Carabid Beetle (Coleoptera: Carabidae) Response to Soil Properties of Urban Wastelands in Warsaw, Poland
Abstract
1. Introduction
2. Materials and Methods
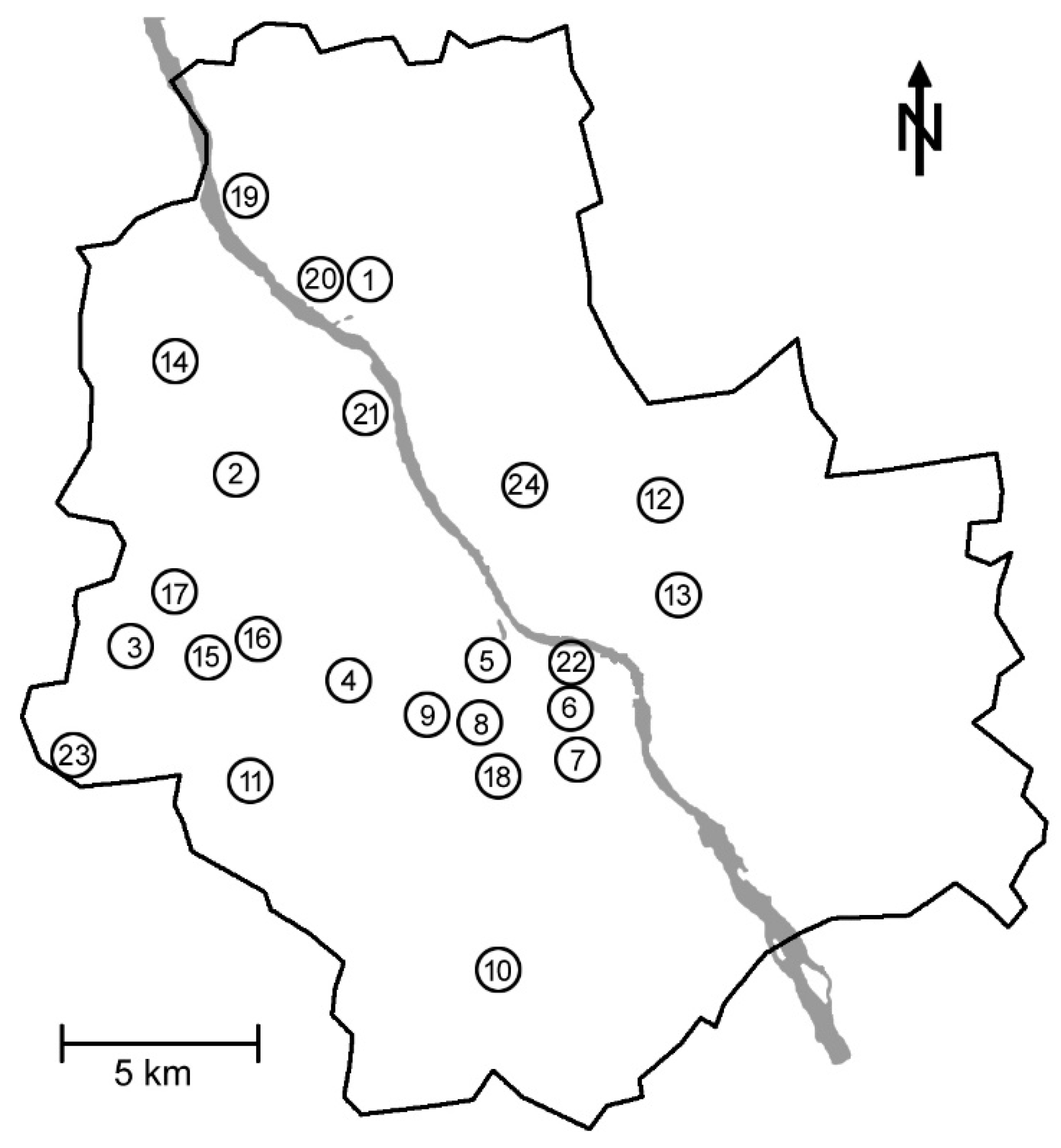
2.1. Study Areas and Sampling Plots
2.2. Field and Laboratory Methods
2.3. Statistical Methods
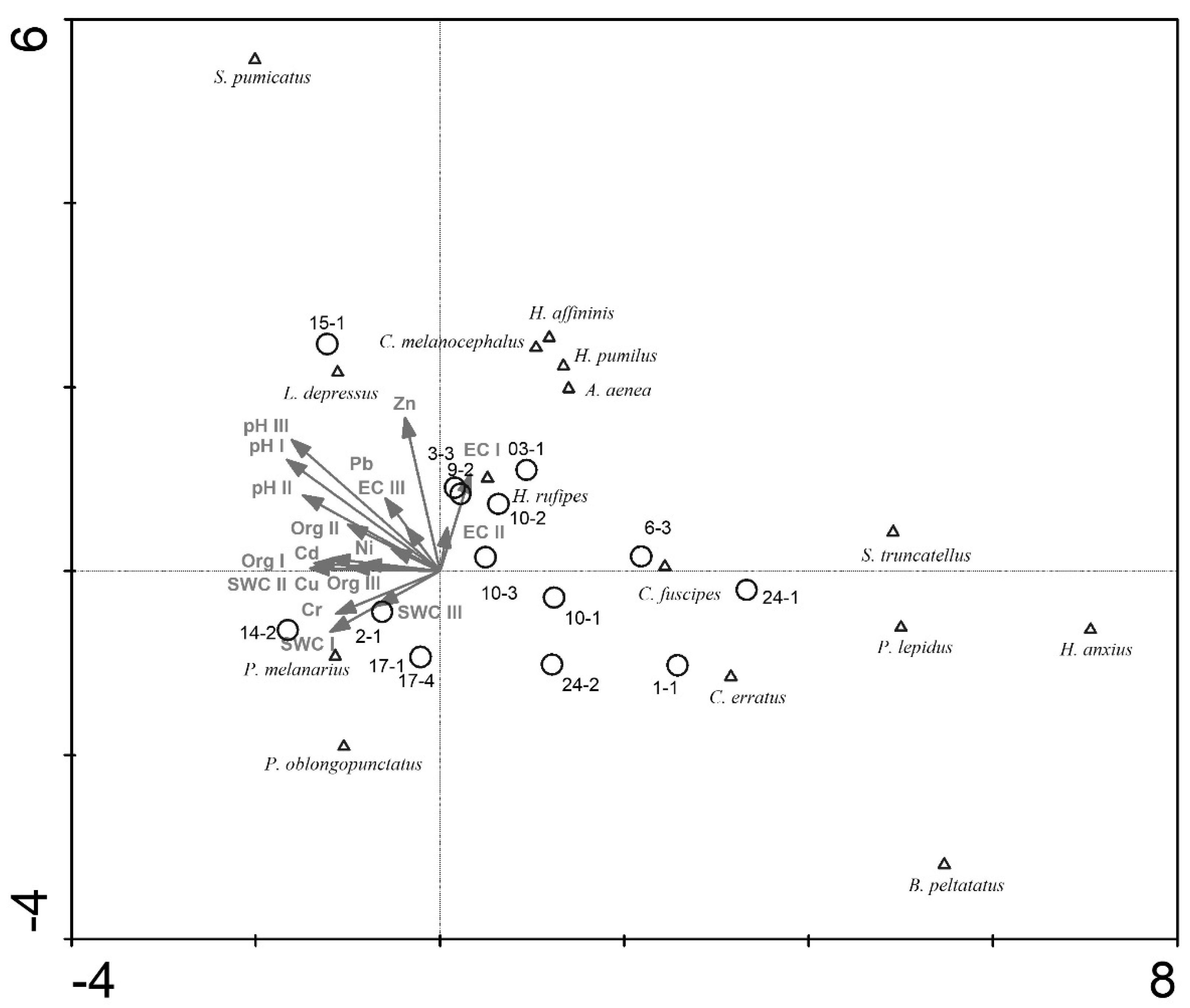
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sampling Plot | pH I | pH II | pH III | EC I | EC II | EC III | Org I | Org II | Org III | SWC I | SWC II | SWC III | Pb | Cd | Ni | Cr | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 6.23 | 6.03 | 5.48 | 0.88 | 0.54 | 0.10 | 1.79 | 2.17 | 1.60 | 3.30 | 7.46 | 11.08 | 10.0 | 0.18 | 8.0 | 8.0 | 5.8 | 34.8 |
| 1-2 | 6.46 | 6.20 | 5.89 | 0.57 | 0.52 | 0.17 | 3.98 | 6.13 | 1.10 | 2.10 | 10.10 | 6.66 | 10.0 | 0.18 | 8.0 | 8.0 | 5.8 | 34.8 |
| 1-3 | 6.46 | 6.20 | 5.89 | 0.57 | 0.52 | 0.17 | 3.98 | 6.13 | 1.10 | 2.10 | 10.10 | 6.66 | 10.0 | 0.18 | 8.0 | 8.0 | 5.8 | 34.8 |
| 1-4 | 6.46 | 6.20 | 5.89 | 0.57 | 0.52 | 0.17 | 3.98 | 6.13 | 1.10 | 2.10 | 10.10 | 6.66 | 10.0 | 0.18 | 8.0 | 8.0 | 5.8 | 34.8 |
| 1-5 | 7.21 | 7.07 | 5.45 | 0.61 | 0.63 | 0.48 | 2.76 | 6.69 | 1.03 | 1.80 | 6.54 | 3.96 | 10.0 | 0.18 | 8.0 | 8.0 | 5.8 | 34.8 |
| 2-1 | 7.03 | 6.97 | 7.30 | 0.38 | 0.39 | 0.24 | 4.29 | 5.51 | 2.64 | 6.00 | 13.64 | 11.55 | 19.7 | 0.24 | 8.0 | 13.2 | 8.5 | 47.7 |
| 2-2 | 7.47 | 6.95 | 7.89 | 0.60 | 0.51 | 0.28 | 2.04 | 6.16 | 2.18 | 5.60 | 14.10 | 12.00 | 19.7 | 0.24 | 8.0 | 13.2 | 8.5 | 47.7 |
| 2-3 | 7.29 | 5.53 | 5.30 | 0.56 | 0.14 | 0.08 | 3.58 | 3.97 | 1.19 | 4.10 | 10.95 | 12.21 | 19.7 | 0.24 | 8.0 | 13.2 | 8.5 | 47.7 |
| 3-1 | 7.7 | 7.58 | 7.48 | 1.42 | 1.00 | 1.29 | 3.04 | 3.94 | 3.41 | 5.10 | 8.72 | 7.32 | 54.8 | 0.32 | 8.0 | 8.0 | 33.3 | 259.0 |
| 3-2 | 7.67 | 7.53 | 7.72 | 1.01 | 1.11 | 0.75 | 2.87 | 5.79 | 4.03 | 6.10 | 8.13 | 5.31 | 54.8 | 0.32 | 8.0 | 8.0 | 33.3 | 259.0 |
| 3-3 | 7.89 | 7.60 | 7.31 | 1.49 | 0.72 | 0.85 | 3.56 | 3.01 | 6.43 | 4.80 | 8.95 | 5.81 | 54.8 | 0.32 | 8.0 | 8.0 | 33.3 | 259.0 |
| 4-1 | 7.25 | 7.20 | 7.33 | 0.96 | 0.66 | 1.41 | 3.25 | 3.60 | 7.26 | 5.00 | 6.05 | 11.45 | 57.8 | 0.41 | 15.2 | 17.1 | 37.0 | 273.0 |
| 4-2 | 7.17 | 7.36 | 7.15 | 0.71 | 0.64 | 1.13 | 2.91 | 4.91 | 4.04 | 12.60 | 10.71 | 4.31 | 57.8 | 0.41 | 15.2 | 17.1 | 37.0 | 273.0 |
| 4-3 | 7.17 | 7.36 | 7.15 | 0.71 | 0.64 | 1.13 | 2.91 | 4.91 | 4.04 | 12.60 | 10.71 | 4.31 | 57.8 | 0.41 | 15.2 | 17.1 | 37.0 | 273.0 |
| 5-1 | 7.04 | 6.31 | 7.11 | 1.29 | 0.63 | 0.34 | 6.68 | 5.73 | 10.93 | 12.70 | 20.35 | 15.09 | 26.4 | 0.38 | 25.5 | 25.9 | 31.7 | 140.0 |
| 5-2 | 7.04 | 6.31 | 7.11 | 1.29 | 0.63 | 0.34 | 6.68 | 5.73 | 10.93 | 12.70 | 20.35 | 15.09 | 26.4 | 0.38 | 25.5 | 25.9 | 31.7 | 140.0 |
| 6-1 | 7.45 | 7.82 | 7.59 | 1.21 | 0.57 | 2.92 | 3.87 | 3.03 | 3.00 | 6.90 | 9.87 | 9.06 | 15.8 | 0.28 | 14.0 | 16.2 | 15.8 | 77.8 |
| 6-2 | 7.47 | 7.23 | 7.36 | 0.82 | 1.55 | 6.00 | 1.94 | 4.32 | 5.71 | 4.60 | 13.64 | 9.93 | 15.8 | 0.28 | 14.0 | 16.2 | 15.8 | 77.8 |
| 6-3 | 7.45 | 7.38 | 7.42 | 0.91 | 2.96 | 0.75 | 2.88 | 2.49 | 2.56 | 6.70 | 11.26 | 9.28 | 15.8 | 0.28 | 14.0 | 16.2 | 15.8 | 77.8 |
| 7-1 | 6.98 | 7.13 | 7.33 | 1.92 | 0.33 | 1.60 | 7.54 | 3.36 | 2.86 | 16.70 | 8.05 | 7.56 | 30.4 | 0.61 | 30.4 | 27.4 | 29.9 | 130.0 |
| 7-2 | 7.23 | 6.81 | 6.96 | 0.63 | 0.88 | 0.70 | 9.40 | 7.26 | 6.65 | 15.00 | 21.09 | 13.56 | 30.4 | 0.61 | 30.4 | 27.4 | 29.9 | 130.0 |
| 8-1 | 7.64 | 7.52 | 7.52 | 0.71 | 4.46 | 6.90 | 8.59 | 19.38 | 19.61 | 8.00 | 27.20 | 41.11 | 19.9 | 0.25 | 8.0 | 8.0 | 12.9 | 87.8 |
| 8-2 | 7.64 | 7.52 | 7.52 | 0.71 | 4.46 | 6.90 | 8.59 | 19.38 | 19.61 | 8.00 | 27.20 | 41.11 | 19.9 | 0.25 | 8.0 | 8.0 | 12.9 | 87.8 |
| 9-1 | 7.39 | 7.16 | 6.96 | 0.82 | 0.80 | 0.54 | 7.35 | 6.47 | 7.11 | 7.80 | 7.87 | 21.61 | 97.8 | 0.71 | 14.2 | 18.7 | 56.7 | 495 |
| 9-2 | 7.39 | 7.16 | 6.96 | 0.82 | 0.80 | 0.54 | 7.35 | 6.47 | 7.11 | 7.80 | 7.87 | 21.61 | 97.8 | 0.71 | 14.2 | 18.7 | 56.7 | 495 |
| 10-1 | 6.38 | 5.17 | 5.04 | 0.56 | 0.14 | 0.30 | 3.31 | 4.84 | 3.73 | 2.70 | 3.69 | 5.81 | 10.00 | 0.08 | 8.0 | 8.0 | 3.5 | 14.9 |
| 10-2 | 7.40 | 7.19 | 7.69 | 1.10 | 0.86 | 0.74 | 3.44 | 7.57 | 3.26 | 3.90 | 4.14 | 9.35 | 10.00 | 0.08 | 12.6 | 12.6 | 5.9 | 32.8 |
| 10-3 | 7.5 | 7.35 | 7.4 | 1.09 | 1.15 | 0.80 | 3.44 | 6.13 | 6.11 | 3.90 | 7.32 | 10.42 | 10.00 | 0.06 | 8.0 | 8.0 | 6.0 | 26.7 |
| 11-1 | 7.9 | 7.48 | 7.95 | 0.46 | 0.07 | 1.00 | 1.96 | 2.84 | 0.44 | 4.30 | 2.38 | 11.20 | 89.3 | 0.22 | 8.0 | 22.3 | 305.0 | 126.0 |
| 11-2 | 7.9 | 7.48 | 7.95 | 0.46 | 0.07 | 1.00 | 1.96 | 2.84 | 0.44 | 4.30 | 2.38 | 11.20 | 89.3 | 0.22 | 8.0 | 22.3 | 305.0 | 126.0 |
| 12-1 | 6.56 | 5.8 | 6.82 | 0.40 | 0.12 | 0.36 | 6.41 | 6.13 | 7.44 | 8.00 | 8.28 | 15.71 | 119.0 | 0.27 | 8.0 | 8.0 | 244.0 | 88.8 |
| 12-2 | 6.56 | 5.8 | 6.82 | 0.40 | 0.12 | 0.36 | 6.41 | 6.13 | 7.44 | 8.00 | 8.28 | 15.71 | 119.0 | 0.27 | 8.0 | 8.0 | 244.0 | 88.8 |
| 12-3 | 6.64 | 6.42 | 7.26 | 0.92 | 0.29 | 0.27 | 8.05 | 7.57 | 7.06 | 13.40 | 17.30 | 13.92 | 119.0 | 0.27 | 8.0 | 8.0 | 244.0 | 88.8 |
| 13-1 | 7.57 | 6.36 | 6.25 | 1.02 | 0.17 | 0.40 | 5.98 | 1.75 | 3.04 | 5.00 | 1.63 | 6.82 | 40.2 | 0.20 | 8.0 | 8.0 | 40.6 | 110.0 |
| 13-2 | 7.57 | 6.36 | 6.25 | 1.02 | 0.17 | 0.40 | 5.98 | 1.75 | 3.04 | 5.00 | 1.63 | 6.82 | 40.2 | 0.20 | 8.0 | 8.0 | 40.6 | 110.0 |
| 14-1 | 7.12 | 7.14 | 6.00 | 1.19 | 1.01 | 0.14 | 8.98 | 7.34 | 2.79 | 5.00 | 4.96 | 11.05 | 54.6 | 0.47 | 8.0 | 18.4 | 42.6 | 86.7 |
| 14-2 | 7.23 | 6.82 | 5.73 | 0.77 | 0.60 | 0.37 | 5.41 | 6.55 | 4.80 | 4.30 | 8.38 | 8.97 | 54.6 | 0.47 | 8.0 | 18.4 | 42.6 | 86.7 |
| 15-1 | 7.64 | 7.3 | 8.42 | 0.84 | 0.95 | 1.12 | 5.39 | 9.96 | 4.71 | 5.30 | 20.94 | 10.64 | 58.9 | 0.67 | 17.2 | 17.6 | 113.0 | 321.0 |
| 16-1 | 7.74 | 7.45 | 8.50 | 0.65 | 0.49 | 0.38 | 6.86 | 5.44 | 1.77 | 7.20 | 16.29 | 12.13 | 196.0 | 0.96 | 14.5 | 20.6 | 131.0 | 277.0 |
| 16-2 | 7.74 | 7.45 | 8.50 | 2.33 | 0.58 | 0.49 | 6.86 | 5.44 | 1.77 | 7.20 | 16.29 | 12.13 | 196.0 | 0.96 | 14.5 | 20.6 | 131.0 | 277.0 |
| 17-1 | 6.28 | 6.99 | 6.25 | 0.60 | 0.52 | 0.53 | 6.30 | 5.14 | 5.94 | 13.10 | 10.01 | 10.96 | 19.6 | 0.58 | 12.6 | 15.9 | 24.8 | 114.0 |
| 17-2 | 6.28 | 6.99 | 6.25 | 0.60 | 0.52 | 0.53 | 6.30 | 5.14 | 5.94 | 13.10 | 10.01 | 10.96 | 19.6 | 0.58 | 12.6 | 15.9 | 24.8 | 114.0 |
| 17-3 | 6.48 | 6.25 | 6.08 | 0.76 | 0.20 | 0.49 | 5.26 | 4.42 | 3.69 | 10.80 | 8.73 | 8.41 | 19.6 | 0.58 | 12.6 | 15.9 | 24.8 | 114.0 |
| 17-4 | 6.28 | 6.99 | 6.25 | 0.60 | 0.52 | 0.53 | 6.30 | 5.14 | 5.94 | 13.10 | 10.01 | 10.96 | 19.6 | 0.58 | 12.6 | 15.9 | 24.8 | 114.0 |
| 17-5 | 6.28 | 6.99 | 6.25 | 0.60 | 0.52 | 0.53 | 6.30 | 5.14 | 5.94 | 13.10 | 10.01 | 10.96 | 19.6 | 0.58 | 12.6 | 15.9 | 24.8 | 114.0 |
| 18-1 | 7.00 | 6.94 | 7.80 | 0.66 | 0.98 | 0.60 | 8.79 | 7.13 | 9.08 | 11.30 | 21.27 | 9.51 | 33.4 | 0.25 | 8.0 | 8.0 | 18.2 | 72.9 |
| 19-1 | 7.68 | 7.41 | 7.25 | 0.75 | 1.19 | 1.00 | 2.79 | 4.28 | 3.68 | 8.50 | 11.26 | 16.40 | 10.0 | 0.88 | 8.0 | 13.5 | 7.4 | 106.0 |
| 20-1 | 7.42 | 7.50 | 7.40 | 0.75 | 0.96 | 0.69 | 4.59 | 0.67 | 2.11 | 4.30 | 17.43 | 15.26 | 33.5 | 0.50 | 8.0 | 15.0 | 13.9 | 109.0 |
| 21-1 | 7.00 | 7.83 | 7.21 | 0.55 | 0.49 | 0.52 | 3.66 | 2.87 | 3.22 | 6.68 | 7.18 | 12.74 | 49.2 | 0.28 | 13.8 | 8.0 | 29.8 | 98.1 |
| 21-2 | 6.88 | 7.38 | 6.97 | 0.62 | 0.85 | 0.66 | 4.69 | 7.23 | 5.03 | 7.13 | 22.77 | 19.90 | 49.2 | 0.28 | 13.8 | 8.0 | 29.8 | 98.1 |
| 22-1 | 7.57 | 7.92 | 7.13 | 0.73 | 0.47 | 0.36 | 4.14 | 5.07 | 4.52 | 4.90 | 11.13 | 8.19 | 10.0 | 0.23 | 8.0 | 8.0 | 3.5 | 60.7 |
| 22-2 | 7.50 | 7.50 | 7.15 | 0.61 | 0.86 | 0.60 | 1.90 | 3.15 | 3.01 | 5.10 | 15.86 | 7.68 | 10.0 | 0.23 | 8.0 | 8.0 | 3.5 | 60.7 |
| 23-1 | 7.63 | 7.44 | 7.32 | 0.67 | 0.60 | 0.92 | 4.60 | 4.67 | 5.16 | 6.67 | 5.66 | 14.96 | 71.9 | 0.12 | 8.0 | 8.0 | 16.5 | 74.9 |
| 23-2 | 7.45 | 7.35 | 7.93 | 0.54 | 0.66 | 0.52 | 3.82 | 2.88 | 1.60 | 5.99 | 4.60 | 11.12 | 71.9 | 0.12 | 8.0 | 8.0 | 16.5 | 74.9 |
| 24-1 | 4.50 | 4.12 | 4.60 | 0.51 | 0.63 | 0.46 | 4.23 | 4.63 | 5.22 | 5.32 | 3.33 | 9.54 | 75.2 | 0.15 | 8.0 | 8.0 | 56.2 | 50.0 |
| 24-2 | 6.30 | 6.70 | 6.33 | 0.35 | 0.31 | 0.43 | 2.87 | 1.30 | 3.64 | 6.49 | 1.69 | 16.00 | 75.2 | 0.15 | 8.0 | 8.0 | 56.2 | 50.0 |
References
- Gałecka-Drozda, A.; Raszeja, E. Useful wasteland—The potential of undeveloped land in modification of urban green infrastructure based on the city of Poznań. Misc. Geogr. 2018, 22, 225–230. [Google Scholar] [CrossRef]
- Yigitcanlar, T.; Kamruzzaman, M.D. Planning, development and management of sustainable cities: A Commentary from the Guest Editors. Sustainability 2015, 7, 14677–14688. [Google Scholar] [CrossRef]
- Tang, H.-T.; Lee, Y.-M. The Making of sustainable urban development: A synthesis framework. Sustainability 2016, 8, 492. [Google Scholar] [CrossRef]
- Sikorska, D.; Łaszkiewicz, E.; Krauze, K.; Sikorski, P. The role of unmanaged informal green spaces in reducing inequalities in urban green spaces availability to children and seniors. Environ. Sci. Policy 2020, 108, 144–154. [Google Scholar] [CrossRef]
- Nejman, R.; Łepkowski, M.; Wilczyńska, A.; Gawryszewska, B.J. The right to wild. Green urban wasteland in the context of urban planning. Urban. Dev. Issues 2018, 59, 43–53. [Google Scholar] [CrossRef]
- Kelcey, J.G. Industrial development and wildlife conservation. Environ. Conserv. 1975, 2, 99–108. [Google Scholar] [CrossRef]
- Gemmell, R.P.; Connell, R.K. Conservation and creation of wildlife habitats on industrial land in Greater Manchester. Landsc. Plan. 1984, 11, 175–186. [Google Scholar] [CrossRef]
- Small, E.C.; Sadler, J.P.; Telfer, M.G. Carabid beetle assemblages on urban derelict sites in Birmingham, UK. J. Insect Conserv. 2003, 6, 233–246. [Google Scholar] [CrossRef]
- Kowarik, I. Urban wilderness: Supply, demand, and access. Urban. For. Urban. Green. 2018, 29, 336–347. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Romano, D.; Malorgio, F.; Benvenuti, S.; Pezzarossa, B. Wildflowers: From conserving biodiversity to urban greening—A review. Urban. For. Urban. Green. 2016, 20, 428–436. [Google Scholar] [CrossRef]
- Hurka, K. Carabidae of Czech and Slovak Republics; Kabourek: Zlin, Czech Republic, 1996; p. 565. [Google Scholar]
- Koivula, M.J. Useful model organisms, indicators, or both? Ground beetles (Coleoptera, Carabidae) reflecting environmental conditions. ZooKeys 2011, 100, 287–317. [Google Scholar] [CrossRef] [PubMed]
- Kędzior, R.; Skalski, T.; Szwalec, A.; Mundała, P. Diversity of carabid beetle assemblages (Coleoptera: Carabidae) in a post-industrial slag deposition area. Baltic J. Coleopterol. 2014, 14, 219–227. [Google Scholar]
- Ghannem, S.; Touaylia, S.; Boumaiza, M. Beetles (Insecta: Coleoptera) as bioindicators of the assessment of environmental pollution. Hum. Ecol. Risk Assess. 2018, 24, 456–464. [Google Scholar] [CrossRef]
- Avgın, S.S.; Luff, M.L. Ground beetles (Coleoptera: Carabidae) as bioindicators of human impact. Munis Entomol. Zool. 2010, 5, 209–215. [Google Scholar]
- Skalski, T.; Stone, D.; Kramarz, P.; Laskowski, R. Ground beetle community responses to heavy metal contamination. Baltic J. Coleopterol. 2010, 10, 1–12. [Google Scholar]
- Skalski, T.; Kędzior, R.; Kolbe, D.; Knutelski, S. Biegaczowate jako wskaźniki zanieczyszczenia lasów metalami ciężkimi. Sylwan 2015, 159, 905–911. [Google Scholar]
- Laskowski, R.; Bednarska, A.J.; Kramarz, P.E.; Loureiro, S.; Scheil, V.; Kudłek, J.; Holmstrup, M. Interactions between toxic chemicals and natural environmental factors—A meta-analysis and case studies. Sci. Total Environ. 2010, 408, 3763–3774. [Google Scholar] [CrossRef]
- Bayley, M.; Baatrup, E.; Heimbach, U.; Bjerregaard, P. Elevated copper levels during larval development cause altered locomotor behaviour in the adult carabid beetle Pterostichus cupreus L. (Coleoptera: Carabidae). Ecotoxicol. Environ. Saf. 1995, 32, 166–170. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Cavaliere, F.; Elliani, R.; Pirrone, N.; Sprovieri, F.; Tagarelli, A.; Giglio, A. Agrochemical treatments as a source of heavy metals and rare earth elements in agricultural soils and bioaccumulation in ground beetles. Sci. Total Environ. 2020, 749, 141438. [Google Scholar] [CrossRef]
- World Population Review. Available online: https://worldpopulationreview.com/world-cities/warsaw-population/ (accessed on 25 April 2020).
- Koivula, M.; Kukkonen, J.; Niemelä, J. Boreal carabid-beetle (Coleoptera, Carabidae) assemblages along the clear-cut originated succession gradient. Biodivers. Conserv. 2002, 11, 1269–1288. [Google Scholar] [CrossRef]
- Skłodowski, J.J.W. Anthropogenic transformation of ground beetle assemblages (Coleoptera: Carabidae) in Białowieża Forest, Poland: From primeval forests to managed woodlands of various ages. Entomol. Fennica 2006, 17, 296–314. [Google Scholar] [CrossRef]
- Schwerk, A.; Szyszko, J. Model of succession in degraded areas based on carabid beetles (Coleoptera, Carabidae). ZooKeys 2011, 100, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Barber, H.S. Traps for cave inhabiting insects. J. Mitchel. Soc. 1931, 46, 259–266. [Google Scholar]
- Freude, H.; Harde, K.-W.; Lohse, G.A.; Klausnitzer, B. Die Käfer Mitteleuropas. Bd. 2, Adephaga 1, Carabidae (Laufkäfer). 2. (erweiterte) Aufl.; Spektrum: Heidelberg/Berlin, Germany, 2004; p. 521. [Google Scholar]
- Larsson, S.G. Entwicklungstypen und Entwicklungszeiten der dänischen Carabiden. Entomol. Meddr. 1939, 20, 277–562. [Google Scholar]
- Lindroth, C.H. Die Fennoscandischen Carabidae: I Spezieller Teil; Elanders Boktryckeri Aktiebolag: Göteborg, Sweden, 1945; p. 709. [Google Scholar]
- Burakowski, B.; Mroczkowski, M.; Stefańska, J. Katalog fauny Polski (Catalogus faunae Poloniae). Część XXIII, tom 2. Chrząszcze (Coleoptera). Biegaczowate (Carabidae), część 1; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1973; p. 232. [Google Scholar]
- Burakowski, B.; Mroczkowski, M.; Stefańska, J. Katalog fauny Polski (Catalogus faunae Poloniae). Część XXIII, tom 3. Chrząszcze (Coleoptera). Biegaczowate (Carabidae), część 2; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1974; p. 430. [Google Scholar]
- Lindroth, C.H. The Carabidae (Coleoptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica. Volume 15, Part II; Scandinavian Science Press, Ltd.: Leiden, The Netherlands, 1986; p. 497. [Google Scholar]
- Dz. U. z 2016 r. poz. 1395. Rozporządzenie Ministra Środowiska z dnia 1 września 2016 w sprawie sposobu prowadzenia oceny zanieczyszczenia powierzchni ziemi. 2016; 86p. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20160001395/O/D20161395.pdf (accessed on 30 September 2020).
- Ter Braak, C.J.F. CANOCO—A FORTRAN Program for Canonical Community Ordination by [Partial][Detrended][Canonical] Correspondence Analysis, Principal Components Analysis and Redundancy Analysis (Version 2.1); DLO Agricultural Mathematics Group: Wageningen, The Netherland, 1987; p. 95. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002; p. 499. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 269. [Google Scholar]
- Przybysz, A.; Wińska-Krysiak, M.; Małecka-Przybysz, M.; Stankiewicz-Kosyl, M.; Skwara, M.; Kłos, A.; Kowalczyk, S.; Jarocka, K.; Sikorski, P. Urban wastelands: On the frontline between air pollution sources and residential areas. Sci. Total Environ. 2020, 721, 137695. [Google Scholar] [CrossRef] [PubMed]
- Van Heerdt, P.F.; Blokhuis, B.; Van Haaften, C. The reproductive cycle and age composition of a population of Pterostichus oblongopunctatus (Fabricius) in The Netherlands (Coleoptera: Carabidae). Tijdschr. Entomol. 1976, 119, 1–15. [Google Scholar]
- Bauer, T. Prey capture and structure of the visual space of an insect that hunts by sight on the litter layer (Notiophilus biguttatus F., Carabidae, Coleoptera). Behav. Ecol. Sociobiol. 1981, 8, 91–97. [Google Scholar] [CrossRef]
- Skłodowski, J. Manual soil preparation and piles of branches can support ground beetles (Coleoptera, carabidae) better than four different mechanical soil treatments in a clear-cut area of a closed-canopy pine forest in northern Poland. Scand. J. For. Res. 2017, 32, 123–133. [Google Scholar] [CrossRef]
- Ermakov, A.I. Structural changes in the carabid fauna of forest ecosystems under a toxic impact. Russ. J. Ecol. 2004, 35, 403–408. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Chudnyavtseva, I.I.; Pokarzhevskii, A.D.; Samonov, A.E.; Slobodyan, V.Y. Arsenic bioaccumulation by beetles in an arsenic-rich region. Bull. Environ. Contam. Toxicol. 2004, 72, 1115–1121. [Google Scholar] [CrossRef]
- Belskaya, E.A.; Zinoviev, E.V. Structure of the complexes of carabid beetles (Coleoptera, Carabidae) in natural and industry-disturbed forest ecosystems in the south-west of the Sverdlovsk region. Siberian J. Ecol. 2007, 4, 533–543. [Google Scholar]
- Ashraf, M.A.; Maah, M.J.; Yusoff, I. Chemical speciation and potential mobility of heavy metals in the soil of former tin mining catchment. Sci. World J. 2012, 125608–125611. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant. Nut. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Chieli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional ecology of heavy metals. Animal 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, A.J.; Laskowski, R.; Pyza, E.; Semik, D.; Świątek, Z.; Woźnicka, O. Metal toxicokinetics and metal-driven damage to the gut of the ground beetle Pterostichus oblongopunctatus. Environ. Sci. Pollut. Res. 2016, 23, 22047–22058. [Google Scholar] [CrossRef] [PubMed]
- Talarico, F.; Brandmayr, P.; Giulianini, P.G.; Ietto, F.; Naccarato, A.; Perrotta, E.; Tagarelli, A.; Giglio, A. Effects of metal pollution on survival and physiological responses in Carabus (Chaetocarabus) lefebvrei (Coleoptera, Carabidae). Eur. J. Soil Biol. 2014, 61, 80–89. [Google Scholar] [CrossRef]
- Sowa, G.; Skalski, T. Effects of chronic metal exposure on the morphology of beetles species representing different ecological niches. Bull. Environ. Contam. Toxicol. 2019, 102, 191–197. [Google Scholar] [CrossRef]
- Šerić Jelaska, L.; Blanuša, M.; Durbešić, P.; Jelaska, S.D. Heavy metal concentrations in ground beetles, leaf litter, and soil of a forest ecosystem. Ecotoxicol. Environ. Saf. 2007, 66, 74–81. [Google Scholar] [CrossRef]
- Tőzsér, D.; Magura, T.; Simon, E.; Mizser, S.; Papp, D.; Tóthmérész, B. Pollution intensity-dependent metal accumulation in ground beetles: A meta-analysis. Environ. Sci. Pollut. Res. 2019, 26, 32092–32102. [Google Scholar] [CrossRef]
- Niemelä, J.; Kotze, D.J.; Venn, S.; Penev, L.; Stoyanov, I.; Spence, J.; Hartley, D.; Montes de Oca, E. Carabid beetle assemblages (Coleoptera, Carabidae) across urban-rural gradients: An international comparison. Landsc. Ecol. 2002, 17, 387–401. [Google Scholar] [CrossRef]
- Bednarska, A.J.; Laskowski, R. Environmental conditions enhance toxicant effects in larvae of the ground beetle Pterostichus oblongopunctatus (Coleoptera: Carabidae). Environ. Pollut. 2009, 157, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, A.J.; Portka, I.; Kramarz, P.E.; Laskowski, R. Combined effect of environmental pollutants (Nickel, Chlorpyrifos) and temperature on the ground beetle, Pterostichus oblongopunctatus (Coleoptera: Carabidae). Environ. Toxicol. Chem. 2009, 28, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, B. Die Biotopstruktur als Raumwiderstand und Raumfülle für die Tierwelt. Zool. Anz. 1957, 20, 332–347. [Google Scholar]
- Greenslade, P.J.M. Pitfall trapping as method for studying populations of Carabidae (Coleoptera). J. Anim. Ecol. 1964, 33, 301–310. [Google Scholar] [CrossRef]
- Szyszko, J.; Gryuntal, S.; Schwerk, A. Differences in locomotory activity between male and female Carabus hortensis (Coleoptera: Carabidae) in a pine forest and a beech forest in relation to feeding state. Environ. Entomol. 2004, 33, 1442–1446. [Google Scholar] [CrossRef]
- Bradshaw, A.D. The reconstruction of ecosystems. J. Appl. Ecol. 1983, 20, 1–17. [Google Scholar] [CrossRef]
- Rebele, F.; Dettmar, J. Industriebrachen. Ökologie und Management; Verlag Eugen Ulmer: Stuttgart (Hohenheim), Germany, 1996; p. 188. [Google Scholar]
- Gandy, M. Marginalia: Aesthetics, Ecology, and Urban Wastelands. Ann. Assoc. Am. Geogr. 2013, 103, 1301–1316. [Google Scholar] [CrossRef]
- Kar, D.; Palit, D. Phytoremediation: An Advance Approach for Stabilization of Coal Mine Wastelands. In Sustainable Agriculture, Forest and Environmental Management; Jhariya, M., Banerjee, A., Meena, R., Yadav, D., Eds.; Springer: Singapore, 2019; pp. 573–606. [Google Scholar]
| Sampling Plot | Phytosociological Units | Stage of Succession | Species | Individuals |
|---|---|---|---|---|
| 1-1 | Regenerative woodland (Populus × canescens association) | Advanced | 1 | 0.39 |
| 1-2 | Grassland (Calamagrostietum epigeji Juraszek 1928) | Early | 10 | 8.98 |
| 1-3 | Grassland (Festuca rubra, association on a slope) | Advanced | 7 | 10.16 |
| 1-4 | Rushes (Phragmition Koch 1926) | Early | 3 | 2.22 |
| 1-5 | Regenerative woodland (Quercus rubra, Tilia cordata association) | Advanced | 6 | 3.91 |
| 2-1 | Regenerative woodland (Quercus robur, Betula pendula association) | Advanced | 11 | 50.78 |
| 2-2 | Regenerative woodland (Betula pendula, Populus tremula association) | Advanced | 7 | 7.81 |
| 2-3 | Bushes (Solidago gigantea association) | Early | 23 | 66.80 |
| 3-1 | Grassland (Lolio-Cynosuretum R.Tx. 1937) | Early | 5 | 8.62 |
| 3-2 | Grassland (Dactylis glomerata association) | Early | 10 | 53.73 |
| 3-3 | Bushes (Prunus cerasifera association) | Early | 14 | 12.31 |
| 4-1 | Eegenerative woodland (Salix caprea, Betula pendula association) | Advanced | 7 | 6.30 |
| 4-2 | Regenerative woodland (Acer platanoides, Acer negundo association) | Early | 7 | 5.51 |
| 4-3 | Herbs (Artemisietea Lohm., Prsg et R. Tx. in R. Tx. 1950) | Early | 12 | 46.85 |
| 5-1 | Regenerative woodland (Acer negundo association) | Early | 7 | 10.23 |
| 5-2 | Bushes (Solidago gigantea association) | Early | 11 | 28.41 |
| 6-1 | Tree coverage of Prunus cerasifera, Acer pseudoplatanus | Advanced | 8 | 17.29 |
| 6-2 | Grassland (Arrhenatherion elatioris (Br.-Bl. 1925) Koch 1926) | Early | 13 | 30.45 |
| 6-3 | Grassland (Lolio-Polygonetum Br-Bl. 1930 em. Lohm. 1975) | Early | 19 | 153.38 |
| 7-1 | Grassland (Lolio-Cynosuretum R.Tx. 1937) | Early | 10 | 124.81 |
| 7-2 | Regenerative woodland (Acer negundo association) | Advanced | 12 | 16.41 |
| 8-1 | Regenerative woodland (Acer negundo association) | Advanced | 11 | 14.02 |
| 8-2 | Herbs (Artemisietea Lohm., Prsg et R. Tx. in R. Tx. 1950) | Early | 8 | 12.88 |
| 9-1 | Regenerative woodlands (Tilia cordata, Acer platanoides association) | Advanced | 6 | 6.53 |
| 9-2 | EGrassland (Lolio-Cynosuretum R.Tx. 1937) | Early | 14 | 71.90 |
| 10-1 | Regenerative woodland (Populus × canescens association) | Early | 13 | 78.41 |
| 10-2 | Grassland (Lolio-Cynosuretum R.Tx. 1937) | Early | 10 | 35.35 |
| 10-3 | Grassland (Calamagrostietum epigeji Juraszek 1928) | Early | 11 | 38.38 |
| 11-1 | Bushes (Prunus cerasifera association) | Early | 6 | 3.70 |
| 11-2 | Bushes (Corylus avellana association) | Early | 7 | 7.41 |
| 12-1 | Bushes (Solidago gigantea association) | Early | 9 | 19.70 |
| 12-2 | Regenerative woodland (Acer negundo association) | Advanced | 10 | 9.09 |
| 12-3 | Regenerative woodland (Populus tremula, Acer negundo association) | Advanced | 5 | 6.82 |
| 13-1 | Regenerative woodlands (Populus nigra-P. × canescens association) | Advanced | 10 | 12.88 |
| 13-2 | Bushes (Cornus sanguinea association) | Early | 10 | 6.44 |
| 14-1 | Regenerative woodland (Quercus rubra, Acer platanoides association) | Advanced | 10 | 9.84 |
| 14-2 | Regenerative woodland (Betula pendula, Agrostis capillaris association) | Advanced | 6 | 14.57 |
| 15-1 | Regenerative woodland (Acer negundo association) | Early | 3 | 1.10 |
| 16-1 | Regenerative woodland (Populus × canescens association) | Early | 0 | 0.00 |
| 16-2 | Regenerative woodland (Acer negundo association) | Early | 0 | 0.00 |
| 17-1 | Regenerative woodland (Robinia pseudoacacia association) | Advanced | 20 | 42.16 |
| 17-2 | Grassland (Lolio-Cynosuretum R.Tx. 1937) | Early | 12 | 14.18 |
| 17-3 | Regenerative woodland (Tilia cordata association) | Advanced | 14 | 10.07 |
| 17-4 | Regenerative woodland (Tilia cordata association) | Advanced | 21 | 41.79 |
| 17-5 | Woodland (Populus × canescens association) | Advanced | 21 | 63.06 |
| 18-1 | Woodland (Acer negundo association) | Early | 19 | 20.08 |
| 19-1 | Regenerative woodlands (Salicetum albo-fragilis R.Tx. 1955) | Advanced | 35 | 187.50 |
| 20-1 | Regenerative woodlands (Salicetum albo-fragilis R.Tx. 1955) | Early | 18 | 44.92 |
| 21-1 | Regenerative woodlands (Salicetum albo-fragilis R.Tx. 1955) | Early | 21 | 53.91 |
| 21-2 | Woodland (Populetum albae Br.-Bl. 1931) | Early | 14 | 48.05 |
| 22-1 | Regenerative woodlands (Salicetum albo-fragilis R.Tx. 1955) | Advanced | 17 | 23.48 |
| 22-2 | Woodland (Populetum albae Br.-Bl. 1931) | Advanced | 10 | 9.09 |
| 23-1 | Grassland (Lolio-Cynosuretum R.Tx. 1937) | Early | 6 | 19.07 |
| 23-2 | Woodland (Populus × canescens association) | Advanced | 8 | 5.56 |
| 24-1 | Regenerative woodland (Quercus robur, Tilia cordata association) | Advanced | 13 | 35.98 |
| 24-2 | Regenerative woodland (Acer platanoides, Robinia pseudoacacia association) | Advanced | 10 | 11.36 |
| Variable | Tested Separately | Forward Selection | ||||
|---|---|---|---|---|---|---|
| Lambda-1 | F | p | Lambda-A | F | p | |
| Pb | 0.18 | 11.868 | 0.0020 | 0.18 | 11.87 | 0.002 |
| Cu | 0.09 | 5.614 | 0.0195 | 0.01 | 1.69 | 0.197 |
| SWC I | 0.06 | 3.152 | 0.0715 | 0.01 | 1.73 | 0.190 |
| pH III | 0.04 | 1.993 | 0.1745 | −0.00 | 0.05 | 0.814 |
| Zn | 0.04 | 1.960 | 0.1560 | 0.02 | 1.48 | 0.242 |
| Org II | 0.02 | 0.967 | 0.2875 | 0.04 | 3.06 | 0.082 |
| pH I | 0.02 | 0.919 | 0.3360 | 0.00 | 0.82 | 0.373 |
| Cd | 0.01 | 0.693 | 0.4100 | 0.10 | 7.04 | 0.011 |
| EC I | 0.01 | 0.519 | 0.4765 | 0.05 | 4.02 | 0.057 |
| EC II | 0.01 | 0.416 | 0.4960 | 0.02 | 1.26 | 0.254 |
| Org III | 0.01 | 0.296 | 0.5765 | 0.09 | 9.20 | 0.004 |
| Cr | 0.00 | 0.246 | 0.6175 | 0.05 | 4.28 | 0.038 |
| SWC III | 0.00 | 0.094 | 0.7500 | 0.01 | 0.80 | 0.366 |
| Ni | 0.00 | 0.069 | 0.7740 | −0.00 | 0.05 | 0.826 |
| SWC II | 0.00 | 0.021 | 0.8855 | 0.01 | 0.77 | 0.401 |
| pH II | 0.00 | 0.015 | 0.9035 | 0.01 | 0.63 | 0.412 |
| EC III | 0.00 | 0.016 | 0.9035 | 0.01 | 0.76 | 0.373 |
| Org I | 0.00 | 0.014 | 0.9110 | −0.00 | 0.00 | 0.945 |
| Variable | Tested Separately | Forward Selection | ||||
|---|---|---|---|---|---|---|
| Lambda-1 | F | p | Lambda-A | F | p | |
| Pb | 0.07 | 4.354 | 0.0445 | 0.07 | 4.35 | 0.045 |
| Cu | 0.06 | 3.423 | 0.0525 | 0.02 | 1.36 | 0.258 |
| Cd | 0.04 | 2.392 | 0.1315 | 0.12 | 7.84 | 0.010 |
| SWC I | 0.04 | 2.249 | 0.1415 | 0.02 | 1.46 | 0.229 |
| Ni | 0.03 | 1.514 | 0.2120 | 0.02 | 1.82 | 0.184 |
| EC II | 0.03 | 1.390 | 0.1995 | 0.10 | 7.49 | 0.014 |
| EC I | 0.02 | 1.327 | 0.2545 | −0.00 | 0.35 | 0.545 |
| Org II | 0.02 | 1.304 | 0.2254 | 0.04 | 2.38 | 0.133 |
| Cr | 0.01 | 0.798 | 0.3790 | 0.02 | 1.17 | 0.296 |
| Org I | 0.01 | 0.603 | 0.4735 | 0.01 | 1.50 | 0.216 |
| Org III | 0.00 | 0.274 | 0.5945 | 0.01 | 1.10 | 0.327 |
| Zn | 0.00 | 0.181 | 0.6710 | 0.03 | 1.85 | 0.190 |
| pH II | 0.00 | 0.108 | 0.7405 | 0.02 | 2.10 | 0.155 |
| SWC II | 0.00 | 0.079 | 0.7760 | 0.01 | 1.16 | 0.290 |
| pH III | 0.00 | 0.077 | 0.7760 | 0.03 | 2.02 | 0.165 |
| EC III | 0.00 | 0.016 | 0.9075 | 0.02 | 1.25 | 0.262 |
| pH I | 0.00 | 0.005 | 0.9455 | 0.01 | 1.08 | 0.299 |
| SWC III | 0.00 | 0.000 | 1.0000 | 0.01 | 1.11 | 0.301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwerk, A.; Wińska-Krysiak, M.; Przybysz, A.; Zaraś-Januszkiewicz, E.; Sikorski, P. Carabid Beetle (Coleoptera: Carabidae) Response to Soil Properties of Urban Wastelands in Warsaw, Poland. Sustainability 2020, 12, 10673. https://doi.org/10.3390/su122410673
Schwerk A, Wińska-Krysiak M, Przybysz A, Zaraś-Januszkiewicz E, Sikorski P. Carabid Beetle (Coleoptera: Carabidae) Response to Soil Properties of Urban Wastelands in Warsaw, Poland. Sustainability. 2020; 12(24):10673. https://doi.org/10.3390/su122410673
Chicago/Turabian StyleSchwerk, Axel, Marzena Wińska-Krysiak, Arkadiusz Przybysz, Ewa Zaraś-Januszkiewicz, and Piotr Sikorski. 2020. "Carabid Beetle (Coleoptera: Carabidae) Response to Soil Properties of Urban Wastelands in Warsaw, Poland" Sustainability 12, no. 24: 10673. https://doi.org/10.3390/su122410673
APA StyleSchwerk, A., Wińska-Krysiak, M., Przybysz, A., Zaraś-Januszkiewicz, E., & Sikorski, P. (2020). Carabid Beetle (Coleoptera: Carabidae) Response to Soil Properties of Urban Wastelands in Warsaw, Poland. Sustainability, 12(24), 10673. https://doi.org/10.3390/su122410673






