractive for microbiologists [31].
Additionally, some studies have demonstrated that acidogenesis is better under limited aeration than under strictly anaerobic conditions; the presence of a small amount of oxygen improves VFA content due to the increase in facultative anaerobes and extracellular hydrolytic enzymes [32,33]. A recent study found that the aerobic sludge containing less strict anaerobic methanogenic archaea may be used to increase VFA accumulation, with an organic conversion to VFAs of 36%, which is higher than that obtained using the anaerobic sludge, due to the longer digestion time required for the anaerobic condition [34]. Therefore, a microbial community including anaerobic and facultative microbes from the soil has the potential to contribute to the anaerobic digestion (AD) process.
In other studies, the purpose of active sludge and manure addition has been to supply the useful microorganisms in the waste treatment [7,18]. Since functional soil bacteria are considered to be a valuable resource with a wide variety of applications, we herein used them as an additive to improve the biodegradation and VFA production from food waste leachate. Such soil microorganisms have been recognized as the greatest contributors to the diversity of terrestrial ecosystems and are the major controllers of almost all global biogeochemical cycles. They maintain plant health through their nutrient cycling roles and relationships with other organisms [35,36]. Therefore, the extreme complexity and high diversity of microbial communities represent a huge source for research and applications in other fields.
Among more than 99% of uncultured soil microorganisms, the bacterial functional group is one of the targets of microbiologists; they are considered as decomposers of organic residues in soil via the enzymes that they release [37,38]. In other words, various kinds of organic wastes could be valuable sources for microbial metabolism. Carbon compounds and simple sugars are easily digested by microbes, and they then tie up soluble nutrients like nitrogen in their cell membranes [39].
On the other hand, one of the potential inhibitors in an anaerobic digester is the imbalance between high nitrogen content and carbon source, which can cause toxicity by generating ammonia. The presence of ammonia-oxidizing bacteria plays a significant role in reducing ammonia stress on other bacteria. Additionally, recent studies have shown that ammonia oxidizers are widely distributed in acidic soil and the soil surrounding roots [36,40].
Numerous molecular biological approaches combined with dependent culture methods have been applied in recent years to explore the diversity of soil bacterial communities [41,42,43,44,45,46]. However, the network interactions among soil microorganisms in each living condition are still an open question for researchers [47]. Significant undiscovered microbes may introduce an incomplete figure of phylogenetic diversity and microbial functions. Therefore, soil microorganisms are still waiting to be cultivated in the laboratory by dependent culture methods and to have their functions explored in the future.
Thus, in considering a combination of promising factors that would be beneficial in terms of VFA production from food waste, this study aims to explore the effective bacterial strain from soil under non-strict anaerobic conditions when used as an additive in conjunction with facultative soil bacteria from the same community. The complex metabolic pathways of soil bacteria in the presence of a dominant potential bacterial strain used in this study might play a crucial role in the whole biological process of food waste leachate to enhance VFA production yield.
2. Materials and Methods
2.1. Substrates and Inocula
The soil used in this study was collected from forest soil at Kyonggi University, South Korea, and bacterial strain was isolated following the method described in a previous study [48]. From a previous study on VFA production and VFA degradation from food waste, we determined that Bacillus sp. AM5 was one of the best VFA-producing bacteria, and it was selected to optimize working conditions in order to increase VFA production.
Bacillus sp. AM5 strain was obtained after 2–3 days of incubation at 35 °C in the following medium (g/L): glucose 10, beef extract 2, peptone 4, yeast extract 1, NaCl2, K2HPO4 1.5, MgCl2·6H2O 0.6, FeSO4·7H2O 0.2, l-cysteine 0.5, trace elements 10 mL, and vitamin solution 10 mL. The trace element solution was composed of (g/L) MnSO4·7H2O 0.01, ZnSO4·7H2SO4 0.05, H3BO3 0.01, N(CH2COOH)3 4.5, CaCl2·2H2O 0.01, Na2MoO4 0.01, CoCl2·6H2O 0.2, and AlK(SO4)2 0.01. The vitamin solution contained riboflavin (g/L) 0.025, citric acid 0.02, folic acid 0.01, and para-aminobenzoic acid 0.01.
The food waste leachate (FWL) was collected from the Suwon Environment Affairs Agency, South Korea, and was diluted in water in a proportion of approximately 1:2 (v/v). This FW was stored in the refrigerator at 4 °C for further use.
2.2. Experimental Set-Up
Serum 150-mL bottles with 100-mL working volume were set up as anaerobic batch reactors in triplicates. The pH was adjusted with 3M HCl or 3M NaOH and monitored using a pH meter (HI 2210, Hanna Instruments, Seoul, Korea). The soil solution was prepared by adding 10 g of sieved soil to 100 mL of water. Next, 1 mL of the soil solution and 1 mL of the pure culture of strain AM5 were then added to 98 mL of food waste leachate diluted two-fold. Reactors with either soil solution or the bacterial culture were included as controls.
Three samples were set up for comparison: FWL with a soil solution only (control), FWL with strain AM5, and FWL inoculated with both the soil solution and enriched culture of strain AM5 (FWL + soil soln., FWL + AM5, and FWL + soil soln. + AM5). Each reactor was flushed with enough N2 to generate non-strict anaerobic conditions. All the bottles were incubated at 55 °C for 15 days, and the VFA concentrations were checked daily. There was no pretreatment in this study.
2.3. Analytical Methods
Samples were centrifuged for 20 min and then filtered through a 0.45-µm membrane filter. The total chemical oxygen demand (TCOD) and soluble chemical oxygen demand (SCOD) were determined using LCK 514 COD cuvette and Hach Lange DR 5000 spectrophotometer (HACH EUROPE, Hach Lange, Germany). Other samples were acidified to pH 2.5 for VFA analysis. VFAs were extracted with ether and determined using a gas chromatograph (GC-6890N, Agilent Inc., Wilmington, DE, USA) equipped with a flame ionization detector and a 30 m × 0.25 mm × 0.25 µm fused-silica capillary column (DB-FFAP, Agilent Inc., Wilmington, DE, USA). The temperatures of the injector and detector were 250 °C and 300 °C, respectively. The oven temperature was initially set at 70 °C for 3 min, followed by a ramp-up of 20 °C/min for 5.5 min, and maintained at a final temperature of 180 °C for 3 min. Nitrogen was used as the carrier gas with a flow rate of 2.6 mL/min. Hydrogen was the fuel gas and synthetic air was the oxidizing gas. Acetic, propionic, isobutyric, butyric, isovaleric, valeric, isocaproic, and caproic acids were detected using a gas chromatograph (GC-6890N, Agilent Inc., Wilmington, DE, USA).
The total solid content (TS) and volatile solid content (VS) of the food waste were measured according to the American Public Health Association (APHA) standard methods [49].
2.4. Phylogenetic Analysis
To determine partial 16S rRNA sequences, the genomic DNA from three VFA-producing strains were extracted according to a previously published method [48] (Pham and Kim, 2014). The 16S rRNA was then amplified through PCR using the universal bacterial primer set 27F and 1492R. A multiscreen filter plate (Millipore Corp, Bedford, MA, USA) was used to purify the PCR product, which was then sequenced using primers 518F (5′-CCA GCA GCC GCG GTA ATA CG-3′) and 800R (5′-TAC CAG GGT ATC TAA TCC-3′) with a PRISM BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA). This process was conducted at 95 °C for 5 min, and then the product was cooled on ice for 5 min and analyzed using an ABI Prism 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA). Finally, SeqMan software (DNASTAR Inc., Madison, WI, USA) was used to assemble the nearly full-length 16S rRNA sequence. This sequence was compared with that of other bacteria using the EZBioCloud server (http://ezbiocloud.net/, accessed on 13 May 2020) [50].
3. Results
3.1. Phylogenetic Analysis
Strain AM5 belongs to the genus Bacillus and its closest strain is Bacillus toyonensis BCT-7112T. This strain was isolated by a modified method used in a previous study conducted at 35 °C under aerobic conditions [48]. Strain AM5 was tested under different temperatures in an anaerobic jar; it was determined that it can grow adequately at 55 °C after a 2-day incubation.
3.2. Hydrolysis Performance
Four trials showed a decrease in TS and VS concentration after a 15-day fermentation period compared to the initial values (Figure 1). In the fermentation system with the contribution of the strain and soil microorganisms, TS and VS decreased to 19,512 mg/L and 12,558 mg/L, with 58.42% and 65.17% removal, respectively.
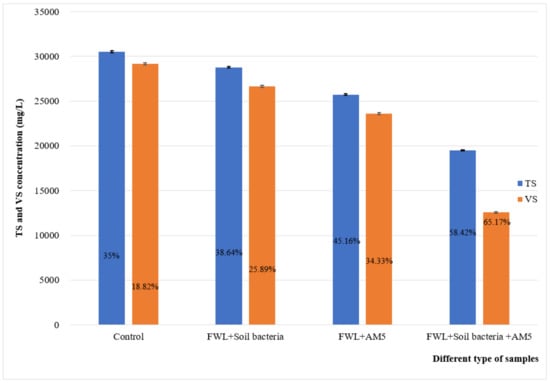
Figure 1.
Total solid content (TS) and volatile solid content (VS) concentrations of different waste samples at 15 days, pH 10.5, and 55 °C. The removal of TS and VS is illustrated as a percentage. FWL: food waste leachate.
The SCOD increased to 60,151 mg/L and 80,131 mg/L in the trial with inocula of the strain or the incorporation of soil bacteria to the strain, respectively. This represented an increase of 70.93% and 127.8%, respectively, compared to the SCOD concentration of the initial FWL, with a concentration of 35,175 mg/L (Table 1 and Figure 2). As a factor that has a significant effect on VFA production, the increase in solubilization allowed a higher VFA yield due to efficient hydrolytic activity in the batch bioreactors.

Table 1.
Characteristics of the original food waste leachate. TCOD: total chemical oxygen demand; SCOD: soluble chemical oxygen demand; VFAs: volatile fatty acids.
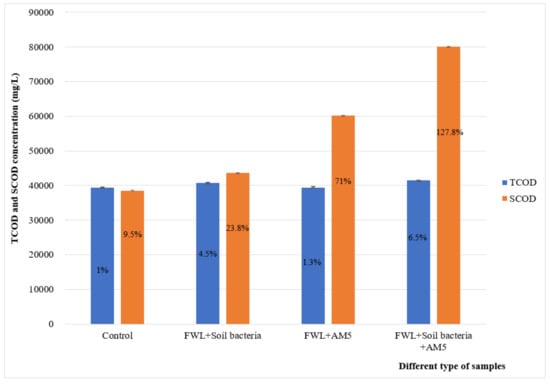
Figure 2.
TCOD and SCOD concentrations of different samples at 15 days, pH 10.5, and 55 °C. The change of TCOD and SCOD concentration in each trial sample was observed as a percentage.
3.3. Effect of the Substrate, the Co-Digester, and the Additives
The total VFA concentrations in three settings (FWL + soil soln., FWL + AM5, and FWL + soil soln. + AM5) were 3760 mg/L, 9485 mg/L, and 33,849 mg/L, respectively.
All of the components of the VFAs were produced in the trial in the presence of strain AM5 and soil bacteria; all compositions except heptanoic acid were observed in the sample inoculated with strain AM5 only, whereas only three components (acetic, propionic, and butyric acid) were observed in the control and samples with soil solution added at thermophilic temperature 55 °C and pH 10.5 (Figure 3).
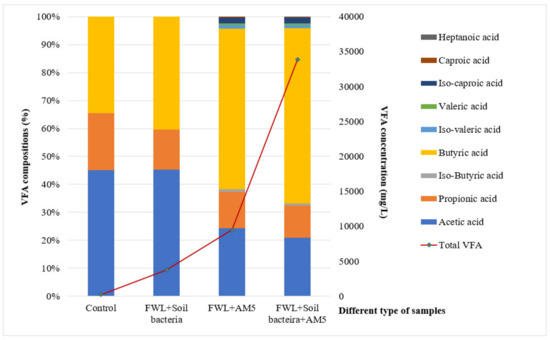
Figure 3.
The concentrations and proportion of each VFA component produced from different samples at 55 °C on the second day of fermentation.
3.4. Reaction Time on VFA Production
Figure 4 shows the profile of the total VFA and components by day of trial FWL + soil soln. + AM5 at 15 days of incubation. The highest VFA production yield peaked at 33,849 mg/L on day 2 and then declined gradually afterwards. Butyric acid was the dominant component of the total VFAs produced on day 2, while acetic acid was observed as the most abundant from day 4 to day 14 and propionic acid was detected with the highest concentration on day 15.
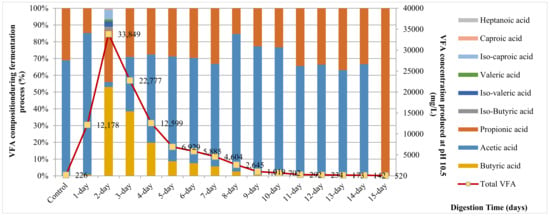
Figure 4.
Total VFA concentration and proportion of VFA components produced at various pH values at 55 °C. Measurements were recorded each day during 15 days of anaerobic fermentation (AF) process.
3.5. Effect of pH
A high VFA production was obtained in trials of enriched cultures of strain AM5 and the presence of the soil microbial community from where this strain was isolated. Notably, the VFA concentration in FWL + soil soln. + AM5 trial was 13,162 g/L at an unadjusted pH compared to the control trial at the initial concentration of 226 mg/L. The second highest value of VFA was observed at pH 10 (30,535 mg/L), with the VFA values decreasing at other pH values (Figure 5).
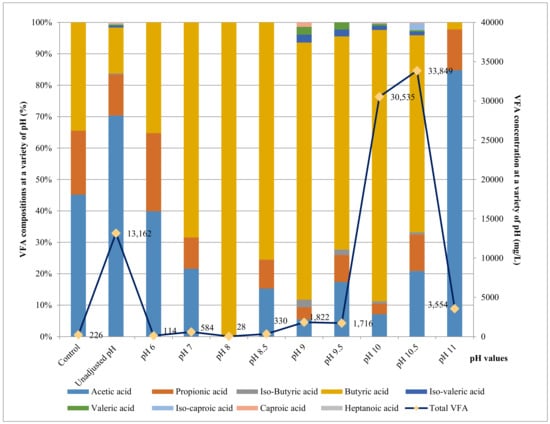
Figure 5.
The total VFA and the proportion of each VFA component produced at different pH values.
Figure 6 shows the VFA production profile at different pH values. There was no significance in the production of butyric acid at pH levels below 9.5 until it reached the highest concentration at pH 10 with 26,368 mg/L and then decreased sharply at alkaline conditions. The second most prominent component observed was acetic acid, which reached its highest production of 9206 mg/L at an unadjusted pH, followed by 7062 mg/L and 2164 mg/L at pH 10.5 and pH 10, respectively. Propionic acid was observed at all pH values. It had a peak of 3937 mg/L at pH 10.5 and decreased to 1717 mg/L and 1027 mg/L at an unadjusted pH and pH 10, respectively. Iso-butyric acid was detected with a low concentration at pH below 9.5 before it increased to 233 mg/L at pH 10 and reached its highest point of 242 mg/L at pH 10.5 before being undetectable at pH 11. Iso-caproic acid reached its highest value of 726 mg/L at pH 10.5, while iso-valeric acid was observed at an unadjusted pH and from pH 9 to 10.5. It peaked at pH 10, with 406 mg/L, and slightly decreased at higher pH levels before becoming zero at pH 11. Valeric acid and caproic reached peaks 192 mg/L and 75 mg/L, respectively, at pH 10 after appearing at unadjusted pH and gradually reduced to 154 mg/L and 60 mg/L, respectively, at pH 10.5. Heptanoic acid was only detected with 30 mg/L at pH no-adjustment and 52 mg/L at pH 10.5. Overall, all VFA components in this study were detected at the unadjusted pH and pH 10.5, and heptanoic acid was the only component that was not detected at pH 10.
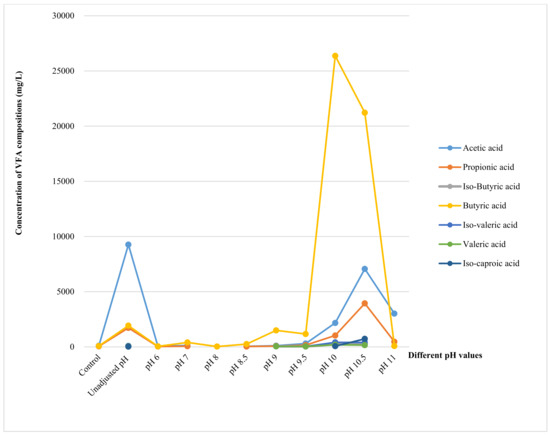
Figure 6.
The concentration of VFA components produced at various pH values.
4. Discussion
Water content is well known as one of the most important parameters that significantly affect the whole AD process. As the initial values showed in Table 1, with TS 46,926 mg/L and VS 35,951 mg/L, the non-strict anaerobic digestion in this study was considered a wet digestion, following the definition established in previous studies, with TS < 15% [51,52]. In another study, regarding VFA production, TS of 20.53 ± 2.04 (%) and VS of 19.95 ± 2.21 (%) was defined as the dry digestion of a food waste disposer containing a mixture of the four following components: 35% rice, 45% cabbage, 16% pork, and 4% tofu [53].
Moreover, previous studies have demonstrated that differences in the characteristics of the original organic waste and various operating conditions lead to a different VFA yields and compositions (Table 2).

Table 2.
VFA production in the current study compared to previous studies. HRT: hydraulic retention time.
As a result, acetate was always dominant in anaerobic fermentation regardless of the substrate type, whereas butyrate and ethanol were increased by adding the carbon-rich substrates. Protein-rich substrates significantly enhanced propionate and valerate production [57]. However, the result of this study showed that the presence of all of the components of VFA, the incorporation of the soil microbial community, and the enriched culture of the AM5 strain influenced changes in the original bacterial community from food waste leachate even though the protein content in the food waste leachate was quite low at 4.3%. The highest VFA yield was observed in the trial of food waste leachate in the presence of an enriched culture of the AM5 strain and soil bacteria as an additive, which reached 33,849 mg/L compared to 9485 mg/L, 3760 mg/L, and 226 mg/L for the FWL + AM5, FWL + soil soln. and FWL only trials, respectively. Therefore, the complicated community introduced in this study may induce the different VFA synthesis pathways.
The difference in the VFA composition suggests that there might be different metabolic pathways taking place. According to another study, glucose, as the main component of FWL, is degraded and then produces acetate and butyrate [57]. However, in the reactor of this study using FWL containing 9.5% carbohydrate in which the soil solution was inoculated, the amount of acetic acid was 45%, while butyric acid became predominant in the presence of strain AM5 in the reactors and accounted for 62.68% of the total volatile fatty acids (TVFAs). Acetic acid was predominant each day of the 15-day period with the exception of day 2 and 3 when butyric acid was present at higher concentrations. Propionate may be produced from the fermentation of amino acids or H2-consuming propionate-producing bacteria [58,59].
Previous investigations have reported that pH is the most critical parameter that influences the growth rate of microorganisms involved in both acidogenic and hydrolysis processes [60,61]. In another study, the AD process was conducted at the same mesophilic condition, pH 6, HRT (hydraulic retention time) of 5 days, and using a combination with mature compost; the VFA yield from food waste obtained was lower than 20 g VFA-COD/L [18]. In contrast, alkaline conditions were found to be optimal for more complex organic materials [62,63]. Additionally, recent studies have speculated that pH affects the metabolic pathways and influences the VFA compositions [64,65]. Jiang et al. indicated that acidic conditions are more favorable for VFA production from more easily degradable materials or sorted food waste. It was found that at an initial pH 6 with no control during the fermentation process conducted at 45 °C and HRT of 5 days, the VFA concentration reached 47.2 g/L due to the high protein content of the organic matter used, whereas valerate component was not produced at an unadjusted pH and accounted for 0.15%, 9.52%, and 3.59% of the VFA content at pH 5, 6, and 7, respectively [53]. In another study, pH 10 seemed to favor acetic acid production, while butyric acid was the main product below pH 5 and valeric acid was detected at the highest concentration of 40 mg/L at pH 6. Propionic acid production was observed to be stable compared to other VFA components when subjected to a change in pH [66]. Herein, the butyric acid in this study reached the highest concentration at pH 10, with 26,368 mg/L, while it was 21,218 mg/L at pH 10.5 and then decreased to 1921 mg/L, 1490 mg/L, and 1164 mg/L at an unadjusted pH, pH 9, and pH 9.5, respectively. This value decreased sharply at acidic and alkaline conditions.
The total VFA obtained the highest concentration on day 2, illustrating that a wet fermentation might contribute significantly as a form of fast reaction for enhancing VFA production. The various acquired metabolites among set-ups resulting from different pH and inocula contribute to the diversity of the VFA compositions in this study.
Motte et al. noted that a butyric acid/acetic acid ratio of 0.4, 0.8, and 2.5 was observed at 10%, 14%, and 33% TS, respectively [67]. However, in the present study, the ratio was 3.0 with 7.1% TS, and the butyric acid metabolism predominantly reached 21,218 mg/L of butyric acid compared to the butyric concentration obtained under highly dry fermentation in the study of Motte and co-workers [67] (Figure 3). Generally, butyric acid can be produced by butyrate oxidation from pyruvate complexes. Given that it excludes major NADH-dependent oxidation/reduction processes, it’s hypothesized to be the metabolic pathway to transform butyric acid to butyryl-CoA and to result in the downregulation of acetate-synthesis pathways.
To date, limited studies have been conducted investigating the effect of pure and mixed bacterial cultures on VFA production and VFA composition [68]. Wang et al. used a mixed bacterial culture from aerobic- and anaerobic activated sludge to evaluate VFA production under acidic conditions [69]. Their results showed the same trend as ours, with the VFA compositions in the order of butyric, acetic, and propionic acids from the highest to the lowest concentration. Co-digestion of the enriched culture of strain AM5 and the microbial community in the soil played a crucial role in VFA production, with all components produced on day 2 and decreasing thereafter (Figure 4). This may have been caused by the competition for carbon and nitrogen sources of microbial community leading to a decreasing abundance of AM5 and soil microbes due to a lack of feeding. Additionally, pH decreased from alkaline to neutral levels during VFA production, which is an optimal condition for the growth of methanogens and may be an important reason for VFA reduction in this stage.
Further studies should analyze the effect of these factors on the overall process. Additionally, the differences between microbial communities in all reactors should be determined. The structure of the microbial community may provide new information regarding the functional microbial groups involved in the fermentation process.
5. Conclusions
Microbes in soil represent an attractive subject for research due to their potential application in many different areas. The contribution of an enriched bacterial culture of strain AM5 in the microbial community of the soil used in this study has opened a new avenue in the current waste treatment methodologies due to the complex metabolic pathways for enhancing VFA production. However, it is well known that the competition between acetogens and methanogens is influenced by numerous factors involved in complex interactions in the microbial community. Thus, in order to further understand the activities of all of the microbes involved, high-throughput sequencing and identification of potentially acid-producing bacteria should be performed; doing so will help in understanding the relationships among microorganisms in this food waste leachate fermentation process.
Author Contributions
Conceptualization: V.H.T.P. and W.C.; methodology: V.H.T.P.; validation: V.H.T.P.; formal analysis and investigation: V.H.T.P., J.A., S.L., I.L. and S.K.; resources: J.A.; data curation: V.H.T.P.; writing—original draft preparation: V.H.T.P.; writing—review and editing: V.H.T.P., J.K. and W.C.; supervision: W.C. and S.C.; funding acquisition: W.C. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Environment Industry & Technology Institute (KEITI) through Project for developing innovative drinking water and wastewater technologies, funded by the Korea Ministry of Environment (MOE) (2021002690003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morales-Polo, C.; Cledera-Castro, M.D.M.; Moratilla Soria, B.Y. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, J.; Xu, Q.; Li, X.; Wang, D.; Yang, Q.; Liu, Y.; Tao, Z. Enhanced volatile fatty acids production from waste activated sludge anaerobic fermentation by adding tofu residue. Bioresour. Technol. 2019, 274, 430–438. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef] [PubMed]
- Issac, O.A.; Elzbieta, P.; Zeynep, C. Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: Effect of substrate proportions and long-term operation. J. Waste Manag. 2020, 112, 30–39. [Google Scholar]
- He, M.; Sun, Y.; Zou, D.; Yuan, H.; Zhu, B.; Li, X.; Pang, Y. Influence of temperature on hydrolysis acidification of food waste. Procedia Environ. Sci. 2012, 16, 85–94. [Google Scholar] [CrossRef]
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile fatty acid production from mesophilic acidogenic fermentation of organic fraction of municipal solid waste and food waste under acidic and alkaline pH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.D.; Brejea, R.; Domuta, C.; Bungau, S.; Cenusa, N.; Tit, D.M. Enzymatic indicators of soil quality. J. Environ. Prot. Ecol. 2017, 18, 871–878. [Google Scholar]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kaminski, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Ji, S.; Huang, D.; Huang, Z.; Huang, Z.; Zeng, Y.; Liu, Y. Impact of alkaline pretreatment to enhance volatile fatty acids (VFAs) production from rice husk. Biochem. Res. Int. 2019, 2019, 8489747. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, H.; Zheng, C. Shifting product spectrum by pH adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef]
- Samuel, A.D.; Bungau, S.; Tit, D.M.; Melinte, C.E.; Purza, L.; Badea, G.E. Effects of long term application of organic and mineral fertilizers on soil enzymes. Rev. Chim. 2018, 69, 2608–2612. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Mata-Alvarez, J.; Dosta, J.; Romero-Guiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Cheah, Y.K.; Dosta, J.; Mata-Álvarez, J. Enhancement of volatile fatty acids production from food waste by mature compost addition. Molecules 2019, 24, 2986. [Google Scholar] [CrossRef]
- Cai, M.; Wilkins, D.; Chen, J.; Ng, S.K.; Lu, H.; Jia, Y.; Lee, P.K. Metagenomic reconstruction of key anaerobic digestion pathways in municipal sludge and industrial wastewater biogas-producing systems. Front. Microbiol. 2016, 7, 778. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, X.; Zhao, J.; Wang, D.; Wang, Q.; Li, X.; Yang, Q.; Zeng, G. Feasibility of enhancing short-chain fatty acids production from sludge anaerobic fermentation at free nitrous acid pretreatment: Role and significance of tea saponin. Bioresour. Technol. 2018, 254, 194–202. [Google Scholar] [CrossRef]
- Nayak, J.; Pal, P. Transforming waste cheese-whey into acetic though a continuous membrane-integrated hybrid process. Ind. Eng. Chem. Res. 2013, 52, 2977–2984. [Google Scholar] [CrossRef]
- Ehsanipour, M.; Suko, A.V.; Bura, R. Fermentation of lignocellulosic sugars to acetic acid by Moorellathermoacetica. J. Ind. Microbiol. Biotechnol. 2016, 43, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, H.; Yin, B.; Zu, Y.; Liu, H.; Fu, B.; Ma, H. Enhanced volatile fatty acid production by a modified biological pretreatment in anaerobic fermentation of waste activated sludge. Chem. Eng. J. 2016, 284, 194–201. [Google Scholar] [CrossRef]
- Baroi, G.N.; Baumann, I.; Westermann, P.; Gavala, H.N. Butyric acid fermentation from pretreated and hydrolysed wheat straw by an adapted Clostridium tyrobutyricum strain. Microb. Biotechnol. 2015, 8, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Liang, S.; Cai, J.; Xu, Z.; Cen, P.; Yang, S.; Li, S. Enhanced butyric acid tolerance and bioproduction by Clostridium tyrobutyricum immobilized in a fibrous bed bioreactor. Biotechnol. Bioeng. 2011, 108, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Woodruff, A.P.; Xiong, M.; Zhou, J.; Dhande, Y.K. A synthetic metabolic pathway for production of the platform chemical isobutyric acid. ChemSusChem 2011, 4, 1068–1070. [Google Scholar] [CrossRef]
- Liang, Z.X.; Li, L.; Li, S.; Cai, Y.H.; Yang, S.T.; Wang, J.F. Enhanced propionic acid production from Jerusalem artichoke hydrolysate by immobilized Propionibacterium acidipropionici in a fibrous-bed bioreactor. Bioprocess Biosyst. Eng. 2012, 35, 915–921. [Google Scholar] [CrossRef]
- Lang, K.; Zierow, J.; Buehler, K.; Schmid, A. Metabolic engineering of Pseudomonas sp. strain VLB120 as platform biocatalyst for the production ofisobutyric acid and other secondary metabolites. Microb. Cell Fact. 2014, 13, 2. [Google Scholar] [CrossRef]
- Thierry, A.; Richoux, R.; Kerjean, J.R. Isovaleric acid is mainly produced by Propionibacterium freudenreichii in Swiss cheese. Int. Dairy J. 2004, 14, 801–807. [Google Scholar] [CrossRef]
- Hou, J.; Li, M.; Mao, X.; Hao, Y.; Ding, J.; Liu, D.; Xi, B.; Liu, H. Response of microbial community of organic-matter-impoverished arable soil to long-term application of soil conditioner derived from dynamic rapid fermentation of food waste. PLoS ONE 2017, 12, e0175715. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J. Cultivation of unculturable soil bacteria. Trends. Biotechnol. 2012, 30, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Sawatdeenarunat, C.; Sung, S.; Khanal, S.K. Enhanced volatile fatty acids production during anaerobic digestion of lignocellulosic biomass via micro-oxygenation. Bioresour. Technol. 2017, 237, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, U.; Deka, D.; Das, G. Enhancing the volatile fatty acid production from agro-industrial waste streams through sludge pretreatment. Environ. Sci. Water Res. Technol. 2019, 5, 334–345. [Google Scholar] [CrossRef]
- Magdalena, J.A.; González-Fernández, C. Archaea inhibition: Strategies for the enhancement of volatile fatty acids production from microalgae. Waste Manag. 2020, 102, 222–230. [Google Scholar] [CrossRef]
- Hassan, W.; Hussain, M.; Bashir, S.; Shah, A.N.; Bano, R.; David, J. ACC-deaminase and/or nitrogen fixing rhizobacteria and growth of wheat (Triticum Aestivum L.). J. Soil Sci. Plant Nutr. 2015, 15, 232–248. [Google Scholar] [CrossRef][Green Version]
- Amoo, A.E.; Babalola, O.O. Ammonia-oxidizing microorganisms: Key players in the promotion of plant growth. J. Soil Sci. Plant Nutr. 2017, 17, 935–947. [Google Scholar] [CrossRef]
- Ingham, E.R. Soil Fungus. In Soil Biology Primer; Soil & Water Conservation Society: Ankeny, IA, USA, 2009; pp. 22–23. [Google Scholar]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.R. Lecture on Soil Physics, Personal Collection of K. Islam; The Ohio State University School of Environment and Natural Resources: Columbus, OH, USA, 2008. [Google Scholar]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Muyzer, G.; Smalla, K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek 1998, 73, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.M.; Triplet, E. Automated approach for ribosomal intergenic spacer analysis of microbial diversity and its application to freshwater bacterial communities. Appl. Environ. Microbiol. 1999, 65, 4630–4636. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef]
- Thies, J.E. Soil microbial community analysis using terminal restriction fragment length polymorphisms. Soil Sci. Soc. Am. J. 2007, 71, 579–591. [Google Scholar] [CrossRef]
- Lef, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmocker, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef]
- Prosser, J.I. Dispersing misconceptions and identifying opportunities for the use of ‘omics’ in soil microbial ecology. Nat. Rev. Microbiol. 2015, 13, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Jansson, J.; Field, D.; Fierer, N.; Desai, N.; Fuhrman, J.A.; Hugenholtz, P.; van der Lelie, D.; Meyer, F.; Stevens, R.; et al. Unlocking the potential of metagenomics through replicated experimental design. Nat. Biotechnol. 2012, 30, 513–520. [Google Scholar] [CrossRef]
- Van Pham, H.T.; Kim, J. Bacillus thaonhiensis sp. nov., a new species, was isolated from the forest soil of Kyonggi University by using a modified culture method. Curr. Microbiol. 2014, 68, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for The Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.A.; Popescu, F. Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Biotechnol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Garcia, N.H.; Strazzera, G.; Frison, N.; Bolzonella, D. Volatile fatty acids production from household food waste. Chem. Eng. Trans. 2018, 64, 103–108. [Google Scholar]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Qiao, D.; Yue, L.; Li, Y.Y.; Zhou, J.; Cen, K. Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresour. Technol. 2017, 241, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, H.; Zhang, L.; Yang, M.; Fu, B.; Liu, H. Novel insight into the relationship between organic substrate composition and volatile fatty acids distribution in acidogenic co-fermentation. Biotechnol. Biofuels 2017, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ding, L.; Xia, A.; Lin, R.; Li, Y.; Zhou, J.; Cen, K. Hydrogen production using amino acids obtained by protein degradation in waste biomass by combined dark- and photo-fermentation. Bioresour. Technol. 2015, 179, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-garcia, R.A.; Mccubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial Propionic Acid Production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Begum, S.; Rao, G.; Sridhar, S.; Bhargava, S.K.; Jegatheesan, V.; Eshtiaghi, N. Evaluation of single and two stage anaerobic digestion of landfill leachate: Effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour. Technol. 2018, 251, 364–373. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.; Liu, Y.; Ngo, H.H.; Guo, W.; Yang, Q.; Li, X. Novel stepwise pH control strategy to improve short chain fatty acid production from sludge anaerobic fermentation. Bioresour. Technol. 2018, 249, 431–438. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Zhou, Q.; Song, Z. The effect of pH on anaerobic fermentation of primary sludge at room temperature. J. Hazard. Mater. 2009, 172, 196–201. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa, L.; Awasthi, M.K.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Mohd-Zaki, Z.; Bastidas-Oyanedel, J.; Lu, Y.; Hoelzle, R.; Pratt, S.; Slater, F.; Batstone, D. Influence of pH regulation mode in glucose fermentation on product selection and process stability. Microorganisms 2016, 4, 2. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, J.; Tan, M.; Du, J.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced carboxylic acids production by decreasing hydrogen partial pressure during acidogenic fermentation of glucose. Bioresour. Technol. 2017, 245, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Motte, J.C.; Trably, E.; Escudié, R.; Hamelin, J.; Steyer, J.P.; Bernet, N.; Delgenes, J.P.; Dumas, C. Total solids content: A key parameter of metabolic pathways in dry anaerobic digestion. Biotechnol. Biofuels 2013, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).