The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica Oleracea L. Var. Capitata L.) Seedlings Grown under Controlled Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
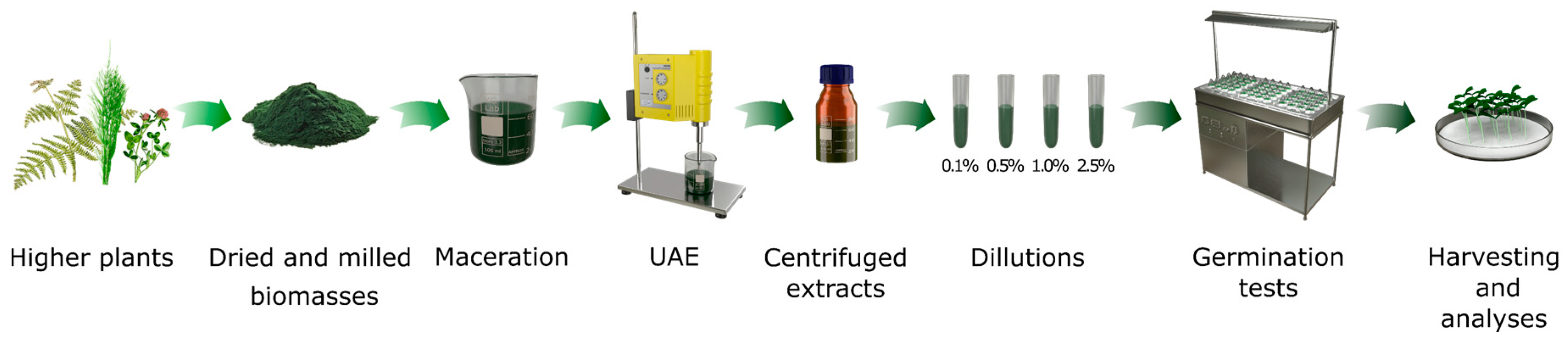
2.3. Extracts Production
2.4. Utilitarian Properties of Extracts
2.4.1. Germination Tests
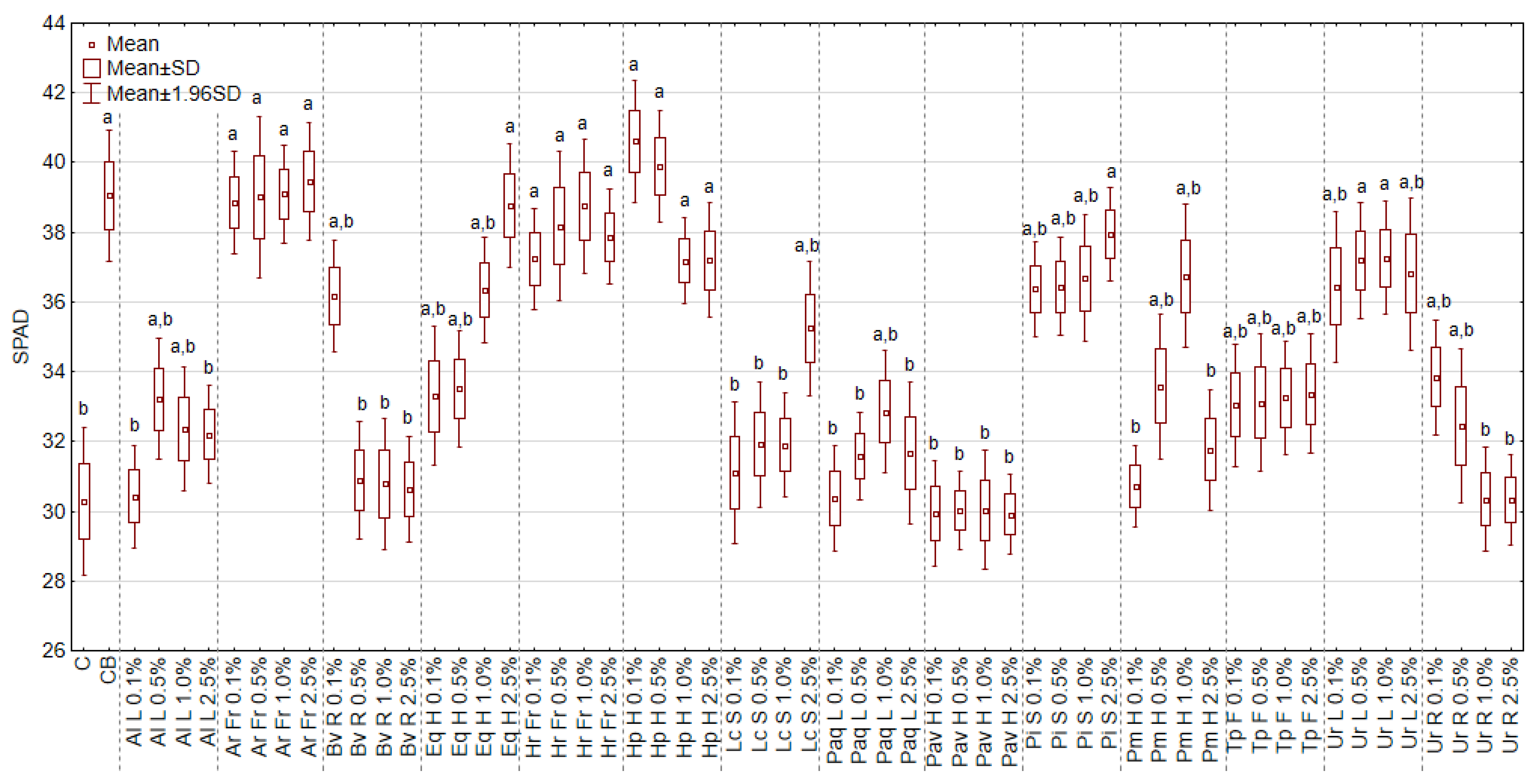
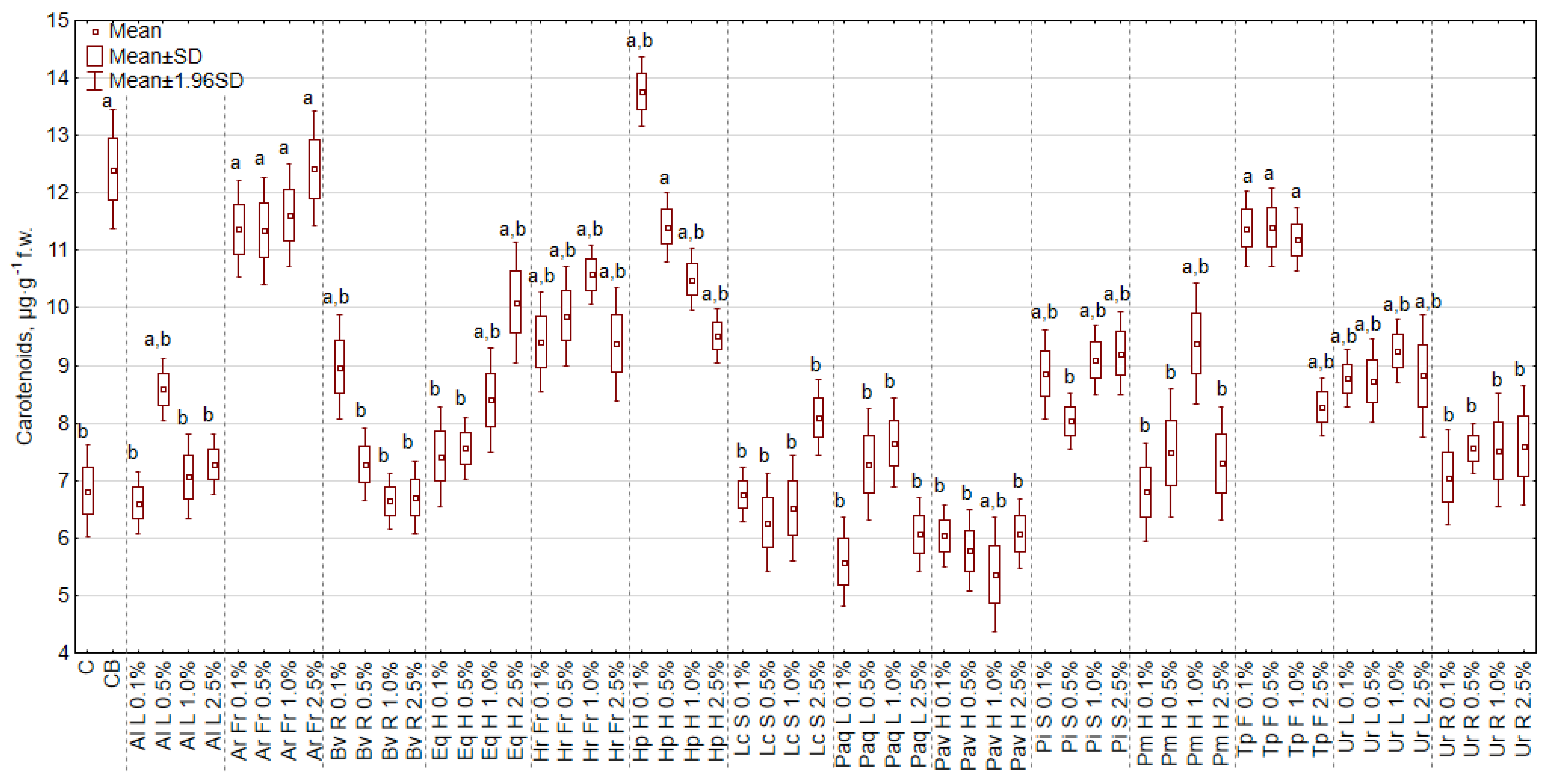
2.4.2. The Concentration of Pigments and Greenness Index of the Leaf
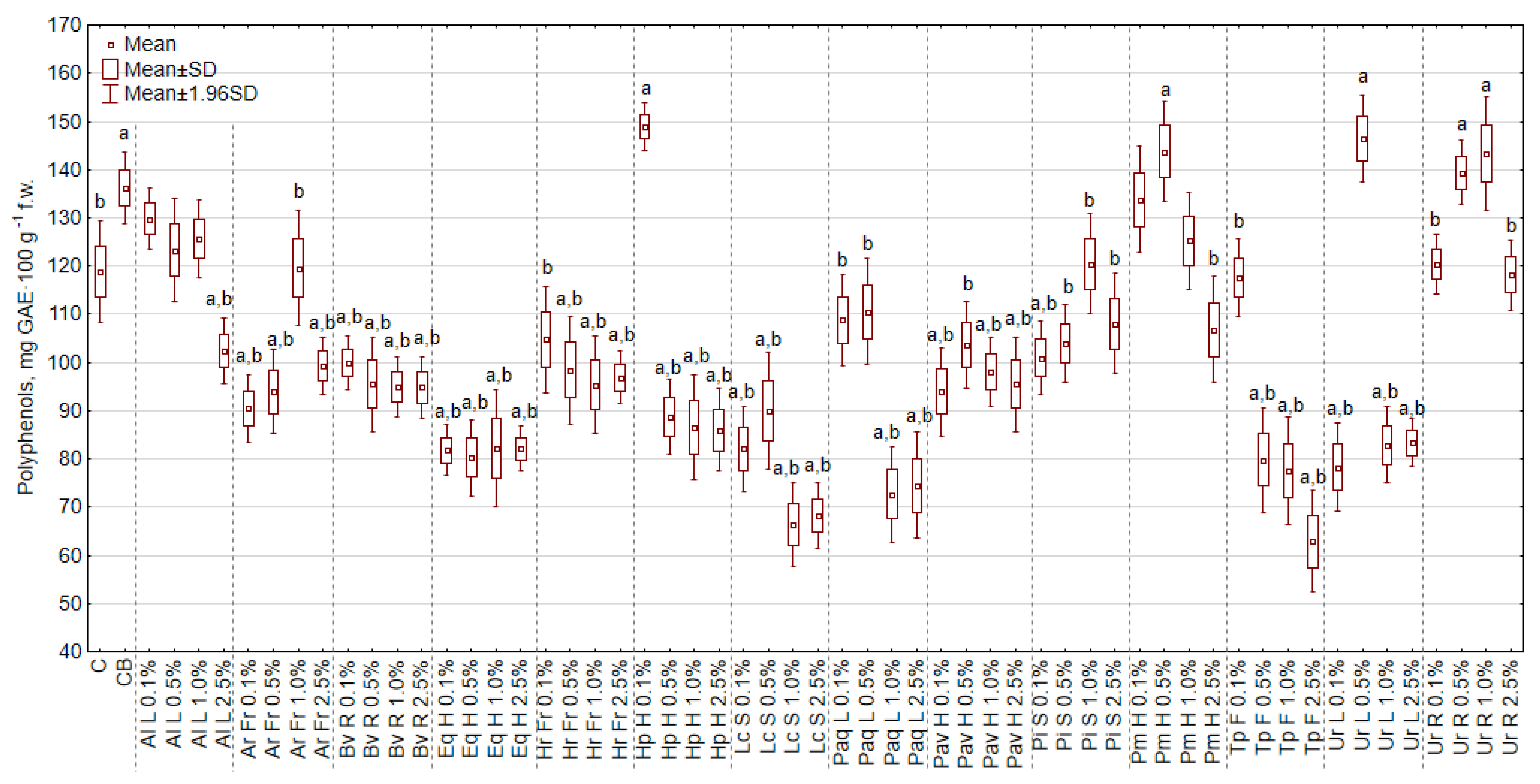
2.4.3. The Concentration of Polyphenols in Cabbage Seedlings
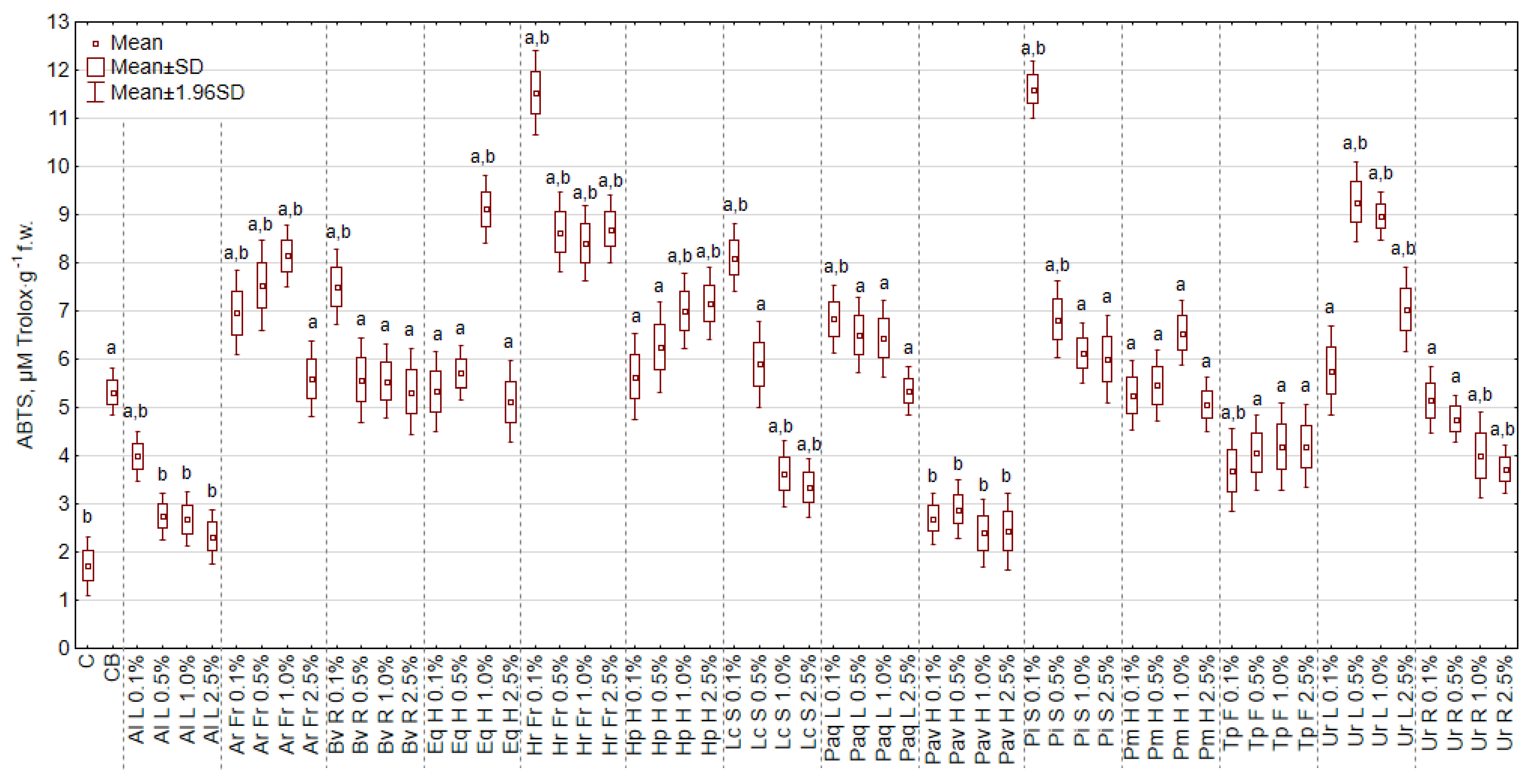
2.4.4. The Antioxidant Activity (DPPH, ABTS, FRAP) of Cabbage Seedlings
2.5. Statistical Analysis
3. Results
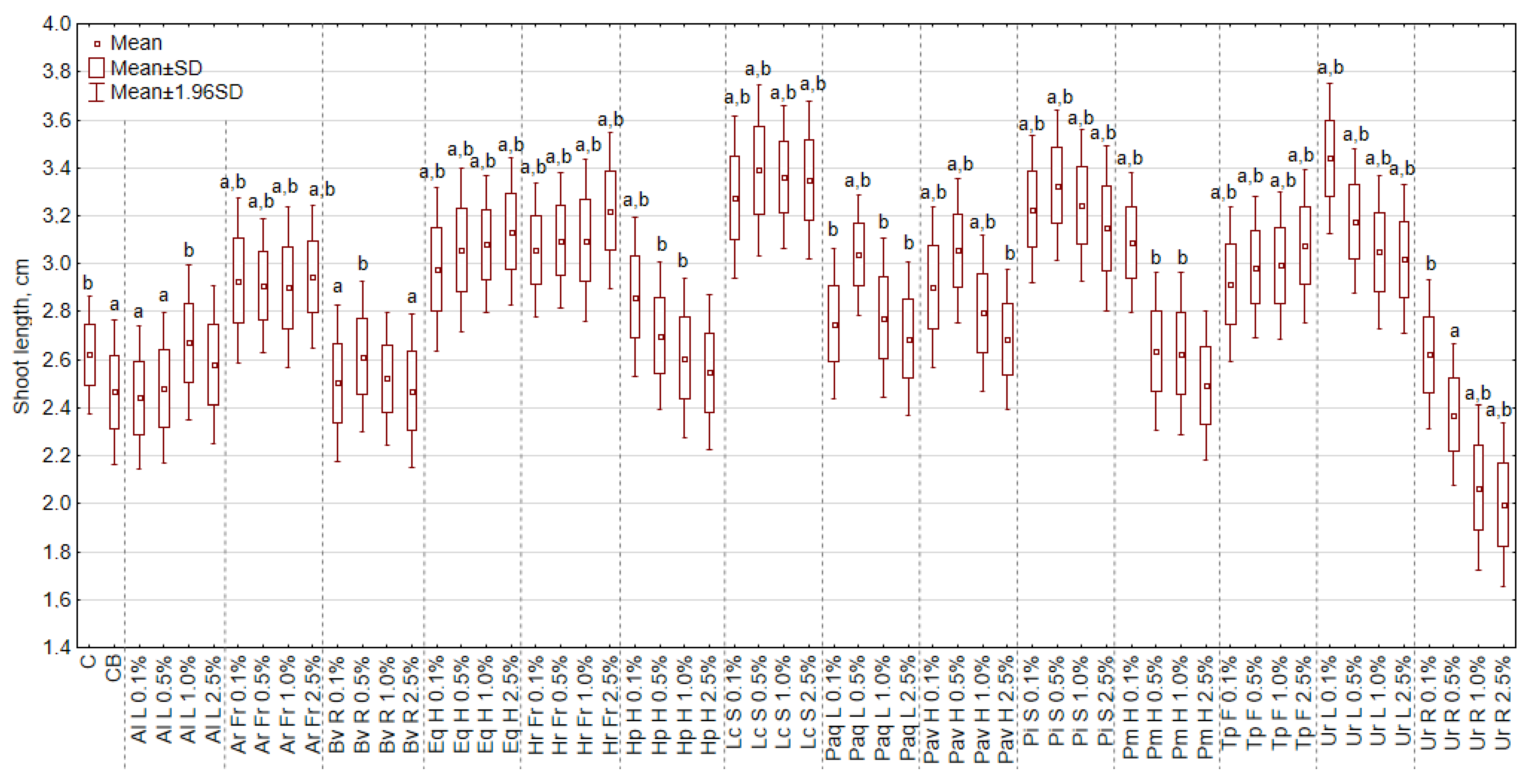
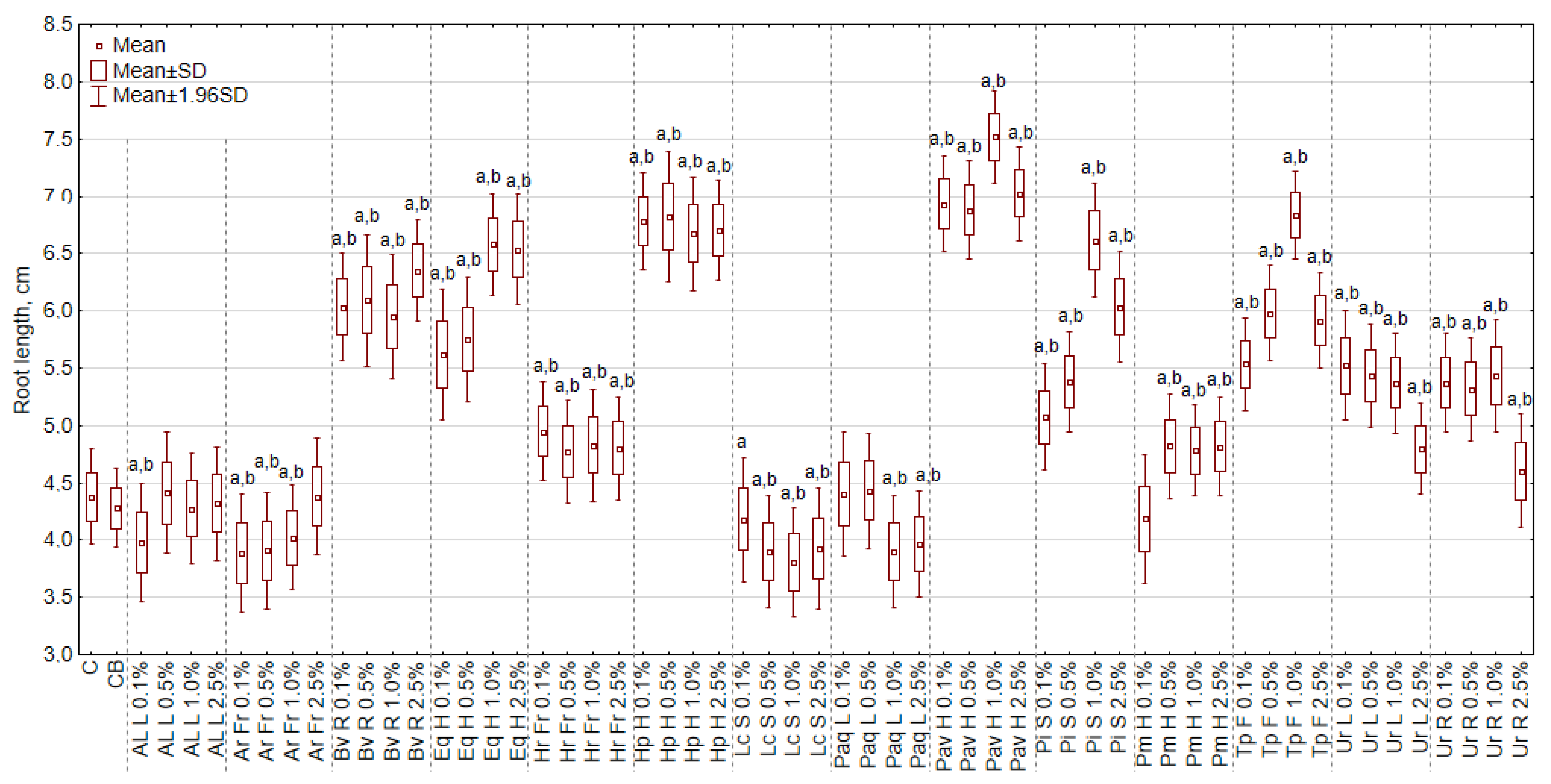
3.1. The Effect of Extracts on the Length of Shoots and Roots
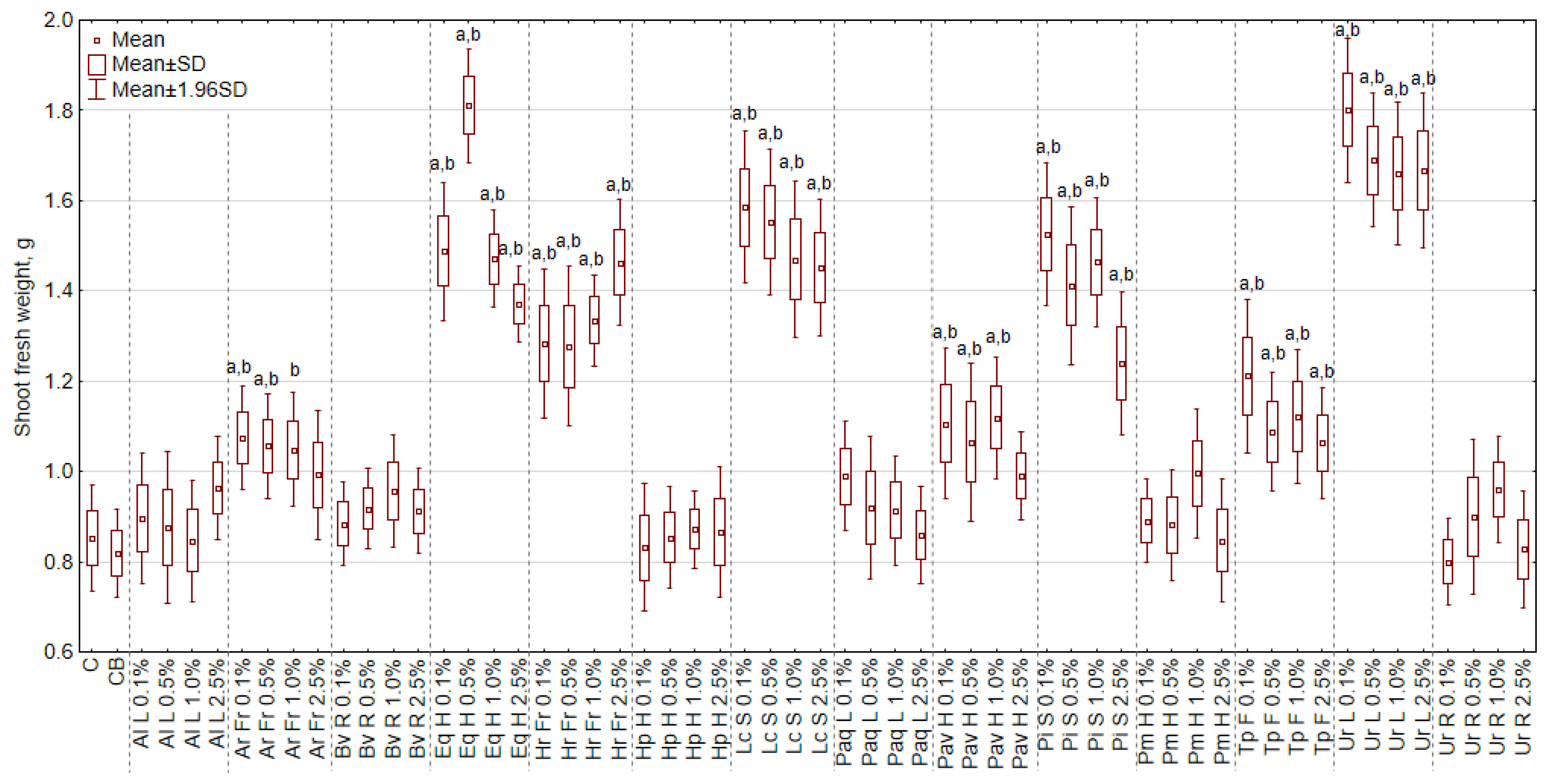
3.2. The Effect of Extracts on the Fresh Weight of Shoots
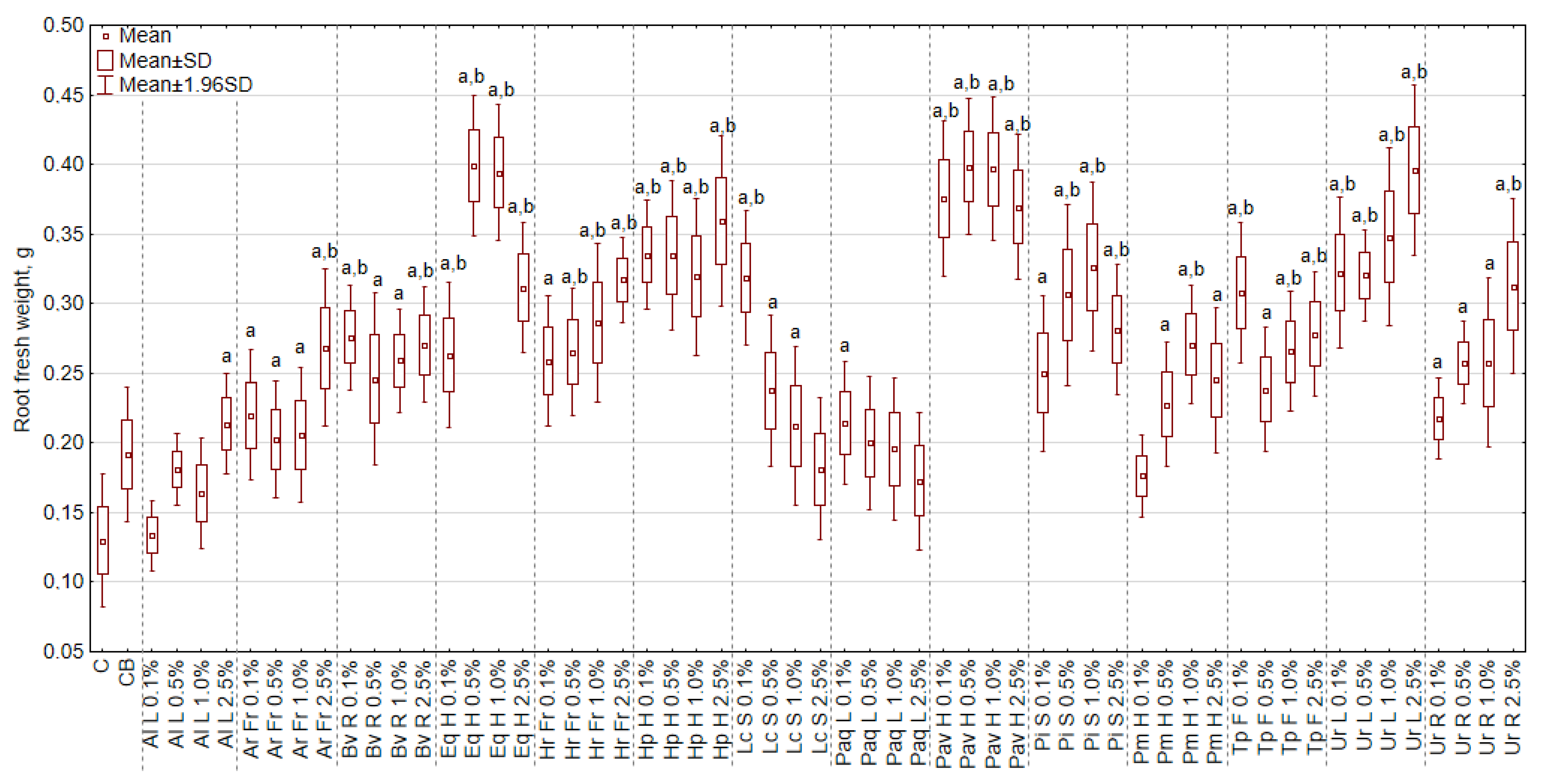
3.3. The Effect of Extracts on the Fresh Weight of Roots
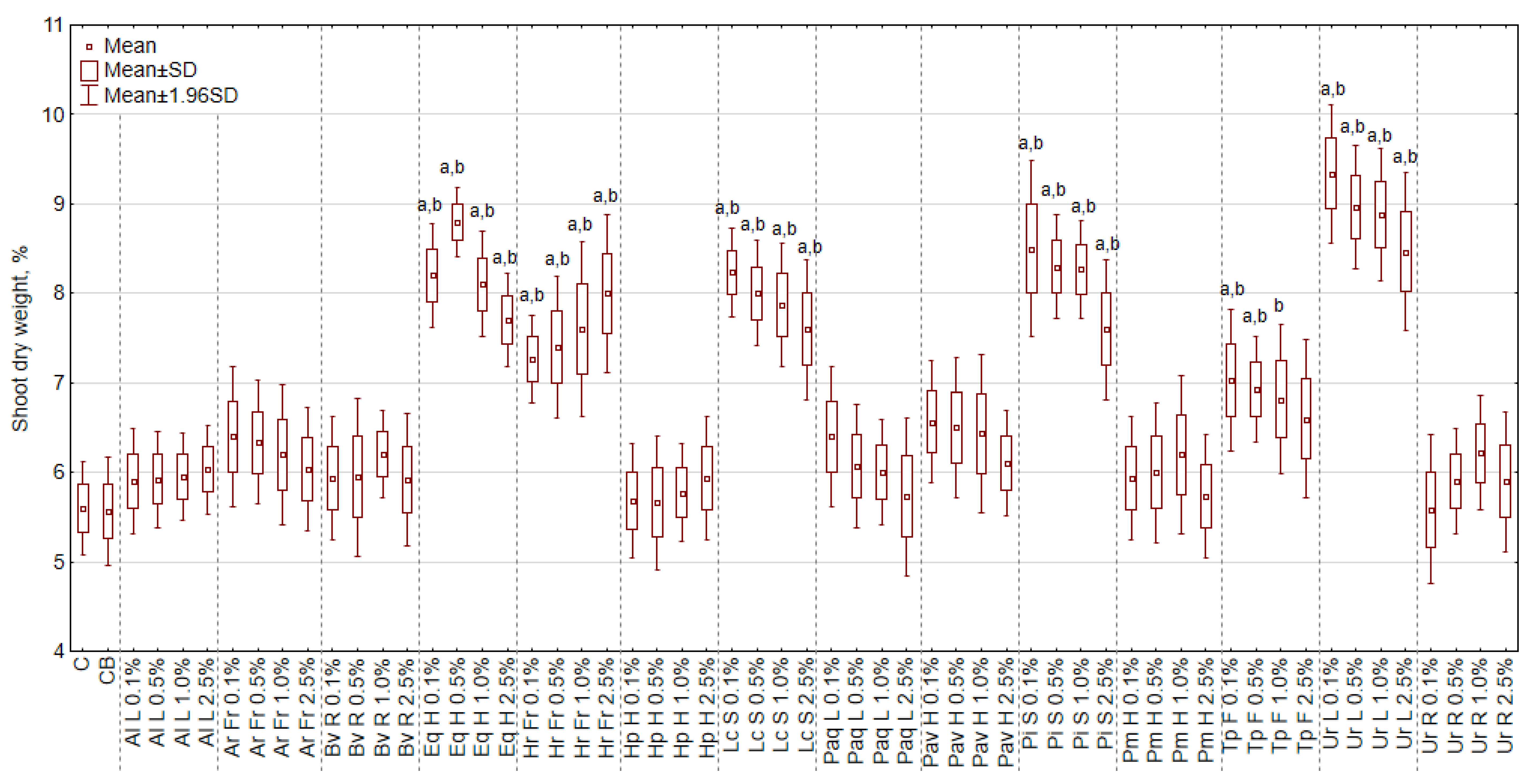
3.4. The Effect of Extracts on the Dry Weight of Shoots
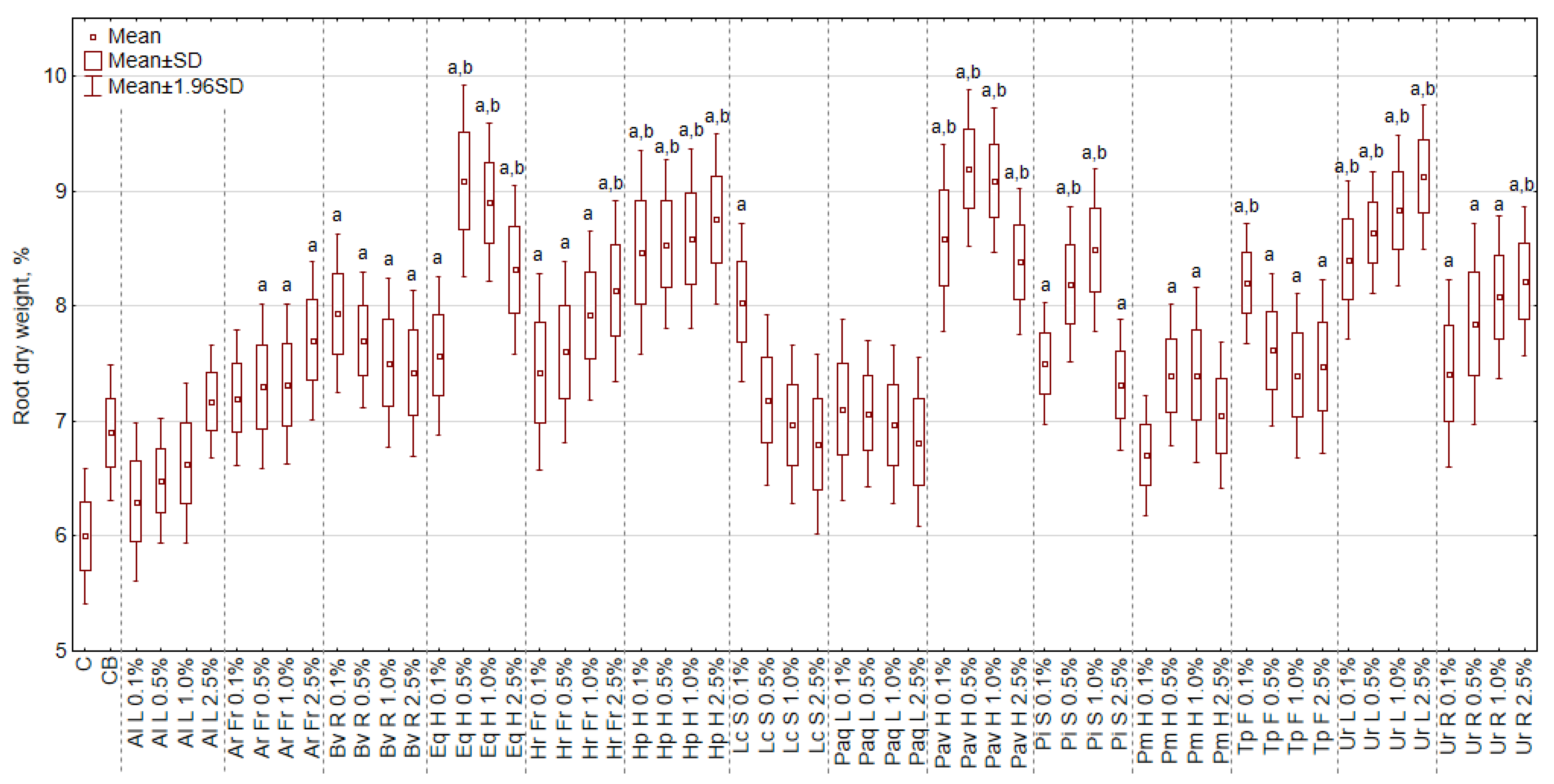
3.5. The Effect of Extracts on the Dry Weight of Roots
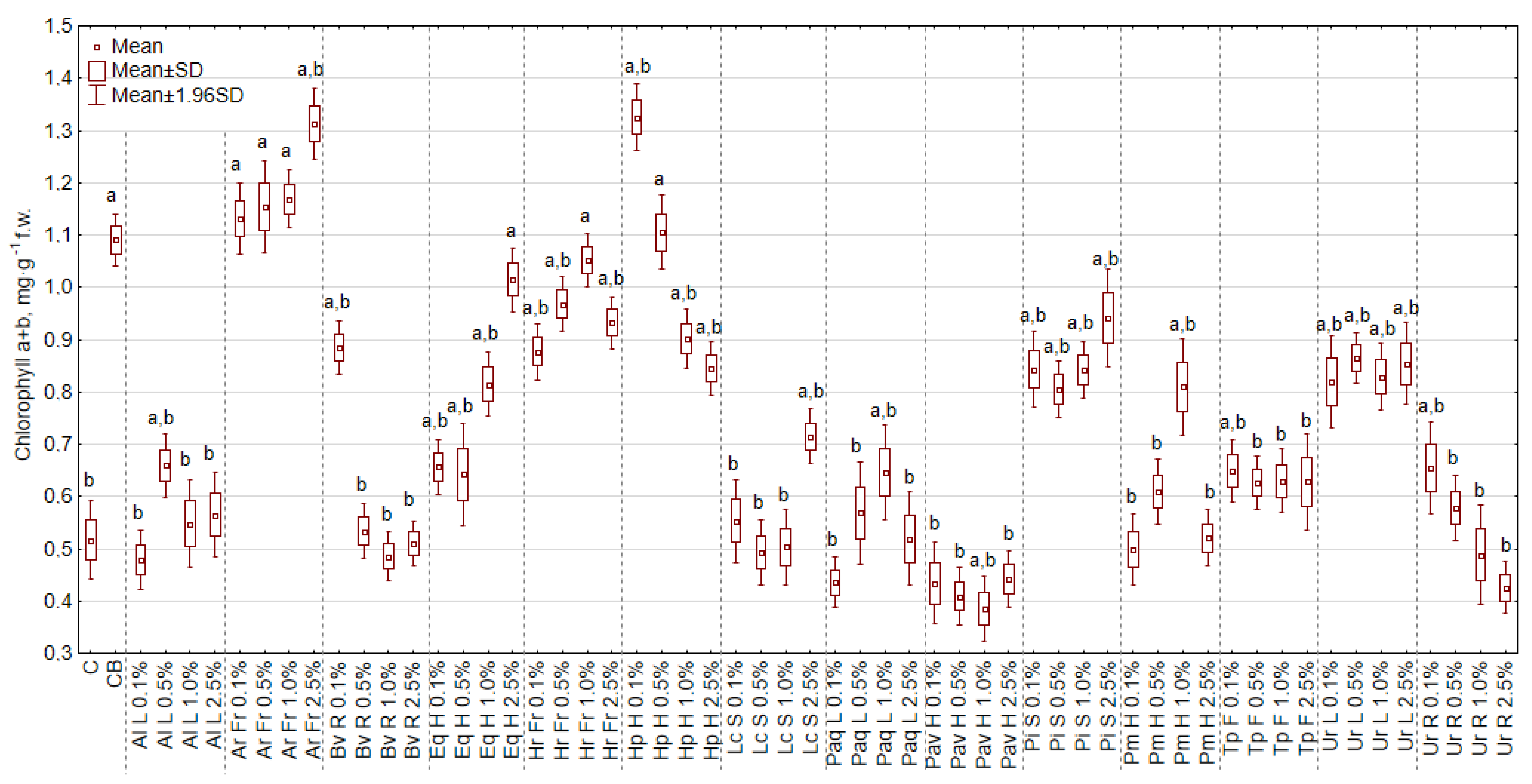
3.6. The Effect of Extracts on the Content of Chlorophyll and Carotenoids, and the Greenness Index of Leaf (SPAD)
3.7. The Effect of Extracts on the Content of Polyphenols
3.8. The Effect of Extracts on the Antioxidant Activity (DPPH, ABTS, FRAP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Challenges and Opportunities in a Global World; FAO: Rome, Italy, 2019. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- FAO, IFAD, UNICEF, WFP and WHO. The State of Food Security and Nutrition in the World 2018. In Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018. [Google Scholar]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hort. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed–based products and humic substances in plant metabolism. Sci. Agric. 2015, 73, 18–23. [Google Scholar] [CrossRef]
- Szalińska, E.; Orlińska-Woźniak, P.; Wilk, P. Nitrate vulnerable zones revision in Poland—Assessment of environmental impact and land use conflicts. Sustainability 2018, 10, 3297. [Google Scholar] [CrossRef]
- EIBC (European Biostimulants Industry Council). Promoting the Biostimulant Industry and the Role of Plant Biostimulants in Making Agriculture More Sustainable. 2013. Available online: www.biostimulants.eu/ (accessed on 10 December 2019).
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kelting, M.; Harris, J.R.; Fanelli, J.; Appleton, B. Humate-based biostimulants affect early post–transplant root growth and sapflow of red maple. Hort. Sci. 1998, 33, 342–344. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Reg. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Behie, S.W.; Bidochka, M.J. Nutrient transfer in plant-fungal symbioses. Trends Plant Sci. 2014, 19, 734–740. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotech. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Matysiak, K.; Miziniak, W.; Kaczmarek, S.; Kierzek, R. Herbicides with natural and synthetic biostimulants in spring wheat. Crop Prod. 2018, 48, 1–10. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Paratu, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Biores. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef]
- Kavipriya, R.; Dhanalakshmi, K.; Jayashree, S.; Thangaraju, N. Seaweed extracts as a biostimulant for legume crop, green gram. J. Ecobiotechnol. 2014, 3, 16–19. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Sarkar, G.; Jatar, N.; Goswami, P.; Cyriac, R.; Suthindhiran, K.; Jayasri, M.A. Combination of different marine algal extracts as biostimulants and biofungicide. J. Plant Nutr. 2018, 41, 1163–1171. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. as biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Pardo-García, A.I.; Martínez-Gil, A.M.; Cadahía, E.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Oak extract application to grapevines as a plant biostimulant to increase wine polyphenols. Food Res. Int. 2014, 55, 150–160. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. Plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Abbas, S.M.; Akladious, S.A. Application of carrot root extract induced salinity tolerance in cowpea (Vigna sinensis L.) seedlings. Pak. J. Bot. 2013, 45, 795–806. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Canterino, S.; Cerutti, A.K.; Bounous, G. Improving the nutritional value of kiwifruit with the application of agroindustry waste extracts. J. Appl. Bot. Food Qual. 2013, 86, 11–15. [Google Scholar]
- Amirkhani, M.; Netravali, A.N.; Huang, W.; Taylor, A.G. Investigation of soy protein-based biostimulant seed coating for broccoli seedling and plant growth enhancement. Hort. Sci. 2016, 51, 1121–1126. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2016, 7, 1–32. [Google Scholar] [CrossRef]
- Surjishe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural sources of antioxidants—A review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical composition and antioxidant activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC–Q/Orbitrap/MS/MS. J. Chem. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Skupień, K.; Oszmiański, J. The effect of mineral fertilization on nutritive value and biological activity of chokeberry fruit. Agric. Food Sci. 2007, 16, 46–55. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, E.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur. Food. Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Roohi, E. Phytochemistry and pharmacological properties of Equisetum arvense L. J. Med. Plants Res. 2012, 6, 3689–3693. [Google Scholar] [CrossRef]
- Fons, F.; Froissard, D.; Bessière, J.-M.; Fruchier, A.; Buatois, B.; Rapior, S. Volatile composition of six horsetails: Prospects and perspectives. Nat. Prod. Commun. 2013, 8, 509–512. [Google Scholar] [CrossRef]
- Uslu, M.E.; Erdogan, I.; Bayraktar, O.; Ates, M. Optimization of extraction conditions for active components in Equisetum arvense extract. Rom. Biotechnol. Lett. 2013, 18, 8115–8131. [Google Scholar]
- Sukhbaatar, B.; Borbaatar, B.; Altangerel, B.; Luvsannyam, L.; Luvsan, K. A dynamic study of some biological active compounds in the sea-buckthorn (Hippophae rhamnoides L.) berries. J. Pharm. Pharmacol. 2017, 5, 366–373. [Google Scholar]
- Krejcarová, J.; Straková, E.; Suchý, P.; Herzig, I.; Karáskova, K. Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceuticals and its therapeutic possibilities—A review. Acta Vet. Brno 2015, 84, 257–268. [Google Scholar] [CrossRef]
- Li, T.S.C.; Schroeder, W.R. Sea buckthorn (Hippophae rhamnoides L.): A multipurpose plant. Hort Technol. 1996, 6, 370–380. [Google Scholar] [CrossRef]
- Patočka, J. The chemistry, pharmacology, and toxicology of the biologically active constituents of the herb Hypericum perforatum L. J. Appl. Biomed. 2003, 1, 61–70. [Google Scholar] [CrossRef]
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s wort (Hypericum perforatum): A review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Saxena, P.K. St. John’s wort (Hypericum perforatum L.): Challenges and strategies for production of chemically consistent plants. Can. J. Plant Sci. 2006, 86, 765–771. [Google Scholar] [CrossRef]
- Shahwar, D.; Bhat, T.M.; Ansari, M.Y.K.; Chaudhary, S.; Aslam, R. Health functional compounds of lentil (Lens culinaris Medik): A review. Int. J. Food Prop. 2017, 20, S1–S15. [Google Scholar] [CrossRef]
- Faris, M.A.-I.E.; Takruri, H.R.; Issa, A.Y. Role of lentils (Lens culinaris L.) in human health and nutrition: A review. Mediterr. J. Nutr. Metab. 2013, 6, 3–16. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, H.; Deng, Z.; Tsao, R. Phytochemicals of lentils (Lens culinaris) and their antioxidant and anti-inflammatory effects. J. Food Bioact. 2018, 1, 93–103. [Google Scholar] [CrossRef]
- Gülsoy, S.K.; Şimşir, S. Chemical composition, fibre morphology, and kraft pulping of bracken stalks (Pteridium aquilinum (l.) Kuhn). Drvna Ind. 2018, 69, 23–33. [Google Scholar] [CrossRef]
- Vetter, J. Chapter 25 Toxicological and medicinal aspects of the most frequent fern species, Pteridium aquilinum (L.) Kuhn. In Working with Ferns: Issues and Applications; Bahillo, M.A.R., Ed.; Springer: New York, NY, USA, 2010; pp. 1–19. [Google Scholar]
- Halarewicz, A.; Szumny, A. Analysis of essential oils in leaf extracts from bracken fern, Pteridium aquilinym (L.) Kuhn. sub. aquilinum. Electron. J. Pol. Agric. 2010, 13, 20. [Google Scholar]
- Shen, B.-B.; Yang, Y.-P.; Yasamin, S.; Liang, N.; Su, W.; Chen, S.-H.; Wang, X.-J.; Wang, W. Analysis of the phytochemistry and bioactivity of the genus Polygonum of Polygonaceae. Dig. Chin. Med. 2018, 1, 19–36. [Google Scholar] [CrossRef]
- Shin, H.; Chung, H.; Park, B.; Lee, K.Y. Identification of antioxidative constituents from Polygonum aviculare using LC-MS coupled with DPPH assay. Nat. Prod. Sci. 2016, 22, 64–69. [Google Scholar] [CrossRef]
- Seo, S.H.; Lee, S.-H.; Cha, P.-H.; Kim, M.-Y.; Min, D.S.; Choi, K.-Y. Polygonum aviculare L. and its active compounds, quercetin hydrate, caffeic acid, and rutin, activate the Wnt/β-catenin pathway and induce cutaneous wound healing. Phytother. Res. 2016, 30, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.I.; Ma, Z. Chapter 8—Impact of processing on bioactive compounds of field peas. Proc. Imp. Act. Comp. Food 2015, 63–70. [Google Scholar] [CrossRef]
- Bastianelli, D.; Grosjean, F.; Peyronnet, C.; Duparque, M.; Régnier, J.M. Feedling value of pea (Pisum sativum, L.) 1. Chemical composition of different categories of pea. Anim. Sci. 1998, 67, 609–619. [Google Scholar] [CrossRef]
- Haymanti, S.; Alok, P.; Venkat, K.S.; Manimegalai, S.; Devi Rajeswari, V. Evaluation of antioxidant activity of Pisum sativum (pod and grain) and detection of its bioactive compounds by GCMS analysis. Der Pharm. Lett. 2014, 6, 359–365. [Google Scholar]
- Najafian, Y.; Hamedi, S.S.; Farshchi, M.K.; Feyzabadi, Z. Plantago major in Traditional Persian Medicine and modern phytotherapy: A narrative review. Electron. Phys. 2018, 10, 6390–6399. [Google Scholar] [CrossRef]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Kudos, M.B.A.; Sulaiman, M.W.A.W.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Sazdanić, D.; Mikulić, M.; Kladar, N.; Hogervorst, J.; Atanacković, K.M. Analysis of the factors influencing red clover (Trifolium pratense L., Fabaceae) isoflavone content. Biol. Serb. 2018, 40, 34–41. [Google Scholar]
- Atiq-ur-Rehman. Biological activities of Trifolium pretense: A review. Acta Sci. Pharm. Sci. 2019, 3, 36–42. [Google Scholar]
- Booth, N.L.; Overk, C.R.; Yao, P.; Burdette, J.E.; Nikolic, D.; Chen, S.-N.; Bolton, J.L.; van Breemen, R.B.; Pauli, G.F.; Farnsworth, N.R. The chemical and biological profile of a red clover (Trifolium pretense) phase II clinical extract. J. Altern. Complement Med. 2006, 12, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Yalcin, B. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rajput, P.; Chaudhary, M.; Sharma, R.A. Phytochemical and pharmacological importance of genus Urtica—A review. Int. J. Pharm. Sci. Res. 2018, 9, 1387–1396. [Google Scholar]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacological review of Urtica doica L. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar]
- Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The effect of plant-derived biostimulants on white heat cabbage seedlings grown under controlled conditions. Sustainability 2019, 11, 5317. [Google Scholar] [CrossRef]
- Jałoszyński, K.; Figiel, A.; Wojdyło, A. Drying kinetics and antioxidant activity of oregano. Acta Agrophys. 2008, 11, 81–90. [Google Scholar]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Almeida, M.M.B.; de Sousa, P.H.M.; Arriaga, Â.M.C.; do Prado, G.M.; de Carvalho Magalhães, C.E.; Maia, G.A.; de Lemos, T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Crouch, I.J.; van Staden, J. Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Babenko, L.M.; Shcherbatiuk, M.M.; Vedenicheva, N.P.; Sheyko, O.A.; Ivanova, A.; Angelova, L.; Maslenkova, L. Adaptive strategy of halophytic plants Polygonum maritimum and Euphorbia paralias. Dopov. Nac. Acad. Nauk Ukr. 2017, 7, 98–105. [Google Scholar] [CrossRef]
- Beck, E.H. Regulation of shoot/root ratio by cytokinins from roots in Urtica dioica: Opinion. Plant Soil 1996, 185, 1–12. [Google Scholar] [CrossRef]
- Sekeroglu, N.; Karaoglan, M.; Gezici, S.; Kulak, M.; Ozkutlu, F.; Kacar, O.; Gul, F. Variation in the composition of the essential oils, hypericin and mineral elements in aerial parts, stem and flower of Hypericum capitatum (CHOISY) growing in Turkey with oxidative DNA damage protective activity. J. Pharm. Res. 2018, 17, 67–77. [Google Scholar]


| Species | Active Compounds | References |
|---|---|---|
| Aloe Aloe vera L. Burm. f. | Vitamins (A, C, E, B1, B2, B6, B9, B12), choline, enzymes (aliiase, alkaline phosphate, amylase, bradykinase, carboxypeptidase, catalase, cellulose, lipase, peroxidase), minerals (Ca, Cr, Cu, Se, Mg, Mn, K, Na, Zn, Fe, P), sugars: monosaccharides (glucose, fructose), polysaccharides (glucomannans/polymannose), anthraquinones (aloin, emodin), fatty acids (cholesterol, campesterol, β-sisosterol, lupeol), hormones (auxins, gibberellins), amino acids (A, R, D, E, G, H, I, L, K, M, F, P, T, Y, V), proteins (lectins), salicylic acid, lignin, saponins, carbohydrates (pure mannan, acetylated mannan, cellulose, pectic substance, xylan) | [32,33,34] |
| Aronia Aronia melanocarpa (Michx) Elliott | Carbohydrate (glucose, fructose, sucrose, sorbitol), dietary fibre (pectins), fat, proteins, organic acids (l-malic acid, citric acid, isocitric acid, tartaric acid, quinic acid, succinic acid, fumaric acid), vitamins (C, B1, B2, B3, B5, B6, B9, E, K), minerals (Na, K, Ca, Mg, Fe, Zn, I), phytochemicals (carotenoids: β-carotene, β-cryptoxanthin; phenols; amygdalin), nitrate, nitrite | [35,36,37] |
| Red beet Beta vulgaris L. subs. vulgaris | Nitrate, phenolics (flavonoids, phenolic acids, phenolic amides), vitamins (A, B), carotenoids, betalains (betacyanins: betanin, isobetanin; betaxanthins: vulgaxanthin I, vulgaxanthin II, indicaxanthin), minerals (Mg, Na, K, P, Ca), sugars, proteins, fibre | [38,39,40] |
| Horsetail Equisetum arvense L. | Alkaloids, phytosterols, tannin, tritepenoids, phenolics (flavonoids, styrylpyrones, phenolic acids), aromatic compounds (benzothiazole, homovanillic acid, isovanillin), terpens (linalool, β–caryphyllene), isoprenoid derivatives (α–ionone, (E, Z)—pseudoionone), silicic acid | [41,42,43] |
| Common sea–buckthorn Hippophae rhamnoides L. | β—carotene, organic acids, essential oils, polyphenols, flavonoids, phytosterols, tocopherols, vitamins (K, C, E, B complex), polyunsaturated fatty acids, coumarins, triterpens, protein (globulins, albumins), amino acids, carbohydrates, minerals (N, Ca, K, Na, Mg, Cu, Fe, Zn, Mn) | [44,45,46] |
| Hypericum Hypericum perforatum L. | Naphthodianthrones, phloroglucinols, flavonoids, biflavones, phenylpropanes, proanthocyanidins, tannins, xanthones, essential oils, amino acids, procyanidins, hypericin, pseudohypericin, hyperforin, melatonin, minerals (B, Cu, Fe, Mg, Mn, Mo, Zn) | [47,48,49] |
| Red lentil Lens culinaris Medik. | Protein (lectins, defensins, protease inhibitors), bioactive peptide, complex carbohydrate fractions, particularly the resistant starches, oligosaccharides, dietary fibre, antioxidants, non-nutritive bioactive phytochemicals, minerals (Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn, Se), vitamins (C, A, E, K, B1, B2, B3, B5, B6, B9), carotene, choline, lipids (fatty acids, cholesterol), polyphenolics, phytic acid, saponins, phytosterols | [50,51,52] |
| Common bracken Pteridium aquilinum L. | Crude fibre, holocellulose, α-cellulose, lignin, crude protein, crude fat, macroelements (N, K, P, S, Ca, Mg), microelements (Fe, Mn, Zn, Cu, Cr), thiaminase, cyanogen glycosides, pterosins, flavonoids (kaempferol, apigenin, quercetin), phenoloids (cinnamic acid, benzoic acid, phenoloid polymers: tannins), volatile compounds (benzaldehyde, limonene, linalool) | [53,54,55] |
| Knotgrass Polygonum aviculare L. | Flavonoids (kaempferol, baicalin, quercetin, myricetin, isoquercetin, luteolin, avicularin, rutin, astragalin), quinones (chrysophanol, emodin, rhein, aloe-emodin), phenylpropanoids, terpenoids (triterpenoids, diterpene), phenolic acids (gallic acid, protocatechuic acid, chlorogenic acid), stilbene glycosides, alkaloids, rosemary acid, caffeic acid, coumaric acid, hirsutine, tadeonal, isotadeonal, apianen lactones, phytohormones | [56,57,58] |
| Pea Pisum sativum L. | Proteins (albumin, vicilin, legumin), amino acids, carotenoids, fibre, enzyme inhibitor, lectin, choline, phytic acid, phenolics, carbohydrates (starch, oligosaccharides, amylose), vitamins (A, B, C), lipids, saponins, tannins, minerals (Ca, Fe, Mg, P, K, Na, Zn, Se) | [59,60,61] |
| Broadleaf plantain Plantago major L. | Phenylethanoid glycosides, triterpenoids, polysaccharides, phenolic acids, alkaloids, phenolic compounds (caffeic acid derivatives), coumarins, fats and oils, mucilage, sterols, volatile substances, flavonoids, vitamins (C, A) | [62,63,64] |
| Red clover Trifolium pratense L. | Isoflavones (biochanin A), pterocarpans, coumestrols, lignans, medicagol, coumarin, soyasaponins, clovamides, flavonoids, afrormosin, daidzein, genistein, methyl orobol, irilin, irilone, formononetin, tyramine, fisetin, calycosin, quercetin, naringenin, pratensein, kaempferol, pseudobaptigenin, irilone, prunetin | [65,66,67] |
| Nettle Urtica dioica L. | Agglutinin, acetophenone, alkaloids, acetylcholine, chlorogenic acid, butyric acid, chlorophyll, caffeic acid, carbonic acid, choline, histamine, coumaric acid, formic acid, pantothenic acid, kaempferol, coproporphyrin, lectin, lecithin, lignan, linoleic and linolenic acids, palmitic acid, quercetin, quinic acid, serotonin, stigmasterol, terpenes, violaxanthin, succinic acid, fixed oil, fatty substance, albumins, protein, vitamins (A, C, B1, K), provitamin A, carotenoids, xanthophyll, oxalate, histamine, acetylcholine, sistosterin, ferric oxide, minerals (N, K, Ca, Si), flavonoids, tannins, volatile compounds, polysaccharides, sterols, amino acids, phytohormones (cytokinins) | [68,69,70] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica Oleracea L. Var. Capitata L.) Seedlings Grown under Controlled Conditions. Sustainability 2020, 12, 1871. https://doi.org/10.3390/su12051871
Godlewska K, Biesiada A, Michalak I, Pacyga P. The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica Oleracea L. Var. Capitata L.) Seedlings Grown under Controlled Conditions. Sustainability. 2020; 12(5):1871. https://doi.org/10.3390/su12051871
Chicago/Turabian StyleGodlewska, Katarzyna, Anita Biesiada, Izabela Michalak, and Paweł Pacyga. 2020. "The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica Oleracea L. Var. Capitata L.) Seedlings Grown under Controlled Conditions" Sustainability 12, no. 5: 1871. https://doi.org/10.3390/su12051871
APA StyleGodlewska, K., Biesiada, A., Michalak, I., & Pacyga, P. (2020). The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica Oleracea L. Var. Capitata L.) Seedlings Grown under Controlled Conditions. Sustainability, 12(5), 1871. https://doi.org/10.3390/su12051871







