Microbiota Management for Effective Disease Suppression: A Systematic Comparison between Soil and Mammals Gut
Abstract
1. Introduction
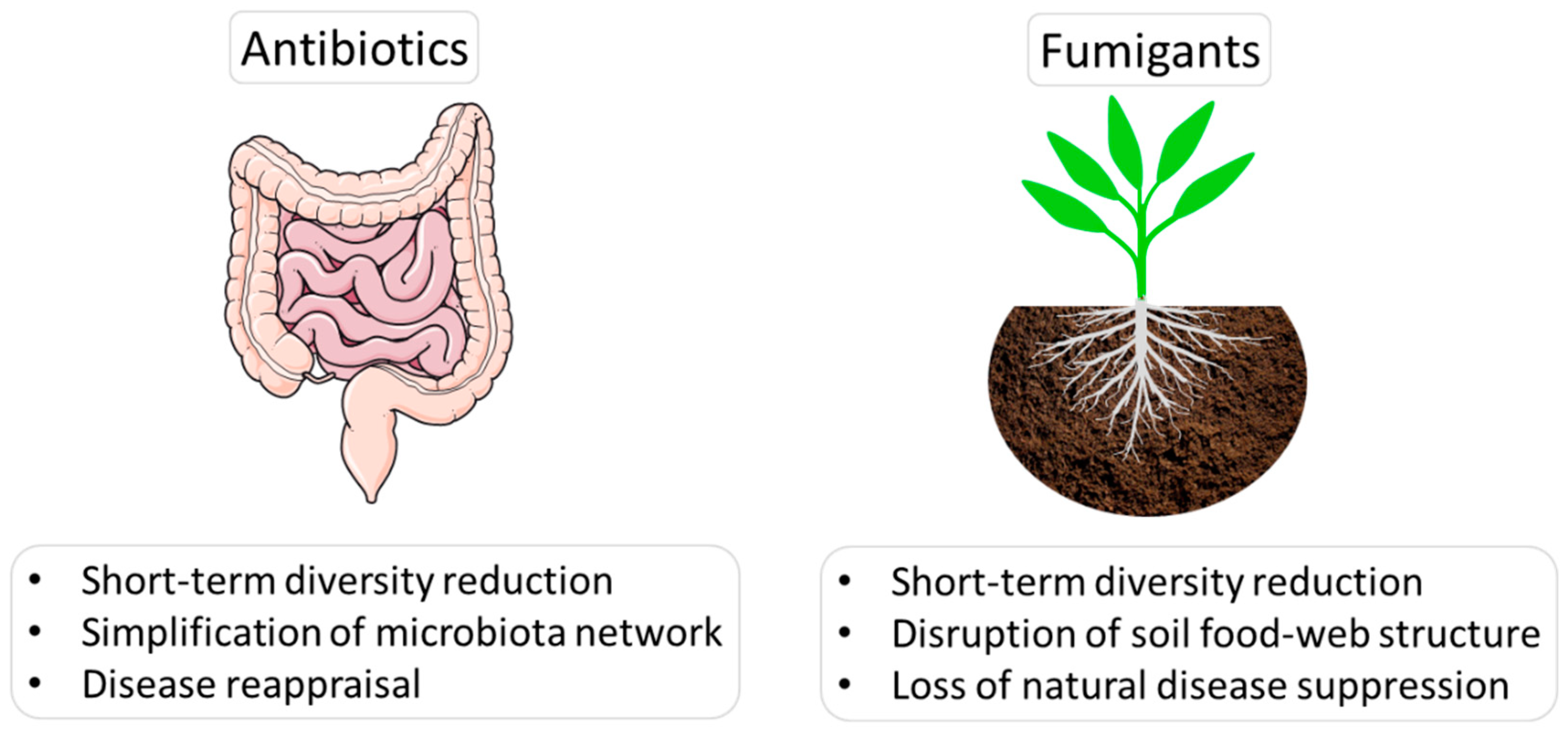
2. Antibiotics, Fumigations, Microbiota Disruption and Disease Reappraisal
3. Microbiome Stirring to Enhance Disease Suppression
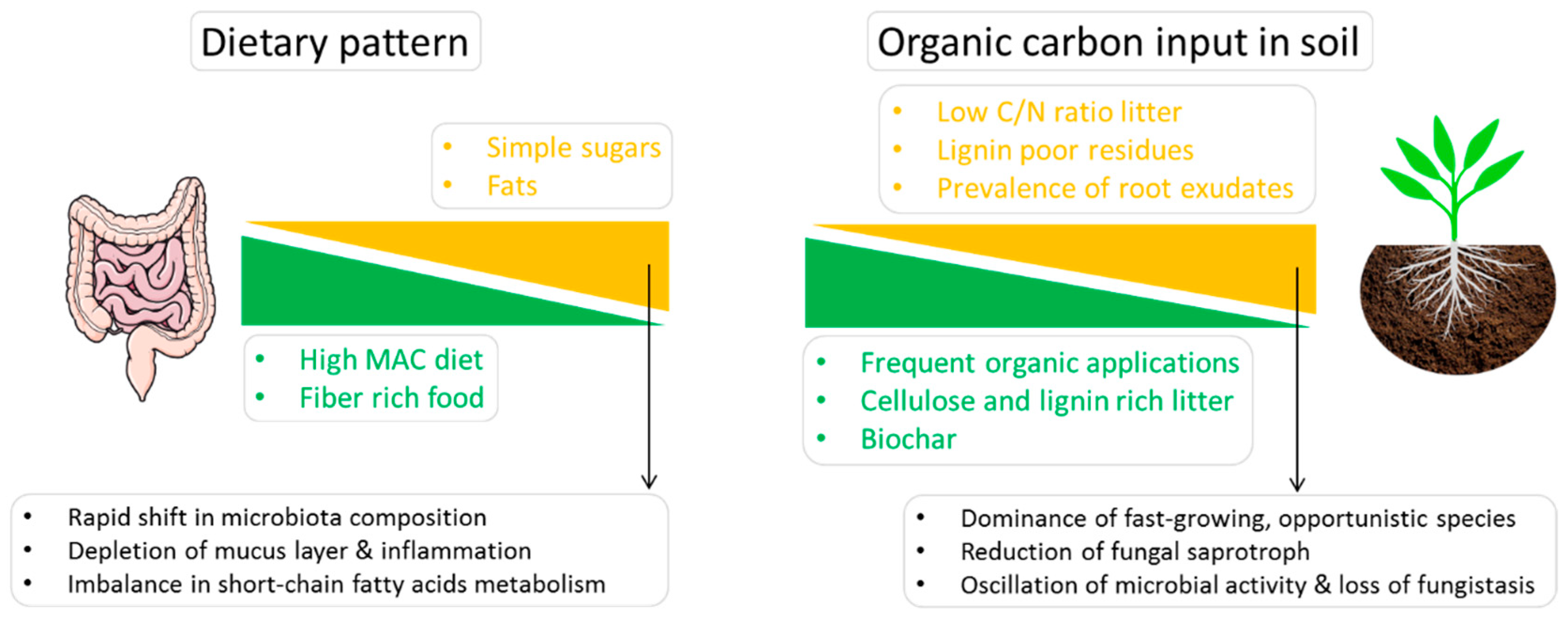
3.1. Gut Microbiota, Diet and Human Health
3.2. Organic Amendment to Feed Soil Microbiota and Promote Disease Suppression
4. Microbiome Transplant: Potentialities and Drawbacks
4.1. Microbiota Transplant in Gut and Vagina
4.2. Microbiota Transplant in Soil Ecosystems
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, F.N. Development of alternative strategies for management of soil-borne pathogens currently controlled with methyl bromide. Annu. Rev. Phytopathol. 2003, 41, 325–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, J.; Zhou, X.; Han, Y.; Zhang, J.; Cai, Z. How green alternatives to chemical pesticides are environmentally friendly and more efficient. Eur. J. Soil Sci. 2019, 70, 518–529. [Google Scholar] [CrossRef]
- Tang, F.H.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Beckie, H.J.; Busi, R.; Lopez-Ruiz, F.J.; Umina, P.A. Herbicide resistance management strategies: How do they compare with those for insecticides, fungicides and antibiotics? Pest Manag. Sci. 2021, 77, 3049–3056. [Google Scholar] [CrossRef]
- Storck, V.; Karpouzas, D.G.; Martin-Laurent, F. Towards a better pesticide policy for the European Union. Sci. Total Environ. 2017, 575, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Zolin, B.; Cassin, M.; Mannino, I. Food Security, Food Safety and Pesticides: China and the EU Compared; University Ca’Foscari of Venice, Dept. of Economics: Venezia, Italy, 2017; Volume 2. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academia Press: San Diego, CA, USA, 2005. [Google Scholar]
- Katan, J.; DeVay, J.E. Soil Solarization; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Mazzola, M.; Hewavitharana, S.S.; Strauss, S.L. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen reinfestation. Phytopathology 2014, 105, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.M.; Kokalis-Burelle, N.; Albano, J.P.; McCollum, T.G.; Muramoto, J.; Shennan, C.; Rosskopf, E.N. Anaerobic soil disinfestation (ASD) combined with soil solarization as a methyl bromide alternative: Vegetable crop performance and soil nutrient dynamics. Plant Soil 2014, 378, 365–381. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. Sustain. Agric. 2011, 2, 761–786. [Google Scholar]
- Sarker, T.C.; Incerti, G.; Spaccini, R.; Piccolo, A.; Mazzoleni, S.; Bonanomi, G. Linking organic matter chemistry with soil aggregate stability: Insight from 13C NMR spectroscopy. Soil Biol. Biochem. 2018, 117, 175–184. [Google Scholar] [CrossRef]
- Hoitink HA, J.; Boehm, M.J. Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446. [Google Scholar] [CrossRef]
- Bonanomi, G.; Alioto, D.; Minutolo, M.; Marra, R.; Cesarano, G.; Vinale, F. Organic amendments modulate soil microbiota and reduce virus disease incidence in the TSWV-tomato pathosystem. Pathogens 2020, 9, 379. [Google Scholar] [CrossRef]
- Lockwood, J.L. Biological control of soil-borne plant pathogens. In Relation of Energy Stress to Behaviour of Soil-Borne Plant Pathogens and to Disease Development; Hornby, D., Ed.; CAB International: Wallingford, UK, 1990; pp. 197–214. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Scheuerell, S.; Mahaffee, W. Compost tea: Principles and prospects for plant disease control. Compost Sci. Util. 2002, 10, 313–338. [Google Scholar] [CrossRef]
- Elad, Y.; Cytryn, E.; Harel, Y.M.; Lew, B.; Graber, E.R. The biochar effect: Plant resistance to biotic stresses. Phytopathol. Mediterr. 2012, 50, 335–349. [Google Scholar]
- Bonanomi, G.; Cesarano, G.; Antignani, V.; Di Maio, C.; De Filippis, F.; Scala, F. Conventional farming impairs Rhizoctonia solani disease suppression by disrupting soil food web. J. Phytopathol. 2018, 166, 663–673. [Google Scholar] [CrossRef]
- De Corato, U. Disease-suppressive compost enhances natural soil suppressiveness against soil-borne plant pathogens: A critical review. Rhizosphere 2020, 13. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Pineda, A.; Kaplan, I.; Bezemer, T.M. Steering soil microbiomes to suppress aboveground insect pests. Trends Plant Sci. 2017, 22, 770–778. [Google Scholar] [CrossRef]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef]
- Croswell, A.; Amir, E.; Teggatz, P.; Barman, M.; Salzman, N.H. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect. Immun. 2009, 77, 2741–2753. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Looft, T.; Allen, H.K. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes 2012, 3, 463–467. [Google Scholar] [CrossRef]
- Alavi, S.; Mitchell, J.D.; Cho, J.Y.; Liu, R.; Macbeth, J.C.; Hsiao, A. Interpersonal gut microbiome variation drives susceptibility and resistance to cholera infection. Cell 2020, 181, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.M.; Ferreyra, J.A.; Higginbottom, S.K.; Lynch, J.B.; Kashyap, P.C.; Gopinath, S.; Naidu, N.; Choudhury, B.; Weimer, B.C.; Monack, D.M.; et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 2013, 502, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of antibiotics on gut microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Van Nood, E.; Tims, S.; Heikamp-de Jong, I.; Ter Braak, C.J.; Keller, J.J.; Zoetendal, E.G.; De Vos, W.M. Reset of a critically disturbed microbial ecosystem: Faecal transplant in recurrent Clostridium difficile infection. ISME J. 2014, 8, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; DiLegge, M.J.; Minas, I.S.; Hamm, A.; Manter, D.; Vivanco, J.M. Soil sterilization leads to re-colonization of a healthier rhizosphere microbiome. Rhizosphere 2019, 12. [Google Scholar] [CrossRef]
- Holmes, G.J.; Mansouripour, S.M.; Hewavitharana, S.S. Strawberries at the Crossroads: Management of soilborne diseases in California without methyl bromide. Phytopathology 2020, 110, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; D’Ascoli, R.; Antignani, V.; Capodilupo, M.; Cozzolino, L.; Marzaioli, R.; Puopolo, G.; Rutigliano, F.A.; Scelza, R.; Scotti, R.; et al. Assessing soil quality under intensive cultivation and tree orchards in Southern Italy. Appl. Soil Ecol. 2011, 47, 184–194. [Google Scholar] [CrossRef]
- Xue, C.; Shen, Z.; Hao, Y.; Yu, S.; Li, Y.; Huang, W.; Chong, Y.; Ran, W.; Li, R.; Shen, Q. Fumigation coupled with bio-organic fertilizer for the suppression of watermelon Fusarium wilt disease re-shapes the soil microbiome. Appl. Soil Ecol. 2019, 140, 49–56. [Google Scholar] [CrossRef]
- Shen, Z.; Xue, C.; Penton, C.R.; Thomashow, L.S.; Zhang, N.; Wang, B.; Ruan, Y.; Li, R.; Shen, Q. Suppression of banana Panama disease induced by soil microbiome reconstruction through an integrated agricultural strategy. Soil Biol. Biochem. 2019, 128, 164–174. [Google Scholar] [CrossRef]
- Smith, K.; McCoy, K.D.; Macpherson, A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2007; Volume 19, pp. 59–69. [Google Scholar]
- Ge, A.H.; Liang, Z.H.; Xiao, J.L.; Zhang, Y.; Zeng, Q.; Xiong, C.; Han, L.; Wang, J.; Zhang, L.M. Microbial assembly and association network in watermelon rhizosphere after soil fumigation for Fusarium wilt control. Agric. Ecosyst. Environ. 2021, 312. [Google Scholar] [CrossRef]
- Genoni, A.; Christophersen, C.T.; Lo, J.; Coghlan, M.; Boyce, M.C.; Bird, A.R.; Lyons-Wall, P.; Devine, A. Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur. J. Nutr. 2020, 59, 1845–1858. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, Z.; Huang, B.; Cao, A.; Wang, Q.; Yan, D.; Ouyang, C.; Wu, J.; Li, Y. Effects of fumigation with allyl isothiocyanate on soil microbial diversity and community structure of tomato. J. Agric. Food Chem. 2020, 68, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Cesarano, G.; La Storia, A.; Ercolini, D.; Scala, F. Organic farming induces changes in soil microbiota that affect agro-ecosystem functions. Soil Biol. Biochem. 2016, 103, 327–336. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Mendes, R.; Raaijmakers, J.M. Cross-kingdom similarities in microbiome functions. ISME J. 2015, 9, 1905–1907. [Google Scholar] [CrossRef] [PubMed]
- Cartenì, F.; Sarker, T.C.; Bonanomi, G.; Cesarano, G.; Esposito, A.; Incerti, G.; Mazzoleni, S.; Lanzotti, V.; Giannino, F. Linking plant phytochemistry to soil processes and functions: The usefulness of 13C NMR spectroscopy. Phytochem. Rev. 2018, 17, 815–832. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- John, G.K.; Mullin, G.E. The gut microbiome and obesity. Curr. Oncol. Rep. 2016, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Hazen, S.L. The gut microbiome and its role in cardiovascular diseases. Circulation 2017, 135, 1008–1010. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Portune, K.J.; Benítez-Páez, A.; Del Pulgar EM, G.; Cerrudo, V.; Sanz, Y. Gut microbiota, diet, and obesity-related disorders—The good, the bad, and the future challenges. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, D.Y.; Dick, W.A.; Davis, K.R.; Hoitink HA, J. Compost and compost water extract-induced systemic acquired resistance in cucumber and Arabidopsis. Phytopathology 1998, 88, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Hryckowian, A.J.; Van Treuren, W.; Smits, S.A.; Davis, N.M.; Gardner, J.O.; Bouley, D.M.; Sonnenburg, J.L. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat. Microbiol. 2018, 3, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Laura, E.; Mason, D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J. Pediatric Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Gasser, P. Soil animals alter plant litter diversity effects on decomposition. Proc. Natl. Acad. Sci. USA 2005, 102, 1519–1524. [Google Scholar] [CrossRef]
- Osnas, J.L.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Cesarano, G.; Sarker, T.C.; Saulino, L.; Saracino, A.; Idbella, M.; Agrelli, D.; D’Ascoli, R.; Rita, A.; et al. Decomposition of woody debris in Mediterranean ecosystems: The role of wood chemical and anatomical traits. Plant Soil 2021, 460, 263–280. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Hardison, J.R. Fire and flame for plant disease control. Annu. Rev. Phytopathol. 1976, 14, 355–379. [Google Scholar] [CrossRef]
- De Vries, F.T.; Bloem, J.; van Eekeren, N.; Brusaard, L.; Hoffland, E. Fungal biomass in pastures increases with age and reduced N input. Soil Biol. Biochem. 2007, 39, 1620–1630. [Google Scholar] [CrossRef]
- Clocchiatti, A. Bringing Soil Fungi into Action: Options for Forward-Looking Agriculture; Wageningen University: Wageningen, The Netherlands, 2021. [Google Scholar]
- Savory, B.M. Specific Replant Diseases; Commonwealth Agricultural Bureaux: Wallingford, UK, 1966. [Google Scholar]
- Troelstra, S.R.; Wagenaar, R.; Smant, W.; Peters, B.A.M. Interpretation of bioassays in the study of interactions between soil organisms and plants: Involvement of nutrient factors. New Phytol. 2001, 150, 697–706. [Google Scholar] [CrossRef]
- Cesarano, G.; Zotti, M.; Antignani, V.; Marra, R.; Scala, F.; Bonanomi, G. Soil sickness and negative plant-soil feedback: A reappraisal of hypotheses. J. Plant Pathol. 2017, 99, 545–570. [Google Scholar]
- Moora, M.; Öpik, M.; Sen, R.; Zobel, M. Native arbuscular mycorrhizal fungal communities differentially influence the seedling performance of rare and common Pulsatilla species. Funct. Ecol. 2004, 18, 554–562. [Google Scholar] [CrossRef]
- Bonanomi, G.; Mingo, A.; Incerti, G.; Mazzoleni, S.; Allegrezza, M. Fairy rings caused by a killer fungus foster plant diversity in species-rich grassland. J. Veg. Sci. 2012, 23, 236–248. [Google Scholar] [CrossRef]
- He, M.; Tian, G.; Semenov, A.M.; Van Bruggen, A.H. Short-term fluctuations of sugar beet damping-off by Pythium ultimum in relation to changes in bacterial communities after organic amendments to two soils. Phytopathology 2012, 102, 413–420. [Google Scholar] [CrossRef][Green Version]
- Van Bruggen, A.H.C.; Semenov, A.M.; van Diepeningen, A.D.; de Vos, O.J.; Blok, W.J. Relation between soil health, wave-like fluctuations in microbial populations, and soil-borne plant disease management. In Plant Disease Epidemiology: Facing Challenges of the 21st Century; Springer: Dordrecht, The Netherlands, 2006; pp. 105–122. [Google Scholar]
- Dick, R.P.; Rasmussen, P.E.; Kerle, E.A. Influence of long-term residue management on soil enzyme activities in relation to soil chemical properties of a wheat-fallow system. Biol. Fertil. Soils 1988, 6, 159–164. [Google Scholar] [CrossRef]
- Kandeler, E.; Stemmer, M.; Klimanek, E.M. Response of soil microbial biomass, urease and xylanase within particle size fractions to long-term soil management. Soil Biol. Biochem. 1999, 31, 261–273. [Google Scholar] [CrossRef]
- Bonanomi, G.; Gaglione, S.A.; Cesarano, G.; Sarker, T.C.; Pascale, M.; Scala, F.; Zoina, A. Frequent applications of organic matter to agricultural soil increase fungistasis. Pedosphere 2017, 27, 86–95. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Hannula, S.E.; van den Berg, M.; Korthals, G.; De Boer, W. The hidden potential of saprotrophic fungi in arable soil: Patterns of short-term stimulation by organic amendments. Appl. Soil Ecol. 2020, 147. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Eizenberg, H.; Plakhine, D.; Ziadne, H.; Tsechansky, L.; Graber, E.R. Non-chemical control of root parasitic weeds with biochar. Front. Plant Sci. 2017, 8, 939. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Kohler, J.; Rillig, M.C. Biochars reduce infection rates of the root-lesion nematode Pratylenchus penetrans and associated biomass loss in carrot. Soil Biol. Biochem. 2016, 95, 11–18. [Google Scholar] [CrossRef]
- Postma, J.; Clematis, F.; Nijhuis, E.H.; Someus, E. Efficacy of four phosphate-mobilizing bacteria applied with an animal bone charcoal formulation in controlling Pythium aphanidermatum and Fusarium oxysporum f. sp. radicis lycopersici in tomato. Biol. Control 2013, 67, 284–291. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Frenkel, O.; Tsechansky, L.; Elad, Y.; Graber, E.R. Immobilization and deactivation of pathogenic enzymes and toxic metabolites by biochar: A possible mechanism involved in soilborne disease suppression. Soil Biol. Biochem. 2018, 121, 59–66. [Google Scholar] [CrossRef]
- Wolf, R.; Wedig, H. Process for Manufacturing a Soil Conditioner. U.S. Patent No WO 2,010,055,139 A1, 20 May 2010. [Google Scholar]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Saracino, A.; Scala, F. Biochar As Plant Growth Promoter: Better Off Alone or Mixed with Organic Amendments? Front. Plant Sci. 2017, 8, 1570. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.K.; Elliott, W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From’omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef]
- Bardin, M.; Pugliese, M. Biocontrol agents against diseases. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Cham, Switzerland, 2020; pp. 385–407. [Google Scholar]
- Ram, R.M.; Singh, H.B. Microbial consortium in biological control: An explicit example of teamwork below ground. J. Eco-Friendly Agric. 2018, 13, 1–12. [Google Scholar]
- Zhang, F.; Luo, W.; Shi, Y.; Fan, Z.; Ji, G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1755. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.S.K.; Joshi, V.; Balamurugan, B.; Gautam, D.; Chethan, G.E.; Lekshman, A. Rumen transfaunation an effective method for treating simple indigestion in ruminants. North-East Vet. 2017, 17, 31–33. [Google Scholar]
- Stagaman, K.; Burns, A.R.; Guillemin, K.; Bohannan, B.J. The role of adaptive immunity as an ecological filter on the gut microbiota in zebrafish. ISME J. 2017, 11, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar] [PubMed]
- Sbahi, H.; Di Palma, J.A. Faecal microbiota transplantation: Applications and limitations in treating gastrointestinal disorders. BMJ Open Gastroenterol. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, T.; Rawla, P.; Ofosu, A.; Gaduputi, V. Fecal microbiota transplant–a new frontier in inflammatory bowel disease. J. Inflamm. Res. 2018, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, B.P.; Vatanen, T.; Allegretti, J.R.; Bai, A.; Xavier, R.J.; Korzenik, J.; Gevers, D.; Ting, A.; Robson, S.C.; Moss, A.C. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm. Bowel Dis. 2016, 22, 2182–2190. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Li, P.; Zhang, T.; Cui, B.; Ji, G.; Lu, X.; Zhang, F. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019, 42, 869–880. [Google Scholar] [CrossRef]
- Pinn, D.M.; Aroniadis, O.C.; Brandt, L.J. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol. Motil. 2015, 27, 19–29. [Google Scholar] [CrossRef]
- Marotz, C.A.; Zarrinpar, A. Focus: Microbiome: Treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J. Biol. Med. 2016, 89, 383. [Google Scholar]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017, 26, 611–619. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Ologun, G.O.; Arora, R.; Wargo, J.A. Gut microbiome modulation via fecal microbiota transplant to augment immunotherapy in patients with melanoma or other cancers. Curr. Oncol. Rep. 2020, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ren, K.; Ning, R.; Li, C.; Zhang, H.; Li, D.; Xu, L.; Sun, F.; Dai, M. Fecal microbiota transplantation provides new insight into wildlife conservation. Glob. Ecol. Conserv. 2020, 24. [Google Scholar] [CrossRef]
- Pereira, G.Q.; Gomes, L.A.; Santos, I.S.; Alfieri, A.F.; Weese, J.S.; Costa, M.C. Fecal microbiota transplantation in puppies with canine parvovirus infection. J. Vet. Intern. Med. 2018, 32, 707–711. [Google Scholar] [CrossRef]
- Niederwerder, M.C.; Constance, L.A.; Rowland, R.R.R.; Abbas, W.; Fernando, S.C.; Potter, M.L.; Sheahan, M.A.; Burkey, T.E.; Hesse, R.A.; Cino-Ozuna, A.G. Fecal microbiota transplantation is associated with reduced morbidity and mortality in porcine circovirus associated disease. Front. Microbiol. 2018, 9, 1631. [Google Scholar] [CrossRef] [PubMed]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Brotman, R.M. Translating the vaginal microbiome: Gaps and challenges. Genome Med. 2016, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Peipert, J.F.; Montagno, A.B.; Cooper, A.S.; Sung, C.J. Bacterial vaginosis as a risk factor for upper genital tract infection. Am. J. Obstet. Gynecol. 1997, 177, 1184–1187. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef]
- Bohbot, J.M.; Daraï, E.; Bretelle, F.; Brami, G.; Daniel, C.; Cardot, J.M. Efficacy and safety of vaginally administered lyophilized Lactobacillus crispatus IP 174178 in the prevention of bacterial vaginosis recurrence. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 81–86. [Google Scholar] [CrossRef]
- DeLong, K.; Bensouda, S.; Zulfiqar, F.; Zierden, H.C.; Hoang, T.M.; Abraham, A.G.; Coleman, J.S.; Cone, R.A.; Gravitt, P.E.; Hendrix, C.W.; et al. Conceptual design of a universal donor screening approach for vaginal microbiota transplant. Front. Cell. Infect. Microbiol. 2019, 9, 306. [Google Scholar] [CrossRef]
- Weller, D.M.; Raaijmakers, J.M.; Gardener BB, M.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef]
- Menzies, J. Occurrence and transfer of a biological factor in soil that suppresses potato scab. Phytopathology 1959, 49, 648–652. [Google Scholar]
- Liu, D.; Anderson, N.A.; Kinkel, L.L. Biological control of potato scab in the field with antagonistic Streptomyces scabies. Phytopathology 1995, 85, 827–831. [Google Scholar] [CrossRef]
- Cook, R.J.; Rovira, A.D. The role of bacteria in the biological control of Gaeumannomyces graminis by suppressive soils. Soil Biol. Biochem. 1976, 8, 269–273. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Alabouvette, C. Fusarium wilt suppressive soils: An example of disease-suppressive soils. Australas. Plant Pathol. 1999, 28, 57–64. [Google Scholar] [CrossRef]
- Martin, F.N.; Hancock, J.G. Association of chemical and biological factors in soils suppressive to Pythium ultimum. Phytopathology 1986, 76, 1221–1231. [Google Scholar] [CrossRef]
- Nishiyama, M.; Shiomi, Y.; Suzuki, S.; Marumoto, T. Suppression of growth of Ralstonia solanacearum, tomato bacterial wilt agent, on/in tomato seedlings cultivated in a suppressive soil. Soil Sci. Plant Nutr. 1999, 45, 79–87. [Google Scholar] [CrossRef]
- Pyrowolakis, A.; Westphal, A.; Sikora, R.A.; Beckerb, J.O. Identification of root-knot nematode suppressive soils. Appl. Soil Ecol. 2002, 19, 51–56. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Xu, Y.; Wei, W.; Yao, Q.; Panet, F. Diversity of parasitic fungi from soybean cyst nematode associated with long-term continuous cropping of soybean in black soil. Acta Agric. Scand. B Soil Plant Sci. 2016, 66, 432–442. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanomi, G.; Idbella, M.; Abd-ElGawad, A.M. Microbiota Management for Effective Disease Suppression: A Systematic Comparison between Soil and Mammals Gut. Sustainability 2021, 13, 7608. https://doi.org/10.3390/su13147608
Bonanomi G, Idbella M, Abd-ElGawad AM. Microbiota Management for Effective Disease Suppression: A Systematic Comparison between Soil and Mammals Gut. Sustainability. 2021; 13(14):7608. https://doi.org/10.3390/su13147608
Chicago/Turabian StyleBonanomi, Giuliano, Mohamed Idbella, and Ahmed M. Abd-ElGawad. 2021. "Microbiota Management for Effective Disease Suppression: A Systematic Comparison between Soil and Mammals Gut" Sustainability 13, no. 14: 7608. https://doi.org/10.3390/su13147608
APA StyleBonanomi, G., Idbella, M., & Abd-ElGawad, A. M. (2021). Microbiota Management for Effective Disease Suppression: A Systematic Comparison between Soil and Mammals Gut. Sustainability, 13(14), 7608. https://doi.org/10.3390/su13147608







