1. Introduction
Grapes are one of the world’s largest fruit crops, with over 75 million tons grown annually, primarily as
Vitis vinifera L. for wine production. According to Food and Agriculture Organization (FAO) statistics, wine production is substantial for the Greek economy, ranking as the country’s second most profitable industry after olives in 2015 and 2016. [
1]. Approximately 35.9 million tons of industry by-products arise from wine, while the rest arise from grape juice processing [
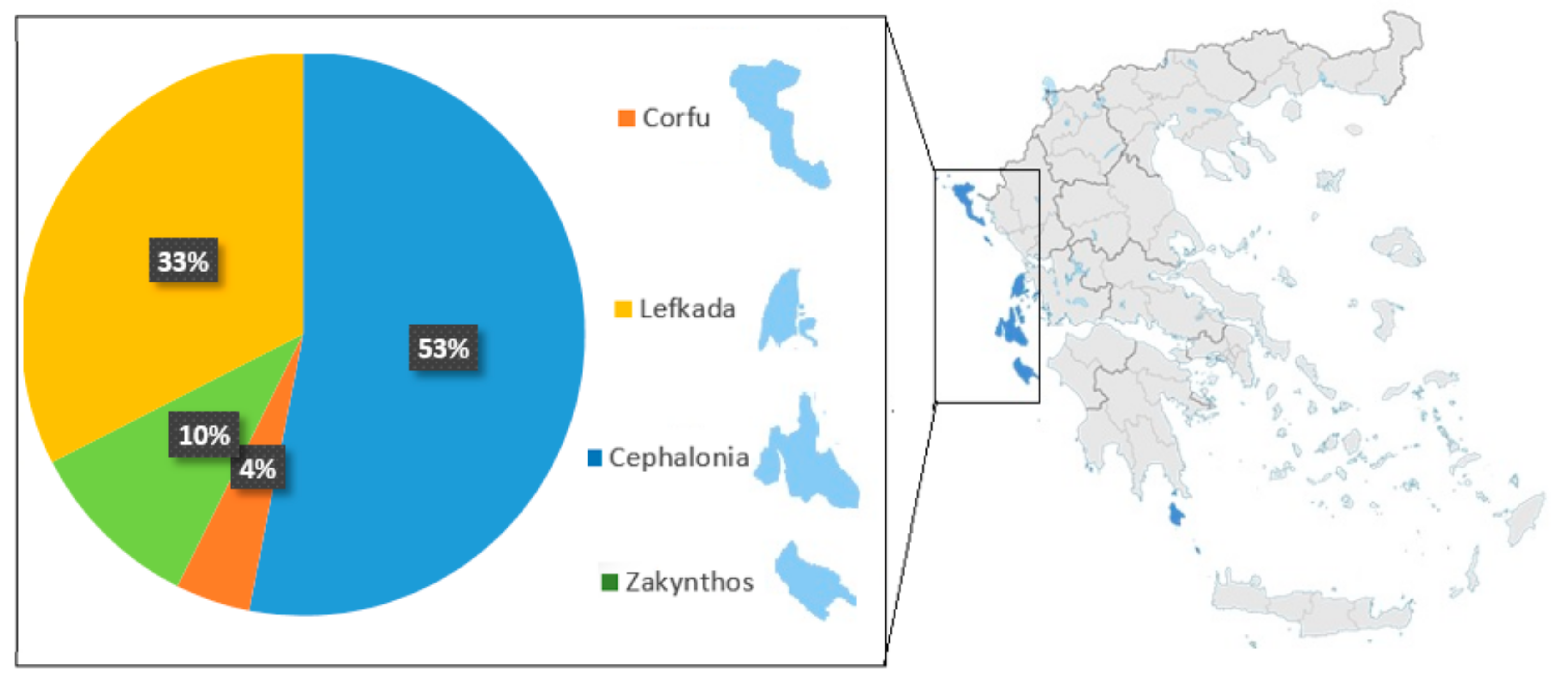
2]. The Ionian Islands produced 1179.25 tons of wine grapes in 2017, according to the Ministry of Rural Development and Food, generating 175.12 tons of grape pomace [
3]. All these by-products could acquire greater potential value if they are valorized properly.
The wine industry generates a significant amount of solid waste, which is primarily disposed of in the environment, causing economic and environmental problems [
4,
5]. Winery by-product wastes are produced continuously throughout the year and may be hazardous to the environment [
6]. By-products are typically characterized by high levels of chemical oxygen demand (COD) and biodegradability [
7]. Specifically, grape pomace consists mainly of grape seeds and skins and they remain after pressing and the fermentation during vinification processes.
Grape seeds are a valuable source of oily constituents like sterols, triglycerides, and fatty acids. Polyunsaturated and monounsaturated fatty acids (PUFAs and MUFAs) are abundant in grape seed oils, with PUFAs accounting for the majority of fatty acids. The yield of oil can range from 5.85 to 22.4 percent (
w/
w), and it is dependent on the cultivar, variety, and year-to-year variations in extraction methods [
8,
9,
10]. Grape skins are characterized by high-phenolic contents. The phenolic composition of grapes varies depending on grape variety and vinification conditions. The major phenolic compounds are anthocyanins, catechins, flavonol glycosides, phenolic acids, and stilbenes [
11]. These compounds are responsible for some of the most essential wine characteristics while also acting as antioxidants. [
12]. Many studies have evaluated the methods for the valorization of winery by-products. Antioxidant and health-promoting practices were the subject of these studies [
4]. Phenolic compounds react to the free radicals and neutralize them with beneficial anti-inflammatory, cardioprotective, and anticarcinogenic effects [
13]. The extraction of phenolic compounds from by-products can be done with many organic solvents such as methanol, ethanol, acetone, and ethyl acetate [
12].
In general, islands are sensitive systems compared to the mainland. This is due to a variety of factors such as their small size, peculiar environment, unique climate, and relative isolation from the mainland. The small size, in terms of area and population, implies a limited variety and quantity of natural resources as well as fewer opportunities for large-scale productive activities. Furthermore, the distance from the urban centers combined with the traffic difficulties caused by the sea has a substantial impact on the degree of isolation. As a result of these characteristics, vulnerable environments with unpredictable environmental factors and minimal development capacity have emerged. A circular economy approach could contribute positively to the solution of the insularity problem. Materials and products must be reused, repaired, renewed, and recycled as part of the transition to a cyclical bioeconomy. Materials which were considered “by-products” can be turned into raw materials. Strengthening collaboration across the supply chain will help eliminate costs, waste, and environmental impact. Developments in environmental innovation ensure new products, processes, technologies, and organizational structure.
Valorization of a by-products’ biomass for the recovery of phytochemicals should include processes that generate far less or even zero further by-products. Otherwise, no concept of “green” or “sustainable” could be substantiated. As a result, research should focus on the discovery and design of extraction processes that allow for the use of alternative solvents and sustainable natural resources while still ensuring a healthy and high-quality extract/product. [
14]. Glycerol is a bio-liquid considered a by-product of the biodiesel industry and simultaneously has not been used widely for extraction purposes. In addition, it constitutes a green and well-established sustainable solvent [
15,
16]. Utilization of grape pomace as a whole product or combination with green solvents to extract bioactive compounds can be used in the food and cosmetic industry resulting in high added-value end products [
17,
18,
19].
The aim of this research was to find a cost-effective solution for managing winery by-products while also supporting the circular bioeconomy. A quality analysis of grape pomace was conducted for this reason. Nontoxic, environmentally friendly solvents were used to extract the extracts, and the fractions were tested for antioxidant activity. For this purpose, traditional grape varieties of the Ionian Islands were selected. Finally, it should be highlighted that analyses were based on by-products just as they were taken from the wineries to provide a realistic view and the promotion of the cyclical bioeconomy. This research will also serve as a valuable contribution to a deeper investigation of the understudied topic of sustainable waste management of agricultural by-products in the Ionian Islands.
2. Materials and Methods
2.1. Chemicals
All the solvents used were extra purity (>99.5%) including water, glycerol and n-hexane, cyclohexane, and methanol. Phenolic standards with a purity of 98–99% (cinnamic acid, gallic acid, caftaric acid, catechin and epicatechin, epicatechin gallate, rutin, quercetin, kaempferol-3-glucoside, p-coumaric acid, and isorharmentin-3-glucoside) were purchased from Aldrich (Steinheim, Germany) and used for identification in MS. Folin–Ciocalteu reagent was used for TPC measurement, as well as caffeic acid. 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), which were used for the free radicals preparation tests, as well as Trolox.
2.2. Plant Material
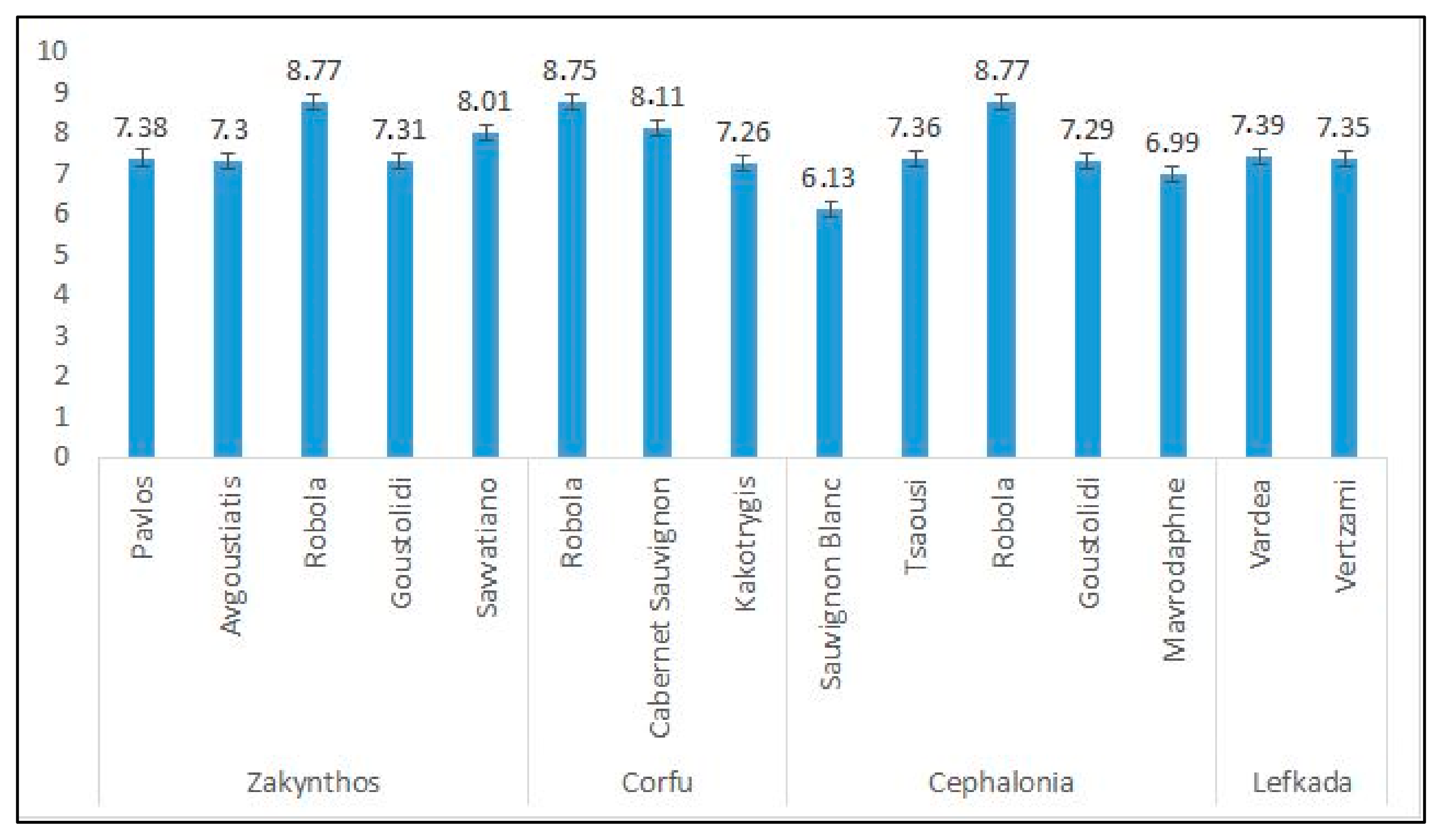
Winery by-products were provided by Gentillini Winery and Vineyards, the Robola Cooperative of Cephalonia, Ktima Grampsa, Ktima Theotoki, and Robotis wineries. Thirty-six samples were analyzed and some of them came from the PDO Robola in Cephalonia. The rest of them were varieties from Pavlos, Avgoustiatis, Robola, Goustolidi, Savvatiano, Cabernet Sauvignon, Kakotrygis, Sauvignon Blanc, Tsaousi, Mavrodaphne, Vardea, and Vertzami.
2.3. Moisture Removal
The initial moisture of crude grape pomace was estimated and expressed in % (w/w). The average moisture was estimated at 73%. The drying process of samples was then carried out in dark on large non-absorbent surfaces at 35 °C assisted by airflow until a final moisture content of 13% was achieved. The duration of the drying process was two days on average and followed by the separation of grape seeds from the skins using a series of sieves. Ratio of skins to seeds for every sample were also specified. Seeds and skins of each sample were sealed in polypropylene bags and stored at −20 °C until their usage.
2.4. Oil Extraction from Grape Seeds
Grape seeds were powdered with a mixer (Philips HR 2074, N.V., Amsterdam, The Netherlands) for 20 s. The crushed grape seed powder was continuously extracted with n-hexane in a soxhlet apparatus at 70 °C for 6 h. The analogy of crushed seeds to n-hexane was 1 to 10 (w/v). Then, the n-hexane fraction was evaporated to dryness under reduced pressure at 35 °C and residuals removed under nitrogen flow.
2.5. Phenolic Extraction from Grape Skins
The extraction of the phenolic compounds was performed using the solid-liquid extraction technique. Before extraction, grape skins were powdered with a mixer (Philips HR 2074, N.V., Amsterdam, The Netherlands) for 2 min with 15 s rest periods to avoid overheating. A defatting process was performed using 1:10 (
w/
v) n-hexane [
12]. Then, defatted samples were extracted with a solvent mixture of water:glycerol (80:20
v/
v) at 600 rpm for 60 min at room temperature (25 °C). All processes were done in triplicate and fractions were filtered through a 0.45 μm filter and stored at −20 °C until further analysis.
2.6. Determination of Total Phenolic Content
TPC was estimated using the Folin–Ciocalteu reagent [
20]. In the case of grape seed oil, 0.1 mL oil was diluted with deionized water to 5 mL in a 10 mL volumetric flask and the addition of 0.5 mL Folin–Ciocalteu reagent. After 3 min, 1 mL of saturated (Na
2CO
3 20%
w/
v) solution was added. The content was mixed and diluted to volume with water and after 1 h measured at 765 nm.
In the case of the grape skin extract, in well plated, 1.5 mL of deionized water, 25 μL of the sample, and 125 μL of Folin–Ciocalteu reagent were added and stirred well. At the end of 3 min we added 375 μL of sodium carbonate solution and 475 μL of deionized water. After 2 h it was measured at 765 nm.
The TPC concentration (C
TPC) was calculated and expressed as mg gallic acid equivalents per mL extract (mg GAE mL
−1) (y = 0.0012x + 0.012; R
2 = 0.9967;
Figure S1). TPC yield (
YTPC) was calculated as mg gallic acid equivalents per g of dry weight (mg GAE g
−1), using the following equation:
where (
V) is the volume of the extraction and (
m) the dry weight of plant material (g).
2.7. Antioxidant Activity of Grape Seed Oils and Grape Skin Extracts
Antioxidant activity was estimated using DPPH and ABTS assays and A
AR was also calculated [
21,
22,
23,
24,
25]. Grape seed oil was diluted in ethyl acetate (1:10) and 1 mL of the solution was added to 4 mL of DPPH solution (0.08 mM). Instead of oil, the control was made with ethyl acetate. After 30 min in the dark, the absorbance was measured at 515 nm. In the case of grape skin extract, 3 mL of DPPH solution were added to 30 μL of each sample. The solutions were vortexed and kept at room temperature in the dark for 30 min. Absorbance was measured in 515 nm as well. A
AR was calculated as described above [
24] and is shown in Equation (3).
The ABTS was prepared by the reaction of 25 mL of ABTS solution (7 mM) with 440 μL of potassium persulfate (140 mM). The solution was left at room temperature for 16–18 h. The solution was then diluted with ethanol to obtain an absorbance of 0.7 ± 0.2 at 734 nm. A total of 100 μL of each grape seed oil was mixed with 2 mL of ABTS, and the absorbance was measured after 6 min at 734 nm. Additionally, 30 μL of each grape skin extract was mixed with 3 mL of ABTS, and the absorbance was measured after 6 min at 734 nm as well.
All measurements were expressed as mg Trolox equivalents g
−1 dry weight (DPPH: y = 11.554x − 2.3777; R
2 = 0.9947;
Figure S2) (ABTS: y = 18.248 + 1.4155; R
2 = 0.9907;
Figure S3). Furthermore, A
AR was calculated as previously described and is shown in Equation (3). A
AR is expressed as μmol of DPPH g
−1 of dry weight. The percentage of inhibition was calculated according to the following formula:
where
CDPPH is the initial molar concentration of DPPH (μmol L
−1),
A515(f) is the sample’s absorbance, and
A515(i) is the absorbance of the blank sample.
2.8. Analysis of FAMEs in Grape Seed Oils by GC-MS
The analysis of FAMEs was performed using a Trace Ultra gas chromatograph (GC) (Thermo Scientific Inc., Waltham, MA, USA), coupled to a mass spectrometer (MS) (DSQII, Thermo Scientific Inc., Waltham, MA, USA). The column used was a TR-5MS (30 m × 0.25 mm i.d., 0.25 μm film thickness) and the carrier gas was helium, at a 1 mL min
−1 rate. The analysis was performed according to the literature [
9] with some modifications. The oven temperature was adapted to 110 °C and then was increased at 205 °C at a rate of 4 °C min
−1, followed by an increment of 1 °C min
−1 up to 215 °C and, up to 250 °C with a step of 4 °C min
−1. Finally, the temperature of 250 °C was kept constant for 15 min. The transfer line and injector temperatures were maintained at 260 and 220 °C, respectively. The injection volume was 1 μL in a split-less mode. The peak identification was carried out with the Wiley 275 mass spectra library, its mass-spectral data, and arithmetic index provided by Adams 0.7 HP.
2.9. Spectroscopic Indices (K232, K268, K270, ΔK)
K232, K268, and K270 extinction coefficients were measured from the absorption of the samples in the UV region at 232, 268, and 270 nm respectively, with a UV-Vis (Cary 60, Agilent spectrophotometer). The samples were prepared according to ISO 3656:2011.
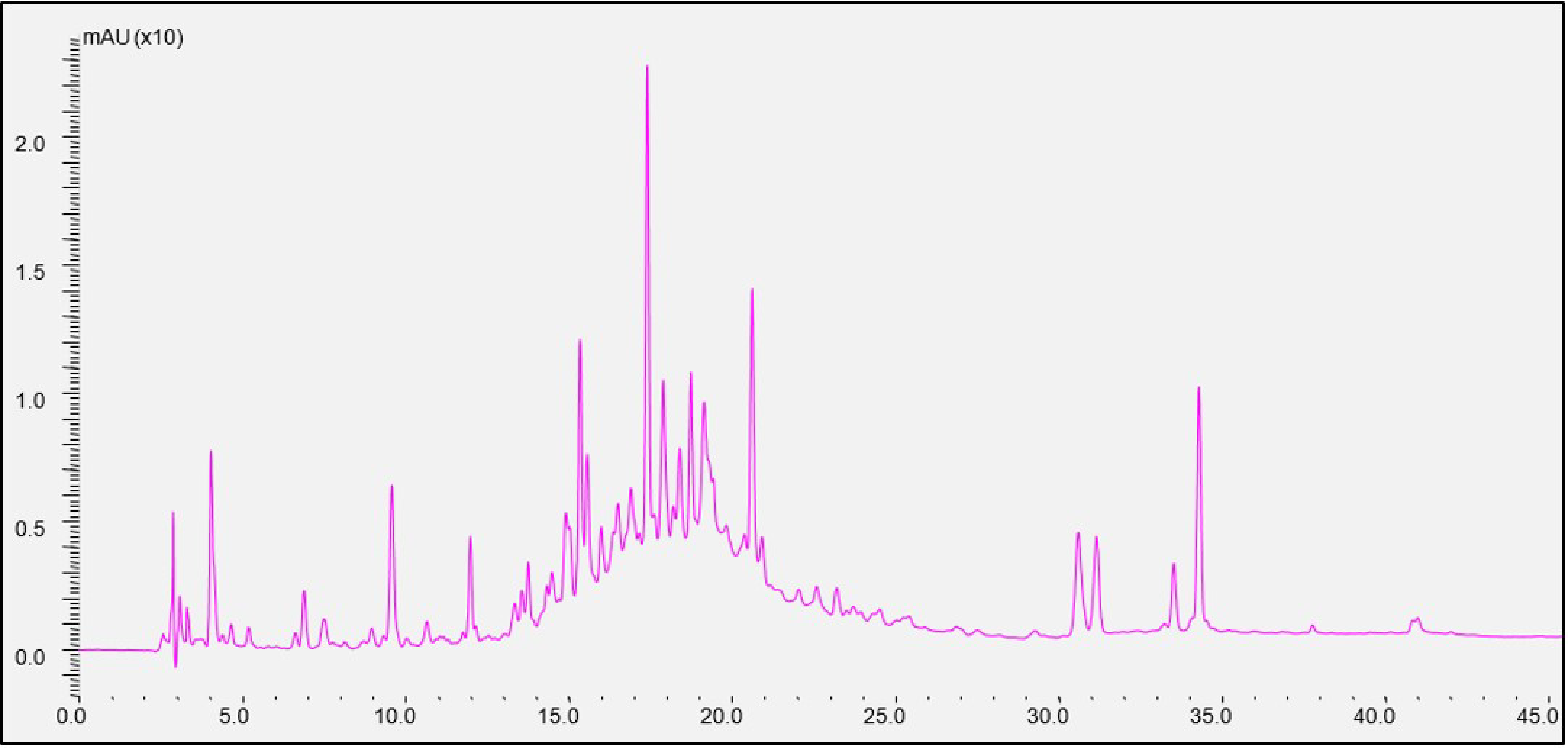
2.10. HPLC-DAD and LC-MS Analysis
Phenolic extracts were analyzed on high-pressure liquid chromatography (HPLC) Agilent 1100 series (Agilent Corporation, California, MA, USA) with a diode array detector (DAD). The system was connected to a computer and HP Chemstation software.
They were also analyzed on a Shimadzu LC/MS-2010A (Kyoto, Japan) equipped with an LC-10ADvp binary pump, a DGU-14A degasser, a SIL-10ADvp autosampler, an SPD-M10Avp Photo Diode Array Detector, and a quadrupole mass detector (MSD) with an electron spray ion source (MS-ESI, Electrospray Ionization). The system was connected to a computer and Shimatzu version 3.40.307 software for chromatographic processing. The detector was set to negative ion operation mode under these conditions: ionization source temperature CDL (curved desolvation line): 300 °C, mist gas flow (N2): 1.5 L min−1, drying gas pressure (N2): 0.1 MPa (10 L min−1 flow), heat block temperature: 300 °C, mist area potential: −2.5 kV, CDL voltage −20 V, detector voltage: −1.55 kV, scan area: 50–1000 m/z, and scan speed: 6000 amu s−1.
A reversed-phase column Supelco (Discovery HS C18) (Darmstadt, Germany), length 250 mm, internal diameter 4 mm with material porosity of 5 μm was used and it eluted the analytes at a flow rate of 1 mL min
−1. The following gradient of mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in methanol) was used for the analysis. The program followed was 0–1 min, 5% solvent (B), 1–5 min, 10% solvent (B), 6–15 min, 33% solvent (B), 16–25 min, 41% solvent (B), 26–35 min, 62% solvent (B), 36–42 min, 66% solvent (B), 43–55 min, 100% solvent (B), 56–65 min, 5% solvent (B). Chromatograms were recorded at wavelengths 280, 320, 360, and 520 nm [
21].
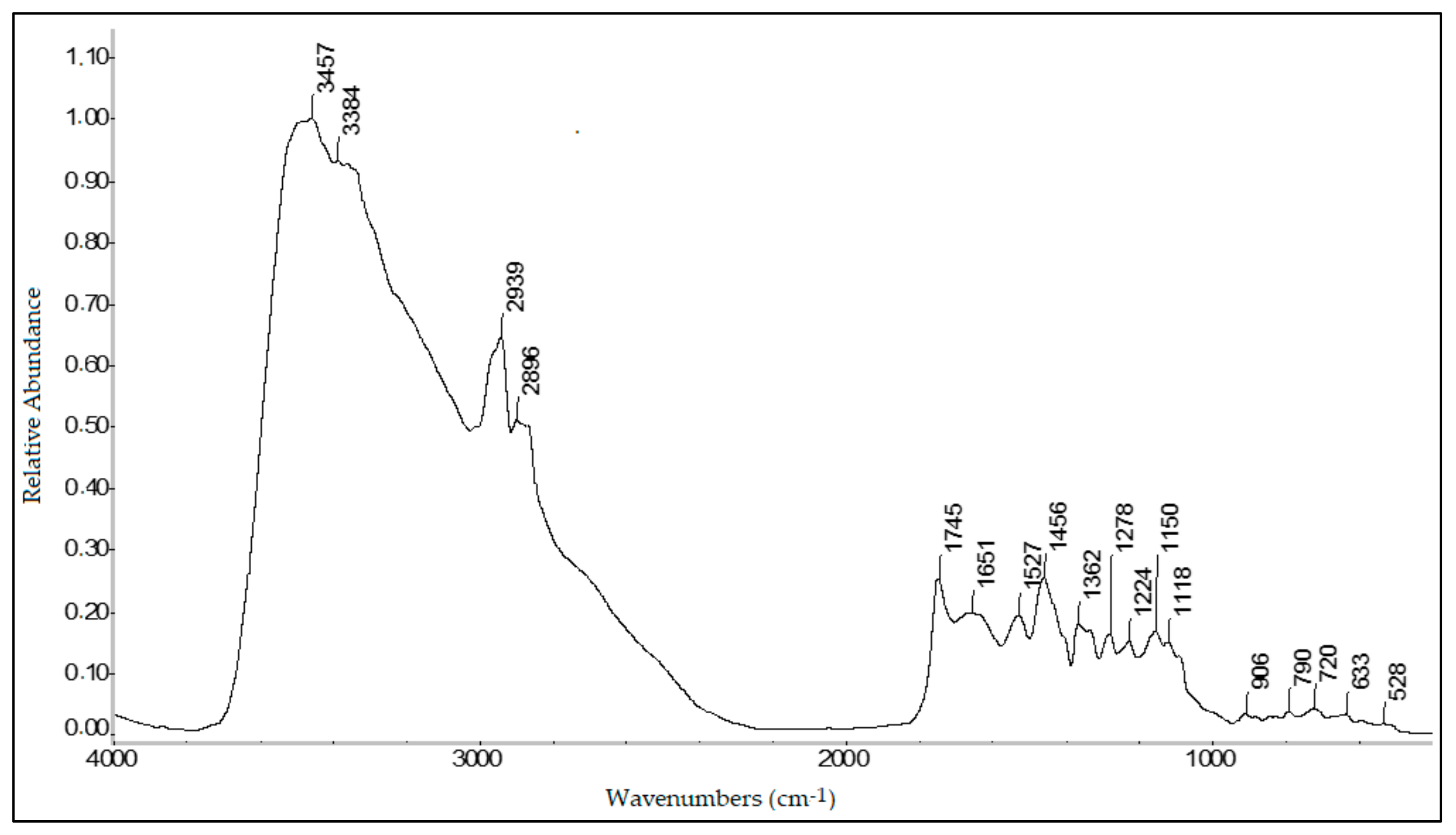
2.11. FTIR Spectroscopy
Fourier-transform infrared (FTIR) spectra were obtained using a Thermo Nicolet 6700 FTIR (Thermo Electron Corporation, Madison, WI, USA) equipped with a deuterated triglycine sulfate (DTGS) detector. The spectra were obtained with the diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS) technique. The speed of the interferometer moving mirror was 0.6329 mm s−1. Spectra were recorded with a resolution of 4 cm−1 and 100 scans. Before the analysis of each sample, the background was recorded. Triple FTIR spectra of each sample were obtained, using a different subsample each time.
FTIR spectra were smoothed using the Savitsky–Golay algorithm and their baselines were corrected. These pre-treatments were performed with “automatic smoothing” (5-point moving second-degree polynomial) and “baseline correction” (second-degree polynomial, twenty iterations) functions. Finally, using the “statistical spectra” function, the mean of three spectra for each sample was calculated and normalized (absorbance maximum value of 1). Spectrum processing was performed using the software OMNIC ver.9.1 (Thermo Fisher Scientific Inc., Waltham, MA, USA).
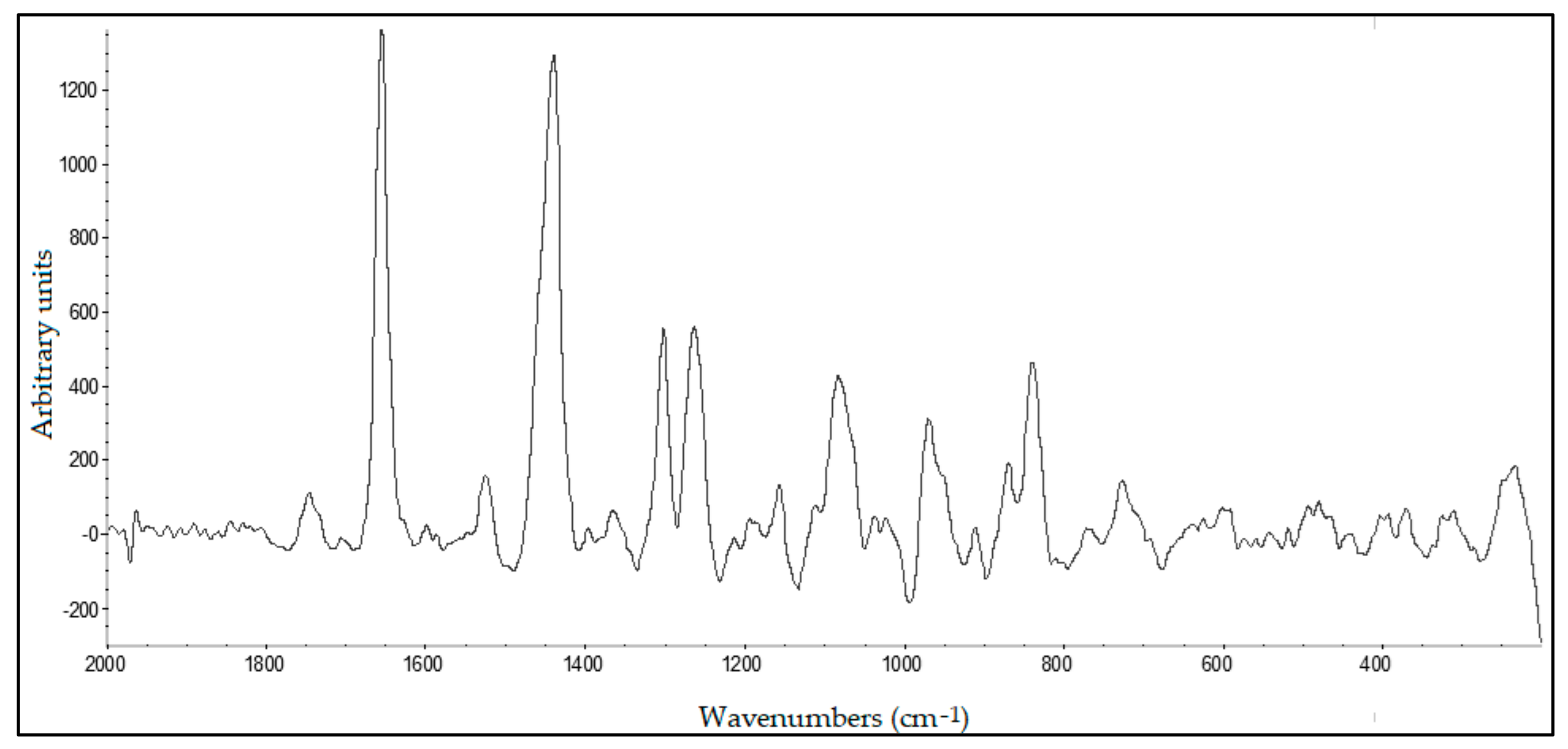
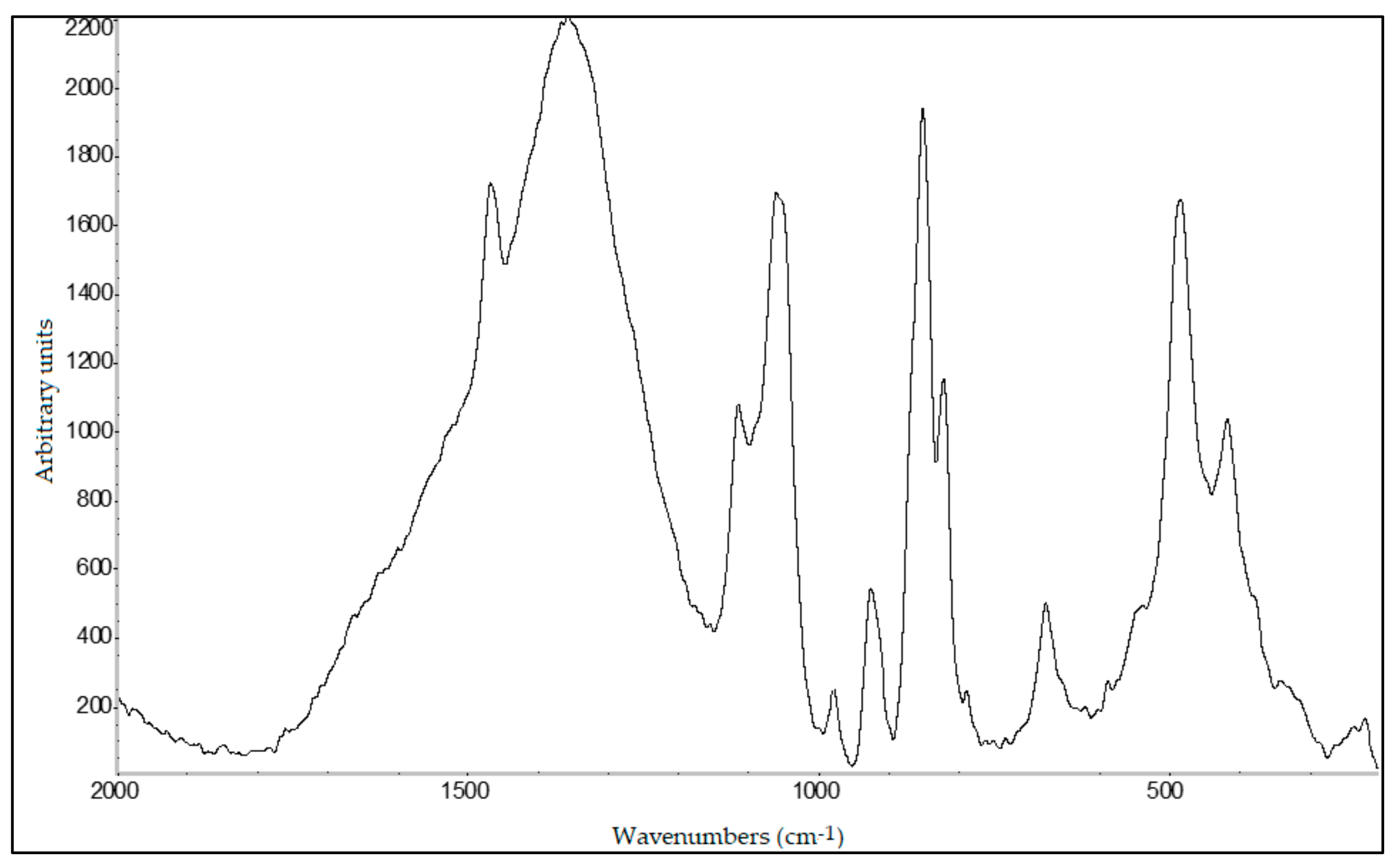
2.12. Raman Spectroscopy
A DeltaNu Advantage 785 visible-infrared Raman spectrometer (DeltaNu Inc., Laramie, WY, USA) equipped with a 785 nm diode laser for excitation with a maximum output power of 71.6 mW was used to record the spectra. Each spectrum was a 10 s acquisition over the spectral range of 2000–200 cm−1 using a resolution of 8 cm−1. The spectrometer was accompanied by NuSpec software. Raman spectra processing was performed as FTIR spectra.
2.13. Statistical Analysis
All the experiments were done in triplicate and the results are given as mean ± standard deviation (SD).
4. Conclusions
The present work outlined the importance of qualitative characteristics of winery by-products especially from traditional PDO grape varieties of Ionian Islands as a critical input to any by-product management plan towards a circular bioeconomy. To provide a realistic representation, by-product samples were collected directly from wineries after the vinification process. The solvents used were nontoxic and environmentally friendly. In addition, the application of green extraction processes ensures that the final products are safe for humans thus increasing the breadth of demand. Grape pomace can be used to empower business sectors of the food industry, beverage, medicine, cosmetics, cooking, feed, and many more. It can also be used as a whole material after a short preprocessing that involves moisture removal and pulverization. Furthermore, due to the ease and speed at which they can be transported, the same handling outcomes are inferred in lowering environmental and economic costs. All the above support the meaning of the circular economy, which states that anything previously classified as “by-products” can now be converted and classified as raw material. After a feasibility analysis, the qualitative data of winery by-products combined with annual production data by variety and per island, allowed for a first mass balance estimation that could form the basis of any management plan. In any case, further research into the optimization of each processing phase is needed to optimize efficiency.