The Correlation between Soil Nutrient and Potato Quality in Loess Plateau of China Based on PLSR
Abstract
1. Introduction
2. Materials and Methods
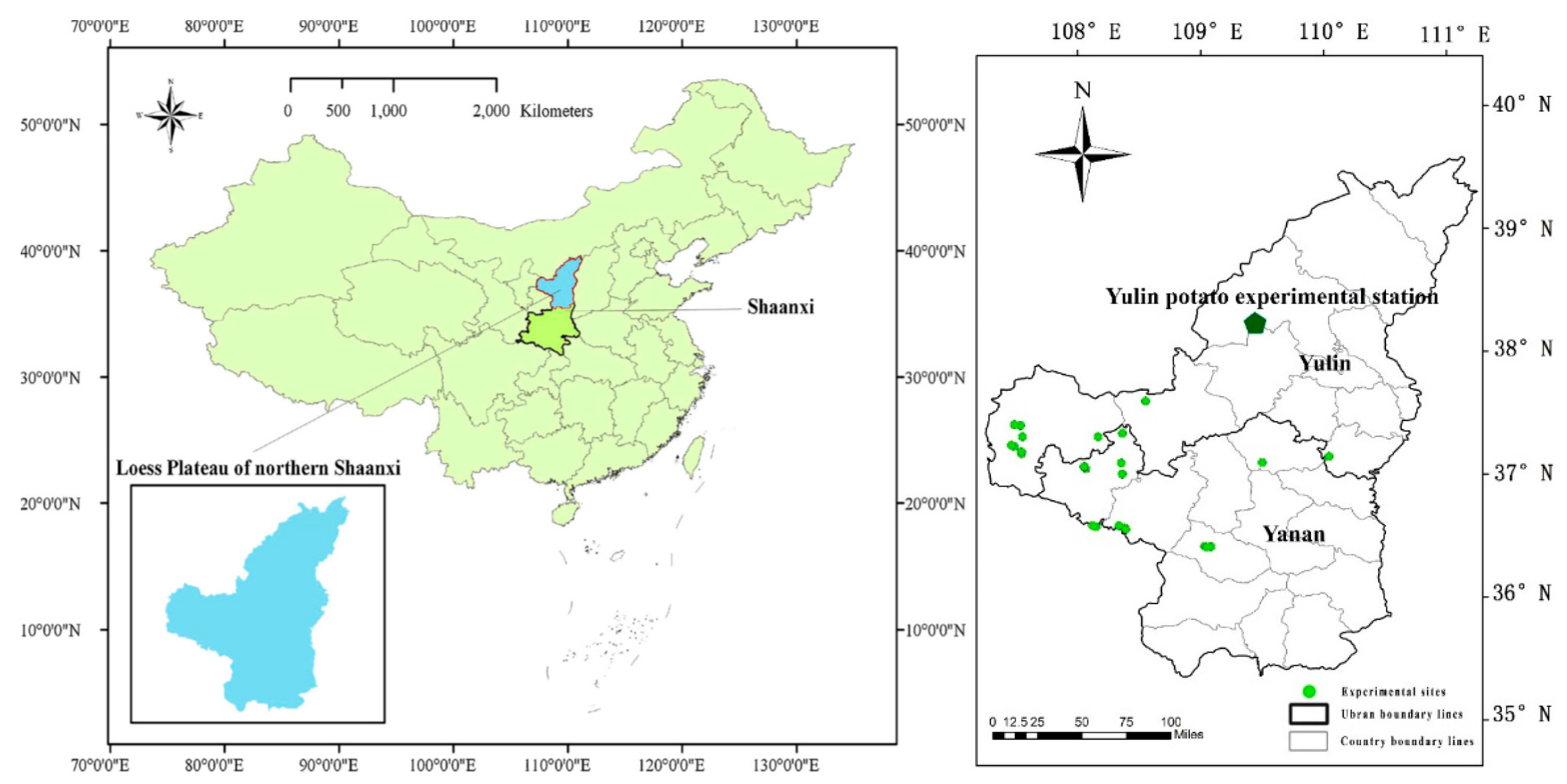
2.1. Site Description
2.2. Collection and Analysis of Soil Samples and Potato Tuber Samples
2.3. Simple Linear Regression
2.4. Partial Least Squares Regression
2.5. Statistical Analysis
3. Results
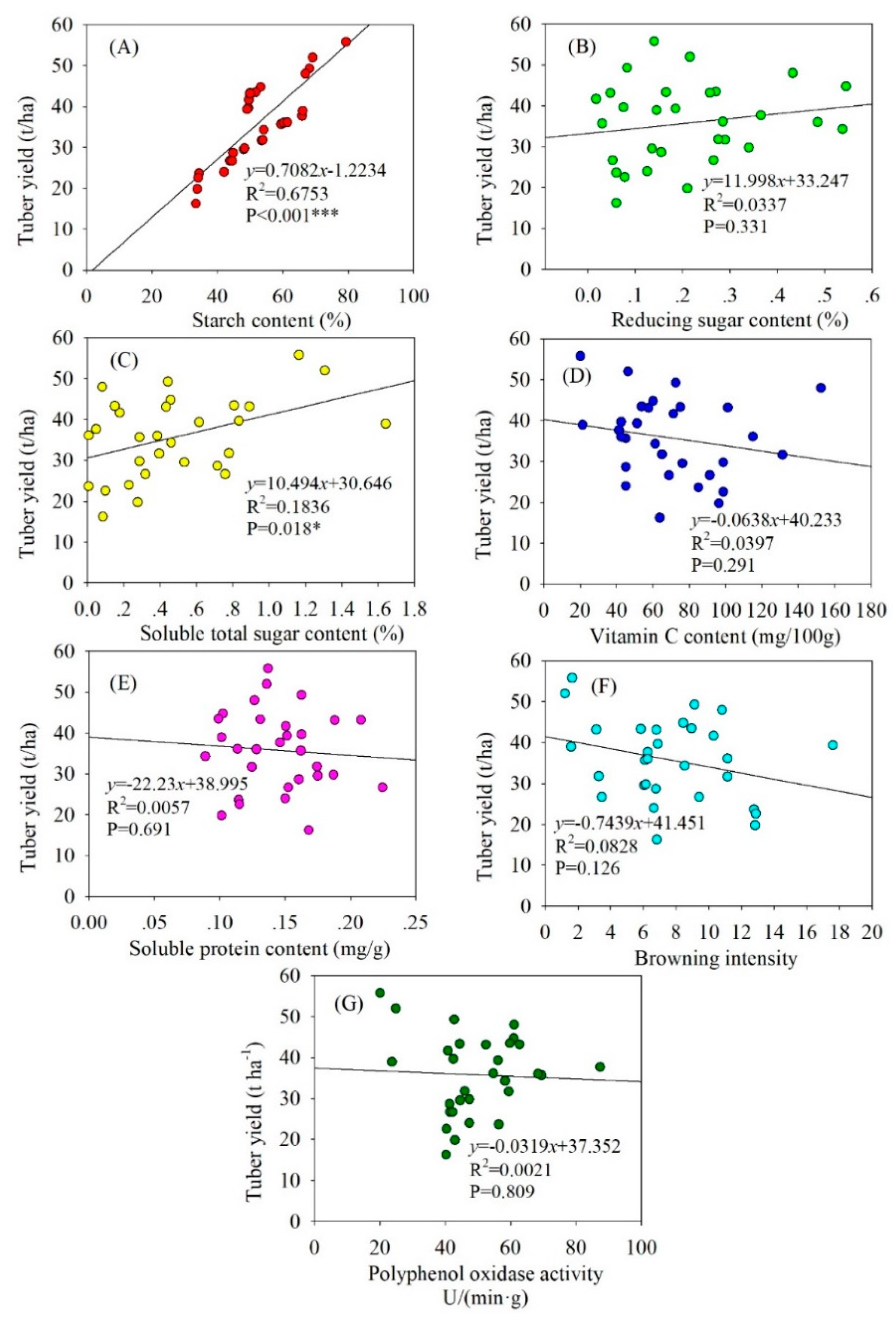
3.1. Linear Regression Analysis of Potato Yield and Soil Nutrient Contents
3.2. Linear Regression Analysis of Potato Yield and Quality
3.3. Differences in Soil Nutrients and Potato Tuber Quality
3.4. Correlation Analysis between Soil Nutrients and Potato Quality
3.5. Variable Importance for Projection of Soil Nutrient Factors Affecting the Potato Tuber Quality
3.6. PLSR of the Relationship between Soil Nutrient Content and Potato Tuber Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Li, H.; Zhang, L.; Zhang, S.; Chen, Y. Mulching Improves Yield and Water-Use Efficiency of Potato Cropping in China: A meta-analysis. Field Crop. Res. 2018, 221, 50–60. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Hamouz, K.; Jaromír, L.; Pulkrábek, J.; Pazderů, K. Effect of Genotype, Flesh Colour and Environment on the Glycoalkaloid Content in Potato Tubers from Integrated Agriculture. Plantsoil Environ. 2018, 64, 186–191. [Google Scholar]
- Zhu, Z.; Guo, W. Frequency, Moisture Content, and Temperature Dependent Dielectric Properties of Potato Starch Related to Drying with Radio-Frequency/Microwave Energy. Sci. Rep. 2017, 7, 9311. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial Phytochemicals in Potato — A review. Food Res. Int. 2013, 50, 487–496. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food Waste Biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y. Evaluation of the Effects of Irrigation and Fertilization on Tomato Fruit Yield and Quality: A principal component analysis. Sci. Rep. 2017, 7, 350. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, N.; Pan, F.; Pan, X.; Wang, X.; Yang, P. Adjusting Sowing Dates Improved Potato Adaptation to Climate Change in Semiarid Region, China. Sustainability 2017, 9, 615. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V.; Cotton, J.; Cano, A. Soil Health Indicators as Affected by Diverse Forage Species and Mixtures in Semi-Arid Pastures. Appl. Soil Ecol. 2018, 132, 179–186. [Google Scholar] [CrossRef]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Manter, D.K.; Buyer, J.S.; Maul, J.E.; Smith, J.L.; Collins, H.P.; Halvorson, J.J.; et al. Understanding and Enhancing Soil Biological Health: The solution for reversing soil degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of Soil Moisture on the Temperature Sensitivity of Heterotrophic Respiration Vary Seasonally in an Old-Field Climate Change Experiment. Glob. Chang. Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V. Assessing the Role of Interseeding Alfalfa into Grass on Improving Pasture Soil Health in Semi-Arid Texas High Plains. Appl. Soil Ecol. 2020, 147, 103399. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Longing, S.D.; Brown, C.P.; Green, P.E. Comparison of Arthropod Communities among Different Forage Types on the Texas High Plains Using Pitfall Traps. Crop. Turfgrass Manag. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Longing, S.D.; Brown, C.P.; Green, P.E.; Barkowsky, E. Pollinator Abundance in Semiarid Pastures as Affected by Forage Species. Crop Sci. 2018, 58, 2665–2671. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Klein, D.; Subbiah, S.; Surowiec, K. Essential Oil Composition of ‘WW-B.Dahl’ Old World Bluestem (Bothriochloa bladhii) Grown in the Texas High Plains. Ind. Crop. Prod. 2019, 133, 1–9. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Longing, S.D. Communities of Canopy-Dwelling Arthropods in Response to Diverse Forages. Agric. Environ. Lett. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Longing, S.D. Fly Densities on Cattle Grazing ‘WW-B. Dahl’old World Bluestem Pasture Systems. Tex. J. Agric. Nat. Resour. 2018, 31, 1–5. [Google Scholar]
- Morier, T.; Cambouris, A.N.; Chokmani, K. In-Season Nitrogen Status Assessment and Yield Estimation Using Hyperspectral Vegetation Indices in a Potato Crop. Agron. J. 2015, 107, 1295–1309. [Google Scholar] [CrossRef]
- Gao, X.; Li, C.; Zhang, M.; Wang, R.; Chen, B. Controlled Release Urea Improved the Nitrogen Use Efficiency, Yield and Quality of Potato (Solanum tuberosum L.) on Silt Loamy Soil. Field Crop. Res. 2015, 181, 60–68. [Google Scholar] [CrossRef]
- Farouk, S. Imroving Growth and Productivity of Potato (Solanum tuberosum L.) by Some Biostimulants and Lithovit with or without Boron. J. Plant Prod. 2015, 6, 2187–2206. [Google Scholar] [CrossRef]
- Gerendás, J.; Heuser, F.; Sattelmacher, B. Influence of Nitrogen and Potassium Supply on Contents of Acrylamide Precursors in Potato Tubers and on Acrylamide Accumulation in French Fries. J. Plant Nutr. 2007, 30, 1499–1516. [Google Scholar] [CrossRef]
- Wilton, M.J.; Karagatzides, J.D.; Tsuji, L.J.S. Nutrient Concentrations of Bush Bean (Phaseolus vulgaris L.) and Potato (Solanum tuberosum L.) Cultivated in Subarctic Soils Managed with Intercropping and Willow (Salix spp.) Agroforestry. Sustainability 2017, 9, 2294. [Google Scholar] [CrossRef]
- Agbede, T.M. Tillage and Fertilizer Effects on Some Soil Properties, Leaf Nutrient Concentrations, Growth and Sweet Potato Yield on an Alfisol in Southwestern Nigeria. Soil Tillage Res. 2010, 110, 25–32. [Google Scholar] [CrossRef]
- Khan, M.Z.; Akhtar, M.E.; Safdar, M.N.; Mahmood, M.M.; Ahmad, S.; Ahmed, N. Effect of Source and Level of Potash on Yield and Quality of Potato Tubers. Pak. J. Bot. 2010, 42, 3137–3145. [Google Scholar]
- Ierna, A.; Pandino, G.; Lombardo, S.; Mauromicale, G. Tuber Yield, Water and Fertilizer Productivity in Early Potato as Affected by a Combination of Irrigation and Fertilization. Agric. Water Manag. 2011, 101, 35–41. [Google Scholar] [CrossRef]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A Review of Variable Selection Methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Wang, N.; Xing, Y.; Wang, X. Exploring Options for Improving Potato Productivity through Reducing Crop Yield Gap in Loess Plateau of China Based on Grey Correlation Analysis. Sustainability 2019, 11, 5621. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Zhao, W.; He, H.; Li, D.; He, W.; Liu, Q.; Yin, H. Seasonal Variations in the Soil Amino Acid Pool and Flux following the Conversion of a Natural Forest to a Pine Plantation on the Eastern Tibetan Plateau, China. Soil Biol. Biochem. 2017, 105, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Qi, J.; Zhang, H.; Tao, F.; Zhang, R. Priming Effects of Soil Organic Matter Decomposition with Addition of Different Carbon Substrates. J. Soils Sediments 2019, 19, 1171–1178. [Google Scholar] [CrossRef]
- Sun, S.; Li, Z.; Wang, R.; Zhang, J.; Pan, C.; Cai, Z. Conversion of Secondary Forests into Chestnut Forests Affects Soil Nutrients in Anji County, China. Sustainability 2019, 11, 2373. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Xing, Y. Effects of Mulching and Nitrogen on Soil Temperature, Water Content, Nitrate-N Content and Maize Yield in the Loess Plateau of China. Agric. Water Manag. 2015, 161, 53–64. [Google Scholar]
- Xiaoli, N.; Hanmi, Z.; Xiukang, W.; Tiantian, H.; Puyu, F.; Ting, L.; Na, Z.; Dongxue, Y. Changes in Root Hydraulic Conductance in Relation to the Overall Growth Response of Maize Seedlings to Partial Root-Zone Nitrogen Application. Agric. Water Manag. 2019, 229, 105839. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Xing, Y.; Liu, X.; Cui, Z.; Yang, L. Enhancing Rice Production by Potassium Management: Recommended reasonable fertilization strategies in different inherent soil productivity levels for a sustainable rice production system. Sustainability 2019, 11, 6522. [Google Scholar] [CrossRef]
- Bilias, F.; Barbayiannis, N. Potassium-Fixing Clay Minerals as Parameters that Define K Availability of K-Deficient Soils Assessed with a Modified Mitscherlich Equation Model. J. Soil Sci. Plant Nutr. 2019, 19, 830–840. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Bi, L.; Wang, Y.; Fan, J.; Zhang, F.; Hou, X.; Cheng, M.; Hu, W.; Wu, L.; et al. Multi-Objective Optimization of Water and Fertilizer Management for Potato Production in Sandy Areas of Northern China Based on TOPSIS. Field Crop. Res. 2019, 240, 55–68. [Google Scholar] [CrossRef]
- Paul, S.; Gogoi, N.; Sarma, B.; Baroowa, B. Biochemical Changes in Potato under Elevated Temperature. Indian J. Plant Physiol. 2014, 19, 36–42. [Google Scholar] [CrossRef]
- Bogucka, B.; Elżbieta, T. Effect of Nitrogen and Potassium Fertilization on Mineral and Amino Acid Content of Colored Flesh Potato Cultivar Blue Congo. J. Plant Nutr. 2018, 41, 856–866. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Q.; Akbar, S.; Zhi, Y.; El Tahchy, A.; Mitchell, M.; Li, Z.; Shrestha, P.; Vanhercke, T.; Ral, J.-P.; et al. Genetic Enhancement of Oil Content in Potato Tuber (Solanum tuberosum L.) through an Integrated Metabolic Engineering Strategy. Plant Biotechnol. J. 2017, 15, 56–67. [Google Scholar] [CrossRef]
- Nguyen, H.T.; van der Fels-Klerx, H.J.; van Boekel, M.A.J.S. Acrylamide and 5-Hydroxymethylfurfural Formation during Biscuit Baking. Part II: Effect of the Ratio of Reducing Sugars and Asparagine. Food Chem. 2017, 230, 14–23. [Google Scholar] [CrossRef]
- Gomes, M.H.; Vieira, T.; Fundo, J.F.; Almeida, D.P.F. Polyphenoloxidase Activity and Browning in Fresh-Cut ‘Rocha’ Pear as Affected by pH, Phenolic Substrates, and Antibrowning Additives. Postharvest Biol. Technol. 2014, 91, 32–38. [Google Scholar] [CrossRef]
- Haitovsky, Y. Missing Data in Regression Analysis. J. R. Stat. Soc. Ser. B (Methodol.) 1968, 30, 67–82. [Google Scholar] [CrossRef]
- Elmasry, G.; Kamruzzaman, M.; Sun, D.-W.; Allen, P. Principles and Applications of Hyperspectral Imaging in Quality Evaluation of Agro-Food Products: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 999–1023. [Google Scholar] [CrossRef] [PubMed]
- Rady, A.; Guyer, D.; Lu, R. Evaluation of Sugar Content of Potatoes Using Hyperspectral Imaging. Food Bioprocess Technol. 2015, 8, 995–1010. [Google Scholar] [CrossRef]
- Cohen, Y.; Alchanatis, V.; Zusman, Y.; Dar, Z.; Bonfil, D.J.; Karnieli, A.; Zilberman, A.; Moulin, A.; Ostrovsky, V.; Levi, A.; et al. Leaf Nitrogen Estimation in Potato Based on Spectral Data and on Simulated Bands of the VENμS Satellite. Precis. Agric. 2010, 11, 520–537. [Google Scholar] [CrossRef]
- Hasan, U.; Sawut, M.; Chen, S. Estimating the Leaf Area Index of Winter Wheat Based on Unmanned Aerial Vehicle RGB-Image Parameters. Sustainability 2019, 11, 6829. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Malhi, S.S. Interactions of Nitrogen with Other Nutrients and Water: Effect on crop yield and quality, nutrient use efficiency, carbon sequestration, and environmental pollution. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Artyszak, A. Effect of Silicon Fertilization on Crop Yield Quantity and Quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- Darabi, A.; Omidvari, S.; Shafiezargar, A.; Rafie, M.; Javadzadeh, M. Impact of Integrated Management of Nitrogen Fertilizers on Yield and Nutritional Quality of Potato. J. Plant Nutr. 2018, 41, 2482–2494. [Google Scholar] [CrossRef]
- Naumann, M.; Koch, M.; Thiel, H.; Gransee, A.; Pawelzik, E. The Importance of Nutrient Management for Potato Production Part II: Plant nutrition and tuber quality. Potato Res. 2019, 63, 1–17. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in Agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Doré, T.; Makowski, D.; Malézieux, E.; Munier-Jolain, N.; Tchamitchian, M.; Tittonell, P. Facing up to the Paradigm of Ecological Intensification in Agronomy: Revisiting methods, concepts and knowledge. Eur. J. Agron. 2011, 34, 197–210. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, M.T.; Bourke, P.M.; Maliepaard, C.; Trindade, L.M. Multi-allelic QTL Analysis of Protein Content in a Bi-Parental Population of Cultivated Tetraploid Potato. Euphytica 2019, 215, 14. [Google Scholar] [CrossRef] [PubMed]
- Herencia, J.F.; García-Galavís, P.A.; Dorado, J.A.R.; Maqueda, C. Comparison of Nutritional Quality of the Crops Grown in an Organic and Conventional Fertilized Soil. Sci. Hortic. 2011, 129, 882–888. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.A.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Luo, W.; Sardans, J.; Dijkstra, F.A.; Peñuelas, J.; Lü, X.-T.; Wu, H.; Li, M.-H.; Bai, E.; Wang, Z.; Han, X.; et al. Thresholds in Decoupled Soil-Plant Elements under Changing Climatic Conditions. Plant Soil 2016, 409, 159–173. [Google Scholar] [CrossRef]
- Hong, S.; Piao, S.; Chen, A.; Liu, Y.; Liu, L.; Peng, S.; Sardans, J.; Sun, Y.; Peñuelas, J.; Zeng, H. Afforestation Neutralizes Soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef]
- Tein, B.; Kauer, K.; Eremeev, V.; Luik, A.; Selge, A.; Loit, E. Farming Systems Affect Potato (Solanum tuberosum L.) Tuber and Soil Quality. Field Crop. Res. 2014, 156, 1–11. [Google Scholar] [CrossRef]
- Ninh, H.T.; Grandy, A.S.; Wickings, K.; Snapp, S.S.; Kirk, W.; Hao, J. Organic Amendment Effects on Potato Productivity and Quality are Related to Soil Microbial Activity. Plant Soil 2015, 386, 223–236. [Google Scholar] [CrossRef]
- Li, S.; Duan, Y.; Guo, T.; Zhang, P.; He, P.; Johnston, A.; Shcherbakov, A. Potassium Management in Potato Production in Northwest Region of China. Field Crop. Res. 2015, 174, 48–54. [Google Scholar] [CrossRef]
- Rosen, C.J.; Kelling, K.A.; Stark, J.C.; Porter, G.A. Optimizing Phosphorus Fertilizer Management in Potato Production. Am. J. Potato Res. 2014, 91, 145–160. [Google Scholar] [CrossRef]
- Wang, X.; Fan, J.; Xing, Y.; Xu, G.; Wang, H.; Deng, J.; Wang, Y.; Zhang, F.; Li, P.; Li, Z. The Effects of Mulch and Nitrogen Fertilizer on the Soil Environment of Crop Plants. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Chietera, G.; Chaillou, S.; Bedu, M.; Marmagne, A.; Masclaux-Daubresse, C.; Chardon, F. Impact of the Genetic–Environment Interaction on the Dynamic of Nitrogen Pools in Arabidopsis. Agriculture 2018, 8, 28. [Google Scholar] [CrossRef]
- Reis Jr, R.D.A.; Monnerat, P.H. Nutrient Concentrations in Potato Stem, Petiole and Leaflet in Response to Potassium Fertilizer. Scientia Agricola 2000, 57, 251–255. [Google Scholar] [CrossRef]
- Ayyub, C.M.; Wasim Haidar, M.; Zulfiqar, F.; Abideen, Z.; Wright, S.R. Potato Tuber Yield and Quality in Response to Different Nitrogen Fertilizer Application Rates under Two Split Doses in an Irrigated Sandy Loam Soil. J. Plant. Nutr. 2019, 42, 1850–1860. [Google Scholar] [CrossRef]
- Alam, S.; Islam, N.; Hossain, J.; Islam, M.; Bhuiyan, S.R.; Hossain, I. Optimizing Crop Geometry for Processing-Grade Tuber Yield, Quality and Economics of Potato in Grey Terrace Soil. Arch. Agron. Soil Sci. 2016, 62, 1496–1507. [Google Scholar] [CrossRef]

| Items | SWC (%) | SOM (g/kg) | SAHN (mg/kg) | SNN (mg/kg) | SAMN (mg/kg) | P (mg/kg) | K (mg/kg) | pH |
|---|---|---|---|---|---|---|---|---|
| Site 1 | 11.18 + 0.35 | 2.17 + 0.09 | 8.44 + 0.36 | 2.14 + 0.87 | 11.72 + 1.26 | 4.97 + 0.63 | 45.26 + 5.68 | 8.93 + 0.04 |
| Site 2 | 15.34 + 0.9 | 9.46 + 0.2 | 29.13 + 1.66 | 4.95 + 0.75 | 11.97 + 1.15 | 6.61 + 2.75 | 73.78 + 17.68 | 8.77 + 0.02 |
| Site 3 | 15.02 + 0.31 | 8.42 + 0.54 | 30.29 + 1.29 | 5.86 + 1.71 | 10.13 + 0.42 | 14.74 + 1.55 | 58.9 + 4.68 | 8.26 + 0.05 |
| Site 4 | 11.94 + 0.25 | 10 + 0.68 | 35.82 + 2.44 | 2.88 + 0.75 | 8.85 + 0.5 | 4.53 + 0.96 | 39.68 + 5.98 | 8.17 + 0.03 |
| Site 5 | 13.28 + 0.5 | 10.6 + 0.59 | 31.62 + 1.68 | 5.11 + 0.6 | 5.45 + 1.05 | 8.54 + 1.24 | 53.94 + 13.41 | 8.6 + 0.06 |
| Site 6 | 8.6 + 0.39 | 3.44 + 0.8 | 19.56 + 2.3 | 20.64 + 1.31 | 9.04 + 2.14 | 24.62 + 0.63 | 47.12 + 9.54 | 8.54 + 0.03 |
| Site 7 | 4.27 + 0.3 | 3.2 + 0.31 | 15.05 + 0.47 | 5.43 + 0.23 | 10.7 + 0.42 | 9.71 + 0.65 | 52.08 + 3.22 | 9.03 + 0.03 |
| Site 8 | 13.75 + 0.4 | 4.18 + 0.46 | 11.08 + 0.4 | 5.42 + 1.06 | 11.64 + 1.1 | 2.08 + 0.66 | 76.88 + 2.84 | 8.94 + 0.01 |
| Site 9 | 8.98 + 1.2 | 10.8 + 0.98 | 45.93 + 11.34 | 5.41 + 0.2 | 8.17 + 0.67 | 12.36 + 3.02 | 59.52 + 3.72 | 8.69 + 0.16 |
| Site 10 | 10.7 + 0.62 | 6.64 + 0.25 | 36.98 + 1.63 | 2.57 + 0.37 | 14.86 + 1.37 | 5.93 + 1.07 | 75.02 + 3.87 | 8.6 + 0.05 |
| Minimum | 3.93 | 2.12 | 8.05 | 1.53 | 4.27 | 1.37 | 35.34 | 8.15 |
| Maximum | 15.94 | 11.62 | 54.25 | 7.99 | 16.08 | 17.90 | 91.14 | 9.06 |
| Mean | 11.31 | 6.88 | 26.39 | 4.71 | 10.25 | 8.68 | 58.22 | 8.65 |
| Standard Deviation | 3.30 | 3.29 | 12.31 | 1.73 | 2.65 | 4.86 | 14.55 | 0.28 |
| Items | SC (%) | RSC (%) | STS (%) | VC mg/(100g) | SPC (mg/g) | BI | PPO U/ (g min) |
|---|---|---|---|---|---|---|---|
| Site 1 | 42.52 + 8.24 | 0.1 + 0.05 | 0.54 + 0.4 | 50.42 + 11.61 | 0.16 + 0 | 6.83 + 0.06 | 41.32 + 1.14 |
| Site 2 | 53.83 + 12.68 | 0.05 + 0.03 | 0.31 + 0.13 | 70.83 + 1.91 | 0.16 + 0.01 | 9.61 + 0.61 | 41.64 + 0.98 |
| Site 3 | 48.79 + 1.08 | 0.21 + 0.11 | 0.32 + 0.19 | 83.33 + 13.37 | 0.16 + 0.03 | 6.01 + 0.15 | 45.44 + 1.7 |
| Site 4 | 34.1 + 0.27 | 0.12 + 0.08 | 0.13 + 0.14 | 93.33 + 7.32 | 0.11 + 0.01 | 12.83 + 0.06 | 46.56 + 8.62 |
| Site 5 | 52.95 + 1.29 | 0.45 + 0.16 | 0.57 + 0.2 | 58.33 + 4.02 | 0.1 + 0.01 | 8.64 + 0.27 | 59.68 + 1.32 |
| Site 6 | 47.01 + 4.33 | 0.12 + 0.07 | 0.43 + 0.19 | 51.25 + 6.25 | 0.16 + 0.02 | 10.34 + 6.28 | 52 + 4.4 |
| Site 7 | 61.84 + 3.51 | 0.29 + 0.24 | 0.24 + 0.17 | 42.92 + 1.91 | 0.15 + 0.02 | 6.21 + 0.09 | 75.1 + 10.65 |
| Site 8 | 60.57 + 6.74 | 0.34 + 0.08 | 0.16 + 0.21 | 132.9 + 18.81 | 0.12 + 0.01 | 11.05 + 0.2 | 58.4 + 3.3 |
| Site 9 | 49.41 + 4.74 | 0.27 + 0.01 | 0.81 + 0.07 | 85.83 + 18.72 | 0.2 + 0.03 | 3.28 + 0.17 | 50.3 + 10.93 |
| Site 10 | 71.46 + 6.94 | 0.17 + 0.04 | 1.37 + 0.25 | 29.17 + 14.81 | 0.12 + 0.02 | 1.48 + 0.24 | 22.84 + 2.5 |
| Minimum | 33.39 | 0.02 | 0.01 | 20.00 | 0.09 | 1.21 | 20.04 |
| Maximum | 79.27 | 0.55 | 1.64 | 152.50 | 0.22 | 17.59 | 87.36 |
| Mean | 52.25 | 0.21 | 0.49 | 69.83 | 0.14 | 7.63 | 49.33 |
| Standard Deviation | 11.41 | 0.15 | 0.40 | 30.70 | 0.03 | 3.80 | 14.17 |
| Items | SC (%) | RSC (%) | STS (%) | VC mg/(100g) | SPC (mg/g) | BI | PPO U/(g min) |
|---|---|---|---|---|---|---|---|
| SWC (%) | −0.156 | −0.08 | −0.141 | 0.447* | −0.207 | 0.254 | −0.382 * |
| SOM (g/kg) | −0.188 | 0.14 | 0.081 | 0.207 | −0.089 | −0.023 | −0.186 |
| SAHN (mg/kg) | −0.075 | 0.017 | 0.396 * | 0.012 | 0.134 | −0.321 | −0.346 |
| SNN (mg/kg) | 0.063 | 0.265 | −0.332 | 0.194 | 0.223 | 0.198 | 0.461 * |
| SAMN (mg/kg) | 0.396 * | −0.427 * | 0.292 | −0.185 | 0.066 | −0.301 | −0.484 ** |
| P (mg/kg) | −0.112 | −0.017 | 0.057 | −0.252 | 0.487 ** | −0.176 | 0.147 |
| K (mg/kg) | 0.640 ** | 0.124 | 0.204 | 0.174 | −0.061 | −0.309 | −0.222 |
| pH | 0.445 * | 0.145 | 0.021 | −0.125 | 0.103 | −0.148 | 0.317 |
| Items | SC (%) | RSC (%) | STS (%) | VC mg/(100g) | SPC (mg/g) | BI | PPO U/(g min) |
|---|---|---|---|---|---|---|---|
| SWC (%) | 0.645 | 0.632 | 1.155 | 1.609 | 1.227 | 1.155 | 1.062 |
| SOM (g/kg) | 0.756 | 0.814 | 0.956 | 0.918 | 0.42 | 0.368 | 0.737 |
| SAHN (mg/kg) | 0.752 | 0.358 | 1.568 | 0.826 | 0.943 | 1.291 | 0.956 |
| SNN (mg/kg) | 0.441 | 1.121 | 1.46 | 1.237 | 0.994 | 1.153 | 1.249 |
| SAMN (mg/kg) | 1.151 | 1.915 | 1.187 | 0.806 | 0.954 | 1.243 | 1.497 |
| P (mg/kg) | 0.457 | 1.009 | 1.043 | 1.485 | 1.892 | 1.12 | 0.81 |
| K (mg/kg) | 1.955 | 1.072 | 0.749 | 0.717 | 1.074 | 1.191 | 0.622 |
| pH | 1.214 | 1.166 | 0.899 | 0.733 | 1.491 | 0.757 | 0.923 |
| Quality Factor | Code | Major Affecting Factor | Regression Equation | R2 | F |
|---|---|---|---|---|---|
| SC | y1 | x5, x7, x8 | y1 = −73.404 + 0.033x5 + 0.412x7 + 11.354x8 | 0.490 | 8.327 ** |
| RSC | y2 | x4, x5, x6, x7, x8 | y2 = −0.358 + 0.015x4 − 0.004x5 − 0.007x6 + 0.003x7 + 0.086x8 | 0.377 | 2.907 * |
| STS | y3 | x1, x3, x4, x5, x6 | y3 = 0.027 − 0.025x1 + 0.014x3 − 0.078x4 + 0.006x5 + 0.019x6 | 0.437 | 3.726 * |
| VC | y4 | x1, x4, x6 | y4 = 12.09 + 3.433x1 + 10.086x4 − 3.289x6 | 0.407 | 5.948 ** |
| SPC | y5 | x1, x6, x7, x8 | y5 = −0.218 + 0.001x1 + 0.004x6 − 0.000125x7 + 0.037x8 | 0.316 | 2.887 * |
| BI | y6 | x1, x3, x4, x5, x6, x7 | y6 = 11.158 + 0.445x1 − 0.069x3 + 1.343x4 − 0.009x5 − 0.421x6 − 0.146x7 | 0.576 | 5.208 ** |
| PPO | y7 | x1, x4, x5 | y7 = 74.727 − 1.512x1 + 2.477x4 − 0.195x5 | 0.450 | 7.091 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Niu, X.; Wang, N.; Jiang, W.; Gao, Y.; Wang, X. The Correlation between Soil Nutrient and Potato Quality in Loess Plateau of China Based on PLSR. Sustainability 2020, 12, 1588. https://doi.org/10.3390/su12041588
Xing Y, Niu X, Wang N, Jiang W, Gao Y, Wang X. The Correlation between Soil Nutrient and Potato Quality in Loess Plateau of China Based on PLSR. Sustainability. 2020; 12(4):1588. https://doi.org/10.3390/su12041588
Chicago/Turabian StyleXing, Yingying, Xiaoli Niu, Ning Wang, Wenting Jiang, Yaguang Gao, and Xiukang Wang. 2020. "The Correlation between Soil Nutrient and Potato Quality in Loess Plateau of China Based on PLSR" Sustainability 12, no. 4: 1588. https://doi.org/10.3390/su12041588
APA StyleXing, Y., Niu, X., Wang, N., Jiang, W., Gao, Y., & Wang, X. (2020). The Correlation between Soil Nutrient and Potato Quality in Loess Plateau of China Based on PLSR. Sustainability, 12(4), 1588. https://doi.org/10.3390/su12041588






