Remote Detection of Cyanobacterial Blooms and Chlorophyll-a Analysis in a Eutrophic Reservoir Using Sentinel-2
Abstract
:1. Introduction
2. Materials and Methods
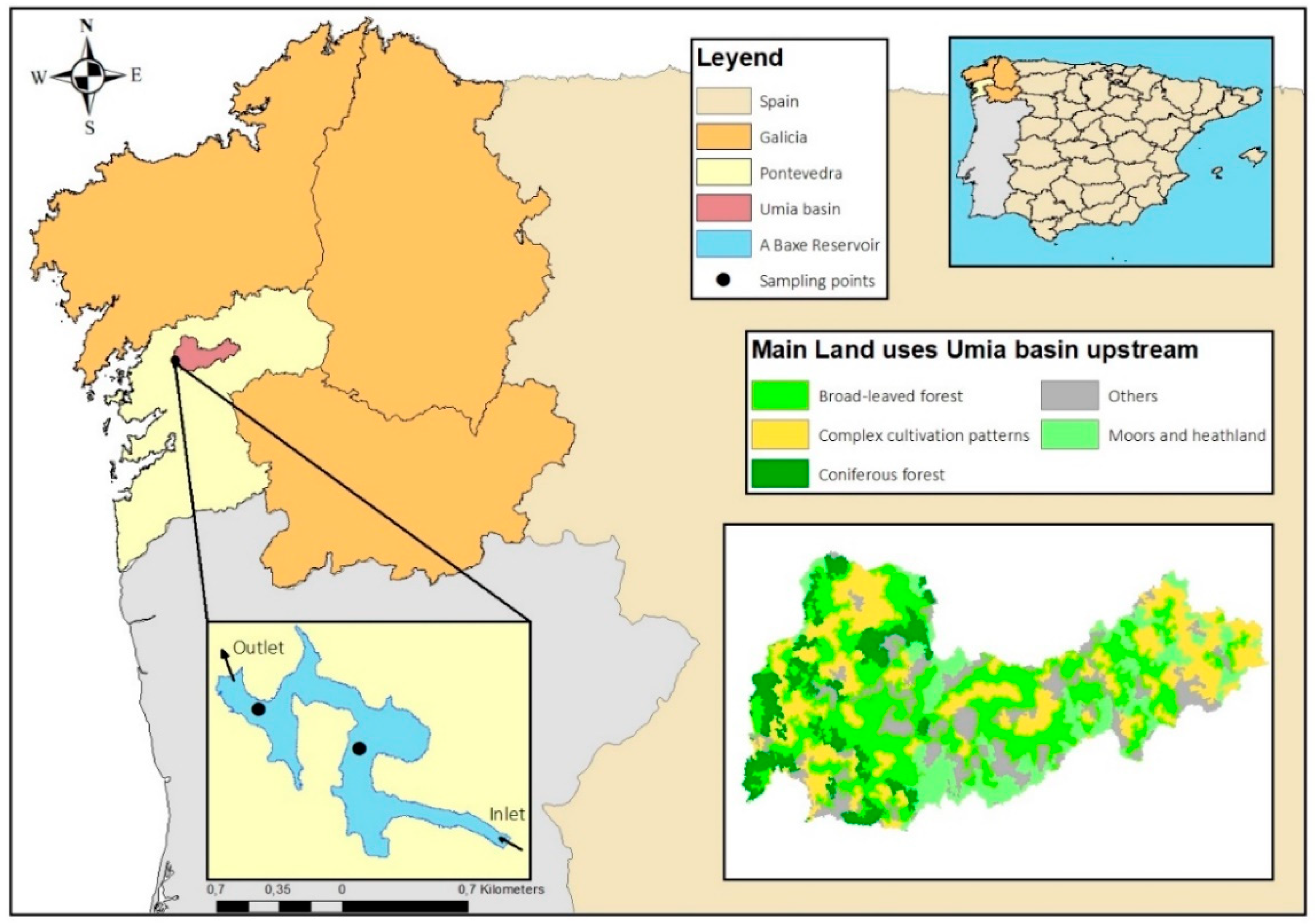
2.1. Study Area and Dataset
2.2. Remote-Sensing Datasets
2.3. Superspectral Satellite Data Pre-Processing and Retrieval of Indices
2.4. Statistical Analysis
3. Results and Discussion
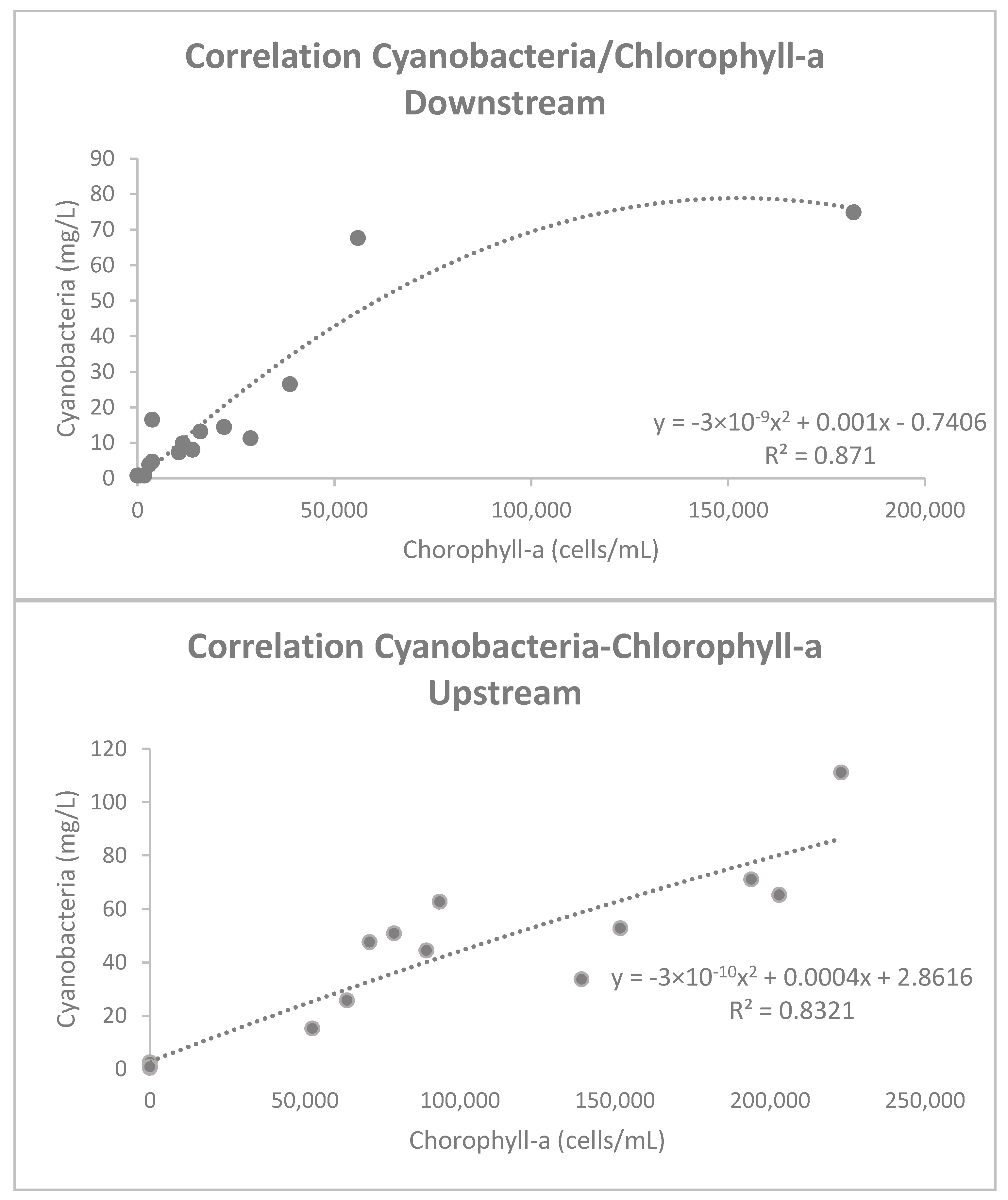
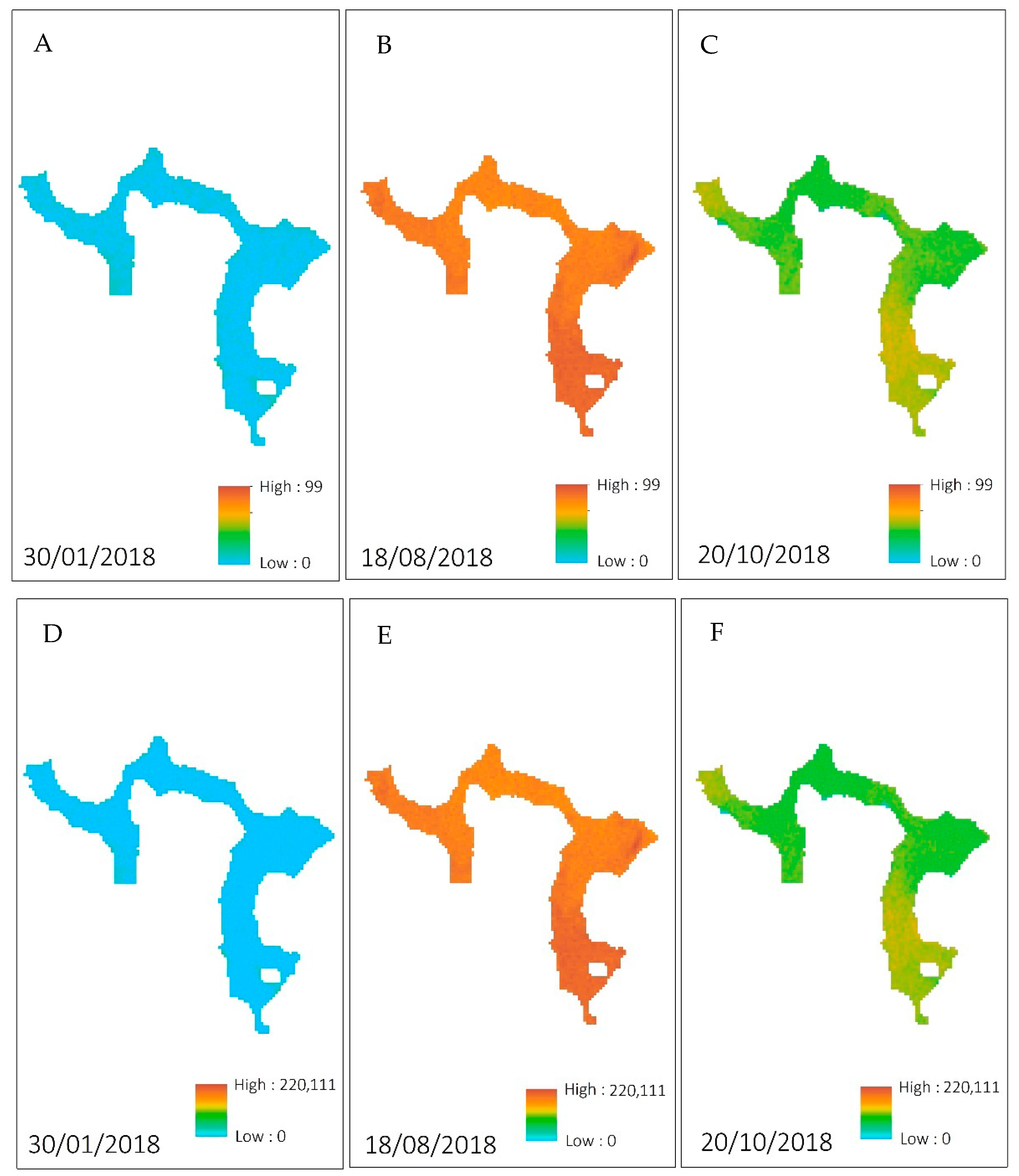
3.1. In Situ Data
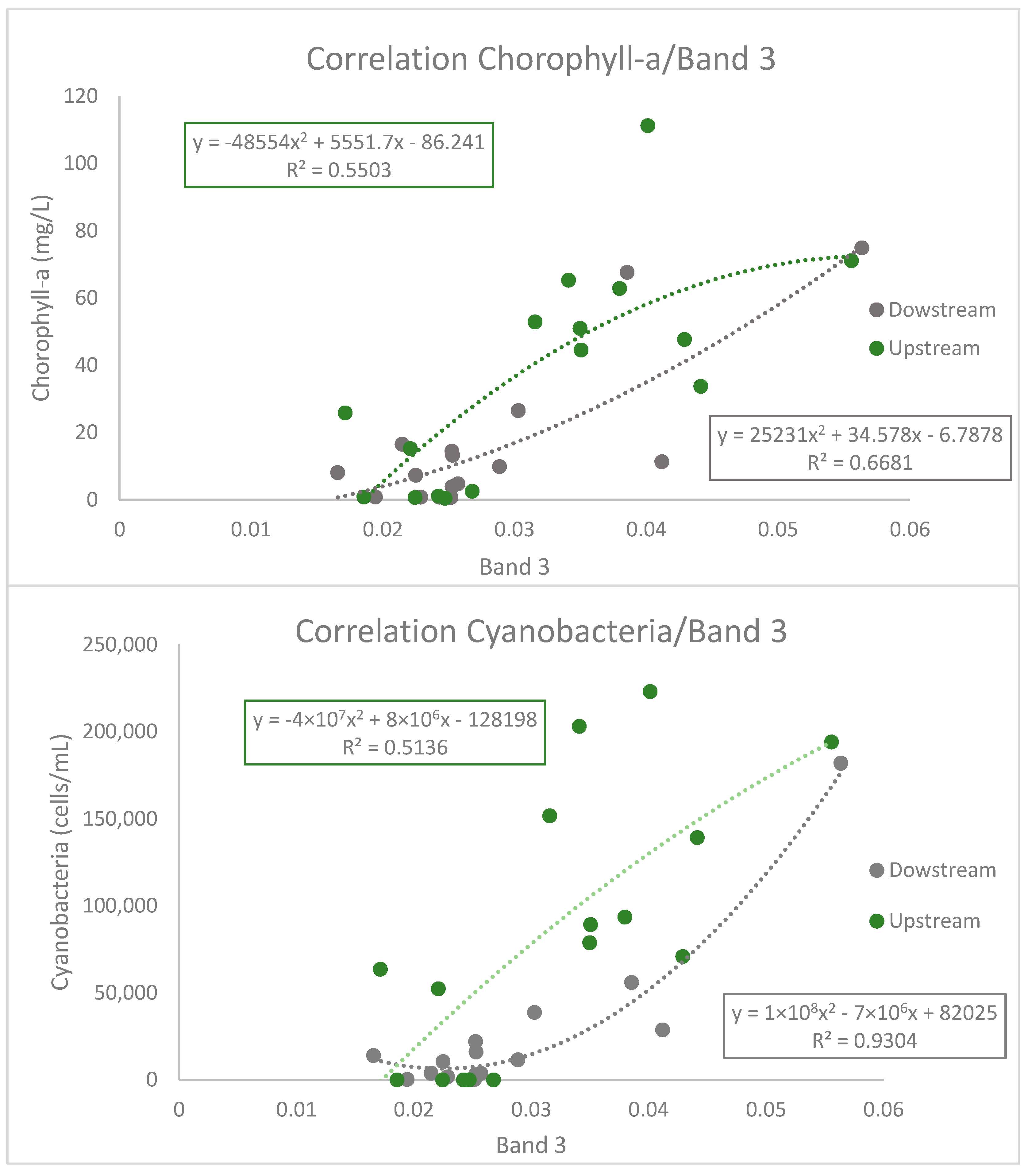
3.2. Sentinel-2 Spectral Band Performance
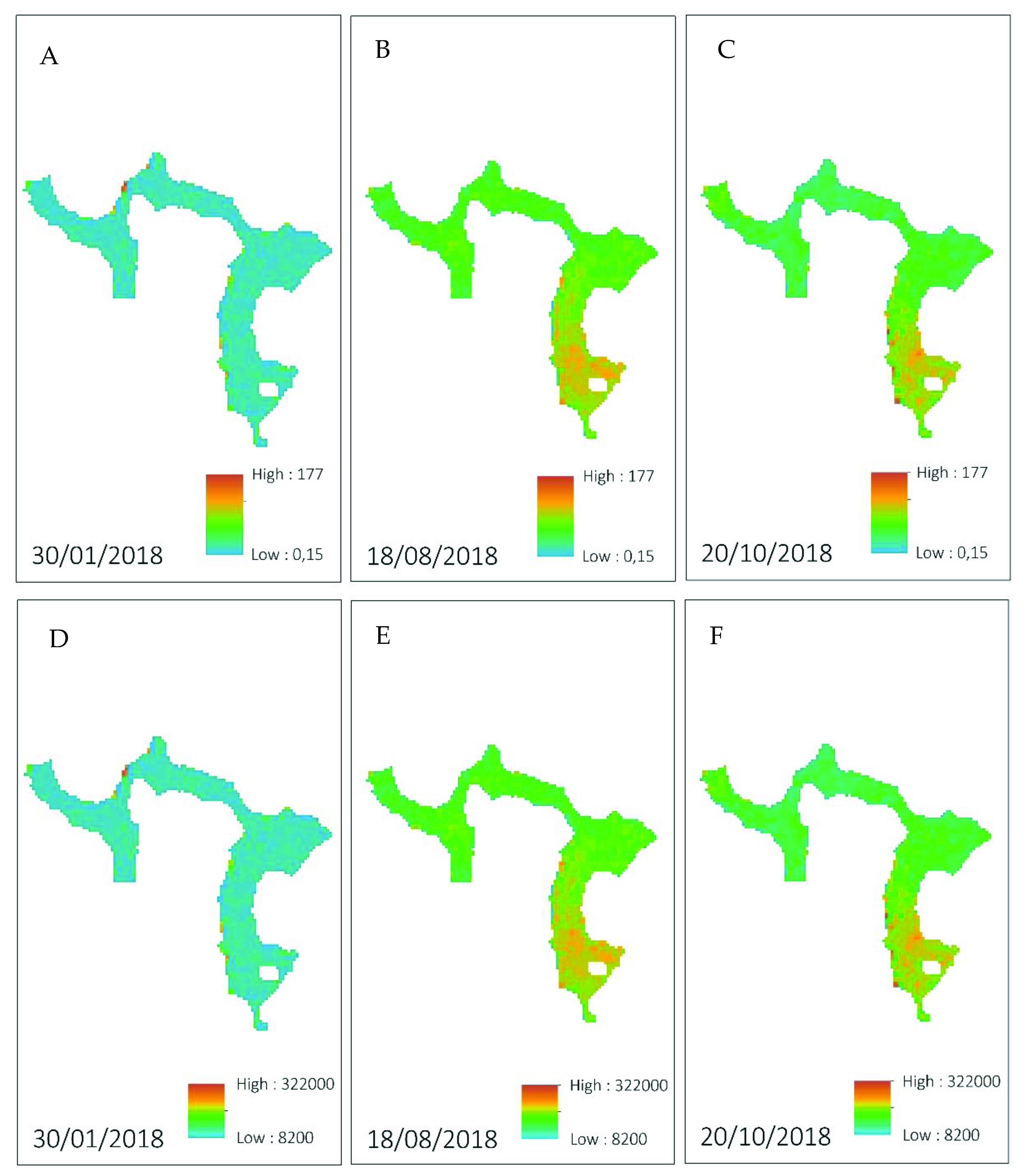
3.3. Water Indices via Sentinel-2
3.4. General Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Date | Chlorophyll-a Dowstream Point (mg/L) | Chlorophyll-a Upstream Point (mg/L) | Cyanobacteria Concentration Downstream Point (cells/mL) | Microcystis sp. Dowstream | Cyanobacteria Concentration Upstream Point | Microcystis sp. Upstream |
|---|---|---|---|---|---|---|
| (cells/mL) | (cells/mL) | (cells/mL) | ||||
| 29 January2018 | 0.82 | 0.81 | 250 | 250 | 0 | 0 |
| 18 June 2018 | 8.05 | 25.79 | 14,000 | 14,000 | 63,600 | 63,600 |
| 25 June 2018 | 16.5 | 15.21 | 3750 | 3750 | 52,400 | 52,400 |
| 09 July 2018 | 11.29 | 33.69 | 28,750 | 28,750 | 139,250 | 139,250 |
| 13 August 2018 | 74.9 | 71.05 | 181,900 | 150,000 | 194,000 | 160,000 |
| 20 August 2018 | 14.42 | 50.93 | 22,000 | 22,000 | 78,800 | 65,000 |
| 27 August 2018 | 4.72 | 47.64 | 3750 | 3750 | 70,900 | 51,250 |
| 10 September2018 | 7.3 | 52.85 | 10,500 | 10,500 | 151,750 | 151,750 |
| 17 September 2018 | 13.21 | 65.26 | 16,000 | 16,000 | 203,000 | 203,000 |
| 24 September 2018 | 9.86 | 62.79 | 11,500 | 11,500 | 93,500 | 93,500 |
| 08 October 2018 | 26.49 | 44.51 | 38,750 | 38,750 | 89,250 | 89,250 |
| 15 October 2018 | 67.6 | 111.17 | 56,000 | 56,000 | 223,000 | 223,000 |
| 19 November 2018 | 3.87 | 0.43 | 3000 | 3000 | 0 | 0 |
| 03 December 2018 | 0.78 | 2.51 | 1750 | 1750 | 0 | 0 |
| 10 December 2018 | 0.74 | 1.08 | 250 | 250 | 0 | 0 |
| 26 December 2018 | 0.79 | 0.69 | 0 | 0 | 0 | 0 |
References
- Bhagowati, B.; Talukdar, B.; Ahamad, K.U. Lake Eutrophication: Causes, Concerns and Remedial Measures. In Emerging Issues in the Water Environment during Anthropocene: A South East Asian Perspective; Kumar, M., Snow, D.D., Honda, R., Eds.; Springer: Singapore, 2020; pp. 211–222. ISBN 978-981-32-9771-5. [Google Scholar]
- Zhao, C.S.; Shao, N.F.; Yang, S.T.; Ren, H.; Ge, Y.R.; Feng, P.; Dong, B.E.; Zhao, Y. Predicting cyanobacteria bloom occurrence in lakes and reservoirs before blooms occur. Sci. Total Environ. 2019, 670, 837–848. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J. Cyanobacteria dynamics in a small tropical reservoir: Understanding spatio-temporal variability and influence of environmental variables. Sci. Total Environ. 2018, 643, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A. Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecol. Eng. 2000, 16, 51–60. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Welkie, D.G.; Rubin, B.E.; Diamond, S.; Hood, R.D.; Savage, D.F.; Golden, S.S. A Hard Day’s Night: Cyanobacteria in Diel Cycles. Trends Microbiol. 2019, 27, 231–242. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Toporowska, M.; Mazur-Marzec, H. Effects of secondary metabolites produced by different cyanobacterial populations on the freshwater zooplankters Brachionus calyciflorus and Daphnia pulex. Environ. Sci. Pollut. Res. 2019, 26, 11793–11804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Dittrich, M. Dynamic polyphosphate metabolism in cyanobacteria responding to phosphorus availability. Environ. Microbiol. 2019, 21, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, X.; Valero, E.; Cancela, Á.; Sánchez, Á. Freshwater algae competition and correlation between their growth and microcystin production. Environ. Sci. Pollut. Res. 2016, 23, 21577–21583. [Google Scholar] [CrossRef]
- Richardson, J.; Feuchtmayr, H.; Miller, C.; Hunter, P.D.; Maberly, S.C.; Carvalho, L. Response of cyanobacteria and phytoplankton abundance to warming, extreme rainfall events and nutrient enrichment. Glob. Chang. Biol. 2019, 25, 3365–3380. [Google Scholar] [CrossRef] [Green Version]
- Sani, F.S.; Azmi, A.S.; Ali, F.; Mel, M. Interactive Effect of Temperature, pH and Light Intensity on Biodesalination of Seawater by Synechococcus sp. PCC 7002 and on the Cyanobacteria Growth. J. Adv. Res. Fluid Mech. Therm. Sci. 2018, 52, 85–93. [Google Scholar]
- Acuña-Alonso, C.; Lorenzo, O.; Álvarez, X.; Cancela, Á.; Valero, E.; Sánchez, Á. Influence of Microcystis sp. and freshwater algae on pH: Changes in their growth associated with sediment. Environ. Pollut. 2020, 263. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, X.; Valero, E.; Picos, J. Evolution of Eutrophication depending on Environmental Conditions: A Case Study in a Reservoir. Eur. J. Sustain. Dev. 2014, 3, 83. [Google Scholar] [CrossRef]
- McFarlane, C.R.; Shah, N.R.; Kabasakal, B.V.; Echeverria, B.; Cotton, C.A.R.; Bubeck, D.; Murray, J.W. Structural basis of light-induced redox regulation in the Calvin–Benson cycle in cyanobacteria. Proc. Natl. Acad. Sci. USA 2019, 116, 20984–20990. [Google Scholar] [CrossRef] [Green Version]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef]
- Schmale, D.G.; Ault, A.P.; Saad, W.; Scott, D.T.; Westrick, J.A. Perspectives on Harmful Algal Blooms (HABs) and the Cyberbiosecurity of Freshwater Systems. Front. Bioeng. Biotechnol. 2019, 7, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sòria-Perpinyà, X.; Vicente, E.; Urrego, P.; Pereira-Sandoval, M.; Ruíz-Verdú, A.; Delegido, J.; Soria, J.M.; Moreno, J. Remote sensing of cyanobacterial blooms in a hypertrophic lagoon (Albufera of València, Eastern Iberian Peninsula) using multitemporal Sentinel-2 images. Sci. Total Environ. 2020, 698, 134305. [Google Scholar] [CrossRef]
- Doerffer, R.; Schiller, H. The MERIS Case 2 water algorithm. Int. J. Remote Sens. 2007, 28, 517–535. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Clayton, T.D.; Swarzenski, P.; Brock, J.C.; Muller–Karger, F.E. Assessment of estuarine water-quality indicators using MODIS medium-resolution bands: Initial results from Tampa Bay, FL. Remote Sens. Environ. 2004, 93, 423–441. [Google Scholar] [CrossRef]
- Carpenter, D.J.; Carpenter, S.M. Modeling inland water quality using Landsat data. Remote Sens. Environ. 1983, 13, 345–352. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, Q.; Wang, Q.; Yang, F.; Wang, S.; Li, J.; Zhang, F.; Yao, Y. Recognition of Water Colour Anomaly by Using Hue Angle and Sentinel 2 Image. Remote Sens. 2020, 12, 716. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Zhang, Y.; Ling, F.; Wang, Q.; Li, W.; Li, X. Water Bodies’ Mapping from Sentinel-2 Imagery with Modified Normalized Difference Water Index at 10-m Spatial Resolution Produced by Sharpening the SWIR Band. Remote Sens. 2016, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Suhet, H.B. Sentinel-2 User Handbook; ESA Standard Document; European Space Agency: Paris, France, 2015. [Google Scholar]
- Kutser, T.; Paavel, B.; Verpoorter, C.; Ligi, M.; Soomets, T.; Toming, K.; Casal, G. Remote Sensing of Black Lakes and Using 810 nm Reflectance Peak for Retrieving Water Quality Parameters of Optically Complex Waters. Remote Sens. 2016, 8, 497. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Alonso, L.; Moreno, J. Evaluation of Sentinel-2 Red-Edge Bands for Empirical Estimation of Green LAI and Chlorophyll Content. Sensors 2011, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Maltese, A.; Capodici, F.; Ciraolo, G.; Corbari, C.; Granata, A.; La Loggia, G. Planktothrix Rubescens in Freshwater Reservoirs: The Sentinel-2 Potentiality for Mapping Phycocyanin Concentration. In Proceedings of the First Sentinel-2 Preparatory Symposium, Frascati, Italy, 23–27 April 2012; Volume 707, p. 37. [Google Scholar]
- Hilborn, E.D.; Beasley, V.R. One Health and Cyanobacteria in Freshwater Systems: Animal Illnesses and Deaths Are Sentinel Events for Human Health Risks. Toxins 2015, 7, 1374. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Benito, C.V.; Navarro, G.; Caballero, I. Using Copernicus Sentinel-2 and Sentinel-3 data to monitor harmful algal blooms in Southern Chile during the COVID-19 lockdown. Mar. Pollut. Bull. 2020, 161, 111722. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Wei, X. Monitoring cyanobacterial harmful algal blooms at high spatiotemporal resolution by fusing Landsat and MODIS imagery. Environ. Adv. 2020, 2, 100008. [Google Scholar] [CrossRef]
- Hagolle, O.; Colin, J. Comment on “Comparison of Cloud Cover Detection Algorithms on Sentinel-2 Images of the Amazon Tropical Forest. Remote Sens. 2021, 13, 1023. [Google Scholar] [CrossRef]
- Pompêo, M.; Moschini-Carlos, V.; Bitencourt, M.D.; Sòria-Perpinyà, X.; Vicente, E.; Delegido, J. Water quality assessment using Sentinel-2 imagery with estimates of chlorophyll a, Secchi disk depth, and Cyanobacteria cell number: The Cantareira System reservoirs (São Paulo, Brazil). Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Ho, M.-Y.; Niedzwiedzki, D.M.; MacGregor-Chatwin, C.; Gerstenecker, G.; Hunter, C.N.; Blankenship, R.E.; Bryant, D.A. Extensive remodeling of the photosynthetic apparatus alters energy transfer among photosynthetic complexes when cyanobacteria acclimate to far-red light. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148064. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Potes, M.; Costa, M.J.; Novais, M.H.; Penha, A.M.; Salgado, R.; Morais, M.M. Temporal and Spatial Variations of Secchi Depth and Diffuse Attenuation Coefficient from Sentinel-2 MSI over a Large Reservoir. Remote Sens. 2020, 12, 768. [Google Scholar] [CrossRef] [Green Version]
- Tao, M.; Duan, H.; Cao, Z.; Loiselle, S.A.; Ma, R. A hybrid EOF algorithm to improve MODIS cyanobacteria phycocyanin data quality in a highly turbid lake: Bloom and nonbloom condition. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 4430–4444. [Google Scholar] [CrossRef]
- Afreen, S.; Fatma, T. Extraction, purification and characterization of phycoerythrin from Michrochaete and its biological activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89. [Google Scholar]
- Caballero, I.; Navarro, G. Monitoring cyanoHABs and water quality in Laguna Lake (Philippines) with Sentinel-2 satellites during the 2020 Pacific typhoon season. Sci. Total Environ. 2021, 788, 147700. [Google Scholar] [CrossRef]
- Toming, K.; Kutser, T.; Laas, A.; Sepp, M.; Paavel, B.; Nõges, T. First Experiences in Mapping Lake Water Quality Parameters with Sentinel-2 MSI Imagery. Remote Sens. 2016, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez, X.Á.; del Olmo, E.V.G.; Carral, Á.C.; Martín, J.P. Modelo Conceptual de la Eutrofización y Proliferación de Cianobacterias. Un Caso de Estudio en el Embalse de A Baxe; Universidade de Vigo: Vigo, Spain, 2015. [Google Scholar]
- Álvarez, X.; Valero, E.; Santos, R.M.B.; Varandas, S.G.P.; Sanches Fernandes, L.F.; Pacheco, F.A.L. Anthropogenic nutrients and eutrophication in multiple land use watersheds: Best management practices and policies for the protection of water resources. Land Use Policy 2017, 69, 1–11. [Google Scholar] [CrossRef]
- Gobierno de España SIOSE. Sistema de Información de Ocupación del Suelo de España. Ministerio de Transportes, Movilidad y Agenda Urbana. 2016. Available online: https://www.siose.es/ (accessed on 12 October 2020).
- Acuña-Alonso, C.; Fernandes, A.C.P.; Álvarez, X.; Valero, E.; Pacheco, F.A.L.; Varandas, S.D.G.P.; Terêncio, D.P.S.; Fernandes, L.F.S. Water security and watershed management assessed through the modelling of hydrology and ecological integrity: A study in the Galicia-Costa (NW Spain). Sci. Total Environ. 2020, 143905. [Google Scholar] [CrossRef]
- Augas de Galicia. Plan Integral de Actuación sobre a Microcystis sp. no Encoro de Caldas de Reis no Río Umia. PLAN UMIA 2011, Santiago de Compostela, Galicia, Spain. Available online: http://7cfe.congresoforestal.es/sites/default/files/actas/7CFE01-311.pdf (accessed on 21 November 2020).
- Kallio, K.; Kutser, T.; Hannonen, T.; Koponen, S.; Pulliainen, J.; Vepsäläinen, J.; Pyhälahti, T. Retrieval of water quality from airborne imaging spectrometry of various lake types in different seasons. Sci. Total Environ. 2001, 268, 59–77. [Google Scholar] [CrossRef]
- Chavez, P.S. An improved dark-object subtraction technique for atmospheric scattering correction of multispectral data. Remote Sens. Environ. 1988, 24, 459–479. [Google Scholar] [CrossRef]
- Aranha, J.; Enes, T.; Calvão, A.; Viana, H. Shrub Biomass Estimates in Former Burnt Areas Using Sentinel 2 Images Processing and Classification. Forests 2020, 11, 555. [Google Scholar] [CrossRef]
- Mcfeeters, S.K. The use of the Normalized Difference Water Index (NDWI) in the delineation of open water features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Third Earth Resources Technology Satellite-1 Symposium: The Proceedings of a Symposium Held by Goddard Space Flight Center at Washington, DC on 10–14 December 1973: Prepared at Goddard Space Flight Center; Scientific and Technical Information Office, National Aeronautics and Space Administration: Washington, DC, USA, 1974; Volume 351, p. 309.
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Haubrock, S.N.; Chabrillat, S.; Lemmnitz, C.; Kaufmann, H. Surface soil moisture quantification models from reflectance data under field conditions. Int. J. Remote Sens. 2008, 29, 3–29. [Google Scholar] [CrossRef]
- Xu, H. Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. Int. J. Remote Sens. 2006, 27, 3025–3033. [Google Scholar] [CrossRef]
- Sun, F.; Sun, W.; Chen, J.; Gong, P. Comparison and improvement of methods for identifying waterbodies in remotely sensed imagery. Int. J. Remote Sens. 2012, 33, 6854–6875. [Google Scholar] [CrossRef]
- Weier, J.; Herring, D. Measuring Vegetation (NDVI & EVI). Earth Observatory; NASA Earth Observatory: Washington, DC, USA, 2000.
- Rodríguez-López, L.; Duran-Llacer, I.; González-Rodríguez, L.; Abarca-del-Rio, R.; Cárdenas, R.; Parra, O.; Martínez-Retureta, R.; Urrutia, R. Spectral analysis using LANDSAT images to monitor the chlorophyll-a concentration in Lake Laja in Chile. Ecol. Inform. 2020, 60, 101183. [Google Scholar] [CrossRef]
- Pandhadha, E.; Aunurrahim, A.; Ayudyanti, A.; Huda, M.; Sabrina, R.; Anindya, S. Total Suspended Solid (TSS) Estimation in Lake Tempe, South Sulawesi Using Sentinel-2B Imagery. EasyChair 2019, 1, 19–22. [Google Scholar]
- Molkov, A.A.; Fedorov, S.V.; Pelevin, V.V.; Korchemkina, E.N. Regional models for high-resolution retrieval of chlorophyll a and TSM concentrations in the Gorky Reservoir by Sentinel-2 imagery. Remote Sens. 2019, 11, 1215. [Google Scholar] [CrossRef] [Green Version]
- Yacobi, Y.Z.; Moses, W.J.; Kaganovsky, S.; Sulimani, B.; Leavitt, B.C.; Gitelson, A.A. NIR-red reflectance-based algorithms for chlorophyll-a estimation in mesotrophic inland and coastal waters: Lake Kinneret case study. Water Res. 2011, 45, 2428–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Salgado, A.A. Modelagem da Dinâmica de Algas e Cianobactérias em um Reservatório de Abastecimento. Ph.D. Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2018. [Google Scholar]
- Saberioon, M.; Brom, J.; Nedbal, V.; Soucek, P.; Císar, P. Chlorophyll-a and total suspended solids retrieval and mapping using Sentinel-2A and machine learning for inland waters. Ecol. Indic. 2020, 113, 106236. [Google Scholar] [CrossRef]
- Ha, N.T.T.; Thao, N.T.P.; Koike, K.; Nhuan, M.T. Selecting the Best Band Ratio to Estimate Chlorophyll-a Concentration in a Tropical Freshwater Lake Using Sentinel 2A Images from a Case Study of Lake Ba Be (Northern Vietnam). ISPRS Int. J. Geo-Inf. 2017, 6, 290. [Google Scholar] [CrossRef]
- Ansper, A.; Alikas, K. Retrieval of Chlorophyll a from Sentinel-2 MSI Data for the European Union Water Framework Directive Reporting Purposes. Remote Sens. 2019, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Binding, C.E.; Zastepa, A.; Zeng, C. The impact of phytoplankton community composition on optical properties and satellite observations of the 2017 western Lake Erie algal bloom. J. Great Lakes Res. 2019, 45, 573–586. [Google Scholar] [CrossRef]
- Germán, A.; Andreo, V.; Tauro, C.; Scavuzzo, C.M.; Ferral, A. A novel method based on time series satellite data analysis to detect algal blooms. Ecol. Inform. 2020, 59, 101131. [Google Scholar] [CrossRef]
- Cremella, B. Puesta a Punto y Validación de un Método Basado en la Fluorescencia in Vivo de Pigmentos Como Indicador de Biomasa de Fitoplancton y Cianobacterias en Cuerpos de Agua de Uruguay. Ph.D. Thesis, Universidad de la República (Uruguay), Montevideo, Uruguay, 2017. [Google Scholar]
- Elhag, M.; Gitas, I.; Othman, A.; Bahrawi, J.; Gikas, P. Assessment of Water Quality Parameters Using Temporal Remote Sensing Spectral Reflectance in Arid Environments, Saudi Arabia. Water 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Chicuazuque Gutiérrez, C.T.; Sarmiento Toro, J.E. Análisis de la Presencia de Partículas Sólidas en Suspensión Sobre el Río Bogotá Generadas Por los Procesos de las Curtiembres, Haciendo Uso de Teledetección, en el Municipio de Villapinzón. Ph.D. Thesis, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia, 2015. [Google Scholar]
- González Caro, M. Análisis Espectral de Solidos Suspendidos en Aguas Continentales con Presencia de Actividades Mineras: Caso de Estudio río Sipí, Pacifico Colombiano. Ph.D. Thesis, Universidad Militar de Granada, Bogotá, Colombia, 2015. [Google Scholar]
- Malahlela, O.E. Spatio-temporal assessment of inland surface water quality using remote sensing data in the wake of changing climate. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 227, p. 62012. [Google Scholar] [CrossRef]
- Soomets, T.; Uudeberg, K.; Jakovels, D.; Brauns, A.; Zagars, M.; Kutser, T. Validation and Comparison of Water Quality Products in Baltic Lakes Using Sentinel-2 MSI and Sentinel-3 OLCI Data. Sensors 2020, 20, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Index | Definition | Definition Based on Sentinel-2 | References |
|---|---|---|---|
| NDWI | [46] | ||
| NDVI | [47] | ||
| gNDVI | [48] | ||
| NSMI | [49] | ||
| Toming’s Index | [24] |
| Band | Point Description | Pearson’s Correlation | |
|---|---|---|---|
| Chlorophyll-a | Cyanobacteria Concentration | ||
| Band 3 (ρGreen, 543–578 nm) | Downstream point | 0.807 (p = 0.011) | 0.882 (p = 0.045) |
| Upstream point | 0.717 (p = 0.000) | 0.713 (p = 0.001) | |
| Band 4 (ρRed, 650–680 nm) | Downstream point | 0.370 (p = 0.011) | 0.263 (p = 0.045) |
| Upstream point | 0.319 (p = (0.011) | 0.453 (p = 0.045) | |
| Band 5 (ρVRE5, 698–713 nm) | Downstream point | 0.614 (p = 0.011) | 0.575 (p = 0.045) |
| Upstream point | 0.568 (p = 0.011) | 0.443 (p = 0.045) | |
| Index | Point Description | Pearson’s Correlation | |
|---|---|---|---|
| Chlorophyll-a | Cyanobacteria Concentration | ||
| NDWI | Downstream point | 0.545 (p = 0.011) | 0.651 (p = 0.045) |
| Upstream point | 0.612 (p = 0.001) | 0.651 (p = 0.011) | |
| NDVI | Downstream point | −0.247 (p = 0.012) | −0.352 (p = 0.045) |
| Upstream point | −0.211 (p = 0.011) | −0.198 (p = 0.011) | |
| gNDVI | Downstream point | −0.545 (p = 0.012) | −0.651 (p = 0.045) |
| Upstream point | −0.609 (p = 0.000) | −0.678 (p = 0.011) | |
| NSMI | Downstream point | 0.505 (p = 0.012) | 0.418 (p = 0.045) |
| Upstream point | 0.767 (p = 0.001) | 0.735 (p = 0.000) | |
| Toming´s Index | Downstream point | 0.768 (p = 0.011) | 0.683 (p = 0.045) |
| Upstream point | 0.682 (p = 0.010) | 0.662 (p = 0.010) | |
| Index | Point Description | Regression Equations | R2 |
|---|---|---|---|
| NDWI | Chlorophyll-a Downstream | y = 354.14x2 + 88.15x + 10.93 | 0.498 |
| Chlorophyll-a Upstream | y = 7.70x2 + 125.64x + 29.64 | 0.442 | |
| Cyanobacteria Downstream | y = 1 × 10 6x2 + 213,239x + 7619.9 | 0.849 | |
| Cyanobacteria Upstream | y = 283,144x2 + 297,113x + 61478 | 0.532 | |
| NSMI | Chlorophyll-a Downstream | y = 1491x2 − 632.08x + 65.449 | 0.356 |
| Chlorophyll-a Upstream | 2903.4x2 − 1332.8x + 152.81 | 0.662 | |
| Cyanobacteria Downstream | y = 2 × 106x2 – 1 × 106X + 101,716 | 0.239 | |
| Cyanobacteria Upstream | y = 4 × 106 + 06x2 – 2 × 106 + 06x + 152,481 | 0.507 | |
| Toming’s Index | Chlorophyll-a Downstream | y = 52,947x2 + 6561x + 20.691 | 0.859 |
| Chlorophyll-a Upstream | y = 85,859x2 + 5397.6x + 35.035 | 0.526 | |
| Cyanobacteria Downstream | y = 1 × 109x2 + 1 × 107x + 31,304 | 0.721 | |
| Cyanobacteria Upstream | y = −1 × 108x2 + 1 × 107x + 86,899 | 0.489 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viso-Vázquez, M.; Acuña-Alonso, C.; Rodríguez, J.L.; Álvarez, X. Remote Detection of Cyanobacterial Blooms and Chlorophyll-a Analysis in a Eutrophic Reservoir Using Sentinel-2. Sustainability 2021, 13, 8570. https://doi.org/10.3390/su13158570
Viso-Vázquez M, Acuña-Alonso C, Rodríguez JL, Álvarez X. Remote Detection of Cyanobacterial Blooms and Chlorophyll-a Analysis in a Eutrophic Reservoir Using Sentinel-2. Sustainability. 2021; 13(15):8570. https://doi.org/10.3390/su13158570
Chicago/Turabian StyleViso-Vázquez, Manuel, Carolina Acuña-Alonso, Juan Luis Rodríguez, and Xana Álvarez. 2021. "Remote Detection of Cyanobacterial Blooms and Chlorophyll-a Analysis in a Eutrophic Reservoir Using Sentinel-2" Sustainability 13, no. 15: 8570. https://doi.org/10.3390/su13158570
APA StyleViso-Vázquez, M., Acuña-Alonso, C., Rodríguez, J. L., & Álvarez, X. (2021). Remote Detection of Cyanobacterial Blooms and Chlorophyll-a Analysis in a Eutrophic Reservoir Using Sentinel-2. Sustainability, 13(15), 8570. https://doi.org/10.3390/su13158570








