Seed Germination in Alpine Meadow Steppe Plants from Central Tibet in Response to Experimental Warming
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Seed Collection
2.2. Germination Experiment Design
2.3. Seed Germination Observation
2.4. Calculations of Germination Percentage and Time
2.5. Statistical Analysis
3. Results
3.1. Seed Germination Percentage
3.2. Mean Germination Time
3.3. Germination Phenological Patterns
4. Discussion
4.1. Effects of Warming on Seed Germination of Alpine Grassland Plants
4.2. Effects of Species Identity on Seed Germination in Alpine Grassland Plants
4.3. Implications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vittoz, P.; Dussex, N.; Wassef, J.; Guisan, A. Diaspore traits discriminate good from weak colonisers on high-elevation summits. Basic Appl. Ecol. 2009, 10, 508–515. [Google Scholar] [CrossRef]
- Parolo, G.; Rossi, G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic Appl. Ecol. 2008, 9, 100–107. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Global Change Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Batllori, E.; Camarero, J.J.; Ninot, J.M.; Gutierrez, E. Seedling recruitment, survival and facilitation in alpine Pinus uncinata tree line ecotones. Implications and potential responses to climate warming. Global Ecol. Biogeogr. 2010, 18, 460–472. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Lamont, B. The Ecology of Seeds and Seedlings. J. Biogeogr. 2001, 28, 547–548. [Google Scholar] [CrossRef]
- Erschbamer, B.; Ruth, N.S.; Winkler, E. Colonization processes on a central Alpine glacier foreland. J. Veg. Sci. 2008, 19, 855–862. [Google Scholar] [CrossRef]
- Marcante, S.; Winkler, E.; Erschbamer, B. Population dynamics along a primary succession gradient: Do alpine species fit into demographic succession theory? Ann. Bot. 2009, 103, 1129–1143. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer Science & Business Media: Berlin, Germany, 2001. [Google Scholar]
- de Kroon, H.; van Groenendael, J. The ecology and evolution of clonal plants. Ecol. Evol. Clonal Plants 1997, 15, 565–582. [Google Scholar]
- Gabrielsen, T.M.; Brochmann, C. Sex after all: High levels of diversity detected in the arctic clonal plant Saxifraga cernua using RAPD markers. Mol. Ecol. 1998, 7, 1701–1708. [Google Scholar] [CrossRef]
- Forbis, T.A. Seedling demography in an alpine ecosystem. Am. J. Bot. 2003, 90, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.; Bosch, P.; Canziani, O.; Chen, Z.; Christ, R.; Davidson, O.; Hare, W.; Huq, S.; Karoly, D.; Kattsov, V. Climate Change 2007 - Synthesis Report. Environ. Policy Collect. 2007, 27, 408. [Google Scholar]
- Li, L.; Zhang, Y.; Wu, J.; Li, S.; Zhang, B.; Zu, J.; Zhang, H.; Ding, M.; Paudel, B. Increasing sensitivity of alpine grasslands to climate variability along an elevational gradient on the Qinghai-Tibet Plateau. Sci. Total Environ. 2019, 678, 21–29. [Google Scholar] [CrossRef]
- Zhang, R.; Su, F.; Jiang, Z.; Gao, X.; Guo, D.; Ni, J.; You, Q.; Lan, C.; Zhou, B. An overview of projected climate and environmental changes across the Tibetan Plateau in the 21st century. Chin. Sci. Bull. 2015, 60, 3036–3047. [Google Scholar]
- Yao, T.; Thompson, L.G.; Mosbrugger, V.; Fan, Z.; Ma, Y.; Luo, T.; Xu, B.; Yang, X.; Joswiak, D.R.; Wang, W. Third Pole Environment (TPE). Environ. Dev. 2012, 3, 52–64. [Google Scholar] [CrossRef]
- Jiao, S.; Wang, L.; Liu, G. Prediction of Tibetan Plateau Permafrost Distribution in Global Warming. Acta Sci. Nat. Univ. Pekin. 2016, 52, 249–256. [Google Scholar]
- Guo, Z.; Niu, F.; Zhan, H.; Wu, Q. Changes of grassland ecosystem due to degradation of permafrost frozen soil in the Qinghai-Tibet Plateau. Acta Ecol. Sin. 2007, 27, 3294–3301. [Google Scholar]
- Gworek, J.R.; Wall, S.B.V.; Brussard, P.F. Changes in biotic interactions and climate determine recruitment of Jeffrey pine along an elevation gradient. For. Ecol. Manag. 2007, 239, 57–68. [Google Scholar] [CrossRef]
- Lloret, F.; Peñuelas, J.; Estiarte, M. Experimental evidence of reduced diversity of seedlings due to climate modification in a Mediterranean-type community. Global Chang. Biol. 2010, 10, 248–258. [Google Scholar] [CrossRef]
- Lesica, P. Arctic-Alpine Plants Decline over Two Decades in Glacier National Park, Montana, U.S.A. Arct. Antarct. Alp. Res. 2018, 46, 327–332. [Google Scholar] [CrossRef]
- Penfield, S. Seed biology—From lab to field. J. Exp. Bot. 2017, 68, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Graae, B.J.; Alsos, I.G.; Ejrnaes, R. The impact of temperature regimes on development, dormancy breaking and germination of dwarf shrub seeds from arctic, alpine and boreal sites. Plant Ecol. 2008, 198, 275–284. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, P.; He, Y.; Zhang, X.; Li, Q. The Climate Feature of Damxung Alpine Meadow Carbon Flux Research Station on the Tibetan Plateau. J. Mt. Sci.-Engl. 2009, 27, 88–95. [Google Scholar]
- Zhou, N.; Fu, G.; Sun, W.; Li, S.; Shen, Z.; He, Y.; Zhang, X.; Wang, J. Initial response of light use efficiency to experimental warming in an alpine meadow in the NorthernTibetan Plateau. Acta Prataculturae Sin. 2016, 25, 251–257. [Google Scholar]
- Shen, Z.X.; Li, Y.L.; Fu, G. Response of soil respiration to short-term experimental warming and precipitation pulses over the growing season in an alpine meadow on the Northern Tibet. Appl. Soil Ecol. 2015, 90, 35–40. [Google Scholar] [CrossRef]
- Hay, F.R.; Smith, R.D.; Dickie, J.; Linington, S.; Pritchard, H.; Probert, R. Seed maturity: When to collect seeds from wild plants. In Seed Conservation: Turning Science into Practice; Royal Botanic Gardens, Kew: London, UK, 2003; pp. 97–133. [Google Scholar]
- Mondoni, A.; Rossi, G.; Orsenigo, S.; Probert, R.J. Climate warming could shift the timing of seed germination in alpine plants. Ann. Bot. 2012, 110, 155–164. [Google Scholar] [CrossRef]
- Hu, X.W.; Fan, Y.; Baskin, C.C.; Baskin, J.M.; Wang, Y.R. Comparison of the effects of temperature and water potential on seed germination of fabaceae species from desert and subalpine grassland. Am. J. Bot. 2015, 102, 649–660. [Google Scholar] [CrossRef]
- Yang, S.H.; Li, X.; Yang, Y.Q.; Yin, X.; Yang, Y.P. Comparing the relationship between seed germination and temperature for Stipa species on the Tibetan Plateau. Botany 2014, 92, 895–900. [Google Scholar] [CrossRef]
- Milbau, A.; Graae, B.J.; Shevtsova, A.; Nijs, I. Effects of a warmer climate on seed germination in the subarctic. Ann. Bot. 2009, 104, 287–296. [Google Scholar] [CrossRef]
- Li, H.; Li, L. Mean and extreme climate change on the Qinghai-Tibetan Plateau with a 2 °C global warming. Progress. Inquisitiones DE Mutat. Clim. 2015, 11, 157–164. [Google Scholar]
- Xu, J.; Li, W.; Zhang, C.; Liu, W.; Du, G. The determinants of seed germination in an alpine/subalpine community on the Eastern Qinghai-Tibetan Plateau. Ecol. Eng. 2017, 98, 114–122. [Google Scholar] [CrossRef]
- Larcher, W.; Kainmuller, C.; Wagner, J. Survival types of high mountain plants under extreme temperatures. Flora 2010, 205, 3–18. [Google Scholar] [CrossRef]
- Ma, X.; Chen, S.; Deng, J.; Feng, Q.; Huang, X. Vegetation phenology dynamics and its response to climate change on the Tibetan Plateau. Acta Prataculturae Sin. 2016, 25, 13–21. [Google Scholar]
- He, T.; Wu, X.; Jia, J. Research advances in morphology and anatomy of alpine plants growing in the Qinghai-Tibet Plateau and their adaptations to environments. Acta Ecol. Sin. 2007, 27, 2574–2583. [Google Scholar]
- Liu, W.; Liu, K.; Zhang, C.; Du, G. Effect of accumulated temperature on seed germination - a case study of 12 Compositae species on the eastern Qinghai-Tibet Plateau of China. Chin. J. Plant Ecol. 2011, 35, 751–758. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Arroyo, M.T.K. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae)—Altitudinal variation in the mediterranean Andes of central Chile. Plant Ecol. 2000, 149, 1–8. [Google Scholar] [CrossRef]
- Wei, Y.; Bai, Y.; Henderson, D.C. Critical conditions for successful regeneration of an endangered annual plant, Cryptantha minima: A modeling approach. J. Arid. Environ. 2009, 73, 872–875. [Google Scholar] [CrossRef]
- Orsenigo, S.; Mondoni, A.; Rossi, G.; Abeli, T. Some like it hot and some like it cold, but not too much: Plant responses to climate extremes. Plant Ecol. 2014, 215, 677–688. [Google Scholar] [CrossRef]
- Cooper, E.J.; Alsos, I.G.; Hagen, D.; Smith, F.M.; Coulson, S.J.; Hodkinson, I.D. Plant recruitment in the High Arctic: Seed bank and seedling emergence on Svalbard. J. Veg. Sci. 2004, 15, 115–124. [Google Scholar] [CrossRef]
- Suter, M.; Luscher, A. Rapid and high seed germination and large soil seed bank of Senecio aquaticus in managed grassland. Sci. World J. 2012, 2012, 723808. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C. Disturbance, Life History Strategies, and Seed Fates in Alpine Herbfield Communities. Am. J. Bot. 1995, 82, 421–433. [Google Scholar] [CrossRef]
- Wookey, P.A.; Robinson, C.H.; Parsons, A.N.; Welker, J.M.; Press, M.C.; Callaghan, T.V.; Lee, J.A. Environmental constraints on the growth, photosynthesis and reproductive development of Dryas octopetala at a high Arctic polar semi-desert, Svalbard. Oecologia 1995, 102, 478–489. [Google Scholar] [CrossRef]
- Mamet, S.D.; Kershaw, G.P. Multi-scale Analysis of Environmental Conditions and Conifer Seedling Distribution Across the Treeline Ecotone of Northern Manitoba, Canada. Ecosystems 2013, 16, 295–309. [Google Scholar] [CrossRef]
- Simons, A.M. Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc. Biol. Sci. 2011, 278, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Gremer, J.R.; Venable, D.L. Bet hedging in desert winter annual plants: Optimal germination strategies in a variable environment. Ecol. Lett. 2014, 17, 380–387. [Google Scholar] [CrossRef]
- Isbell, F.I.; Polley, H.W.; Wilsey, B.J. Species interaction mechanisms maintain grassland plant species diversity. Ecology 2009, 90, 1821–1830. [Google Scholar] [CrossRef]
- Ronnenberg, K.; Wesche, K.; Pietsch, M.; Hensen, I. Seed germination of five mountain steppe species of Central Asia. J. Arid Environ. 2007, 71, 404–410. [Google Scholar] [CrossRef]
- Probert, R.J.; Fenner, M. The role of temperature in the regulation of seed dormancy and germination. Seeds Ecol. Regen. Plant Communities 2000, 2, 261–292. [Google Scholar]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D. Asexual and sexual reproductive strategies in clonal plants. J. Plant Ecol. 2006, 30, 174–183. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, J.; Mu, X.; Lin, J. Advances on the Mechanism of Low Sexual Reproductivity of Leymus Chinensis. Chin. J. Grassl. 2002, 24, 55–60. [Google Scholar]
- Wang, H.; Wang, Z.; Li, L.; Chen, Y.; Ren, L. Reproductive tendency of clonal plants in various habitats. Chin. J. Ecol. 2005, 24, 670–676. [Google Scholar]
- Evette, A.; Bedecarrats, A.; Bornette, G. Environmental Constraints Influence Clonal Traits of Herbaceous Plant Communities in an Alpine Massif. Folia Geobot. 2009, 44, 95–108. [Google Scholar] [CrossRef]
- Herben, T.; Sera, B.; Klimesova, J. Clonal growth and sexual reproduction: Tradeoffs and environmental constraints. Oikos 2015, 124, 469–476. [Google Scholar] [CrossRef]
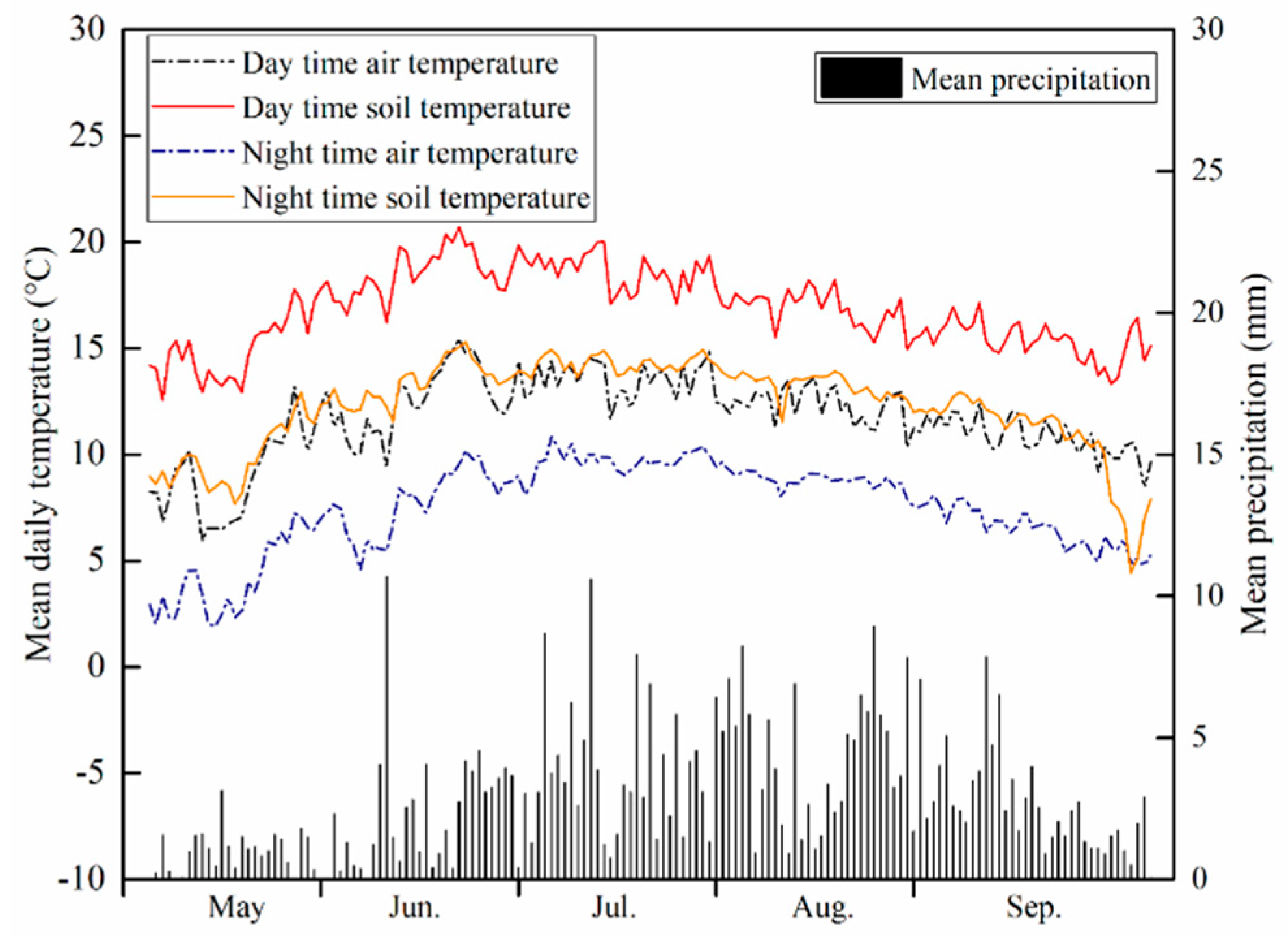
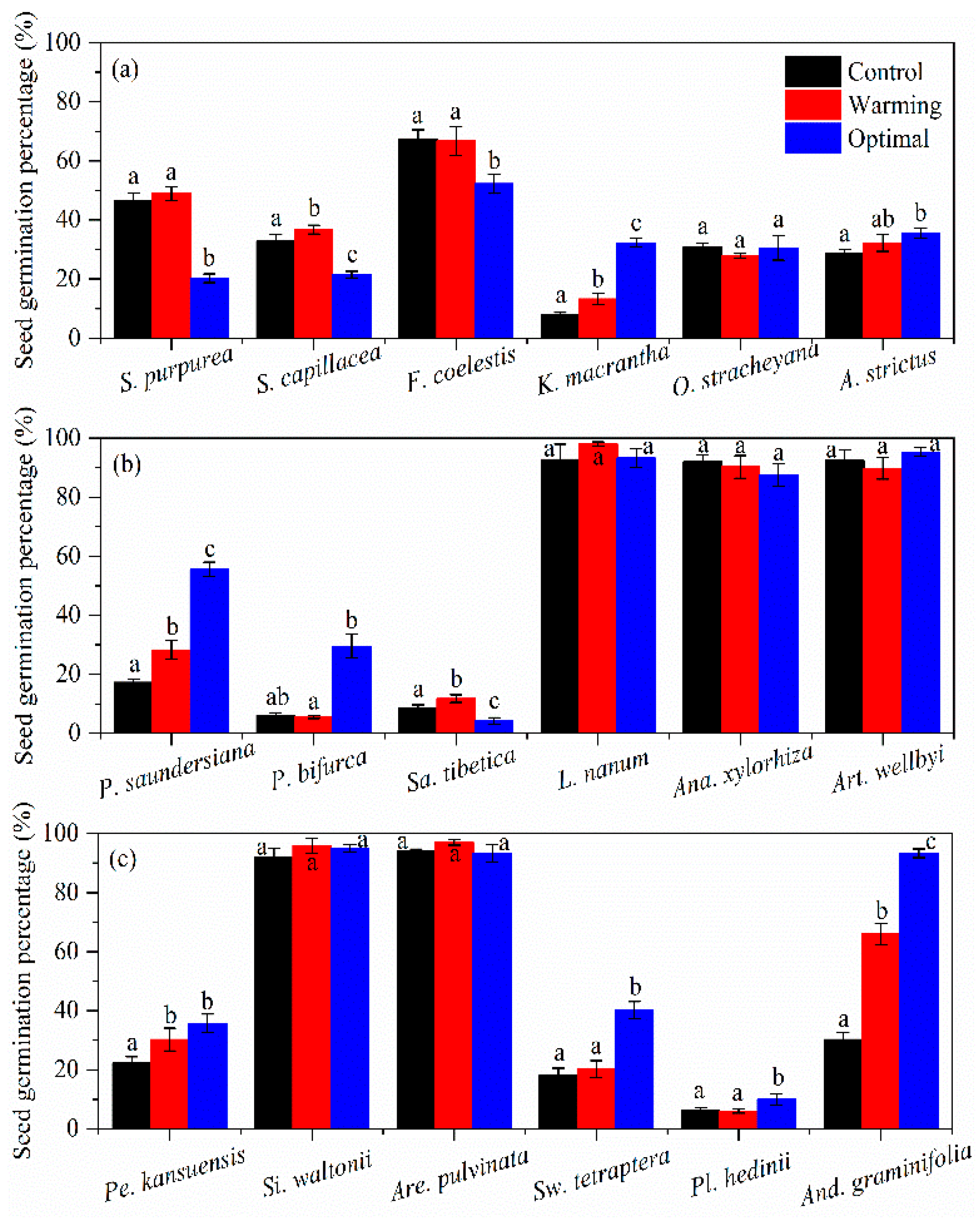
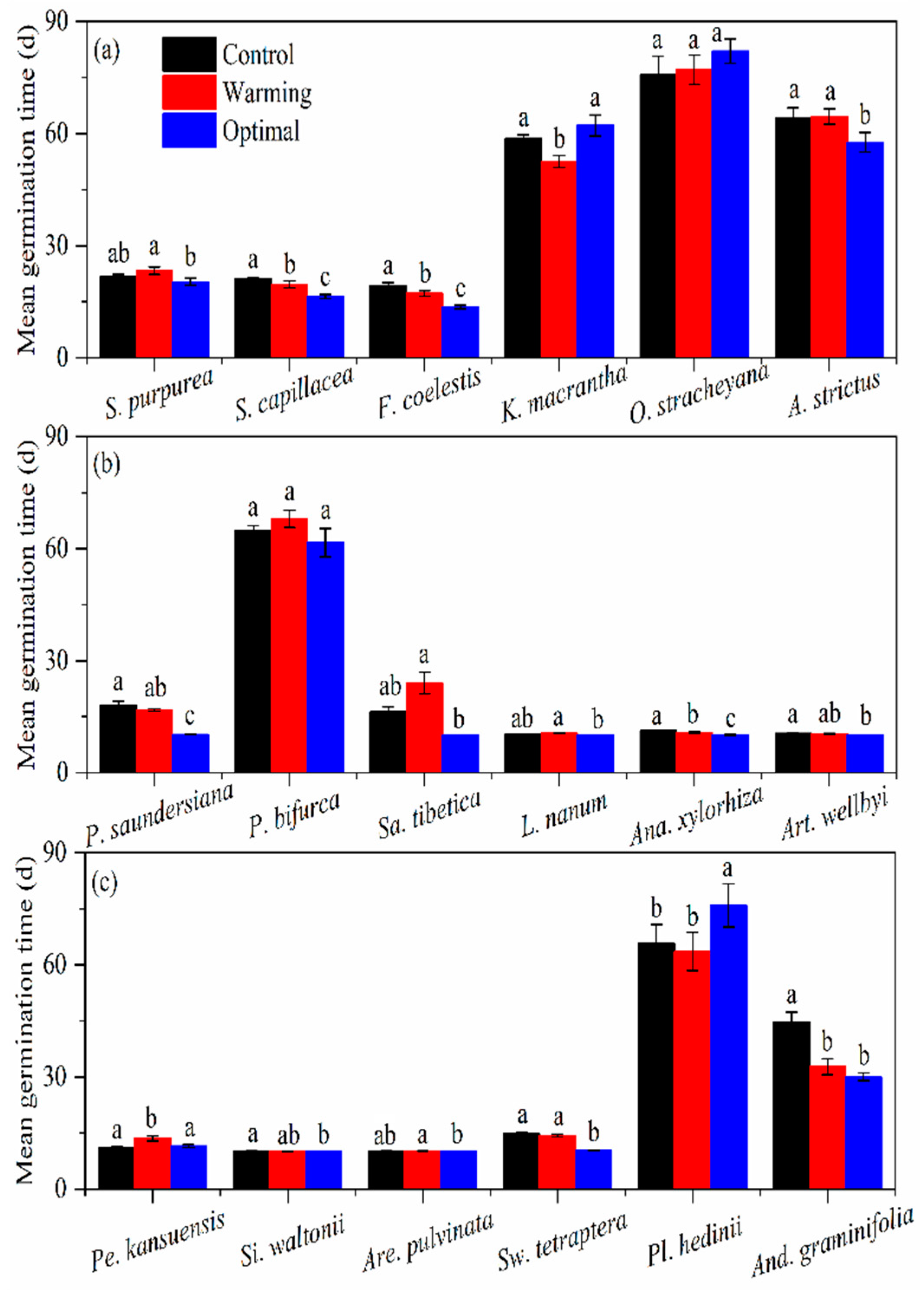
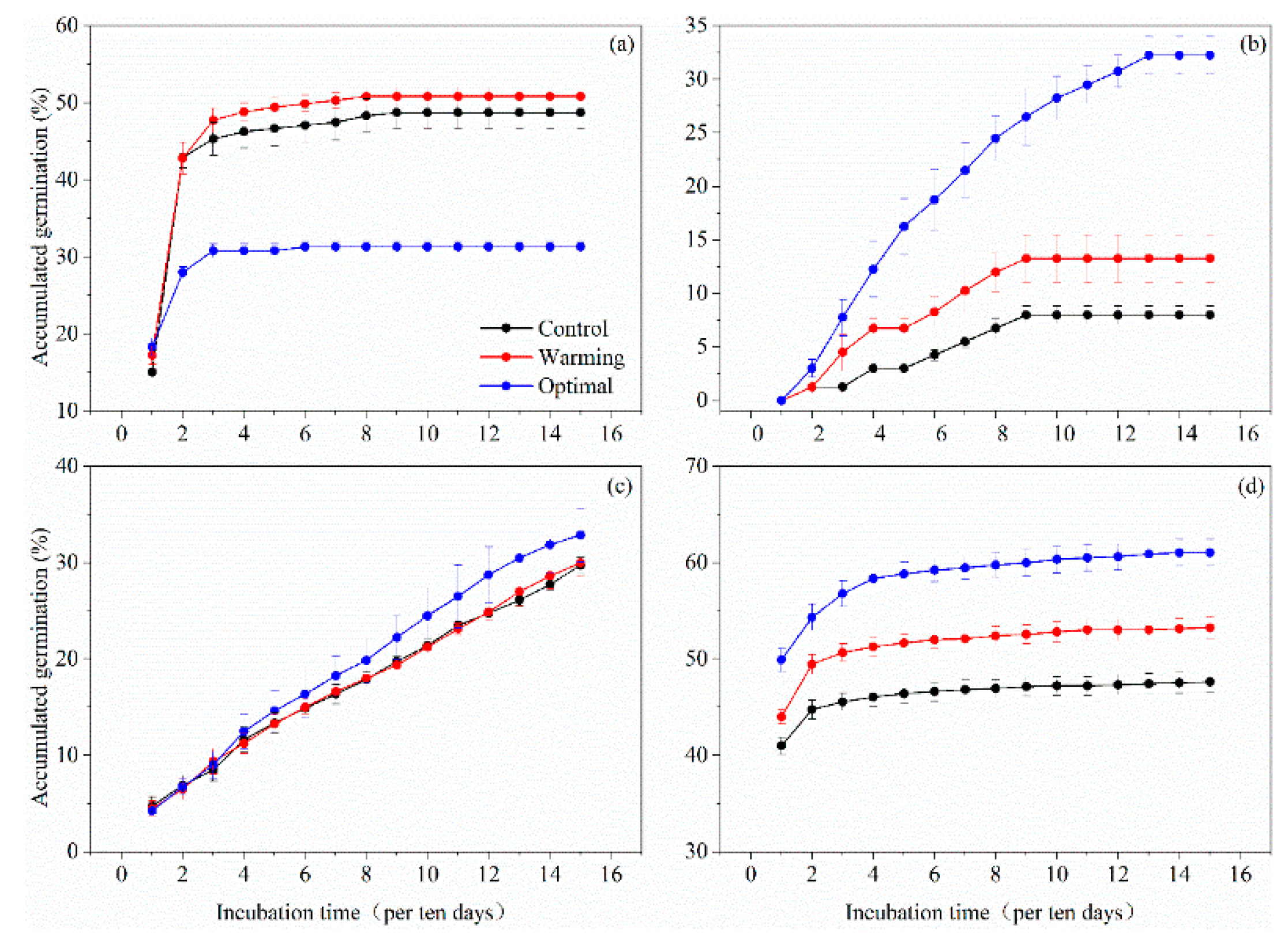
| Species | Family | Functional Type | Abbreviations ‡ |
|---|---|---|---|
| Stipa purpurea Griseb. | Gramineae | Grass | S. purpurea |
| Stipa capillacea Keng | Gramineae | Grass | S. capillacea |
| Festuca coelestis Krecz. et Bobr. | Gramineae | Grass | F. coelestis |
| Kobresia pygmaea C. B. Clarke † | Cyperaceae | Sedge | K. pygmaea |
| Kobresia macrantha Bocklr. | Cyperaceae | Sedge | K. macratha |
| Oxytropis stracheyana Benth. ex Baker | Leguminosae | Legume | O. stracheyana |
| Astragalus strictus Grah. Ex Bend. | Leguminosae | Legume | A. strictus |
| Potentilla saundersiana Royle | Rosaceae | Forb | P. saundersiana |
| Potentilla bifurca Linn. | Rosaceae | Forb | P. bifurca |
| Saussurea tibetica C. Winkl. | Compositae | Forb | Sa. tibetica |
| Leontopodium nanum Hand.-Mazz. | Compositae | Forb | L. nanum |
| Anaphalis xylorhiza Sch.-Bip. ex Hook. f. | Compositae | Forb | Ana. xylorhiza |
| Artemisia wellbyi Hemsl. et Pears. ex Deasy | Compositae | Forb | Art. wellbyi |
| Pedicularis kansuensis Maxim. | Scrophulariaceae | Forb | Pe. kansuensis |
| Silene waltonii Williams | Caryophyllaceae | Forb | Si. waltonii |
| Arenaria pulvinata Edgew. | Caryophyllaceae | Forb | Are. pulvinata |
| Swertia tetraptera Maxim. | Gentianaceae | Forb | Sw. tetraptera |
| Pleurospermum hedinii Diels | Umbelliferae | Forb | Pl. hedinii |
| Androsace graminifolia C. E. C. Fisch. | Primulaceae | Forb | And. graminifolia |
| Incubation Temperature (°C) | ||||
|---|---|---|---|---|
| Phase | Dates † | Control | Warming (+3 ℃) | Optimum |
| 1 | 1st May–10th May | 14.9/9.2 | 17.9/12.2 | 25/15 |
| 2 | 11th May–20th May | 15.1/9.3 | 18.1/12.3 | 25/15 |
| 3 | 21st May–30th May | 17.7/12.0 | 20.7/15.0 | 25/15 |
| 4 | 31st May–9th June | 18.6/12.5 | 21.6/15.5 | 25/15 |
| 5 | 10th June–19th June | 20.2/14.3 | 23.2/17.3 | 25/15 |
| 6 | 20th June–29th June | 19.6/13.8 | 22.6/16.8 | 25/15 |
| 7 | 30th June–9th July | 19.7/14.4 | 22.7/17.4 | 25/15 |
| 8 | 10th July–19th July | 18.8/14.2 | 21.8/17.2 | 25/15 |
| 9 | 20th July–29th July | 18.3/14.1 | 21.3/17.1 | 25/15 |
| 10 | 30th July–8th August | 17.6/13.5 | 20.6/16.5 | 25/15 |
| 11 | 9th August–18th August | 17.7/13.7 | 20.7/16.7 | 25/15 |
| 12 | 19th August–28th August | 17.4/13.5 | 20.4/16.5 | 25/15 |
| 13 | 29th August–7th September | 17.4/13.4 | 20.4/16.4 | 25/15 |
| 14 | 8th September–17th September | 17.2/12.7 | 20.2/15.7 | 25/15 |
| 15 | 18th September–27th September | 15.9/11.6 | 18.9/14.6 | 25/15 |
| Levene’s Test | Control_SW | Warming_SW | Optimal_SW | |
|---|---|---|---|---|
| S. purpurea | 0.587 | 0.240 | 0.062 | 0.850 |
| S. capillacea | 0.059 | 0.123 | 0.850 | 0.972 |
| F. coelestis | 0.123 | 0.262 | 0.233 | 0.976 |
| K. macrantha | 0.149 | 0.683 | 0.798 | 0.850 |
| O. stracheyana | 0.081 | 0.850 | 0.272 | 0.662 |
| A. strictus | 0.457 | 0.224 | 0.556 | 0.272 |
| P. saundersiana | 0.241 | 0.406 | 0.584 | 0.962 |
| P. bifurca | 0.024 | 0.024 | 0.024 | 0.998 |
| Si. waltonii | 0.312 | 0.024 | 0.952 | 0.161 |
| Are. pulvinata | 0.002 | 0.683 | 0.024 | 0.513 |
| Sw. tetraptera | 0.814 | 0.734 | 0.513 | 0.900 |
| Pl. hedinii | 0.274 | 0.272 | 0.683 | 0.577 |
| And. graminifolia | 0.314 | 0.650 | 0.995 | 0.850 |
| Pe. kansuensis | 0.184 | 0.911 | 0.332 | 0.584 |
| Sa. tibetica | 0.674 | 0.972 | 0.224 | 0.406 |
| L. nanum | 0.024 | 0.467 | 0.683 | 0.584 |
| Ana. xylorhiza | 0.197 | 0.972 | 0.332 | 0.798 |
| Art. wellbyi | 0.449 | 0.899 | 0.650 | 0.850 |
| Levene’s test | Control_SW | Warming_SW | Optimal_SW | |
|---|---|---|---|---|
| S. purpurea | 0.787 | 0.972 | 0.216 | 0.103 |
| S. capillacea | 0.056 | 0.272 | 0.504 | 0.515 |
| F. coelestis | 0.403 | 0.848 | 0.187 | 0.343 |
| K. macrantha | 0.201 | 0.599 | 0.503 | 0.030 |
| O. stracheyana | 0.843 | 0.442 | 0.432 | 0.420 |
| A. strictus | 0.599 | 0.599 | 0.609 | 0.201 |
| P. saundersiana | 0.102 | 0.790 | 1.000 | 0.001 |
| P. bifurca | 0.074 | 0.011 | 0.223 | 0.318 |
| Si. waltonii | 0.013 | 0.272 | 0.001 | -- |
| Are. pulvinata | 0.075 | 0.272 | 0.683 | -- |
| Sw. tetraptera | 0.341 | 0.079 | 0.414 | 0.086 |
| Pl. hedinii | 0.738 | 0.598 | 0.437 | 0.431 |
| And. graminifolia | 0.380 | 0.217 | 0.392 | 0.610 |
| Pe. kansuensis | 0.613 | 0.605 | 0.948 | 0.798 |
| Sa. tibetica | 0.040 | 0.125 | 0.118 | -- |
| L. nanum | 0.029 | 0.272 | 0.001 | -- |
| Ana. xylorhiza | 0.248 | 0.850 | 0.798 | 0.272 |
| Art. wellbyi | 0.033 | 0.001 | 0.972 | -- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Niu, B.; Zhang, X.; He, Y.; Shi, P.; Miao, Y.; Cao, Y.; Li, M.; Wang, Z. Seed Germination in Alpine Meadow Steppe Plants from Central Tibet in Response to Experimental Warming. Sustainability 2020, 12, 1884. https://doi.org/10.3390/su12051884
Wang X, Niu B, Zhang X, He Y, Shi P, Miao Y, Cao Y, Li M, Wang Z. Seed Germination in Alpine Meadow Steppe Plants from Central Tibet in Response to Experimental Warming. Sustainability. 2020; 12(5):1884. https://doi.org/10.3390/su12051884
Chicago/Turabian StyleWang, Xiangtao, Ben Niu, Xianzhou Zhang, Yongtao He, Peili Shi, Yanjun Miao, Yanan Cao, Meng Li, and Zhipeng Wang. 2020. "Seed Germination in Alpine Meadow Steppe Plants from Central Tibet in Response to Experimental Warming" Sustainability 12, no. 5: 1884. https://doi.org/10.3390/su12051884
APA StyleWang, X., Niu, B., Zhang, X., He, Y., Shi, P., Miao, Y., Cao, Y., Li, M., & Wang, Z. (2020). Seed Germination in Alpine Meadow Steppe Plants from Central Tibet in Response to Experimental Warming. Sustainability, 12(5), 1884. https://doi.org/10.3390/su12051884







