Effects of Dietary Tannins’ Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Data Extraction
2.3. Calculations and Statistical Analysis
2.4. Heterogeneity
2.5. Publication Bias
2.6. Meta-Regression
3. Results
3.1. Study Attributes and Excluded Studies
3.2. Growth Performance and Nutrient Intake
3.3. Digestibility, Ruminal Parameters, and Methane Emissions
3.4. Analysis of Publication Bias
3.5. Meta-Regression
3.6. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Author | Country | Tannin Source | Tannin Type | Method of Inclusion |
| Aboagye et al. [17] | Canada | CH, CH, BL, BL | HT, HT, BL, BL | E, E, E, E |
| Aboagye et al. [21] | Canada | TA, CH | HT, HT | E, E |
| Ávila et al. [81] | Brazil | AM, AM | CT, CT | E, E |
| Avila et al. [20] | Brazil | AM, AM, AM | CT, CT, CT | E, E, E |
| Caetano et al. [82] | Australia | Grape | CT | NAT |
| Dickhoefer et al. [83] | Germany | QU, QU, QU, QU | BL, BL, BL, BL | E, E, E, E |
| Ebert et al. [84] | US | QU (n = 10) | CT (n = 10) | E (n = 10) |
| Jolazadeh et al. [85] | Iran | PIST, PIST, PIST | BL, BL, BL | E, E, E |
| Koenig and Beauchemin [86] | Canada | AM | CT | E |
| Koenig et al. [87] | Canada | AM, AM, AM, AM | CT, CT, CT, CT | E, E, E, E |
| Krueger et al. [88] | US | CH, AM | HT, CT | E, E |
| Martello et al. [89] | Brazil | BL, BL | BL, BL | E, E |
| Mezzomo et al. [90] | Brazil | QU, QU | CT, CT | E, E |
| Mezzomo et al. [91] | Brazil | BL, BL, BL | BL, BL, BL | E, E, E |
| Norris et al. [22] | US | QU, QU, QU | CT, CT, CT | E, E, E |
| Norris et al. [92] | US | QU, QU, QU | CT, CT, CT | E, E, E |
| Orlandi et al. [11] | Brazil | AM, AM, AM | BL, BL, BL | E, E, E |
| Piñeiro-Vázquez et al. [19] | Mexico | QU, QU, QU, QU | CT, CT, CT, CT | E, E, E, E |
| Piñeiro-Vázquez et al. [93] | Mexico | LEU, LEU, LEU, LEU | CT, CT, CT, CT | N, N, N, N |
| Piñeiro-Vázquez et al. [94] | Mexico | QU, QU, QU, QU | CT, CT, CT, CT | E, E, E, E |
| Piñeiro-Vázquez et al. [95] | Mexico | LEU, LEU, LEU, LEU | CT, CT, CT, CT | N, N, N, N |
| Poblete et al. [96] | Philippines | AM, AM | BL, BL | E, E |
| Rivera-Méndez et al. [97] | Mexico | QU, QU | CT, CT | E, E |
| Rivera-Méndez et al. [18] | Mexico | QU (n = 4), CH, BL | CT (n = 4), HT, BL | E, E, E, E, E, E |
| Shakeri et al. [98] | Iran | PIST, PIST, PIST | BL, BL, BL | N, N, N |
| Shakeri et al. [99] | Iran | PIST (n = 9) | BL (n = 9) | N (n = 9) |
| Suybeng et al. [24] | Australia | BL, BL, BL | CT, CT, CT | N, N, N |
| Tabke et al. [100] | US | TA, TA | HT, HT | E, E |
| Tseu et al. [101] | Brazil | AM, AM, AM | BL, BL, BL | E, E, E |
| Yang et al. [73] | China | TA, TA, TA | HT, HT, HT | E, E, E |
| Yuste et al. [102] | Spain | BL | BL | E |
| Zhou et al. [103] | China | TA, TA | HT, HT | E, E |
References
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannin mitigation options. Anim. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.M.; Bayer, C.; Lassalas, M.; Michelon, G.M.; Schaitz, L.H.; Biasolo, R.; Civiero, M.; Mendonça, H.; Ribeiro-Filho, N. Effects of ground corn and Acacia mearnsii tannin extract supplementation on nitrogen excretion and nitrous oxide emissions from sheep. Livest. Sci. 2021, 246, 104458. [Google Scholar] [CrossRef]
- Batista, E.D.; Detmann, E.; Valadares, F.S.C.; Titgemeyer, E.C.; Valadares, R.F.D. The effect of CP concentration in the diet on urea kinetics and microbial usage of recycled urea in cattle: A meta-analysis. Animal 2014, 11, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.A.; Clough, T.J.; Cameron, K.C.; Di, H.J.; Edwards, G.R.; de Klein, C.A.M. Potential for forage diet manipulation in New Zealand pasture ecosystems to mitigate ruminant urine derived N2O emissions: A review. N. Z. J. Agric. Res. 2016, 59, 301–317. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef] [Green Version]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M. Effect of Lipid-Encapsulated Acacia Tannin Extract on Feed Intake, Nutrient Digestibility and Methane Emission in Sheep. Animals 2019, 9, 863. [Google Scholar] [CrossRef] [Green Version]
- Seoni, E.; Rothacher, M.; Arrigo, Y.; Ampuero Kragten, S.; Bee, G.; Dohme-Meier, F. The Fate of Tannins from Birdsfoot Trefoil and Their Effect on the Nitrogen Balance in Growing Lambs Fed Diets Varying in Protein Level. Animals 2021, 11, 190. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Cao, Z.; Wang, Y.; Yang, H.; Azarfar, A.; Li, S. Effect of Different Tannin Sources on Nutrient Intake, Digestibility, Performance, Nitrogen Utilization, and Blood Parameters in Dairy Cows. Animals 2019, 9, 507. [Google Scholar] [CrossRef] [Green Version]
- Fagundes, G.M.; Benetel, G.; Santos, K.C.; Welter, K.C.; Melo, F.A.; Muir, J.P.; Bueno, I.C.S. Tannin-Rich Plants as Natural Manipulators of Rumen Fermentation in the Livestock Industry. Molecules 2020, 25, 2943. [Google Scholar] [CrossRef]
- Orlandi, T.; Kozloski, G.V.; Alves, T.P.; Mesquita, F.R.; Ávila, S.C. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Anim. Feed Sci. Technol. 2015, 210, 37–45. [Google Scholar] [CrossRef]
- Sarnataro, C.; Spanghero, M. In vitro rumen fermentation of feed substrates added with chestnut tannins or an extract from Stevia rebaudiana Bertoni. Anim. Nutr. 2020, 6, 54–60. [Google Scholar] [CrossRef]
- Santos, J.D.C.d.; Saraiva, E.P.; Gonzaga Neto, S.; Saraiva, C.A.S.; Pinheiro, A.d.C.; Fonsêca, V.d.F.C.; Santos, S.G.C.G.d.; Souza, C.G.d.; Almeida, M.E.V.; Veríssimo, T.N.S.; et al. Feeding Behavior of Lactating Dairy Cattle Fed Sorghum-Based Diets and Increasing Levels of Tannic Acid. Agriculture 2021, 11, 172. [Google Scholar] [CrossRef]
- Verma, S.; Taube, F.; Malisch, C.S. Examining the Variables Leading to Apparent Incongruity between Antimethanogenic Potential of Tannins and Their Observed Effects in Ruminants—A Review. Sustainability 2021, 13, 2743. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M.; Morgavi, D.P. Replacing urea with nitrate as a non-protein nitrogen source increases lambs’ growth and reduces methane production, whereas acacia tannin has no effect. Anim. Feed Sci. Technol. 2020, 259, 114360. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolysable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high forage diet. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef]
- Rivera-Mendez, C.; Plascencia, A.; Torrentera, N.; Zinn, R.A. Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. J. Appl. Anim. Res. 2017, 45, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Piñeiro-Vázquez, A.T.; Canul-Solis, J.R.; Alayón-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Solorio-Sánchez, F.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Energy utilization, nitrogen balance and microbial protein supply in cattle fed Pennisetum purpureum and condensed tannins. J. Anim. Physiol. Anim. Nutr. 2017, 101, 159–169. [Google Scholar] [CrossRef]
- Avila, A.S.; Zambom, M.A.; Faccenda, A.; Fischer, M.L.; Anschau, F.A.; Venturini, T.; Tinini, R.C.R.; Dessbesell, J.G.; Faciola, A.P. Effects of black wattle (Acacia mearnsii) condensed tannins on intake, protozoa population, ruminal fermentation, and nutrient digestibility in Jersey steers. Animals 2020, 10, 1011. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of gallic acid and hydrolysable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage. J. Anim. Sci. 2019, 97, 2230–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norris, A.B.; Tedeschi, L.O.; Foster, J.L.; Muir, J.P.; Pinchak, W.E.; Fonseca, M.A. AFST: Influence of quebracho tannin extract fed at differing rates within a high-roughage diet on the apparent digestibility of dry matter and fiber, nitrogen balance and fecal gas flux. Anim. Feed Sci. Technol. 2020, 260, 114365. [Google Scholar] [CrossRef]
- Fagundes, G.M.; Benetel, G.; Welter, K.C.; Melo, F.A.; Muir, J.P.; Carriero, M.M.; Souza, R.L.M.; Meo-Filho, P.; Frighetto, R.T.S.; Berndt, A.; et al. Tannin as a natural rumen modifier to control methanogenesis in beef cattle in tropical systems: Friend or foe to biogas energy production? Res. Vet. Sci. 2020, 132, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Suybeng, B.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O. Supplementing northern Australian beef cattle with Desmanthus tropical legume reduces in-vivo methane emissions. Animals 2020, 10, 2097. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Zangeneh, M.; Banaeian, N.; Clark, S. Meta-Analysis on Energy-Use Patterns of Cropping Systems in Iran. Sustainability 2021, 13, 3868. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis, 1st ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2009; p. 413. [Google Scholar]
- Doré, T.; Makowski, D.; Malézieux, E.; Munier-Jolain, N.; Tchamitchian, M.; Tittonell, P. Facing up to the paradigm of ecological intensification in agronomy: Revisiting methods, concepts and knowledge. Eur. J. Agron. 2011, 34, 197–210. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analysis. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Abhijith, A.; Dunshea, F.R.; Warner, R.D.; Leury, B.J.; Ha, M.; Chauhan, S.S. A Meta-Analysis of the Effectiveness of High, Medium, and Low Voltage Electrical Stimulation on the Meat Quality of Small Ruminants. Foods 2020, 9, 1587. [Google Scholar] [CrossRef]
- Herremans, S.; Vanwindekens, F.; Decruyenaere, V.; Beckers, Y.; Froidmont, E. Effect of dietary tannins on milk yield and composition, nitrogen partitioning and nitrogen use efficiency of lactating dairy cows: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1209–1218. [Google Scholar] [CrossRef]
- Lean, I.J.; Thompson, J.M.; Dunshea, F.R. A Meta-Analysis of Zilpaterol and Ractopamine Effects on Feedlot Performance, Carcass Traits and Shear Strength of Meat in Cattle. PLoS ONE 2014, 9, e115904. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2019; pp. 143–176. [Google Scholar]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, G.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J. OpenMEE: Intuitive, open-source software for metaanalysis in ecology and evolutionary biology. Methods Ecol. Evol. 2016, 8, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Viechtbauer, W. Conducting meta-analysis in R with the metaphor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Hedges, L.V. Distribution theory for Glass´s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Lean, I.J.; Rabiee, A.R.; Duffield, T.F.; Dohoo, I.R. Invited review: Use of meta-analysis in animal health and reproduction: Methods and applications. J. Dairy Sci. 2009, 92, 3545–3565. [Google Scholar] [CrossRef] [Green Version]
- SAS (Statistical Analysis System). SAS/STAT User’s Guide (Release 6.4); SAS Institute: Cary, NC, USA, 2017. [Google Scholar]
- Torres, R.N.S.; Moura, D.C.; Ghedini, C.P.; Ezequiel, J.M.B.; Almeida, M.T.C. Meta-analysis of the effects of essential oils on ruminal fermentation and performance of sheep. Small Rumin. Res. 2020, 189. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Altman, D.G. Systematic Reviews in Health Care, 2nd ed.; MBJ Publishing Group: London, UK, 2001; pp. 109–121. [Google Scholar]
- Littell, J.H.; Corcoran, J.; Pillai, V. Systematic Reviews and Meta-Analysis, 1st ed.; Oxford University Press: Oxford, UK, 2008; pp. 111–132. [Google Scholar]
- Sterne, J.A.C.; Harbord, R.M. Funnel plots in meta-analysis. Stata J. 2004, 4, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Trikalinos, T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. CMAJ 2007, 176, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Amer. Statist. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Capper, J.L.; Hayes, D.J. The environmental and economic impact of removing growth-enhancing technologies from U.S. beef production. J. Anim. Sci. 2012, 90, 3527–3537. [Google Scholar] [CrossRef] [Green Version]
- Hassan, F.; Arshad, M.A.; Ebeid, H.M.; Rehman, M.S.; Khan, M.S.; Shahid, S.; Yang, C. Phytogenic Additives Can Modulate Rumen Microbiome to Mediate Fermentation Kinetics and Methanogenesis Through Exploiting Diet–Microbe Interaction. Front. Vet. Sci. 2020, 7, 575801. [Google Scholar] [CrossRef]
- Mendez-Ortiz, F.A.; Sandoval-Castro, C.A.; Ventura-Cordero, J.; Sarmiento-Franco, L.A.; Torres-Acosta, J.F.J. Condensed tannin intake and sheep performance: A meta-analysis on voluntary intake and live weight change. Anim. Feed Sci. Technol. 2018, 245, 67–76. [Google Scholar] [CrossRef]
- Kenny, D.; Fitzsimons, C.; Waters, S.; McGee, M. Invited review: Improving feed efficiency of beef cattle–the current state of the art and future challenges. Animal 2018, 12, 1815–1826. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional Aspects of Ecologically Relevant Phytochemicals in Ruminant Production. Front. Vet. Sci. 2021, 8, 628445. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Q.; Liu, Y.; Yan, T.; Li, F.; Cao, Y.; Yao, J. Dynamics of methanogenesis, ruminal fermentation and fiber digestibility in ruminants following elimination of protozoa: A meta-analysis. J. Anim. Sci. Biotech. 2018, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Salami, S.A.; Valenti, B.; Bella, M.; O’Grady, M.N.; Luciano, G.; Kerry, J.P.; Jones, E.; Priolo, A.; Newbold, C.J. Characterisation of the ruminal fermentation and microbiome in lambs supplemented with hydrolysable and condensed tannins. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.; Becker, K. Divergence between Purified Hydrolysable and Condensed Tannin Effects on Methane Emission, Rumen Fermentation and Microbial Population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Huang, X.D.; Liang, J.B.; Tan, H.Y.; Yahya, R.; Khamseekhiew, B.; Ho, Y.W. Molecular weight and protein binding affinity of Leucaena condensed tannins and their effects on in vitro fermentation parameters. Anim. Feed Sci. Technol. 2010, 159, 81–87. [Google Scholar] [CrossRef]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Jouany, J.P. Effect of rumen protozoa on nitrogen utilization by ruminants. J. Nutr. 1996, 126, 1335S–1346S. [Google Scholar] [CrossRef] [Green Version]
- Belanche, A.; Newbold, C.J.; Morgavi, D.P.; Bach, A.; Zweifel, B.; Yáñez-Ruiz, D.R. A meta-analysis describing the effects of the essential oils blend agolin ruminant on performance, rumen fermentation and methane emissions in dairy cows. Animals 2020, 10, 620. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Faciola, A.P. Evaluating strategies to reduce ruminal protozoa and their impacts on nutrient utilization and animal performance in ruminants—A meta-analysis. Front Microbiol. 2019, 10, 2648. [Google Scholar] [CrossRef] [Green Version]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the Nature of Tannins on in vitro Ruminal Methane and Volatile Fatty Acid Production and on Methanogenic Archaea and Protozoal Populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [Green Version]
- Saminathan, M.; Gan, H.M.; Abdullah, N.; Wong, C.M.V.L.; Ramiah, S.K.; Tan, H.Y.; Sieo, C.C.; Ho, Y.W. Changes in rumen protozoal community by condensed tannin fractions of different molecular weights from a Leucaena leucocephala hybrid in vitro. J. Appl. Microbiol. 2017, 123, 41–53. [Google Scholar] [CrossRef]
- Saminathan, M.; Ramiah, K.S.; Gan, H.M.; Abdullah, N.; Wong, C.M.V.L.; Ho, Y.W.; Idrus, Z. In vitro study on the effects of condensed tannins of different molecular weights on bovine rumen fungal population and diversity. Ital. J. Anim. Sci. 2019, 18, 1451–1462. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; FAO: Rome, Italy, 2013. [Google Scholar]
- Ugbogu, E.A.; Elghandour, M.M.M.Y.; Ikpeazu, V.O.; Buendía, G.R.; Molina, O.M.; Arunsi, U.O.; Emmanuel, O.; Salem, A.Z.M. The potential impacts of dietary plant natural products on the sustainable mitigation of methane emission from livestock farming. J. Clean. Prod. 2019, 213, 915–925. [Google Scholar] [CrossRef]
- Saminathan, M.; Sieo, C.C.; Gan, H.M.; Abdullah, N.; Wong, C.M.V.L.; Ho, Y.W. Effects of condensed tannin fractions of different molecular weights on population and diversity of bovine rumen methanogenic archaea in vitro, as determined by high-throughput sequencing. Anim. Feed Sci. Technol. 2016, 216, 146–160. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Jiménez-Ocampo, R.; Valencia-Salazar, S.S.; Montoya-Flores, M.D.; Molina-Botero, I.C.; Arango, J.; Gómez-Bravo, C.A.; Aguilar-Pérez, C.F.; Solorio-Sánchez, F.J. Role of Secondary Plant Metabolites on Enteric Methane Mitigation in Ruminants. Front. Vet. Sci. 2020, 7, 584. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem. Rev. 2021. [Google Scholar] [CrossRef]
- Yang, K.; Wei, C.; Zhao, G.Y.; Xu, Z.W.; Lin, S.X. Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. J. Anim. Physiol. Anim. Nutr. 2016, 101, 302–310. [Google Scholar] [CrossRef]
- Kardel, M.; Taube, F.; Schulz, H.; Schütze, W.; Gierus, M. Di_erent approaches to evaluate tannin content and structure of selected plant extracts–review and new aspects. J. Appl. Bot. Food Qual. 2013, 86, 154–166. [Google Scholar] [CrossRef]
- Venter, P.B.; Sisa, M.; Van Der Merwe, M.J.; Bonnet, S.L.; Van Der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 1: The chemical composition of quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phytochemistry 2012, 73, 95–105. [Google Scholar] [CrossRef]
- Guyader, J.; Eugène, M.; Nozière, P.; Morgavi, D.P.; Doreau, M.; Martin, C. Influence of rumen protozoa on methane emission in ruminants: A meta-analysis approach. Animal 2014, 8, 1816–1825. [Google Scholar] [CrossRef]
- Nichols, K.L.; Del Grosso, S.J.; Derner, J.D.; Follett, R.F.; Archibeque, S.L.; Stewart, C.E.; Paustian, K.H. Nitrous oxide and methane fluxes from cattle excrement on C3 pasture and C4-dominated shortgrass steppe. Agric. Ecosyst. Environ. 2016, 225, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Erickson, G.; Klopfenstein, T. Nutritional and management methods to decrease nitrogen losses from beef feedlots. J. Anim. Sci. 2010, 88, E172–E180. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.P.; Cowie, A.L.; Chan, K.Y. Soil Health and Climate Change, 1st ed.; Springer: Berlin, Germany, 2011; p. 403. [Google Scholar]
- Wecking, A.R.; Wall, A.M.; Liáng, L.L.; Lindsey, S.B.; Luo, J.; Campbell, D.I.; Schipper, L.A. Reconciling annual nitrous oxide emissions of an intensively grazed dairy pasture determined by eddy covariance and emission factors. Agric. Ecosyst. Environ. 2020, 287, 106646. [Google Scholar] [CrossRef]
- Avila, S.C.; Kozloski, G.V.; Orlandi, T.; Mezzomo, M.P.; Stefanello, S. Impact of a tannin extract on digestibility, ruminal fermentation and duodenal flow of amino acids in steers fed maize silage and concentrate containing soybean meal or canola meal as protein source. J. Agric. Sci. 2015, 153, 943–953. [Google Scholar] [CrossRef]
- Caetano, M.; Wilkes, M.J.; Pitchford, W.S.; Lee, S.J.; Hynd, P.I. Effect of ensiled crimped grape marc on energy intake, performance and gas emissions of beef cattle. Anim. Feed Sci. Technol. 2019, 247, 166–172. [Google Scholar] [CrossRef]
- Dickhoefer, U.; Ahnert, S.; Susenbeth, A. Effects of quebracho tannin extract on rumen fermentation and yield and composition of microbial mass in heifers. J. Anim. Sci. 2016, 94, 1561–1575. [Google Scholar] [CrossRef] [Green Version]
- Ebert, P.J.; Bailey, E.A.; Shreck, A.L.; Jennings, J.S.; Cole, N.A. Effect of condensed tannin extract supplementation on growth performance, nitrogen balance, gas emissions, and energetic losses of beef steers. J. Anim. Sci. 2017, 95, 1345–1355. [Google Scholar] [CrossRef]
- Jolazadeh, A.; Dehghan-Banadaky, M.; Rezayazdi, K. Effects of soybean meal treated with tannins extracted from pistachio hulls on performance, ruminal fermentation, blood metabolites and nutrient digestion of Holstein bulls. Anim. Feed Sci. Technol. 2015, 203, 33–40. [Google Scholar] [CrossRef]
- Koenig, K.M.; Beauchemin, K.A. Effect of feeding condensed tannins in high protein finishing diets containing corn distillers’ grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 2018, 96, 4398–4413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, K.M.; Beauchemin, K.A.; McGinn, S.M. Feeding condensed tannins to mitigate ammonia emissions from beef feedlot cattle fed high-protein finishing diets containing distillers grains. J. Anim. Sci. 2018, 96, 4414–4430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, W.K.; Gutierrez-Bañuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Martello, H.F.; De Paula, N.F.; Teobaldo, R.W.; Zervoudakis, J.T.; Fonseca, M.A.; Cabral, L.S.; Rocha, J.K.L.; Mundim, A.T.; Moraes, E.H.B.K. Interaction between tannin and urea on nitrogen utilization by beef cattle grazing during the dry season. Livest. Sci. 2020, 234, 103988. [Google Scholar] [CrossRef]
- Mezzomo, R.; Paulino, P.V.R.; Detmann, E.; Valadares Filho, S.C.; Paulino, M.F.; Monnerat, J.P.I.S.; Duarte, M.S.; Silva, L.H.P.; Moura, L.S. Influence of condensed tannin on intake, digestibility, and efficiency of protein utilization in beef steers fed high concentrate diet. Livest. Sci. 2011, 141, 1–11. [Google Scholar] [CrossRef]
- Mezzomo, R.; Paulino, P.V.R.; Barbosa, M.M.; da Silva-Martins, T.; Paulino, M.F.; Alves, K.S.; Gomes, D.I.; Monnerat, J.P.I.S. Performance and carcass characteristics of young cattle fed with soybean meal treated with tannins. Anim. Sci. J. 2016, 87, 775–782. [Google Scholar] [CrossRef]
- Norris, A.B.; Crossland, W.L.; Tedeschi, L.O.; Foster, J.L.; Muir, J.P.; Pinchak, W.E.; Fonseca, M.A. Inclusion of quebracho tannin extract in a high-roughage cattle diet alters digestibility, nitrogen balance, and energy partitioning. J. Anim. Sci. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Pineiro-Vázquez, A.T.; Canul-Solis, J.R.; Jiménez-Ferrer, G.O.; Alayón-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effect of condensed tannins from Leucaena leucocephala on rumen fermentation, methane production and population of rumen protozoa in heifers fed low-quality forage. Asian Australas J. Anim. Sci. 2018, 31, 1738–1746. [Google Scholar] [CrossRef] [Green Version]
- Piñeiro-Vázquez, A.T.; Jiménez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effects of quebracho tannin extract on intake, digestibility, rumen fermentation, and methane production in crossbred heifers fed low-quality tropical grass. Trop. Anim. Health Prod. 2018, 50, 29–36. [Google Scholar] [CrossRef]
- Piñeiro-Vázquez, A.T.; Jiménez-Ferrer, G.O.; Chay-Canul, A.J.; Casanova-Lugo, F.; Díaz-Echeverría, V.F.; Ayala-Burgos, A.J.; Solorio-Sánchez, F.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Intake; digestibility; nitrogen balance and energy utilization in heifers fed low-quality forage and Leucaena leucocephala. Anim. Feed Sci. Technol. 2017, 228, 194–201. [Google Scholar] [CrossRef]
- Poblete, J.B.; Angeles, A.A.; Agbisit, J.E.M.; Espaldon, M.V.O. Response of Growing Dairy Bulls to Dietary Tannin in Rations with Varying Energy Levels. Trop. Anim. Sci. J. 2020, 43, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Méndez, C.R.; Plascencia, A.; Torrentera, N.; Zinn, R.A. Influence of tannins supplementation on growth performance, dietary net energy and carcass characteristics of yearling steers fed finishing diet containing dried distillers grains with solubles. Indian J. Anim. Sci. 2016, 86, 108–111. [Google Scholar]
- Shakeri, P.; Riasi, A.; Alikhani, M.; Fazaeli, H.; Ghorbani, G.R. Effects of feeding pistachio by-products silage on growth performance, serum metabolites and urine characteristics in Holstein male calves. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, P.; Riasi, A.; Alikhani, M. Effects of long period feeding pistachio by-product silage on chewing activity, nutrient digestibility and ruminal fermentation parameters of Holstein male calves. Animal 2014, 8, 1826–1831. [Google Scholar] [CrossRef] [Green Version]
- Tabke, M.C.; Sarturi, J.O.; Galyean, M.L.; Trojan, S.J.; Brooks, J.C.; Johnson, B.J.; Martin, J.; Baggerman, J.; Thompson, J. Effects of tannic acid on growth performance, carcass characteristics, digestibility, nitrogen volatilization, and meat lipid oxidation of steers fed steam-flaked corn–based finishing diets. J. Anim. Sci. 2017, 11, 5124–5136. [Google Scholar] [CrossRef] [Green Version]
- Tseu, R.J.; Junior, F.P.; Carvalho, R.F.; Sene, G.A.; Tropaldi, C.B.; Peres, A.H.; Rodrigues, P.H.M. Effect of tannins and monensin on feeding behaviour, feed intake, digestive parameters and microbial efficiency of nellore cows. Ital. J. Anim. Sci. 2020, 19, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Yuste, S.; Amanzougarene, Z.; de la Fuente, G.; de Vega, A.; Fondevila, M. Rumen protozoal dynamics during the transition from milk/grass to high-concentrate based diet in beef calves as affected by the addition of tannins or medium-chain fatty acids. Anim. Feed Sci. Technol. 2019, 257, 114273. [Google Scholar] [CrossRef]
- Zhou, K.; Bao, Y.; Zhao, G. Effects of dietary crude protein and tannic acid on rumen fermentation, rumen microbiota and nutrient digestion in beef cattle. Arch. Anim. Nutr. 2019, 73, 30–43. [Google Scholar] [CrossRef]






| Parameter | NC | Mean | Median | Minimum | Maximum | SD | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Features | Control | Tannin | Control | Tannin | Control | Tannin | Control | Tannin | Control | Tannin | |
| Forage g/kg DM | 105 | 506.9 | 509.1 | 498.0 | 425.0 | 50.0 | 30.0 | 1000 | 1000 | 358.9 | 372.5 |
| DM, g/kg | 80 | 647.8 | 645.7 | 700.0 | 702.5 | 256.0 | 256.0 | 927.0 | 928.0 | 211.7 | 210.5 |
| OM, g/kg DM | 50 | 927.5 | 928.3 | 936.0 | 936.4 | 835.1 | 835.8 | 953.0 | 953.0 | 30.24 | 30.10 |
| CP, g/kg DM | 105 | 124.4 | 129.0 | 132.5 | 134.5 | 30.10 | 30.10 | 204.0 | 205.0 | 40.60 | 36.46 |
| EE, g/kg DM | 61 | 38.31 | 39.52 | 32.10 | 35.50 | 17.50 | 17.50 | 61.0 | 61.0 | 13.98 | 13.68 |
| NDF, g/kg DM | 97 | 430.5 a | 423.7 b | 409.0 | 404.4 | 163.0 | 163.0 | 763.5 | 770.0 | 177.2 | 172.8 |
| ADF, g/kg DM | 73 | 259.1 | 259.7 | 226.5 | 224.5 | 82.10 | 82.10 | 468.5 | 487.0 | 117.7 | 120.2 |
| Starch, g/kg DM | 31 | 364.8 | 362.9 | 415.8 | 422.6 | 48.0 | 23.0 | 575.0 | 575.0 | 180.6 | 183.3 |
| Ca, g/kg DM | 41 | 6.18 | 6.33 | 5.55 | 6.15 | 5.30 | 5.30 | 7.50 | 7.50 | 0.836 | 0.792 |
| P, g/kg DM | 41 | 4.11 | 4.10 | 4.20 | 4.10 | 3.60 | 3.60 | 4.50 | 4.50 | 0.319 | 0.313 |
| Tannin, g/kg DM | 105 | - | 14.61 | - | 12.10 | - | 0.46 | - | 60 | - | 12.29 |
| Duration, days | 99 | 93 | 90 | 28 | 180 | 33.38 | |||||
| Extracted response parameters | |||||||||||
| FBW, kg | 31 | 457.5 | 458.2 | 443.5 | 437 | 189.5 | 204.3 | 621 | 616 | 122.1 | 122.6 |
| DMI, kg/d | 73 | 8.357 | 8.136 | 8.20 | 7.84 | 3.80 | 3.60 | 12.60 | 12.76 | 2.267 | 2.456 |
| OMI, kg/d | 46 | 6.837 | 6.820 | 6.540 | 6.690 | 1.185 | 1.155 | 12.440 | 12.480 | 2.321 | 2.391 |
| CPI, kg/d | 26 | 0.828 | 0.957 | 0.705 | 0.990 | 0.194 | 0.167 | 2.090 | 2.200 | 0.513 | 0.494 |
| EEI, kg/d | 8 | 0.232 | 0.232 | 0.170 | 0.180 | 0.160 | 0.150 | 0.410 | 0.390 | 0.096 | 0.098 |
| NDFI, kg/d | 38 | 3.679 | 3.524 | 3.760 | 3.740 | 1.810 | 1.900 | 4.630 | 4.730 | 0.959 | 0.835 |
| ADFI, kg/d | 17 | 2.521 | 2.453 | 2.850 | 2.500 | 1.260 | 1.280 | 3.500 | 3.620 | 0.674 | 0.664 |
| ADG, kg/d | 37 | 1.258 | 1.273 | 1.370 | 1.320 | 0.018 | 0.120 | 2.080 | 2.140 | 0.589 | 0.545 |
| FE, kg/kg | 22 | 0.153 | 0.150 | 0.163 | 0.159 | 0.092 | 0.092 | 0.206 | 0.198 | 0.037 | 0.033 |
| DMD, g/kg DM | 49 | 622.0 | 594.3 | 628.0 | 601.9 | 411.9 | 428.5 | 810.5 | 797.7 | 77.57 | 95.08 |
| OMD, g/kg DM | 59 | 660.1 | 632.0 | 660.0 | 646.3 | 451.7 | 442.0 | 820.0 | 810.0 | 84.48 | 100.4 |
| CPD, g/kg DM | 43 | 571.6 | 541.2 | 679.0 | 635.0 | 276.2 | 79.57 | 767.6 | 770.9 | 183.9 | 226.1 |
| EED, g/kg DM | 23 | 689.4 | 679.4 | 713.0 | 699.0 | 447.0 | 435.0 | 857.3 | 891.0 | 112.7 | 116.7 |
| NDFD, g/kg DM | 47 | 561.4 | 534.9 | 576.0 | 518.1 | 385.0 | 405.0 | 771.0 | 776.9 | 90.11 | 80.54 |
| ADFD, g/kg DM | 24 | 494.1 | 415.4 | 532.0 | 413.6 | 403.0 | 219.1 | 549.1 | 561.0 | 54.72 | 88.33 |
| Ruminal pH | 57 | 6.637 | 6.621 | 6.700 | 6.680 | 5.810 | 5.890 | 7.190 | 7.430 | 0.337 | 0.356 |
| NH3-N, mg/dL | 57 | 11.25 | 10.59 | 10.63 | 8.16 | 2.48 | 1.73 | 30.40 | 36.50 | 6.338 | 7.673 |
| Total VFA, mM | 54 | 84.72 | 86.49 | 74.01 | 78.42 | 35.80 | 32.72 | 158 | 141 | 29.30 | 28.81 |
| Acetate, % molar | 54 | 60.39 | 60.67 | 67.80 | 66.09 | 31.42 | 38.41 | 74.10 | 74.40 | 11.54 | 10.41 |
| Propionate, % molar | 54 | 19.39 | 19.85 | 18.74 | 18.47 | 6.58 | 9.25 | 36.80 | 38.0 | 6.987 | 6.505 |
| Butyrate, % molar | 54 | 11.94 | 12.38 | 10.33 | 11.70 | 6.10 | 5.30 | 19.40 | 19.77 | 3.799 | 3.866 |
| Protozoa, log10/mL | 26 | 5.508 | 5.306 | 5.480 | 5.595 | 1.310 | 0.930 | 11.90 | 10.60 | 3.540 | 3.161 |
| CH4, L/d | 26 | 150.6 | 135.7 | 128.8 | 107.0 | 44.16 | 29.20 | 331.7 | 302.4 | 80.86 | 88.44 |
| CH4, L/DMI | 28 | 19.93 | 18.76 | 20.10 | 14.78 | 5.60 | 5.43 | 31.22 | 51.80 | 9.35 | 12.38 |
| UNE, g/d | 35 | 56.64 | 54.95 | 54.80 | 46.0 | 4.30 | 9.0 | 168.0 | 167.0 | 47.88 | 44.48 |
| FNE, g/d | 31 | 57.10 | 66.73 | 49.88 | 62.0 | 16.20 | 19.50 | 126.0 | 146.0 | 32.64 | 38.04 |
| NUE, % | 22 | 25.76 | 20.75 | 25.34 | 16.45 | 16.89 | 6.20 | 39.15 | 39.0 | 7.43 | 11.62 |
| Variable | N | NC | SMD | SE | 95% CI | p-Value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Q | p-Value | I2 (%) | ||||||
| Final bodyweight | 11 | 31 | −0.041 | 0.102 | −0.241 | 0.158 | 0.68 | 38.642 | 0.13 | 22.36 |
| Dry matter intake | 25 | 73 | −0.010 | 0.078 | −0.163 | 0.144 | 0.90 | 102.879 | <0.05 | 30.01 |
| Organic matter intake | 16 | 46 | 0.062 | 0.086 | −0.106 | 0.230 | 0.47 | 22.526 | 0.99 | 0 |
| Crude protein intake | 9 | 26 | 0.321 | 0.171 | −0.014 | 0.657 | 0.06 | 46.693 | <0.05 | 46.46 |
| Ether extract intake | 3 | 8 | −0.026 | 0.241 | −0.499 | 0.447 | 0.91 | 6.723 | 0.45 | 0 |
| Neutral detergent fiber intake | 14 | 38 | −0.167 | 0.096 | −0.355 | 0.022 | 0.08 | 20.042 | 0.99 | 0 |
| Acid detergent fiber intake | 6 | 17 | −0.189 | 0.135 | −0.453 | 0.075 | 0.16 | 4.241 | 0.99 | 0 |
| Average daily gain | 13 | 37 | 0.059 | 0.083 | −0.104 | 0.222 | 0.47 | 35.49 | 0.49 | 0 |
| Feed efficiency | 7 | 22 | −0.287 | 0.150 | −0.581 | 0.007 | 0.06 | 43.045 | <0.05 | 51.21 |
| Parameter | N | NC | SMD | SE | 95% CI | p-Value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Q | p-Value | I2 (%) | ||||||
| Dry matter digestibility | 17 | 49 | −0.589 | 0.124 | −0.833 | −0.346 | <0.001 | 97.833 | <0.001 | 50.94 |
| Organic matter digestibility | 21 | 59 | −0.612 | 0.108 | −0.825 | −0.400 | <0.001 | 108.599 | <0.001 | 46.59 |
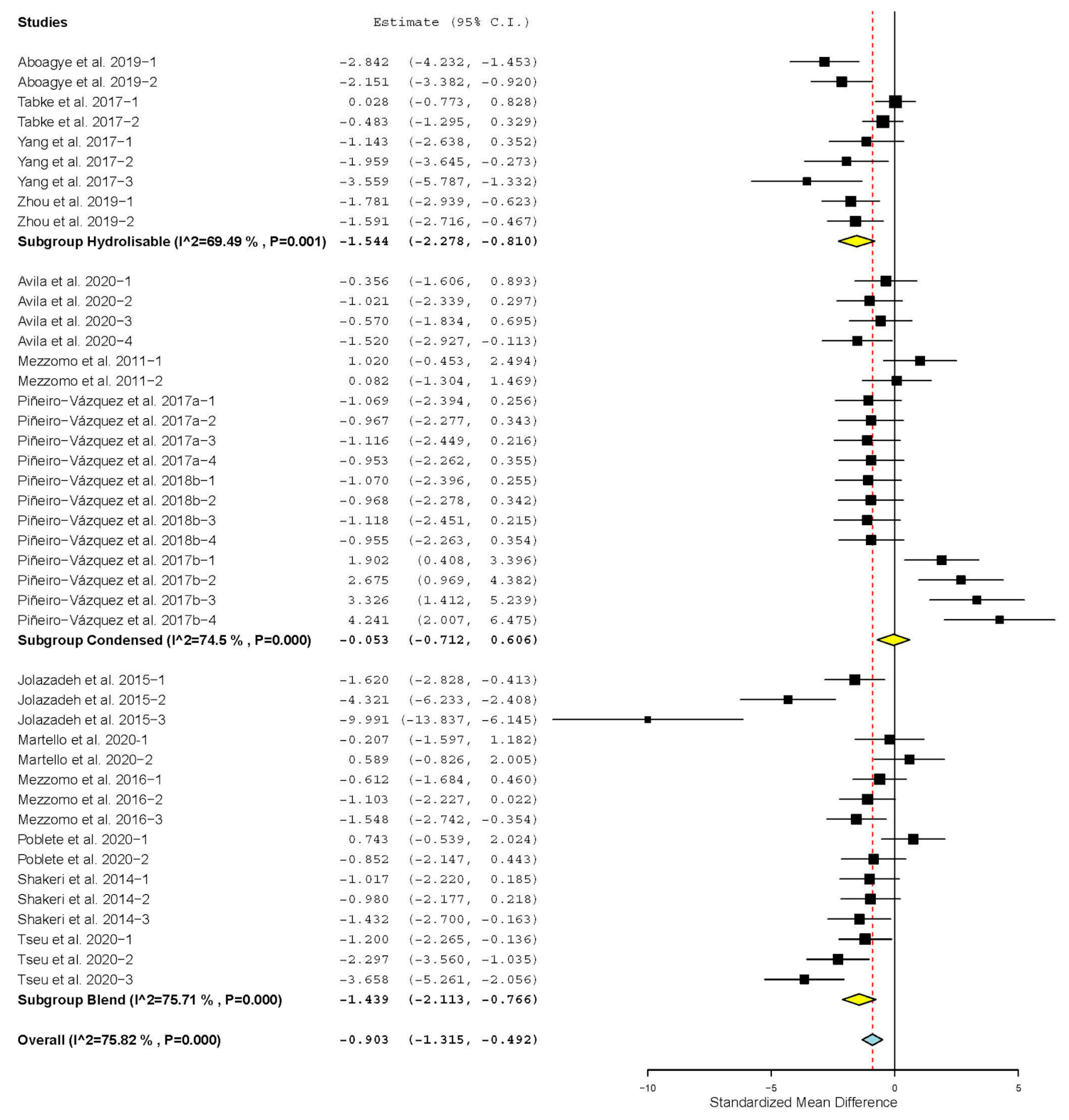
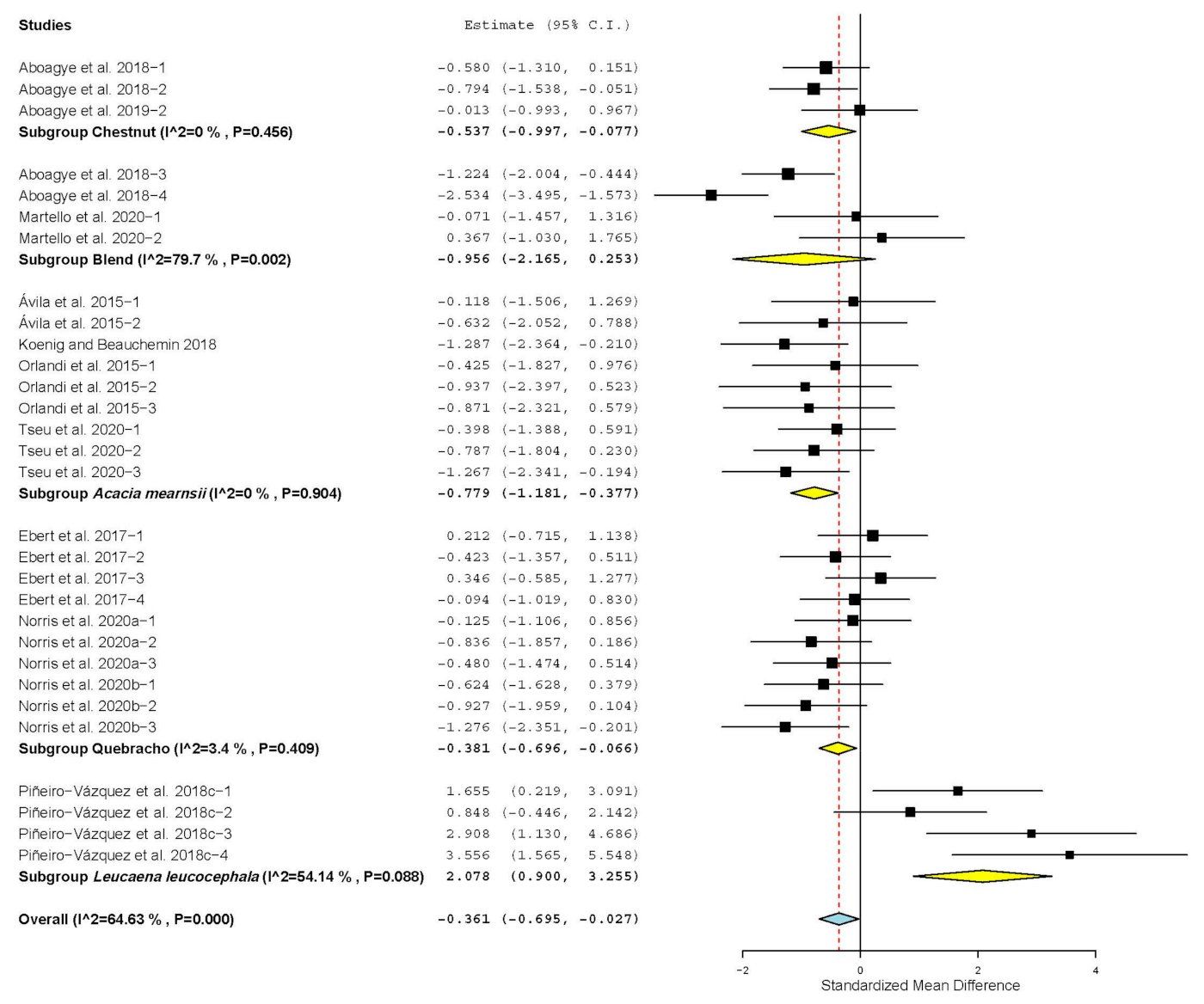
| Crude protein digestibility | 15 | 43 | −0.903 | 0.210 | −1.315 | −0.492 | <0.001 | 173.687 | <0.001 | 75.82 |
| Ether extract digestibility | 8 | 23 | −0.328 | 0.215 | −0.750 | 0.094 | 0.12 | 61.615 | <0.001 | 64.29 |
| NDFD | 18 | 47 | −0.370 | 0.150 | −0.644 | −0.076 | 0.01 | 127.334 | <0.001 | 63.87 |
| ADFD | 9 | 24 | −0.716 | 0.151 | −1.012 | −0.419 | <0.001 | 37.107 | <0.05 | 38.02 |
| Ruminal pH | 20 | 57 | −0.171 | 0.099 | −0.364 | 0.022 | 0.08 | 98.287 | <0.001 | 43.02 |
| Ruminal NH3-N | 20 | 57 | −0.508 | 0.128 | −0.759 | −0.258 | <0.001 | 148.223 | <0.001 | 62.22 |
| Total VFA | 19 | 54 | 0.021 | 0.124 | −0.223 | 0.265 | 0.86 | 139.359 | <0.001 | 61.97 |
| Acetate | 19 | 54 | 0.041 | 0.115 | −0.184 | 0.267 | 0.72 | 120.090 | <0.001 | 55.87 |
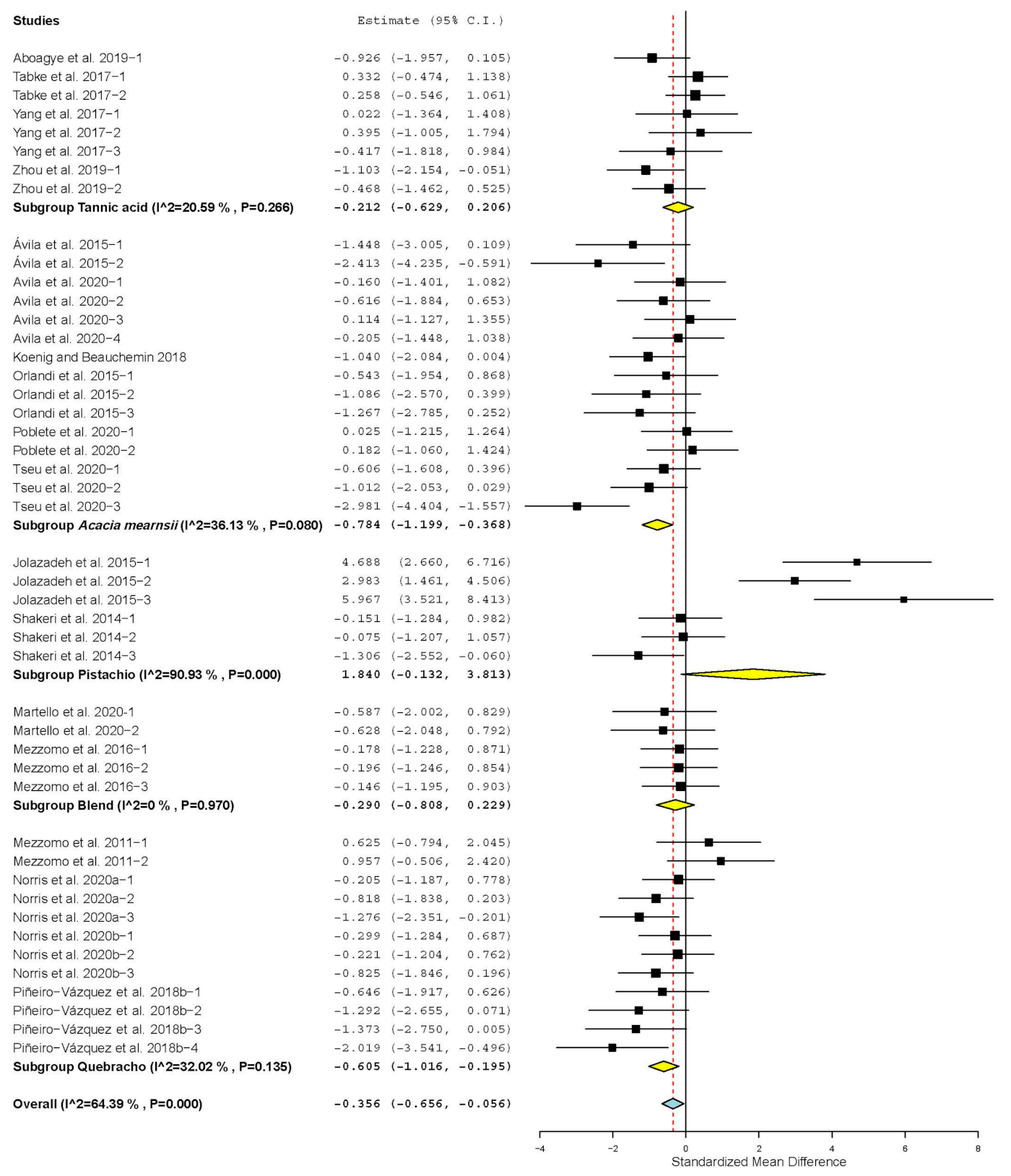
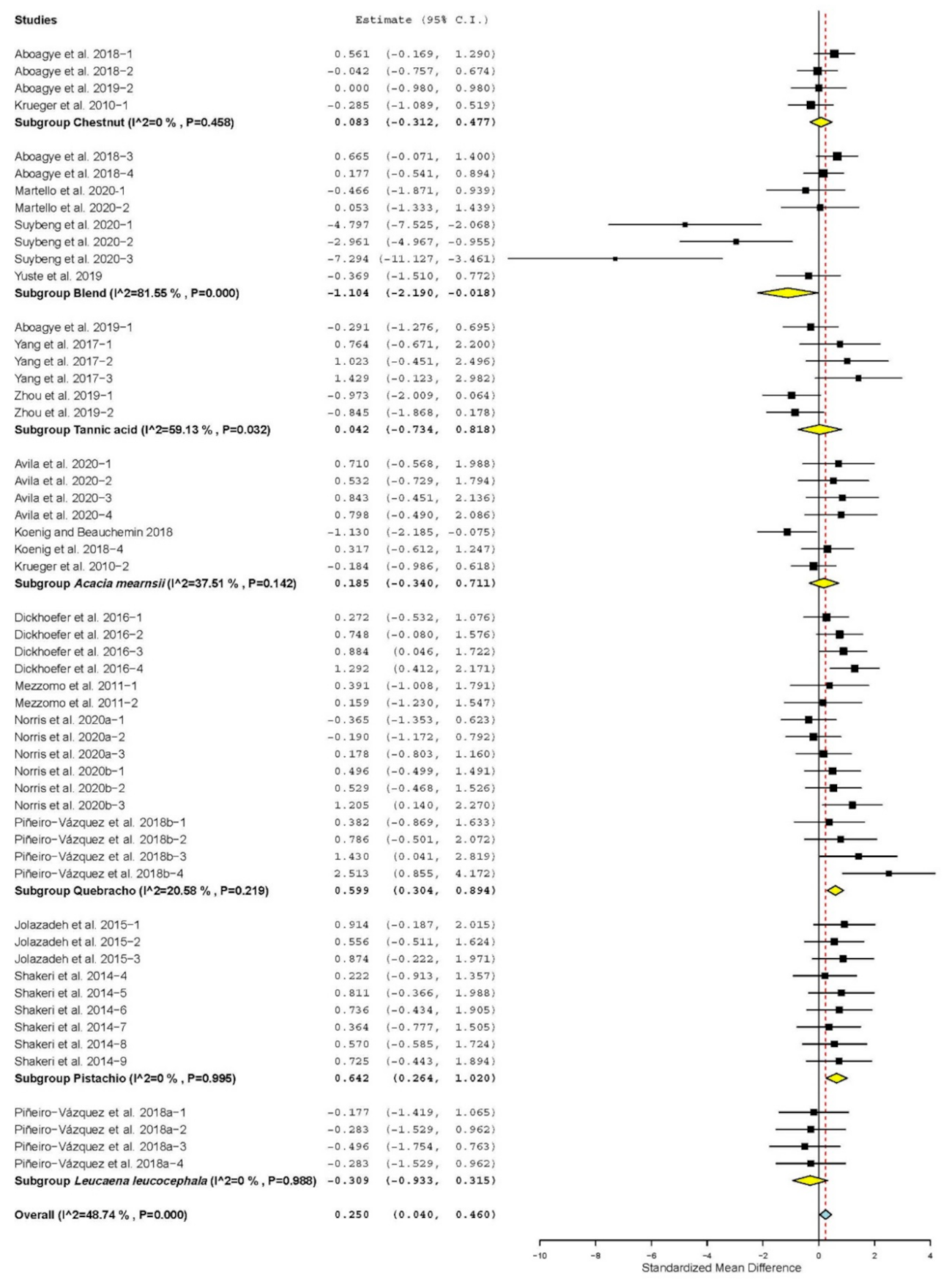
| Propionate | 19 | 54 | 0.250 | 0.107 | 0.040 | 0.460 | 0.02 | 103.404 | <0.001 | 48.74 |
| Butyrate | 19 | 54 | 0.198 | 0.079 | 0.042 | 0.354 | 0.01 | 61.204 | 0.20 | 13.40 |
| Protozoa | 8 | 26 | −0.745 | 0.397 | −1.523 | 0.033 | 0.06 | 235.732 | <0.001 | 89.39 |
| Methane emissions/day | 9 | 26 | −0.474 | 0.155 | −0.178 | −0.171 | 0.002 | 50.007 | <0.05 | 48.01 |
| Methane emissions/unit of DMI | 10 | 28 | −0.408 | 0.155 | −0.712 | −0.105 | 0.008 | 56.848 | <0.001 | 52.50 |
| Urinary nitrogen excretion | 12 | 35 | −0.338 | 0.149 | −0.630 | −0.046 | 0.023 | 83.931 | <0.001 | 59.49 |
| Fecal nitrogen excretion | 11 | 31 | 0.860 | 0.138 | 0.589 | 1.131 | <0.001 | 48.304 | 0.018 | 37.89 |
| Nitrogen use efficiency | 8 | 22 | −0.273 | 0.262 | −0.786 | 0.239 | 0.296 | 75.726 | <0.001 | 72.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Hernández-García, P.A. Effects of Dietary Tannins’ Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis. Sustainability 2021, 13, 7410. https://doi.org/10.3390/su13137410
Orzuna-Orzuna JF, Dorantes-Iturbide G, Lara-Bueno A, Mendoza-Martínez GD, Miranda-Romero LA, Hernández-García PA. Effects of Dietary Tannins’ Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis. Sustainability. 2021; 13(13):7410. https://doi.org/10.3390/su13137410
Chicago/Turabian StyleOrzuna-Orzuna, José Felipe, Griselda Dorantes-Iturbide, Alejandro Lara-Bueno, Germán David Mendoza-Martínez, Luis Alberto Miranda-Romero, and Pedro Abel Hernández-García. 2021. "Effects of Dietary Tannins’ Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis" Sustainability 13, no. 13: 7410. https://doi.org/10.3390/su13137410
APA StyleOrzuna-Orzuna, J. F., Dorantes-Iturbide, G., Lara-Bueno, A., Mendoza-Martínez, G. D., Miranda-Romero, L. A., & Hernández-García, P. A. (2021). Effects of Dietary Tannins’ Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis. Sustainability, 13(13), 7410. https://doi.org/10.3390/su13137410






