Ironmaking and Steelmaking Slags as Sustainable Adsorbents for Industrial Effluents and Wastewater Treatment: A Critical Review of Properties, Performance, Challenges and Opportunities
Abstract
1. Introduction
2. Basic Principles of the Adsorption Process
2.1. Adsorption Isotherms
2.2. Kinetic Models
3. Sources, Types and Properties of Metallurgical Slags
3.1. Chemical Properties of Ironmaking and Steelmaking Slags
3.1.1. Blast Furnace Slags
3.1.2. Steelmaking Slags
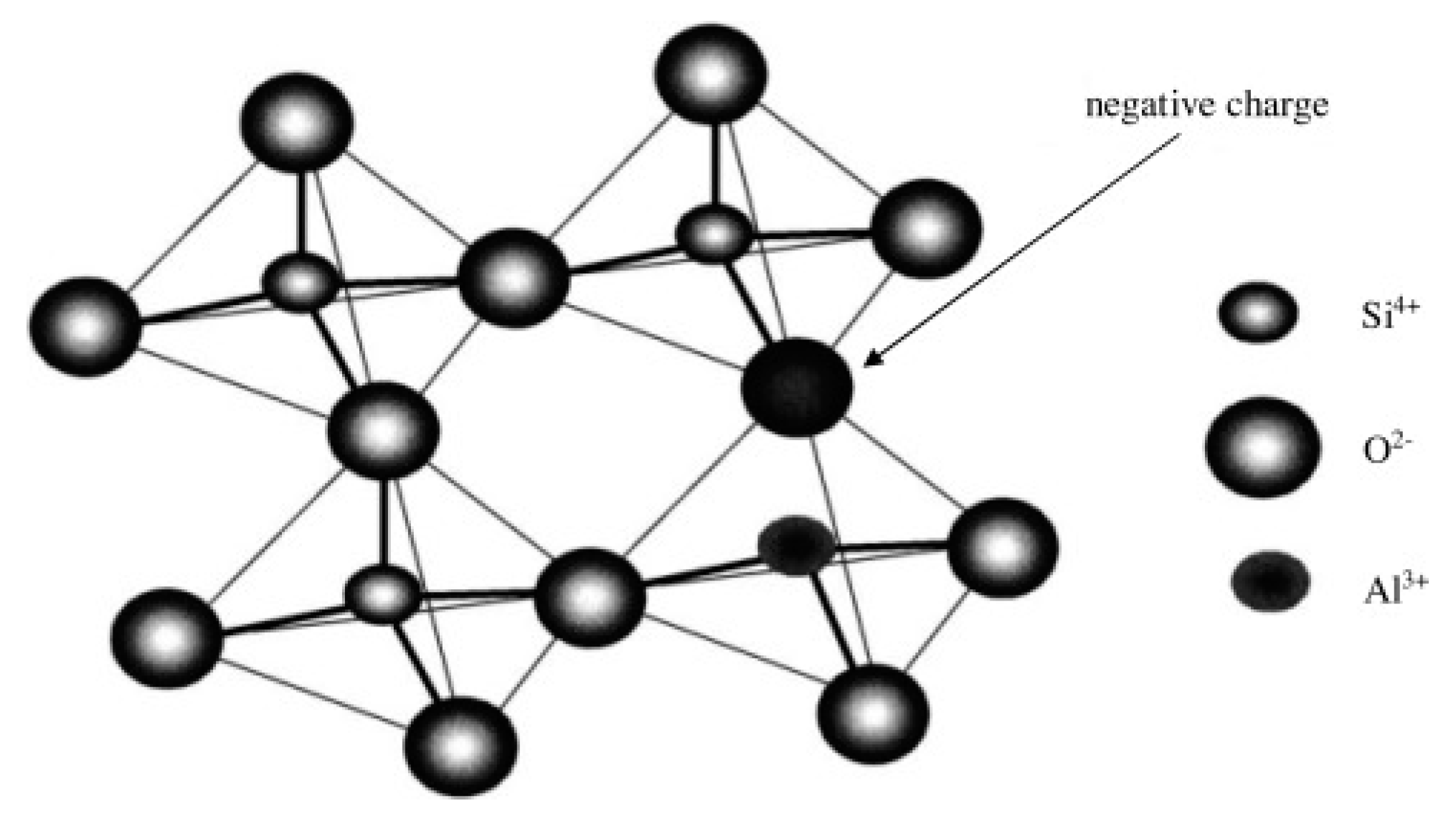
3.2. Phase Chemical Properties of Ironmaking and Steelmaking Slags
3.3. Physical Properties of Ironmaking and Steelmaking Slags
4. Applications and Performance of Slags as Low-cost Adsorbents
4.1. Acid Neutralisation and Precipitation of Metal Ions
4.2. Adsorption Capacity of Slags
4.3. Parameters that Affect the Adsorption Capacity of Slags
4.3.1. Effect of Solution pH
4.3.2. Effect of Initial Concentration of Metal Ions
4.3.3. Effect of slag loading ratio
4.3.4. Effect of Particle Size
4.3.5. Competitive Multi-Solute Adsorption
5. Opportunities to Enhance the Adsorption Performance of Slags
5.1. Activated Slags and Their Performance as Adsorbents
5.1.1. Thermal Activation
5.1.2. Mechanochemical Activation
5.1.3. Alkali Activation and Geopolymerisation of Slags
5.2. Slag-Based Permeable Reactive Barrier Materials
5.3. Synthesis of Zeolites from Slags
5.4. Synthesis of Layered Double Hydroxides from Slags
6. Environmental Challenges of Steelmaking-Slag-Based Adsorbents
6.1. Toxicity and Environmental Challenges of Chromium Bearing Slags
6.2. Stabilisation of Chromium in Slags
6.3. Integrating Metal Recovery from Steelmaking Slags
7. Mitigating the Environmental Challenges of Spent Slag Adsorbents
7.1. Regeneration, Recycling and Reuse of Spent-Slag-Based Adsorbents
7.1.1. Regeneration of Spent-Slag-Based Adsorbents
7.1.2. Challenges to Regenerability of Slag-Based Adsorbents
7.2. Immobilisation and Stabilisation of Spent-Slag-Based Adsorbents
7.2.1. Immobilisation using Cementitious Materials
7.2.2. Immobilisation by Thermal Vitrification
8. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Gupta, V.K.; Carrott, P.; Ribeiro Carrott, M.; Suhas. Low-cost adsorbents: Growing approach to wastewater treatment—A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.-C.; Zhang, Q.-J.; Zhang, W.-M.; Zhang, Q.-X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Crittenden, J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 2018, 131, 246–254. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Chang, Y.-R.; Chen, M.-L.; Lo, Y.-K.; Lai, J.-Y.; Lee, D.-J. Poly (vinyl alcohol) and alginate cross-linked matrix with immobilized Prussian blue and ion exchange resin for cesium removal from waters. Bioresour. Technol. 2016, 214, 192–198. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- El-Naas, M.H.; Alhaija, M.A. Modeling of Adsorption Processes. In Mathematical Modelling; Christopher Brennan, R., Ed.; Nova Publishers Inc.: Hauppauge, NY, USA, 2011. [Google Scholar]
- Buekens, A.; Zyaykina, N. Adsorbents and adsorption processes for pollution control. Pollut. Control Technol. 2001, 2, 1–10. [Google Scholar]
- Menendez-Diaz, J.; Martín-Gullón, I. Types of Carbon Adsorbents and Their Production. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 7, pp. 1–47. [Google Scholar]
- Zheng, C.; Zhao, L.; Zhou, X.; Fu, Z.; Li, A. Treatment technologies for organic wastewater. Water Treat. 2013, 11, 249–286. [Google Scholar]
- Simate, G.S.; Maledi, N.; Ochieng, A.; Ndlovu, S.; Zhang, J.; Walubita, L.F. Coal-based adsorbents for water and wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 2291–2312. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Dimitrova, S.; Nikolov, V.; Mehandjiev, D. Effect of the heat treatment on the morphology and sorption ability to metal ions of metallurgical slag. J. Mater. Sci. 2001, 36, 2639–2643. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-H.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Dimitrova, S.; Mehanjiev, D. Interaction of blast-furnace slag with heavy metal ions in water solutions. Water Res. 2000, 34, 1957–1961. [Google Scholar] [CrossRef]
- Gaskell, D.R. The Determination of Phase Diagrams for Slag Systems. In Methods for Phase Diagram Determination; Elsevier: Amsterdam, The Netherlands, 2007; pp. 442–458. [Google Scholar]
- Reuter, M.; Xiao, Y.; Boin, U. Recycling and Environmental Issues of Metallurgical Slags and Salt Fluxes. In Proceedings of the VII International Conference on Molten Slags Fluxes and Salts, The South African Institute of Mining and Metallurgy, Johannesburg, South African, 25–28 January 2004; pp. 349–356. [Google Scholar]
- Matinde, E.; Simate, G.; Ndlovu, S. Mining and metallurgical wastes: A review of recycling and re-use practices. J. S. Afr. Inst. Min. Metall. 2018, 118, 825–844. [Google Scholar] [CrossRef]
- Ndlovu, S.; Simate, G.S.; Matinde, E. Waste Production and Utilization in the Metal Extraction Industry; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R., II. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Mercado-Borrayo, B.; González-Chávez, J.; Ramírez-Zamora, R.; Schouwenaars, R. Valorization of metallurgical slag for the treatment of water pollution: An emerging technology for resource conservation and re-utilization. J. Sustain. Metall. 2018, 4, 50–67. [Google Scholar] [CrossRef]
- Tan, K.; Hameed, B. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Verhaverbeke, S. Cleaning of Trace Metallic Impurities from Solid Substrates Using Liquid Media. In Handbook for Cleaning/Decontamination of Surfaces; Elsevier: Amsterdam, The Netherlands, 2007; pp. 485–538. [Google Scholar]
- Ho, Y.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Knaebel, K.S. Adsorbent selection. Accessed 2011, 6, 1–23. [Google Scholar]
- El-Khaiary, M.I.; Malash, G.F.; Ho, Y.-S. On the use of linearized pseudo-second-order kinetic equations for modeling adsorption systems. Desalination 2010, 257, 93–101. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Al-Anber, M.A. Thermodynamics Approach in the Adsorption of Heavy Metals. In Thermodynamics-Interaction Studies-Solids, Liquids and Gases; IntechOpen: London, UK, 2011. [Google Scholar]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 2011. [Google Scholar] [CrossRef]
- Association, W.S. Steel Industry by-Products. World Steel Association, 2018; Volume 2018. Available online: https://www.worldsteel.org/en/dam/jcr:1b916a916d-906fd-914e984-b935d-c911d911d918df914/Fact_By-products_2018.pdf (accessed on 6 November 2018).
- Barati, M.; Esfahani, S.; Utigard, T. Energy recovery from high temperature slags. Energy 2011, 36, 5440–5449. [Google Scholar] [CrossRef]
- Shi, C. Steel slag—Its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Zheng, G.H.; Kozinsk, J.A. Solid waste remediation in the metallurgical industry: Application and environmental impact. Environ. Prog. 1996, 15, 283–292. [Google Scholar] [CrossRef]
- Talling, B.; Krivenko, P. Blast furnace slag-the ultimate binder. In Waste Materials Used in Concrete Manufacturing; Elsevier: Amsterdam, The Netherlands, 1996; pp. 235–289. [Google Scholar]
- Dimitrova, S.; Mehandgiev, D. Lead removal from aqueous solutions by granulated blast-furnace slag. Water Res. 1998, 32, 3289–3292. [Google Scholar] [CrossRef]
- Rao, S.R. Resource Recovery and Recycling from Metallurgical Wastes; Elsevier: Amsterdam, The Netherlands, 2011; Volume 7. [Google Scholar]
- EUROSLAG. Ferrous slag: General information. Available online: https://www.euroslag.com/products/ (accessed on 6 November 2018).
- Rao, S.R. Metallurgical Slags, Dust and Fumes. In Waste Management Series; Elsevier: Amsterdam, The Netherlands, 2006; Volume 7, pp. 269–327. [Google Scholar]
- Johansson, L. Blast furnace slag as phosphorus sorbents—Column studies. Sci. Total Environ. 1999, 229, 89–97. [Google Scholar] [CrossRef]
- About Iron and Steel Slag. Available online: http://www.slg.jp/e/slag/character.html (accessed on 6 November 2018).
- Das, B.; Prakash, S.; Reddy, P.; Misra, V. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Waligora, J.; Bulteel, D.; Degrugilliers, P.; Damidot, D.; Potdevin, J.; Measson, M. Chemical and mineralogical characterizations of LD converter steel slags: A multi-analytical techniques approach. Mater. Charact. 2010, 61, 39–48. [Google Scholar] [CrossRef]
- Setién, J.; Hernández, D.; González, J. Characterization of ladle furnace basic slag for use as a construction material. Constr. Build. Mater. 2009, 23, 1788–1794. [Google Scholar] [CrossRef]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Larsson, M.L.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Durinck, D.; Engström, F.; Arnout, S.; Heulens, J.; Jones, P.T.; Björkman, B.; Blanpain, B.; Wollants, P. Hot stage processing of metallurgical slags. Resour. Conserv. Recycl. 2008, 52, 1121–1131. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E.; Nordström, U. Physicochemical and mineralogical properties of stainless steel slags oriented to metal recovery. Resour. Conserv. Recycl. 2004, 40, 245–271. [Google Scholar] [CrossRef]
- Huaiwei, Z.; Xin, H. An overview for the utilization of wastes from stainless steel industries. Resour. Conserv. Recycl. 2011, 55, 745–754. [Google Scholar] [CrossRef]
- Mihailova, I.; Dimitrova, S.; Mehandjiev, D. Effect of the thermal treatment on the Pb (II) adsorption ability of blast furnace slag. J. Chem. Technol. Metall. 2013, 48, 72–79. [Google Scholar]
- Rađenović, A.; Malina, J.; Sofilić, T. Characterization of ladle furnace slag from carbon steel production as a potential adsorbent. Adv. Mater. Sci. Eng. 2013, 2013. [Google Scholar] [CrossRef]
- Xue, Y.; Hou, H.; Zhu, S. Adsorption removal of reactive dyes from aqueous solution by modified basic oxygen furnace slag: Isotherm and kinetic study. Chem. Eng. J. 2009, 147, 272–279. [Google Scholar] [CrossRef]
- Islam, M.; Patel, R. Thermal activation of basic oxygen furnace slag and evaluation of its fluoride removal efficiency. Chem. Eng. J. 2011, 169, 68–77. [Google Scholar] [CrossRef]
- Gupta, V.K. Equilibrium uptake, sorption dynamics, process development, and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag, a low-cost adsorbent. Ind. Eng. Chem. Res. 1998, 37, 192–202. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, S.; Zhou, M. Adsorption characterization of Cu (II) from aqueous solution onto basic oxygen furnace slag. Chem. Eng. J. 2013, 231, 355–364. [Google Scholar] [CrossRef]
- Yu, J.; Liang, W.; Wang, L.; Li, F.; Zou, Y.; Wang, H. Phosphate removal from domestic wastewater using thermally modified steel slag. J. Environ. Sci. 2015, 31, 81–88. [Google Scholar] [CrossRef]
- Yang, L.; Qian, X.; Wang, Z.; Li, Y.; Bai, H.; Li, H. Steel slag as low-cost adsorbent for the removal of phenanthrene and naphthalene. Adsorpt. Sci. Technol. 2018, 36, 1160–1177. [Google Scholar] [CrossRef]
- Li, J.; Wu, B.; Zhou, T.; Chai, X. Preferential removal of phosphorus using modified steel slag and cement combination for its implications in engineering applications. Environ. Technol. Innov. 2018, 10, 264–274. [Google Scholar] [CrossRef]
- Duan, J.; Su, B. Removal characteristics of Cd (II) from acidic aqueous solution by modified steel-making slag. Chem. Eng. J. 2014, 246, 160–167. [Google Scholar] [CrossRef]
- Curković, L.; Cerjan-Stefanović, S.; Rastovean-Mioe, A. Batch Pb2+ and Cu2+ removal by electric furnace slag. Water Res. 2001, 35, 3436–3440. [Google Scholar] [CrossRef]
- Deng, S. Sorbent technology. Encycl. Chem. Process. 2006, 1, 2825–2845. [Google Scholar]
- Liu, S.-Y.; Gao, J.; Yang, Y.-J.; Yang, Y.-C.; Ye, Z.-X. Adsorption intrinsic kinetics and isotherms of lead ions on steel slag. J. Hazard. Mater. 2010, 173, 558–562. [Google Scholar] [CrossRef]
- Dimitrova, S. Metal sorption on blast-furnace slag. Water Res. 1996, 30, 228–232. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.; Aldrich, C. Removal of pollutants from acid mine wastewater using metallurgical by-product slags. Sep. Purif. Technol. 2004, 40, 61–67. [Google Scholar] [CrossRef]
- Chen, X.; Hou, W.; Song, G.; Wang, Q. Adsorption of Cu, Cd, Zn and Pb ions from aqueous solutions by electric arc furnaceslag and the effects of pH and grain size. Chem. Biochem. Eng. Q 2011, 25, 105–114. [Google Scholar]
- Zahar, M.S.M.; Kusin, F.M.; Muhammad, S.N. Adsorption of manganese in aqueous solution by steel slag. Procedia Environ. Sci. 2015, 30, 145–150. [Google Scholar] [CrossRef]
- Runtti, H.; Luukkonen, T.; Niskanen, M.; Tuomikoski, S.; Kangas, T.; Tynjälä, P.; Tolonen, E.-T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J. Sulphate removal over barium-modified blast-furnace-slag geopolymer. J. Hazard. Mater. 2016, 317, 373–384. [Google Scholar] [CrossRef]
- Bodurtha, P.; Brassard, P. Neutralization of acid by steel-making slags. Environ. Technol. 2000, 21, 1271–1281. [Google Scholar] [CrossRef]
- Yan, J.; Moreno, L.; Neretnieks, I. The long-term acid neutralizing capacity of steel slag. Waste Manag. 2000, 20, 217–223. [Google Scholar] [CrossRef]
- Xue, Y.; Hou, H.; Zhu, S. Competitive adsorption of copper (II), cadmium (II), lead (II) and zinc (II) onto basic oxygen furnace slag. J. Hazard. Mater. 2009, 162, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Kuyucak, N. Role of microorganisms in mining: Generation of acid rock drainage and its mitigation and treatment. EJMP EP 2002, 2, 179–196. [Google Scholar]
- Robb, G.A.; Robinson, J.D. Acid drainage from mines. Geogr. J. 1995, 161, 47–54. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 2006, 83, 106–113. [Google Scholar] [CrossRef]
- Kim, D.-H.; Shin, M.-C.; Choi, H.-D.; Seo, C.-I.; Baek, K. Removal mechanisms of copper using steel-making slag: Adsorption and precipitation. Desalination 2008, 223, 283–289. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Tian, S.; Li, K.; Yan, F.; Liu, N.; Yang, M.; Chen, X. BOF steel slag as a low-cost sorbent for vanadium (V) removal from soil washing effluent. Sci. Rep. 2017, 7, 11177. [Google Scholar] [CrossRef]
- Han, C.; Jiao, Y.; Wu, Q.; Yang, W.; Yang, H.; Xue, X. Kinetics and mechanism of hexavalent chromium removal by basic oxygen furnace slag. J. Environ. Sci. 2016, 46, 63–71. [Google Scholar] [CrossRef]
- Gao, Y.-M.; Sengupta, A.K.; Simpson, D. A new hybrid inorganic sorbent for heavy metals removal. Water Res. 1995, 29, 2195–2205. [Google Scholar] [CrossRef]
- Yamashita, K.; Ikehata, T.; Tate, K.; Nakahara, K. Method of Removing Dissolved Heavy Metals from Aqueous Waste Liquids. US Patent 4,377,483, 22 March 1983. [Google Scholar]
- Dushina, A.; Aleskovski, V. Ion exchange as first stage of the solid substance transformation into electrolytic solutions. J. Appl. Chem. 1976, 49, 41. [Google Scholar]
- Liu, S.-Y.; Gao, J.; Qu, B.; Yang, Y. Adsorption Behaviors of Heavy Metal Ions by Steel Slag-An Industrial Solidwaste. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–16 June 2009; pp. 1–4. [Google Scholar]
- Name, T.; Sheridan, C. Remediation of acid mine drainage using metallurgical slags. Miner. Eng. 2014, 64, 15–22. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Westholm, L.J.; Sillanpää, M. Steel slag as a low-cost sorbent for metal removal in the presence of chelating agents. J. Ind. Eng. Chem. 2015, 27, 115–125. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Kandasamy, J.; Naidu, R.; Vigneswaran, S. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ. Sci. Pollut. Res. 2018, 25, 20430–20438. [Google Scholar] [CrossRef]
- Huang, C.; Rhoads, E.A. Adsorption of Zn (II) onto hydrous aluminosilicates. J. Colloid Interface Sci. 1989, 131, 289–306. [Google Scholar] [CrossRef]
- Gadde, R.R.; Laitinen, H.A. Heavy metal adsorption by hydrous iron and manganese oxides. Anal. Chem. 1974, 46, 2022–2026. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH-dependent surface charging and the points of zero charge. J. Colloid Interface Sci. 2002, 253, 77–87. [Google Scholar] [CrossRef]
- Ortiz, N.; Pires, M.; Bressiani, J. Use of steel converter slag as nickel adsorber to wastewater treatment. Waste Manag. 2001, 21, 631–635. [Google Scholar] [CrossRef]
- Huifen, Y.; Wen, M.; Weina, Z.; Zhiyong, W. Steel Slag as Multi-Functional Material for Removal of Heavy Metal Ions in Wastewater. In Proceedings of the 2011 International Conference on Computer Distributed Control and Intelligent Environmental Monitoring, Changsha, China, 19–20 February 2011; pp. 1287–1290. [Google Scholar]
- Choi, K.; Lee, S.; Park, J.O.; Park, J.-A.; Cho, S.-H.; Lee, S.Y.; Lee, J.H.; Choi, J.-W. Chromium removal from aqueous solution by a PEI-silica nanocomposite. Sci. Rep. 2018, 8, 1438. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’Eb, M. Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Leckie, J.O. Competitive adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J. Colloid Interface Sci. 1981, 83, 410–419. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Kaczmarski, K.; Bellot, J.C. Effect of particle-size distribution and particle porosity changes on mass-transfer kinetics. Acta Chromatogr. 2003, 13, 22–37. [Google Scholar]
- Müller, B.R. Effect of particle size and surface area on the adsorption of albumin-bonded bilirubin on activated carbon. Carbon 2010, 48, 3607–3615. [Google Scholar] [CrossRef]
- Pang, Y.; Yu, J.; Tang, L.; Zeng, G.; Zhu, C.; Wei, X. Magnetic Nanohybrid Materials for Water-Pollutant Removal. In Nanohybrid and Nanoporous Materials for Aquatic Pollution Control; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–30. [Google Scholar]
- Plazinski, W.; Rudzinski, W. Kinetics of adsorption at solid/solution interfaces controlled by intraparticle diffusion: A theoretical analysis. J. Phys. Chem. C 2009, 113, 12495–12501. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: Adsorption isotherm study. Arab. J. Chem. 2012, 5, 439–446. [Google Scholar] [CrossRef]
- Mihajlović, M.T.; Lazarević, S.S.; Janković-Častvan, I.M.; Jokić, B.M.; Janaćković, D.T.; Petrović, R.D. A comparative study of the removal of lead, cadmium and zinc ions from aqueous solutions by natural and Fe (III)-modified zeolite. Chem. Ind. Chem. Eng. Q. 2014, 20, 283–293. [Google Scholar] [CrossRef]
- Borhade, A.V.; Kshirsagar, T.A.; Dholi, A.G.; Agashe, J.A. Removal of heavy metals Cd2+, Pb2+, and Ni2+ from aqueous solutions using synthesized azide cancrinite, Na8 [AlSiO4] 6 (N3) 2.4 (H2O) 4.6. J. Chem. Eng. Data 2015, 60, 586–593. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions. Part 5—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Chen, S.; Ma, Y.; Chen, L.; Xian, K. Adsorption of aqueous Cd2+, Pb2+, Cu2+ ions by nano-hydroxyapatite: Single-and multi-metal competitive adsorption study. Geochem. J. 2010, 44, 233–239. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, X.; Jiang, W. Study on adsorption of heavy metal ion in metallurgical wastewater by sepiolite. In Proceedings of the 2011 2nd International Conference on Environmental Science and Development IPCBEE, Singapore, 26–28 February 2011. [Google Scholar]
- Neris, J.B.; Luzardo, F.H.M.; da Silva, E.G.P.; Velasco, F.G. Evaluation of adsorption processes of metal ions in multi-element aqueous systems by lignocellulosic adsorbents applying different isotherms: A critical review. Chem. Eng. J. 2019, 357, 404–420. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Boyd, G.; Adamson, A.; Myers, L., Jr. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Motsi, T.; Rowson, N.; Simmons, M. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Bingxue, Z.; Guarecuco, R.; Thomas, T.; Minghui, Y. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2018, 213, 42–58. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Liu, L.C.; Xu, Y.; Wang, Y.C. A new alkali-activated steel slag-based cementitious material for photocatalytic degradation of organic pollutant from waste water. J. Hazard. Mater. 2012, 209, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Runtti, H.; Niskanen, M.; Tolonen, E.-T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; Lassi, U. Simultaneous removal of Ni (II), As (III), and Sb (III) from spiked mine effluent with metakaolin and blast-furnace-slag geopolymers. J. Environ. Manag. 2016, 166, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- El-Eswed, B.I. Solidification Versus Adsorption for Immobilization of Pollutants in Geopolymeric Materials: A Review; InTech Open: London, UK, 2018. [Google Scholar]
- Ahn, J.S.; Chon, C.-M.; Moon, H.-S.; Kim, K.-W. Arsenic removal using steel manufacturing byproducts as permeable reactive materials in mine tailing containment systems. Water Res. 2003, 37, 2478–2488. [Google Scholar] [CrossRef]
- Pratt, C.; Shilton, A.; Pratt, S.; Haverkamp, R.G.; Bolan, N.S. Phosphorus removal mechanisms in active slag filters treating waste stabilization pond effluent. Environ. Sci. Technol. 2007, 41, 3296–3301. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Drizo, A.; Rizzo, D.M.; Druschel, G.; Hayden, N.; Twohig, E. Evaluating the efficiency and temporal variation of pilot-scale constructed wetlands and steel slag phosphorus removing filters for treating dairy wastewater. Water Res. 2010, 44, 4077–4086. [Google Scholar] [CrossRef] [PubMed]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Barca, C.; Meyer, D.; Liira, M.; Drissen, P.; Comeau, Y.; Andrès, Y.; Chazarenc, F. Steel slag filters to upgrade phosphorus removal in small wastewater treatment plants: Removal mechanisms and performance. Ecol. Eng. 2014, 68, 214–222. [Google Scholar] [CrossRef]
- Barca, C.; Troesch, S.; Meyer, D.; Drissen, P.; Andres, Y.; Chazarenc, F. Steel slag filters to upgrade phosphorus removal in constructed wetlands: Two years of field experiments. Environ. Sci. Technol. 2012, 47, 549–556. [Google Scholar] [CrossRef]
- Claveau-Mallet, D.; Courcelles, B.; Comeau, Y. Phosphorus removal by steel slag filters: Modeling dissolution and precipitation kinetics to predict longevity. Environ. Sci. Technol. 2014, 48, 7486–7493. [Google Scholar] [CrossRef]
- Claveau-Mallet, D.; Wallace, S.; Comeau, Y. Model of phosphorus precipitation and crystal formation in electric arc furnace steel slag filters. Environ. Sci. Technol. 2012, 46, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Claveau-Mallet, D.; Wallace, S.; Comeau, Y. Removal of phosphorus, fluoride and metals from a gypsum mining leachate using steel slag filters. Water Res. 2013, 47, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Sugano, Y.; Sahara, R.; Murakami, T.; Narushima, T.; Iguchi, Y.; Ouchi, C. Hydrothermal synthesis of zeolite A using blast furnace slag. ISIJ Int. 2005, 45, 937–945. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Ohmichi, T.; Mori, K.; Katayama, I.; Yamashita, H. Synthesis of zeolite from steel slag and its application as a support of nano-sized TiO 2 photocatalyst. J. Mater. Sci. 2008, 43, 2407–2410. [Google Scholar] [CrossRef]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal synthesis of mixtures of NaA zeolite and sodalite from Ti-bearing electric arc furnace slag. Rsc Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Ohmichi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. A novel conversion process for waste slag: Synthesis of a hydrotalcite-like compound and zeolite from blast furnace slag and evaluation of adsorption capacities. J. Mater. Chem. 2010, 20, 5052–5062. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Tamagawa, S.; Fujitani, T.; Yamashita, H. A novel conversion process for waste slag: Synthesis of calcium silicate hydrate from blast furnace slag and its application as a versatile adsorbent for water purification. J. Mater. Chem. A 2013, 1, 7199–7210. [Google Scholar] [CrossRef]
- Shao, N.; Tang, S.; Liu, Z.; Li, L.; Yan, F.; Liu, F.; Li, S.; Zhang, Z. Hierarchically structured calcium silicate hydrate-based nanocomposites derived from steel slag for highly efficient heavy metal removal from wastewater. Acs Sustain. Chem. Eng. 2018, 6, 14926–14935. [Google Scholar] [CrossRef]
- Yang, L.; Yang, M.; Xu, P.; Zhao, X.; Bai, H.; Li, H. Characteristics of nitrate removal from aqueous solution by modified steel slag. Water 2017, 9, 757. [Google Scholar] [CrossRef]
- Allahverdi, A.; Maleki, A.; Mahinroosta, M. Chemical activation of slag-blended Portland cement. J. Build. Eng. 2018, 18, 76–83. [Google Scholar] [CrossRef]
- Sobolev, K. Mechano-chemical modification of cement with high volumes of blast furnace slag. Cem. Concr. Compos. 2005, 27, 848–853. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006, 75, 177. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Bandopadhyay, A.; Alex, T.; Kumar, B.R.; Das, S.K.; Mehrotra, S. Mechanical activation of granulated blast furnace slag and its effect on the properties and structure of portland slag cement. Cem. Concr. Compos. 2008, 30, 679–685. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Effect of mechanochemical processing on adsorptive properties of blast furnace slag. J. Environ. Eng. 2013, 139, 1446–1453. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Liu, L.C.; Ni, L.L.; Wang, B.L. A facile and low-cost synthesis of granulated blast furnace slag-based cementitious material coupled with Fe2O3 catalyst for treatment of dye wastewater. Appl. Catal. B Environ. 2013, 138, 9–16. [Google Scholar] [CrossRef]
- Sarkar, C.; Basu, J.K.; Samanta, A.N. Removal of Ni2+ ion from waste water by Geopolymeric Adsorbent derived from LD Slag. J. Water Process Eng. 2017, 17, 237–244. [Google Scholar] [CrossRef]
- Waybrant, K.; Blowes, D.; Ptacek, C. Selection of reactive mixtures for use in permeable reactive walls for treatment of mine drainage. Environ. Sci. Technol. 1998, 32, 1972–1979. [Google Scholar] [CrossRef]
- Cocos, I.A.; Zagury, G.J.; Clément, B.; Samson, R. Multiple factor design for reactive mixture selection for use in reactive walls in mine drainage treatment. Water Res. 2002, 36, 167–177. [Google Scholar] [CrossRef]
- Shabalala, A.N.; Ekolu, S.O.; Diop, S.; Solomon, F. Pervious concrete reactive barrier for removal of heavy metals from acid mine drainage− column study. J. Hazard. Mater. 2017, 323, 641–653. [Google Scholar] [CrossRef]
- Sasaki, K.; Nukina, S.; Wilopo, W.; Hirajima, T. Removal of arsenate in acid mine drainage by a permeable reactive barrier bearing granulated blast furnace slag: Column study. Mater. Trans. 2008, 0803030343. [Google Scholar] [CrossRef]
- Bowden, L.I.; Jarvis, A.P.; Younger, P.L.; Johnson, K.L. Phosphorus removal from waste waters using basic oxygen steel slag. Environ. Sci. Technol. 2009, 43, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Westholm, L.J.; Repo, E.; Sillanpää, M. Filter materials for metal removal from mine drainage—A review. Environ. Sci. Pollut. Res. 2014, 21, 9109–9128. [Google Scholar] [CrossRef] [PubMed]
- Bothe, J.V.; Brown, P.W. Arsenic immobilization by calcium arsenate formation. Environ. Sci. Technol. 1999, 33, 3806–3811. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Xie, Q.; Wang, D.; Cheng, G. Solubility and Stability of Calcium Arsenates at 25 °C. Water Air Soil Pollut. 2006, 169, 221–238. [Google Scholar] [CrossRef]
- Pascua, C.S.; Minato, M.; Yokoyama, S.; Sato, T. Uptake of dissolved arsenic during the retrieval of silica from spent geothermal brine. Geothermics 2007, 36, 230–242. [Google Scholar] [CrossRef]
- Sullivan, C.; Tyrer, M.; Cheeseman, C.R.; Graham, N.J. Disposal of water treatment wastes containing arsenic—A review. Sci. Total Environ. 2010, 408, 1770–1778. [Google Scholar] [CrossRef]
- Riley, A.L.; Mayes, W.M. Long-term evolution of highly alkaline steel slag drainage waters. Environ. Monit. Assess. 2015, 187, 463. [Google Scholar] [CrossRef]
- De Windt, L.; Chaurand, P.; Rose, J. Kinetics of steel slag leaching: Batch tests and modeling. Waste Manag. 2011, 31, 225–235. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.T.; Alastuey, A.; Hernández, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. Fly Ash Zeolites: Innovations, Applications and Directions; Springer: Berlin/Heidelberg, Germany, 2016; Volume 78. [Google Scholar]
- Jha, B.; Singh, D. A review on synthesis, characterization and industrial applications of flyash zeolites. J. Mater. Educ. 2011, 33, 65. [Google Scholar]
- Gupta, V.; Imran, A. Water treatment: Low-cost alternatives to carbon adsorbents. In Encyclopedia of Surface and Colloid Science; CRC Press: Boca Raton, FL, USA, 2015; pp. 7618–7652. [Google Scholar]
- Çoruh, S. The removal of zinc ions by natural and conditioned clinoptilolites. Desalination 2008, 225, 41–57. [Google Scholar] [CrossRef]
- Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Peter, A.; Nicula, C.; Tutu, H.; Silipas, D.; Indrea, E. Adsorption of heavy metal cations by Na-clinoptilolite: Equilibrium and selectivity studies. J. Environ. Manag. 2014, 137, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.V.; Dal Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Li, Y.; Bai, P.; Yan, Y.; Yan, W.; Shi, W.; Xu, R. Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamashita, H. Synthesis of Ca-based layered double hydroxide from blast furnace slag and its catalytic applications. ISIJ Int. 2015, 55, 1531–1537. [Google Scholar] [CrossRef]
- Bagherifam, S.; Komarneni, S.; Lakzian, A.; Fotovat, A.; Khorasani, R.; Huang, W.; Ma, J.; Wang, Y. Evaluation of Zn–Al–SO4 layered double hydroxide for the removal of arsenite and arsenate from a simulated soil solution: Isotherms and kinetics. Appl. Clay Sci. 2014, 95, 119–125. [Google Scholar] [CrossRef]
- Baliarsingh, N.; Parida, K.; Pradhan, G. Influence of the nature and concentration of precursor metal ions in the brucite layer of LDHs for phosphate adsorption–a review. Rsc Adv. 2013, 3, 23865–23878. [Google Scholar] [CrossRef]
- Tóth, V.; Sipiczki, M.; Pallagi, A.; Kukovecz, Á.; Kónya, Z.; Sipos, P.; Pálinkó, I. Synthesis and properties of CaAl-layered double hydroxides of hydrocalumite-type. Chem. Pap. 2014, 68, 633–637. [Google Scholar] [CrossRef]
- He, X.; Qiu, X.; Hu, C.; Liu, Y. Treatment of heavy metal ions in wastewater using layered double hydroxides: A review. J. Dispers. Sci. Technol. 2018, 39, 792–801. [Google Scholar] [CrossRef]
- Matusik, J.; Rybka, K. Removal of Chromates and Sulphates by Mg/Fe LDH and Heterostructured LDH/Halloysite Materials: Efficiency, Selectivity, and Stability of Adsorbents in Single-and Multi-Element Systems. Materials 2019, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, F.; Liu, D.-L.; Shi, P. Highly efficient removal of Cr (VI) from wastewater via adsorption with novel magnetic Fe3O4@ C@ MgAl-layered double-hydroxide. Chin. Chem. Lett. 2015, 26, 1137–1143. [Google Scholar] [CrossRef]
- Ling, F.; Fang, L.; Lu, Y.; Gao, J.; Wu, F.; Zhou, M.; Hu, B. A novel CoFe layered double hydroxides adsorbent: High adsorption amount for methyl orange dye and fast removal of Cr (VI). Microporous Mesoporous Mater. 2016, 234, 230–238. [Google Scholar] [CrossRef]
- Gong, J.; Liu, T.; Wang, X.; Hu, X.; Zhang, L. Efficient removal of heavy metal ions from aqueous systems with the assembly of anisotropic layered double hydroxide nanocrystals@ carbon nanosphere. Environ. Sci. Technol. 2011, 45, 6181–6187. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.; Gao, J.; Wu, F.; Zhang, X. High performance NiFe layered double hydroxide for methyl orange dye and Cr (VI) adsorption. Chemosphere 2016, 152, 415–422. [Google Scholar] [CrossRef]
- Outokumpu Stainless, A. Handbook of Stainless Steel; Espoo: Outokumpu OYJ: Helsinki, Finland, 2013. [Google Scholar]
- Rai, D.; Eary, L.; Zachara, J.M. Environmental chemistry of chromium. Sci. Total Environ. 1989, 86, 15–23. [Google Scholar] [CrossRef]
- Ščančar, J.; Milačič, R. A critical overview of Cr speciation analysis based on high performance liquid chromatography and spectrometric techniques. J. Anal. At. Spectrom. 2014, 29, 427–443. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Bolan, N.; Wang, H. A critical review on bioremediation technologies for Cr (VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1027–1078. [Google Scholar] [CrossRef]
- Apte, A.D.; Tare, V.; Bose, P. Extent of oxidation of Cr (III) to Cr (VI) under various conditions pertaining to natural environment. J. Hazard. Mater. 2006, 128, 164–174. [Google Scholar] [CrossRef]
- Ball, J.W.; Nordstrom, D.K. Critical evaluation and selection of standard state thermodynamic properties for chromium metal and its aqueous ions, hydrolysis species, oxides, and hydroxides. J. Chem. Eng. Data 1998, 43, 895–918. [Google Scholar] [CrossRef]
- Chaurand, P.; Rose, J.; Briois, V.; Olivi, L.; Hazemann, J.-L.; Proux, O.; Domas, J.; Bottero, J.-Y. Environmental impacts of steel slag reused in road construction: A crystallographic and molecular (XANES) approach. J. Hazard. Mater. 2007, 139, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, J. Occurrences, uses, and properties of chromium. Regul. Toxicol. Pharmacol. 1997, 26, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, J. Chromium chemistry and implications for environmental fate and toxicity. Soil Sediment Contam. 1997, 6, 561–568. [Google Scholar] [CrossRef]
- García-Ramos, E.; Romero-Serrano, A.; Zeifert, B.; Flores-Sánchez, P.; Hallen-López, M.; Palacios, E.G. Immobilization of chromium in slags using MgO and Al2O3. Steel Res. Int. 2008, 79, 332–339. [Google Scholar] [CrossRef]
- Cabrera-Real, H.; Romero-Serrano, A.; Zeifert, B.; Hernandez-Ramirez, A.; Hallen-Lopez, M.; Cruz-Ramirez, A. Effect of MgO and CaO/SiO2 on the immobilization of chromium in synthetic slags. J. Mater. Cycles Waste Manag. 2012, 14, 317–324. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.; Cao, L.; Zheng, X.; Jiang, M. Stability of Chromium in Stainless Steel Slag during Cooling. Minerals 2018, 8, 445. [Google Scholar] [CrossRef]
- Barbieri, L.; Leonelli, C.; Manfredini, T.; Pellacani, G.C.; Siligardi, C.; Tondello, E.; Bertoncello, R. Solubility, reactivity and nucleation effect of Cr2O3 in the CaO-MgO-Al2O3-SiO2 glassy system. J. Mater. Sci. 1994, 29, 6273–6280. [Google Scholar] [CrossRef]
- Kühn, M.; Mudersbach, D. Treatment of Liquid EAF-Slag from Stainless Steelmaking to Produce Environmental Friendly Construction Materials. In Proceedings of the 2nd International Conference on Process Development in Iron and Steelmaking (ScanMet II), Lulea, Sweden, 6–9 June 2004. [Google Scholar]
- Tanskanen, P.A.; Makkonen, H.T. Design of Slag Mineralogy and Petrology. Univ. Oulu Outocompu Finl. Glob. Slag Mag. 2007, 5, 16–20. [Google Scholar]
- Albertsson, G. Abatement of Chromium Emissions from Steelmaking Slags-Cr Stabilization by Phase Separation. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, December 2013. [Google Scholar]
- Engström, F.; Larsson, M.L.; Samuelsson, C.; Sandström, Å.; Robinson, R.; Björkman, B. Leaching behavior of aged steel slags. Steel Res. Int. 2014, 85, 607–615. [Google Scholar] [CrossRef]
- Primavera, A.; Pontoni, L.; Mombelli, D.; Barella, S.; Mapelli, C. EAF slag treatment for inert materials’ production. J. Sustain. Metall. 2016, 2, 3–12. [Google Scholar] [CrossRef]
- Sahu, N.; Biswas, A.; Kapure, G.U. A short review on utilization of ferrochromium slag. Miner. Process. Extr. Metall. Rev. 2016, 37, 211–219. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Gruttadauria, A.; Baldizzone, C.; Magni, F.; Levrangi, P.L.; Simone, P. Analisys of electric arc furnace slag. Steel Res. Int. 2012, 83, 1012–1019. [Google Scholar] [CrossRef]
- Abdullah, M.O. Applied Energy: An Introduction; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Lata, S.; Singh, P.; Samadder, S. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, J.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Verbinnen, B.; Block, C.; Van Caneghem, J.; Vandecasteele, C. Recycling of spent adsorbents for oxyanions and heavy metal ions in the production of ceramics. Waste Manag. 2015, 45, 407–411. [Google Scholar] [CrossRef]
- Conner, J.R.; Hoeffner, S.L. A critical review of stabilization/solidification technology. Crit. Rev. Environ. Sci. Technol. 1998, 28, 397–462. [Google Scholar] [CrossRef]
- Colombo, P.; Brusatin, G.; Bernardo, E.; Scarinci, G. Inertization and reuse of waste materials by vitrification and fabrication of glass-based products. Curr. Opin. Solid State Mater. Sci. 2003, 7, 225–239. [Google Scholar] [CrossRef]
- Bai, Z.; Qiu, G.; Peng, B.; Guo, M.; Zhang, M. Synthesis and characterization of glass-ceramics prepared from high-carbon ferrochromium slag. RSC Adv. 2016, 6, 52715–52723. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A. Immobilization and leaching characteristics of arsenic from cement and/or lime solidified/stabilized spent adsorbent containing arsenic. J. Hazard. Mater. 2008, 153, 434–443. [Google Scholar] [CrossRef]
- Shi, C.; Fernandez-Jimenez, A. Stabilization/solidification of hazardous and radioactive wastes with alkali-activated cements. J. Hazard. Mater. 2006, 137, 1656–1663. [Google Scholar] [CrossRef]
- Wiles, C.C. A review of solidification/stabilization technology. J. Hazard. Mater. 1987, 14, 5–21. [Google Scholar] [CrossRef]
- Rawlings, R. Glass-ceramic matrix composites. Composites 1994, 25, 372–379. [Google Scholar] [CrossRef]
- Ponsot, I.; Bernardo, E. Self glazed glass ceramic foams from metallurgical slag and recycled glass. J. Clean. Prod. 2013, 59, 245–250. [Google Scholar] [CrossRef]
- Ponsot, I.; Detsch, R.; Boccaccini, A.; Bernardo, E. Waste derived glass ceramic composites prepared by low temperature sintering/sinter-crystallisation. Adv. Appl. Ceram. 2015, 114, S17–S25. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Haynes, R.J. A comparison of inorganic solid wastes as adsorbents of heavy metal cations in aqueous solution and their capacity for desorption and regeneration. Water Air Soil Pollut. 2011, 218, 457–470. [Google Scholar] [CrossRef]
- Shahadat, M.; Isamil, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. R SC Adv. 2018, 8, 24571–24587. [Google Scholar]
- Rathore, V.K.; Mondal, P. Stabilization of arsenic and fluoride bearing spent adsorbent in clay bricks: Preparation, characterization and leaching studies. J. Environ. Manag. 2017, 200, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Malviya, R.; Chaudhary, R. Factors affecting hazardous waste solidification/stabilization: A review. J. Hazard. Mater. 2006, 137, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Paria, S.; Yuet, P.K. Solidification–stabilization of organic and inorganic contaminants using portland cement: A literature review. Environ. Rev. 2006, 14, 217–255. [Google Scholar] [CrossRef]
- Laforest, G.; Duchesne, J. Immobilization of chromium (VI) evaluated by binding isotherms for ground granulated blast furnace slag and ordinary Portland cement. Cem. Concr. Res. 2005, 35, 2322–2332. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A.K.; Jeon, B.H.; Park, J.M. Adsorptive removal of cobalt from aqueous solutions by utilizing industrial waste and its cement fixation. Sep. Sci. Technol. 2007, 42, 1255–1266. [Google Scholar] [CrossRef]
- Karayannis, V.; Ntampegliotis, K.; Lamprakopoulos, S.; Papapolymerou, G.; Spiliotis, X. Novel sintered ceramic materials incorporated with EAF carbon steel slag. Mater. Res. Express 2017, 4, 015505. [Google Scholar] [CrossRef]
- Nel, A. Use of South African blast-furnace slag for ceramic purposes. J. S. Afr. Inst. Min. Metall. 1970, 70, 366–368. [Google Scholar]
- Chinnam, R.; Francis, A.; Will, J.; Bernardo, E.; Boccaccini, A. Functional glasses and glass-ceramics derived from iron rich waste and combination of industrial residues. J. Non Cryst. Solids 2013, 365, 63–74. [Google Scholar] [CrossRef]

| Adsorbent | Nature | Pore Diameter, dp (Ǻ) | Particle Porosity, εp | Particle Density (g cm−3) | Surface Area (m2 g−1) |
|---|---|---|---|---|---|
| Activated alumina | Hydrophilic; amorphous | 10–75 | 0.50 | 1.25 | 320 |
| Silica gel (small pore) | Hydrophilic/ hydrophobic; amorphous | 22–26 | 0.47 | 1.09 | 750–850 |
| Silica gel (large pore) | 100–150 | 0.71 | 0.62 | 300–350 | |
| Activated carbon (small pore) | Hydrophobic; amorphous | 10–25 | 0.4–0.6 | 0.5–0.9 | 400–1200 |
| Activated carbon (large pore) | >30 | - | 0.6–0.8 | 200–600 | |
| Molecular sieve carbon | Hydrophobic | 2–10 | - | 0.98 | 400 |
| Molecular sieve zeolites | Polar hydrophilic; crystalline | 3–10 | 0.2–0.5 | 1.4 | 600–700 |
| Polymeric adsorbents | - | 40–25 | 0.4–0.55 | - | 80–700 |
| Composition (wt. %) | |||||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | MnO | FeO | S | References |
| 30–50 | 28–40 | 8–24 | 1–18 | na | na | na | [41] |
| 36–45 | 33–42 | 10–16 | 3–16 | ≤2 | na | na | [40] |
| 33.7 | 35.1 | 10.6 | 14.4 | 0.5 | 0.4 | 0.8 | [46] |
| 41.7 | 33.8 | 13.4 | 7.4 | 0.3 | 0.5 | 0.8 | [47] |
| 35–42 | 35–40 | 8–15 | 8–9 | 0.3–1.0 | 0.5–0.8 | 0.7–1.5 | [21] |
| 34–43 | 27–38 | 7–12 | 7–15 | 0.15–0.76 | 0.2–1.6 | 1.0–1.9 | [45] |
| 30–56 | 28–38 | 8–24 | 1–18 | 0.5–2 | 0.5–1 | na | [38] |
| 35–42 | 33–38 | 10–15 | 7–12 | ≤1 | ≤1 | 1–1.5 | [44] |
| Slag Type | Chemical Composition (wt. %) | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | FeO | Fe2O3 | MnO | P2O5 | TiO2 | Cr2O3 | ||
| BOF | 45–60 | 10–15 | 1–5 | 3–13 | 7–20 | 3–9 | 2–6 | 1–4 | na | na | [49] |
| BOF | 39.40 | 11.97 | 2.16 | 9.69 | 30.23 | na | 2.74 | 1.00 | 0.40 | 0.20 | [36] |
| BOF | 47.71 | 13.25 | 3.04 | 6.37 | na | 24.36 | 2.64 | 1.47 | 0.67 | 0.19 | [50] |
| BOF | 47.9 | 12.2 | 1.2 | 0.8 | 26.3 | na | 0.3 | 3.3 | na | na | [48] |
| BOF | 30–55 | 8–20 | 1–6 | 5–15 | 10–35 | na | 2-8 | ≤2 | 0.4–2 | ≤0.73 | [39] |
| BOF | 42–55 | 12–18 | ≤3 | ≤8 | na | na | ≤5 | ≤2 | na | ≤10 | [40] |
| EAF | 30–50 | 11–20 | 10–18 | 8–13 | 8–22 | 5–6 | 5–10 | 2–5 | na | na | [49] |
| EAF (carbon steel) | 30–60 | 9–20 | 5–15 | 15–30 | na | 3-8 | ≤0.58 | na | 0.15–1.5 | [39] | |
| EAF | 45.5 | 32.2 | 3.7 | 5.2 | 3.3 | 1.0 | 2.0 | na | 0.13 | 0.05 | [52] |
| EAF | 46.9 | 33.5 | 2.30 | 6.22 | 1.07 | 0.36 | 2.60 | 0.02 | 0.16 | 2.92 | [53] |
| EAF (stainless steel) | 39–45 | 24–32 | 3–7.5 | 8–15 | 1–6 | na | 0.4–2 | ≤0.16 | na | 0.15–29.2 | [39] |
| EAF | 25–40 | 10–17 | 4–7 | 4–15 | na | na | ≤6 | ≤1.5 | na | ≤3 | [40] |
| Ladle | 30–60 | 2–35 | 5–35 | 1–10 | 0.1–1.5 | na | 0-5 | ≤0.9 | na | ≤0.73 | [39] |
| Ladle | 55 | 15 | 12.5 | 7.5 | na | 2.1 | 0.36 | na | 0.33 | 0.01 | [51] |
| Slag | Phase Chemical Properties | Comments | References |
|---|---|---|---|
| Granulated-Blast Furnace Slag (g-BFS) | Glassy-amorphous and vitrified phases | In addition to conventional uses as construction additives, g-BFS and s-BFS can be adopted as adsorbents and geopolymers in the treatment and remediation of industrial effluents and wastewater (Section 3). Activation of BFS is conducted in order to increase their adsorptive capabilities (Section 5). | [21,22,23,44] |
| Slow-cooled Blast Furnace Slag (s-BFS) | Crystallized mineral composition mainly consisting of melilite (Ca2MgSi2O7–Ca2Al2SiO7), and merwinite (Ca3MgSi2O8). | ||
| Basic Oxygen Furnace (BOF) | Ca3SiO5; α-Ca2SiO4; β-Ca2SiO4; 2CaO·Fe2O3; CaO; MgO; 4CaO·Al2O3·Fe2O3; CaO-FeO-MnO-MgO solid solution; wustite solid solution; entrained metallic-Fe | The presence of free CaO and crystalline Ca-Al-Mg silicates imparts acid neutralisation and adsorption capacities, respectively. The SiO2 and Al2O3 are precursors to the synthesis of geopolymers, zeolites and layered double hydroxides from steelmaking slags (Section 5). | [22,23,39,50,52] |
| Electric Arc Furnace (EAF) | FeCr2O4; FeFe2O4;Ni-Cr-Fe solid solution; Ca2SiO4; SiO2; Ca14Mg2(SiO4)8; CaCrO4; Ca3Mg(SiO4)2; Ca2MgSi2O7; Ca2Al2SiO7; (Ca,Mn)2SiO4; Fe-Cr -Ni intermetallic solid solution; (Mg,Mn)(Cr,Al,Fe)2O4 spinel solid solution; (Fe,Mg,Mn)O wustite type solid solution; | The presence of dissolved and/or entrained toxic alloying elements (e.g., Cr and Ni) limits the direct application of steelmaking slag adsorbents. Thus, the long-term toxicity characteristic leaching properties (TCLPs) of slags are largely unknown and require further research (Section 6). Their utilisation could thus be enhanced by integrating with metal recovery and/or slag-cleaning steps to produce environmentally benign residues for use as adsorbents (Section 6). | [22,23,52,53,54,55,56,58,67] |
| Ladle Furnace (stainless steel) | FeCr2O4; FeFe2O4;Ni-Cr-Fe solid solution; Ca2SiO4; CaF2; CaCrO4;Ca14Mg2(SiO4)8; Ca2SiO4; Ca4Si2O7F2; MgO; Fe-Cr; Fe-Ni |
| Material | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Mean Pore Diameter, dp (nm) | Particle Size (d) | References |
|---|---|---|---|---|---|
| BFS | 4.93 | na | na | 0.063–0.25 mm | [70] |
| Granulated BFS | 1.63 | na | na | d < 0.25 mm | [15] |
| Ungranulated BFS | 1.83 | na | na | d < 0.25 mm | |
| Granulated BFS | ~1 | na | na | d < 0.25 mm & 0.50–1.25 mm | [15] |
| Granulated BFS | 0.3–0.5 | na | na | d < 0.25 mm and 0.25–0.80 mm | [42] |
| EAF | 0.142 | na | 12.58 | 0.05–0.630 mm | [67] |
| Ironmaking slag | 31.93 | na | na | 24.5 µm | [71] |
| Steelmaking Slag | 22.33 | na | na | 24.1 µm | |
| Steelmaking slag | 0.82–1.85 | na | 17–27 | 0.85–0.08 mm | [69] |
| Steelmaking slag | 0.29 | na | na | 0.5–1.0 mm | |
| EAFBS slag | 3.42 | na | na | 0.9–2.0 mm | [72] |
| BFS | 0.66 | 0.032 | 18.53 | 44–149 µm | [25] |
| Steelmaking (BOF) slag | 7.34 | 0.028 | 15.9 | 44–149 µm | |
| Steelmaking slags (unmodified) | 7.52 | 11 000 | 5.91 | 2000–4000 µm | [63] |
| Steelmaking slag (modified) | 8.62 | 112 000 | 5.58 | 2000–4000 µm | |
| Steelmaking slag | 30.27 | 0.028 | 30.72 | d < 1 mm | [73] |
| LF slag | 3.04 | 0.0026 | 3.21 | 0.125–0.063 mm | [58] |
| Steelmaking slag (unmodified) | 2.854 | 0.013 | na | <0.25 mm | [64] |
| Steelmaking slag (modified) | 9.53 | 0.025 | na | <0.25 mm | |
| BFS | 2.79 | 0.008 | 12.7 | na | [74] |
| BFS geopolymer | 64.5 | 0.070 | 5.93 | na | |
| Barium-modified BFS geopolymer | 63.1 | 0.070 | 6.32 | na |
| Slag Type | Crystalline/ Amorphous State | Experimental Conditions | Adsorption Capacity (mg/g) | Adsorption Efficiency (%) | Selectivity Series | References |
|---|---|---|---|---|---|---|
| BFS | - | Co = 10−4–10−3 mol/L Me2+ *; pH = 4.0–4.2; T = 25 °C; dp = 0.063–0.25 mm; Phase ratio = 1g/L BFS | 2.1 × 10−3 mol/g Cu2+; 0.95 × 10−3 mol/g Ni2+ 1.58 × 10−3 mol/g Zn2+ | 100% Cu2+; 100% Zn2+ 72% Ni2+ | Cu2+ > Zn2+ > Ni2+ | [70] |
| BFS | - | Co = 13–93 mg/L Pb2+; pH = 2–9; T = 25 °C; dp = 0.1–5 mm; Phase ratio = 1g/L BFS | ~ 37 mg/g Pb2+ | 98% Pb2+ | [42] | |
| BFS | Crystalline | Co = 10−3 mol/L Me2+ (Cu, Ni and Zn); pH = 4; T = 25 °C; dp = 0.1–5 mm; Phase ratio = 1g/L BFS | - | 94–100% Me2+ (Cu, Ni and Zn) | Cu2+ > Ni2+ > Zn2+ | [19] |
| BFS | Mainly amorphous | Co = 16.5 mg/L Cu2+; pH = 4; T = 25 °C; dp < 0.25 mm; Phase ratio = 1g/L BFS | 16.52 mg/g Cu2+ | - | - | [15] |
| EAF | Crystalline | Co = 100–1000 mg/L Pb2+ and Cu2+; pH = 4.5; T = 20–40 °C; dp = 0.05–0.63 mm; Phase ratio = 10 g/L EAF | 37.04 mg/g Pb2+ 39.22 mg/g Cu2+ | - | Cu2+ > Pb2+ | [67] |
| Ironmaking slag | - | Co = 15–170 mg/L Pb2+ and Cu2+; pH = 5.5; p = 24.1–24.5 μm; Phase ratio = 2 g/L slag; | 95.34 mg/g Pb2+ 88.50 mg/g Cu2+ | - | Cu2+ > Pb2+ | [71] |
| Steelmaking slag | - | Co = 25 mg/L Cr3+ = Zn2+ = Cu2+ = Pb2+; pH = 1.3–7.5; T = -; dp = 420–177 μm; Phase ratio = 10 g/L slag; Time = 1 h; | - | 97.87% Cr3+; 96.79% Zn2+; 85.41% Cu2+; 82.08% Pb2+; | Cr3+ > Zn2+ > Cu2+ > Pb2+ | [89] |
| EAF (SG slag) | Crystalline | Co = 0.2–3.2 mmol/L Me2+; pH = 5; T = 25 °C; dp = 45–90 μm; Phase ratio = 10 g/L slag | 0.156 mmol/g Cu2+; 0.166 mmol/g Cd2+; 0.148 mmol/g Zn2+; 0.145 mmol/g Pb2+; | - | Cd2+ > Cu2+ > Zn2+ > Pb2+ | [5] |
| EAF | - | Co = 6 mg/L Arsenic; pH = 10; T = 25 °C; dp = -; Phase ratio = 7 g/L EAF | 1.99 mg/g As | 100% As | - | [6] |
| BOF | Crystalline | Co = 1000–4000 mg/L Cu2+; pH = 3–5; T = 20 °C; dp < 0.6 mm; Phase ratio = 5 g/L BOF | 245.2 mg/g Cu2+ | 99.9% Cu2+ (from 1000 mg/L) | - | [62] |
| BOF | - | ; pH = 2.5; T = 25 °C; dp < 75 μm; Phase ratio = 20–140 g/L BOF (100 g/L optimum) | - | 99.7% Fe2+; ; | - | [90] |
| Steelmaking slag | Crystalline | 1–100 mg/L Co2+ & Ni2+; 2–170 mg/L Cd2+; 3.5–350 mg/L Pb2+; pH = 2–5.5; T = ambient; dp = -; Phase ratio = 5 g/L slag | 0.04 mmol/g Co2+; 0.05 mmol/g Ni2+; 0.04 mmol/g Cd2+; 0.08 mmol/g Pb2+; | - | Pb2+ > Ni2+ > Cd2+ > Co2+ | [91] |
| Steelmaking slag | - | Co = 100 mg/L Mn2+; pH = 3–8 (6 optimum); T = 25 °C; dp = 1 mm; Phase ratio = 0.1–2 g/L slag | ~1.5 mg/g Mn2+ | >95% Mn2+ | - | [73] |
| BOF | Crystalline | 75–150 mg/L Cr6+; pH = -; T = 25 °C; dp < 0.180 mm; Phase ratio = 10 g/L BOF | 11.9 mg/g Cr6+ | 100% Cr6+ (≤100 mg/L); 64.8% Cr6+ (~150 mg/L) | - | [85] |
| BOF | Crystalline | 5-100 mg/L Vanadium; pH = 1–11; T = 25 °C; dp < 0.15 mm; Phase ratio = 50 g/L BOF | 5.45 mg/g | 97.1% V (100 mg/L; pH = 6 | - | [84] |
| Steelmaking slag | Crystalline | 0.05–1 mg/L Phenanthrene; 3–5 mg/L Naphthalene; pH = 3–11; T = 25 °C; dp < 150 μm; Phase ratio = 1–10 g/L slag | 0.043 mg/g phenanthrene; 0.041 mg/g naphthalene | - | - | [64] |
| BFS | More amorphous than crystalline | 5 mg/L Pb = Cu = Cd = Cr = Zn; pH = 2–9; T = 25 °C; dp = 150-300 μm; Phase ratio = 5 g/L BFS | 5.05 mg/g Cd 4.83 mg/g Cr 5.22 mg/g Cu 4.93 mg/g Pb 4.26 mg/g Zn | ~88–95% for all metal ions (5 mg/L; pH = 6.5) | Pb, Cu, Cd > Cr > Zn | [92] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manchisi, J.; Matinde, E.; Rowson, N.A.; Simmons, M.J.H.; Simate, G.S.; Ndlovu, S.; Mwewa, B. Ironmaking and Steelmaking Slags as Sustainable Adsorbents for Industrial Effluents and Wastewater Treatment: A Critical Review of Properties, Performance, Challenges and Opportunities. Sustainability 2020, 12, 2118. https://doi.org/10.3390/su12052118
Manchisi J, Matinde E, Rowson NA, Simmons MJH, Simate GS, Ndlovu S, Mwewa B. Ironmaking and Steelmaking Slags as Sustainable Adsorbents for Industrial Effluents and Wastewater Treatment: A Critical Review of Properties, Performance, Challenges and Opportunities. Sustainability. 2020; 12(5):2118. https://doi.org/10.3390/su12052118
Chicago/Turabian StyleManchisi, James, Elias Matinde, Neil A. Rowson, Mark J. H. Simmons, Geoffrey S. Simate, Sehliselo Ndlovu, and Brian Mwewa. 2020. "Ironmaking and Steelmaking Slags as Sustainable Adsorbents for Industrial Effluents and Wastewater Treatment: A Critical Review of Properties, Performance, Challenges and Opportunities" Sustainability 12, no. 5: 2118. https://doi.org/10.3390/su12052118
APA StyleManchisi, J., Matinde, E., Rowson, N. A., Simmons, M. J. H., Simate, G. S., Ndlovu, S., & Mwewa, B. (2020). Ironmaking and Steelmaking Slags as Sustainable Adsorbents for Industrial Effluents and Wastewater Treatment: A Critical Review of Properties, Performance, Challenges and Opportunities. Sustainability, 12(5), 2118. https://doi.org/10.3390/su12052118









