Plastic Bottle Cap Recycling—Characterization of Recyclate Composition and Opportunities for Design for Circularity
Abstract
1. Introduction
1.1. Changing Policies and Regulatory Requirements Affecting Plastic Bottles and Their Caps
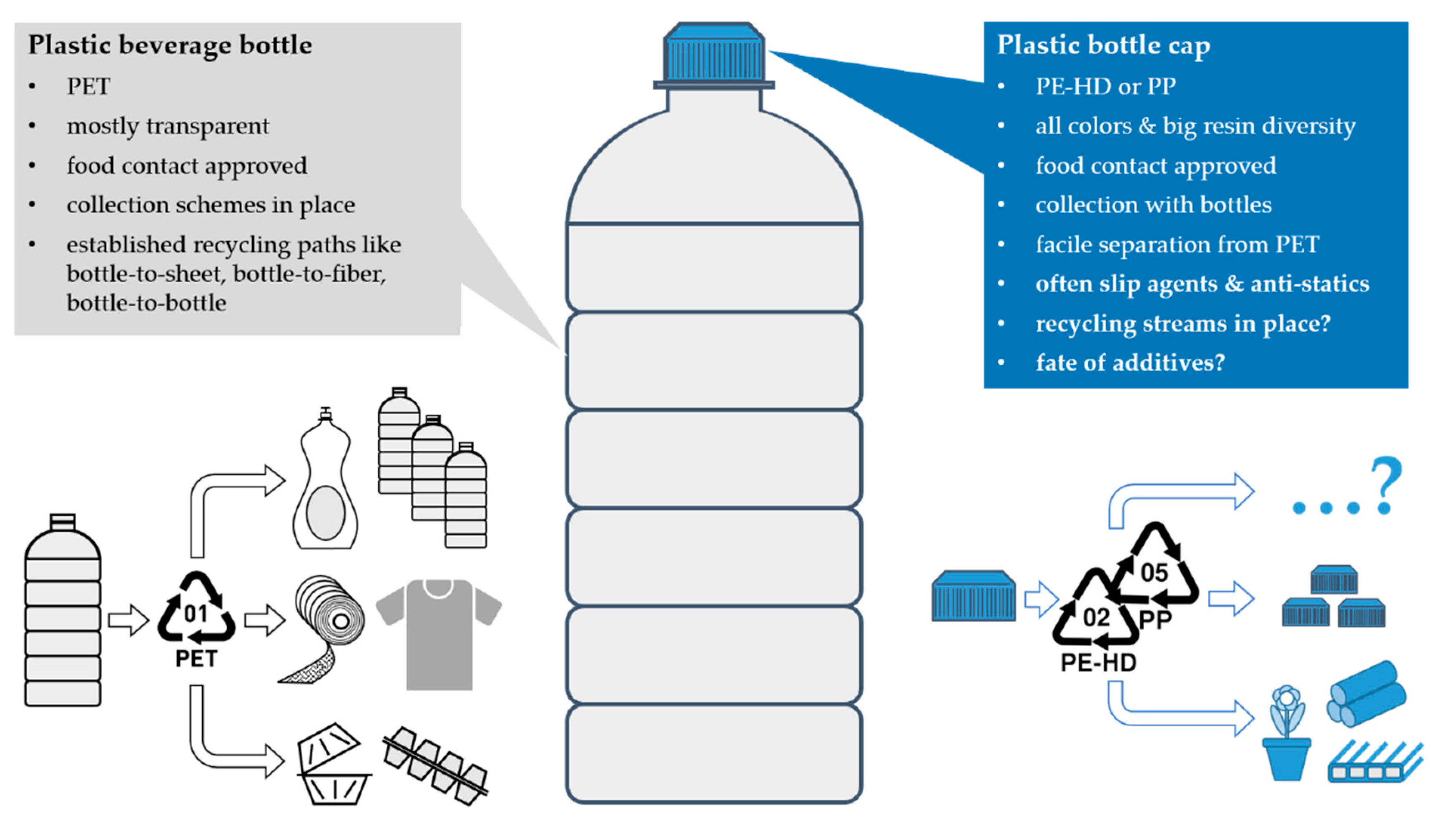
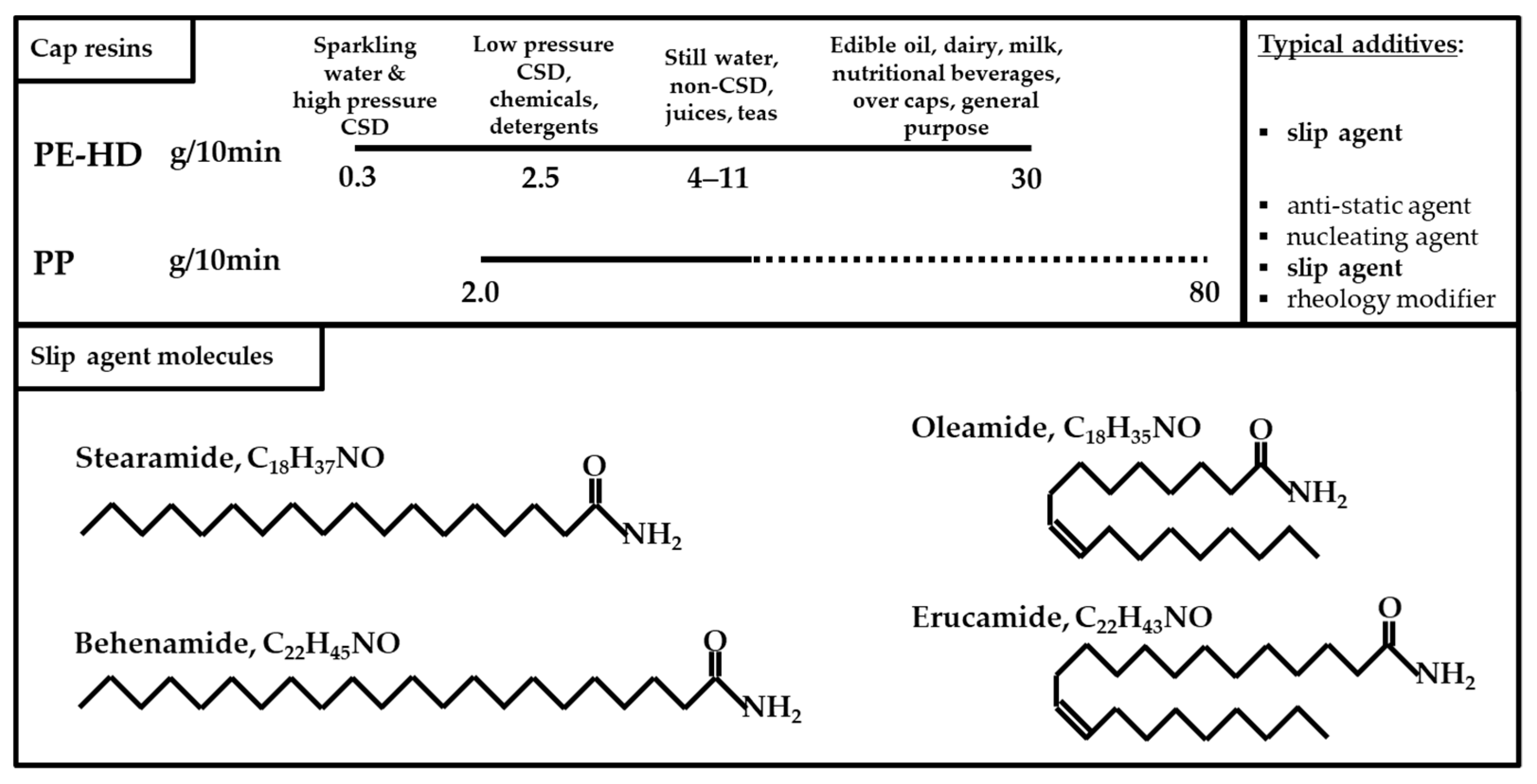
1.2. Characteristics of Plastic Bottle Cap Materials
1.3. Goal and Scope
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods
2.3.1. Infrared Spectroscopy (ATR-FTIR)
2.3.2. High-Performance Liquid Chromatography (HPLC-UV)
2.3.3. Differential Scanning Calorimetry (DSC)
3. Results
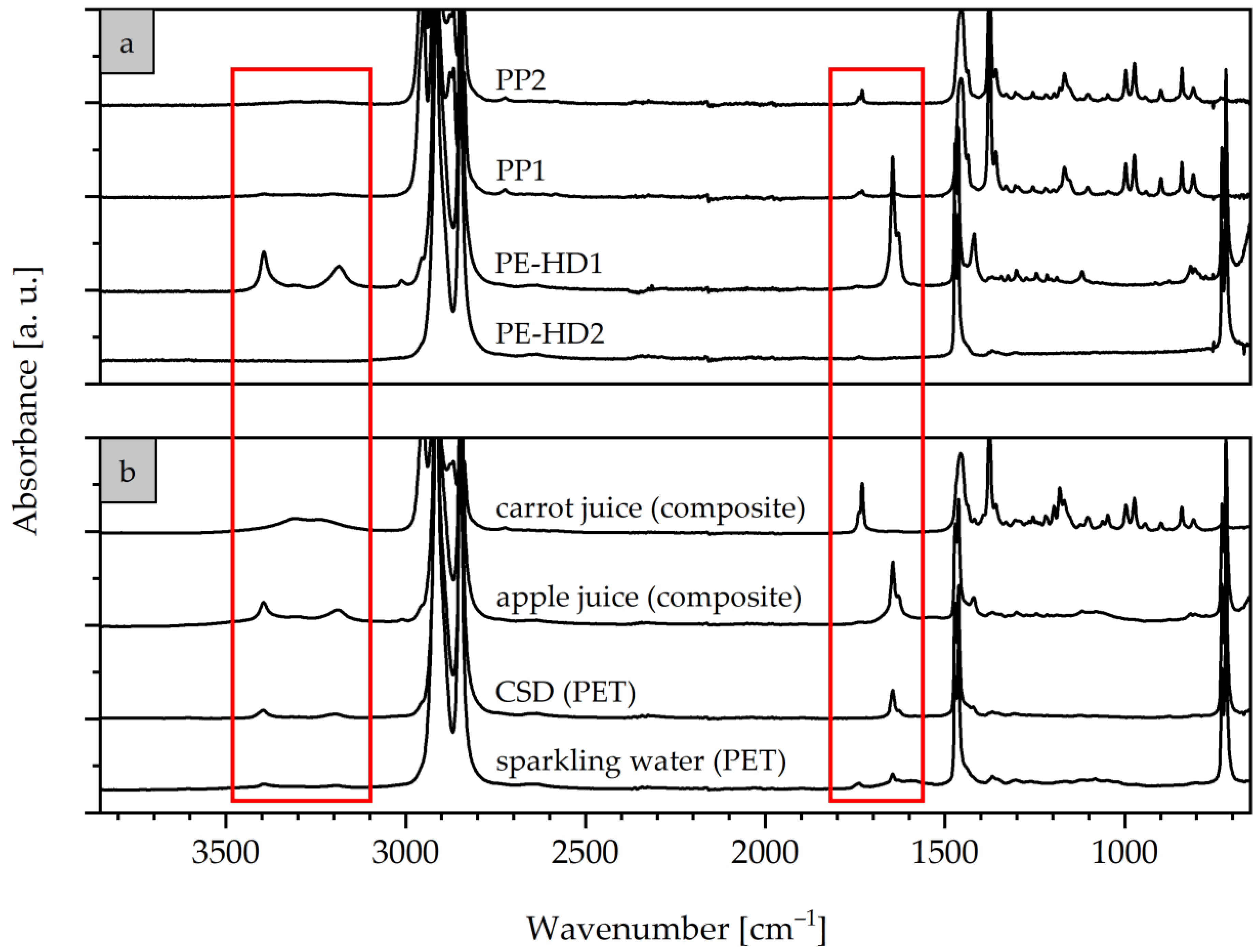
3.1. Spectroscopic Surface Characteristics of Selected Commercial Resins and Caps
3.2. Bottle Cap Recyclates (BCRs)
3.2.1. Polymeric Constituents of Bottle Cap Recyclates (BCRs)
3.2.2. Evidence for Legacy Additives in Plastic Bottle Cap Recyclates
3.2.3. Identification and Quantification of Selected Slip Agents as Examples of Product-Specific Legacy Additives
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eriksen, M.K.; Astrup, T.F. Characterisation of source-separated, rigid plastic waste and evaluation of recycling initiatives: Effects of product design and source-separation system. Waste Manag. 2019, 87, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Simon, B. What are the most significant aspects of supporting the circular economy in the plastic industry? Resour. Conserv. Recycl. 2019, 141, 299–300. [Google Scholar] [CrossRef]
- World Economic Forum; Ellen MacArthur Foundation; McKinsey & Company. The New Plastics Economy. Rethinking the Future of Plastics. 2016. Available online: http://www.ellenmacarthurfoundation.org/publications (accessed on 12 November 2020).
- Kaur, G.; Uisan, K.; Ong, K.L.; Ki Lin, C.S. Recent Trends in Green and Sustainable Chemistry & Waste Valorisation: Rethinking Plastics in a circular economy. Curr. Opin. Green Sustain. Chem. 2018, 9, 30–39. [Google Scholar] [CrossRef]
- van Eygen, E.; Laner, D.; Fellner, J. Circular economy of plastic packaging: Current practice and perspectives in Austria. Waste Manag. 2018, 72, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kuczenski, B.; Geyer, R. Material flow analysis of polyethylene terephthalate in the US, 1996–2007. Resour. Conserv. Recycl. 2010, 54, 1161–1169. [Google Scholar] [CrossRef]
- Ciacci, L.; Passarini, F.; Vassura, I. The European PVC cycle: In-use stock and flows. Resour. Conserv. Recycl. 2017, 123, 108–116. [Google Scholar] [CrossRef]
- Brooks, A.L.; Wang, S.; Jambeck, J.R. The Chinese import ban and its impact on global plastic waste trade. Sci. Adv. 2018, 4, eaat0131. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Lazarevic, D.; Aoustin, E.; Buclet, N.; Brandt, N. Plastic waste management in the context of a European recycling society: Comparing results and uncertainties in a life cycle perspective. Resour. Conserv. Recycl. 2010, 55, 246–259. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics—The Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/de/resources/publications/2154-plastics-facts-2019 (accessed on 10 April 2020).
- PlasticsEurope. The Circular Economy for Plastics. A European Overview. 2019. Available online: https://www.plasticseurope.org/de/resources/publications/2606-circular-economy-plastics-european-overview (accessed on 10 April 2020).
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Laville, S.; Taylor, M. A Million Bottles a Minute: World’s Plastic Binge ‘as Dangerous as Climate Change’. The Guardian [Online], 28 June 2018. Available online: https://www.theguardian.com/environment/2017/jun/28/a-million-a-minute-worlds-plastic-bottle-binge-as-dangerous-as-climate-change (accessed on 21 February 2019).
- Shen, L.; Worrell, E.; Patel, M.K. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recycl. 2010, 55, 34–52. [Google Scholar] [CrossRef]
- Kang, D.H.; Auras, R.; Vorst, K.; Singh, J. An exploratory model for predicting post-consumer recycled PET content in PET sheets. Polym. Test. 2011, 30, 60–68. [Google Scholar] [CrossRef][Green Version]
- Boonstra, M.; van Hest, F. The Plastic Bottle Cap Report. Findings of the First Survey into Plastic Bottle Cap Pollution on Beaches in The Netherlands. 2017. Available online: https://www.noordzee.nl/project/userfiles//SDN_Doppenrapport_EN_2017_DEF_small_2.pdf (accessed on 26 March 2020).
- Future Market Insights. Plastic Caps and Closures Market—Global Industry Analysis, Size and Forecast, 2017 to 2027. Available online: https://www.futuremarketinsights.com/reports/plastic-caps-and-closures-market (accessed on 21 February 2019).
- Plastics News Europe. Healthy Growth Forecast for Plastics Caps and Closures. Available online: https://www.plasticsnewseurope.com/article/20180123/PNE/180129980/healthy-growth-forecast-for-plastics-caps-and-closures (accessed on 21 February 2019).
- A European Strategy for Plastics in a Circular Economy; COM(2018): Brussels, Belgium, 2018.
- Gambino, I.; Bagordo, F.; Coluccia, B.; Grassi, T.; de Filippis, G.; Piscitelli, P.; Galante, B.; de Leo, F. PET-Bottled Water Consumption in View of a Circular Economy: The Case Study of Salento (South Italy). Sustainability 2020, 12, 7988. [Google Scholar] [CrossRef]
- Directive (EU) 2019/904 of the European Parliament and of the Council. On the Reduction of the Impact of Certain Plastic Products on the Environment; Directive (EU) 2019/904 of the European Parliament and of the Council: Brussels, Belgium, 2019. [Google Scholar]
- Haque, M.S. Sustainable use of plastic brick from waste PET plastic bottle as building block in Rohingya refugee camp: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 36163–36183. [Google Scholar] [CrossRef]
- Oyinlola, M.; Whitehead, T.; Abuzeinab, A.; Adefila, A.; Akinola, Y.; Anafi, F.; Farukh, F.; Jegede, O.; Kandan, K.; Kim, B.; et al. Bottle house: A case study of transdisciplinary research for tackling global challenges. Habitat Int. 2018, 79, 18–29. [Google Scholar] [CrossRef]
- Taaffe, J.; O’Sullivan, S.; Rahman, M.E.; Pakrashi, V. Experimental characterisation of Polyethylene Terephthalate (PET) bottle Eco-bricks. Mater. Des. 2014, 60, 50–56. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Panzera, T.H.; Freire, R.T.; Scarpa, F. Sustainable sandwich structures made from bottle caps core and aluminium skins: A statistical approach. Thin-Walled Struct. 2018, 130, 362–371. [Google Scholar] [CrossRef]
- Siodłak, D. Building Molecular Models Using Screw-On Bottle Caps. J. Chem. Educ. 2013, 90, 1247–1249. [Google Scholar] [CrossRef]
- Siodłak, D. Building Large Molecular Models with Plastic Screw-On Bottle Caps and Sturdy Connectors. J. Chem. Educ. 2017, 94, 256–259. [Google Scholar] [CrossRef]
- Unal, N.I.; Mertdinc, S.; Haykiri-Acma, H.; Yaman, S. Comparison of the fuel properties and the combustion behavior of PET bottle caps with lignite. Energy Procedia 2017, 136, 22–26. [Google Scholar] [CrossRef]
- Corvaglia, R.; Brandau, O. Closures for PET Bottles. In Bottles, Preforms and Closures, 2nd ed.; Brandau, O., Ed.; William Andrew: Amsterdam, The Netherlands; Boston, MA, USA, 2012; pp. 115–163. ISBN 9781437735260. [Google Scholar]
- Plastics News. Polyethylene to See More Use during Switch to 1-Piece Caps. Available online: https://www.plasticsnews.com/article/20121005/NEWS/310059974/polyethylene-to-see-more-use-during-switch-to-1-piece-caps (accessed on 3 December 2020).
- Maier, R.-D.; Schiller, M. Handbuch Kunststoff-Additive, 4, Vollständig Neu Bearbeitete Auflage; Hanser: München, Germany, 2016; ISBN 9783446432918. [Google Scholar]
- Plastics Additives Handbook, 6th ed.; Zweifel, H., Maier, R.-D., Schiller, M., Eds.; Hanser: München, Germany, 2009; ISBN 9783446408012. [Google Scholar]
- Dulal, N.; Shanks, R.; Gengenbach, T.; Gill, H.; Chalmers, D.; Adhikari, B.; Pardo Martinez, I. Slip-additive migration, surface morphology, and performance on injection moulded high-density polyethylene closures. J. Colloid Interface Sci. 2017, 505, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Dulal, N.; Shanks, R.; Chalmers, D.; Adhikari, B.; Gill, H. Migration and performance of erucamide slip additive in high-density polyethylene bottle caps. J. Appl. Polym. Sci. 2018, 135, 46822. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food; COMMISSION REGULATION (EU) No 10/2011; Commission Regulation: Brussels, Belgium, 2011.
- Strube, A.; Buettner, A.; Groetzinger, C. Characterization and identification of a plastic-like off-odor in mineral water. Water Supply 2009, 9, 299–309. [Google Scholar] [CrossRef]
- Song, Y.S.; Al-Taher, F.; Sadler, G. Migration of volatile degradation products into ozonated water from plastic packaging materials. Food Addit. Contam. 2003, 20, 985–994. [Google Scholar] [CrossRef]
- Kort, M.; Nijssen, B. Experiences with Off-Flavor Research over the Last Decade. In Flavour Science: Proceedings from XIII Weurman Flavour Research Symposium; Zaragoza in September 2011; Ferreira, V., Lopez, R., Eds.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2014; pp. 459–463. ISBN 9780123985491. [Google Scholar]
- Couturier, M. Erucamide-Free Closure and Liner Composition. WIPO Patent WO2004078833A2, 5 March 2003. Available online: https://patentimages.storage.googleapis.com/ed/c7/71/532b443c0528cd/WO2004078833A2.pdf (accessed on 4 December 2020).
- Hahladakis, J.N.; Iacovidou, E. Closing the loop on plastic packaging materials: What is quality and how does it affect their circularity? Sci. Total Environ. 2018, 630, 1394–1400. [Google Scholar] [CrossRef]
- Shuler, C.A.; Janorkar, A.V.; Hirt, D.E. Fate of erucamide in polyolefin films at elevated temperature. Polym. Eng. Sci. 2004, 44, 2247–2253. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2004; ISBN 9780470011140. [Google Scholar]
- Socrates, G. Infrared and Raman characteristic group frequencies. In Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2010; ISBN 978-0-470-09307-8. [Google Scholar]
- Hummel, D.O. Atlas of Plastics Additives; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-540-42414-7. [Google Scholar]
- Wallner, G.M.; Resch, K.; Teichert, C.; Gahleitner, M.; Binder, W. Effect of Material Structure and Additives on the Optical Properties of PP Cast Films. Monatsh. Chem. 2006, 137, 887–897. [Google Scholar] [CrossRef]
- Schweighuber, A.; Gall, M.; Fischer, J.; Liu, Y.; Braun, H.; Buchberger, W. Development of an LC-MS method for the semiquantitative determination of polyamide 6 contaminations in polyolefin recyclates. Anal. Bioanal. Chem. 2020. [Google Scholar] [CrossRef]
- PubChem. Oleamide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5283387 (accessed on 4 December 2020).
- PubChem. Erucamide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Erucamide (accessed on 4 December 2020).
- PubChem. Docosanamide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/76468 (accessed on 4 December 2020).
- PubChem. Octadecanamide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/31292 (accessed on 4 December 2020).
- Ehrenstein, G.W.; Riedel, G.; Trawiel, P. Thermal analysis of plastics. In Theory and Practice; Hanser: Munich, Germany, 2004; ISBN 978-3-446-22673-9. [Google Scholar]
- Vyncke, G.; Fiorio, R.; Cardon, L.; Ragaert, K. The effect of polyethylene on the properties of talc-filled recycled polypropylene. Plast. Rubber Compos. 2020, 1–8. [Google Scholar] [CrossRef]
- Gall, M.; Lang, R.W.; Fischer, J.; Niehoff, A.; Schmidt, S. Characterization of post-use Polyethylene and Polypropylene Recyclate Blends for Pipe Applications. In Proceedings of the 19th Plastic Pipes Conference PPXIX, Las Vegas, NV, USA, 24–26 September 2018. [Google Scholar]
- Grabmayer, K.; Wallner, G.M.; Beißmann, S.; Braun, U.; Steffen, R.; Nitsche, D.; Röder, B.; Buchberger, W.; Lang, R.W. Accelerated aging of polyethylene materials at high oxygen pressure characterized by photoluminescence spectroscopy and established aging characterization methods. Polym. Degrad. Stab. 2014, 109, 40–49. [Google Scholar] [CrossRef]
- Grabmayer, K.; Wallner, G.M.; Beißmann, S.; Schlothauer, J.; Steffen, R.; Nitsche, D.; Röder, B.; Buchberger, W.; Lang, R.W. Characterization of the aging behavior of polyethylene by photoluminescence spectroscopy. Polym. Degrad. Stab. 2014, 107, 28–36. [Google Scholar] [CrossRef]
- Vilaplana, F.; Karlsson, S. Quality Concepts for the Improved Use of Recycled Polymeric Materials: A Review. Macromol. Mater. Eng. 2008, 293, 274–297. [Google Scholar] [CrossRef]
- Jansson, A.; Möller, K.; Hjertberg, T. Chemical degradation of a polypropylene material exposed to simulated recycling. Polym. Degrad. Stab. 2004, 84, 227–232. [Google Scholar] [CrossRef]
- Wallner, G.M.; Povacz, M.; Hausner, R.; Lang, R.W. Lifetime modeling of polypropylene absorber materials for overheating protected hot water collectors. Sol. Energy 2016, 125, 324–331. [Google Scholar] [CrossRef]
- Resch, K.; Wallner, G.M.; Teichert, C.; Gahleitner, M. Highly transparent polypropylene cast films: Relationships between optical properties, additives, and surface structure. Polym. Eng. Sci. 2007, 47, 1021–1032. [Google Scholar] [CrossRef]
- Brouwer, M.T.; van Thoden Velzen, E.U.; Augustinus, A.; Soethoudt, H.; de Meester, S.; Ragaert, K. Predictive model for the Dutch post-consumer plastic packaging recycling system and implications for the circular economy. Waste Manag. 2018, 71, 62–85. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Christiansen, J.D.; Daugaard, A.E.; Astrup, T.F. Closing the loop for PET, PE and PP waste from households: Influence of material properties and product design for plastic recycling. Waste Manag. 2019, 96, 75–85. [Google Scholar] [CrossRef]
- Gall, M.; Wiener, M.; Chagas de Oliveira, C.; Lang, R.W.; Hansen, E.G. Building a circular plastics economy with informal waste pickers: Recyclate quality, business model, and societal impacts. Resour. Conserv. Recycl. 2020, 156, 104685. [Google Scholar] [CrossRef]
- Jmal, H.; Bahlouli, N.; Wagner-Kocher, C.; Leray, D.; Ruch, F.; Munsch, J.-N.; Nardin, M. Influence of the grade on the variability of the mechanical properties of polypropylene waste. Waste Manag. 2018, 75, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Archodoulaki, V.M. Upcycling of polypropylene-the influence of polyethylene impurities. Polym. Eng. Sci. 2017, 57, 1374–1381. [Google Scholar] [CrossRef]
- Teh, J.W.; Rudin, A.; Keung, J.C. A review of polyethylene–polypropylene blends and their compatibilization. Adv. Polym. Technol. 1994, 13, 1–23. [Google Scholar] [CrossRef]
- Jose, S.; Aprem, A.S.; Francis, B.; Chandy, M.C.; Werner, P.; Alstaedt, V.; Thomas, S. Phase morphology, crystallisation behaviour and mechanical properties of isotactic polypropylene/high density polyethylene blends. Eur. Polym. J. 2004, 40, 2105–2115. [Google Scholar] [CrossRef]
- van Belle, A.; Demets, R.; Mys, N.; van Kets, K.; Dewulf, J.; van Geem, K.; de Meester, S.; Ragaert, K. Microstructural Contributions of Different Polyolefins to the Deformation Mechanisms of Their Binary Blends. Polymers 2020, 12, 1171. [Google Scholar] [CrossRef]
- Lang, R.W.; Bradler, P.R.; Fischer, J.; Poehlmann, D.; Schmidt, S.; Niehoff, A. Quality and performance assessment of in-plant and post-use PE recyclates for pipe applications. In Proceedings of the 18th Plastic Pipes Conference PPXVIII, Berlin, Germany, 12–14 September 2016. [Google Scholar]
- Wagner, S.; Schlummer, M. Legacy additives in a circular economy of plastics: Current dilemma, policy analysis, and emerging countermeasures. Resour. Conserv. Recycl. 2020, 158, 104800. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Identification of non volatile migrant compounds and NIAS in polypropylene films used as food packaging characterized by UPLC-MS/QTOF. Talanta 2018, 188, 750–762. [Google Scholar] [CrossRef]
- Moreta, C.; Tena, M.-T. Determination of plastic additives in packaging by liquid chromatography coupled to high resolution mass spectrometry. J. Chromatogr. A 2015, 1414, 77–87. [Google Scholar] [CrossRef]
- Naumoska, K.; Jug, U.; Metličar, V.; Vovk, I. Oleamide, a Bioactive Compound, Unwittingly Introduced into the Human Body through Some Plastic Food/Beverages and Medicine Containers. Foods 2020, 9, 549. [Google Scholar] [CrossRef]
- Jug, U.; Naumoska, K.; Metličar, V.; Schink, A.; Makuc, D.; Vovk, I.; Plavec, J.; Lucas, K. Interference of oleamide with analytical and bioassay results. Sci. Rep. 2020, 10, 2163. [Google Scholar] [CrossRef]
- Getachew, P.; Getachew, M.; Joo, J.; Choi, Y.S.; Hwang, D.S.; Hong, Y.-K. The slip agents oleamide and erucamide reduce biofouling by marine benthic organisms (diatoms, biofilms and abalones). Toxicol. Environ. Health Sci. 2016, 8, 341–348. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, D.; Wadsworth, L.C. Corona treatment of polyolefin films? A review. Adv. Polym. Technol. 1999, 18, 171–180. [Google Scholar] [CrossRef]
- Dai, L.; Xu, D. Polyethylene surface enhancement by corona and chemical co-treatment. Tetrahedron Lett. 2019, 60, 1005–1010. [Google Scholar] [CrossRef]
- Izdebska, J. Corona Treatment. In Printing on Polymers: Fundamentals and Applications, 1st ed.; Izdebska, J., Thomas, S., Eds.; Elsevier Reference Monographs: Amsterdam, The Netherlands, 2015; pp. 123–142. ISBN 9780323374682. [Google Scholar]
- Peloso, C.W.; O’Connor, M.J.; Bigger, S.W.; Scheirs, J. Characterising the degradation of the polymer slip additive erucamide in the presence of inorganic antiblock agents. Polym. Degrad. Stab. 1998, 62, 285–290. [Google Scholar] [CrossRef]
- Juan, R.; Domínguez, C.; Robledo, N.; Paredes, B.; García-Muñoz, R.A. Incorporation of recycled high-density polyethylene to polyethylene pipe grade resins to increase close-loop recycling and Underpin the circular economy. J. Clean. Prod. 2020, 276, 124081. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Azapagic, A.; Emsley, A.; Hamerton, I. Polymers. In The Environment and Sustainable Development; J. Wiley: West Sussex, UK; Hoboken, NJ, USA, 2003; ISBN 0471877417. [Google Scholar]
- Kerscher, U. Towards a Sustainable Future? The EU Policies Concerning Plastics and Their Didactical Potential for Primary and Secondary Teaching. Discourse Commun. Sustain. Educ. 2019, 10, 47–62. [Google Scholar] [CrossRef]
- Mülhaupt, R. Green Polymer Chemistry and Bio-based Plastics: Dreams and Reality. Macromolecular Chemistry and Physics. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef]
- Lang, R.W. Kunststoffe—Schlüsselwerkstoffe für Eine Nachhaltige Entwicklung. Available online: https://www.kunststoff-cluster.at/partnerunternehmen/unser-nachrichtenportal-ihre-medienpraesenz/detail/news/kunststoffe-schluesselwerkstoffe-fuer-eine-nachhal/ (accessed on 29 March 2019).

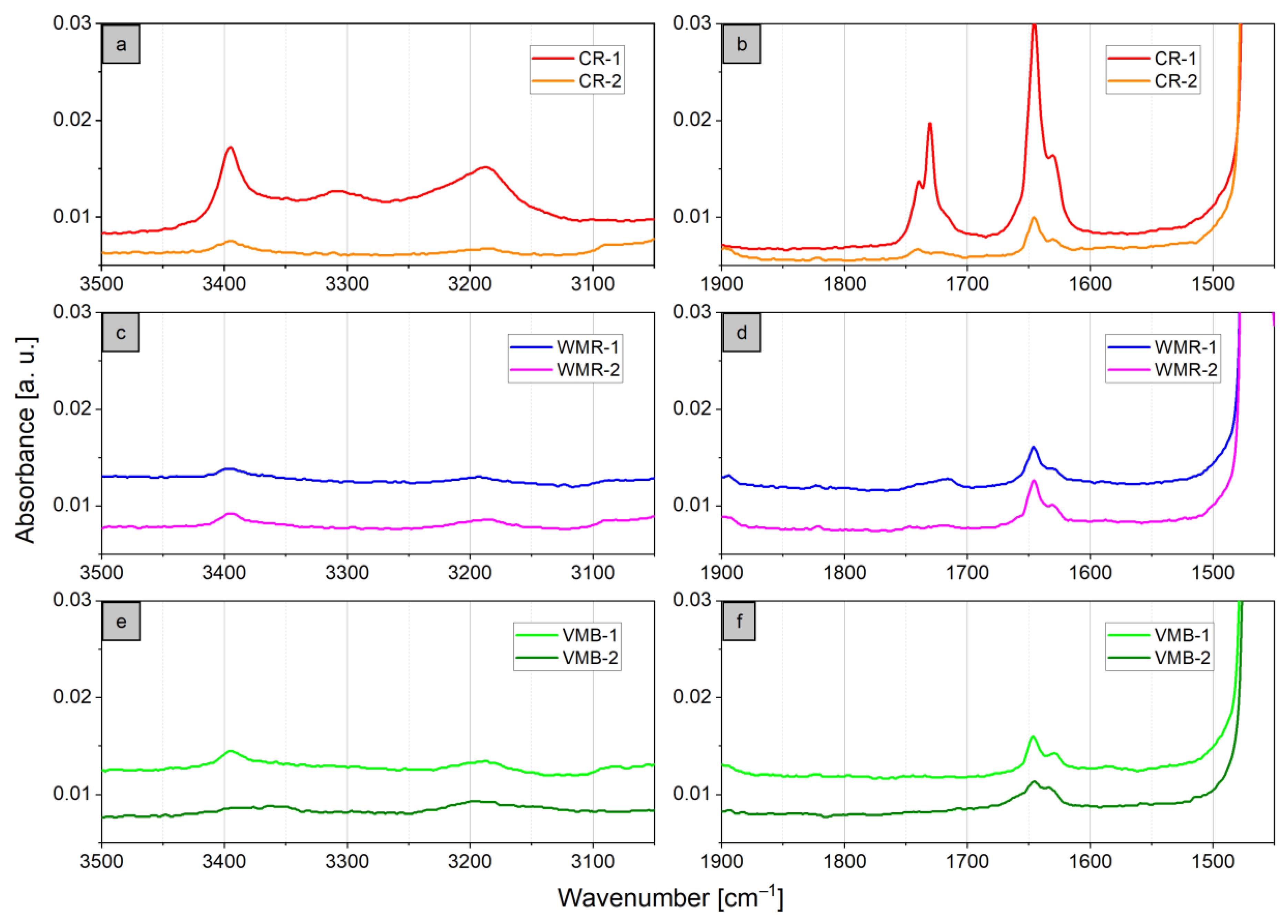
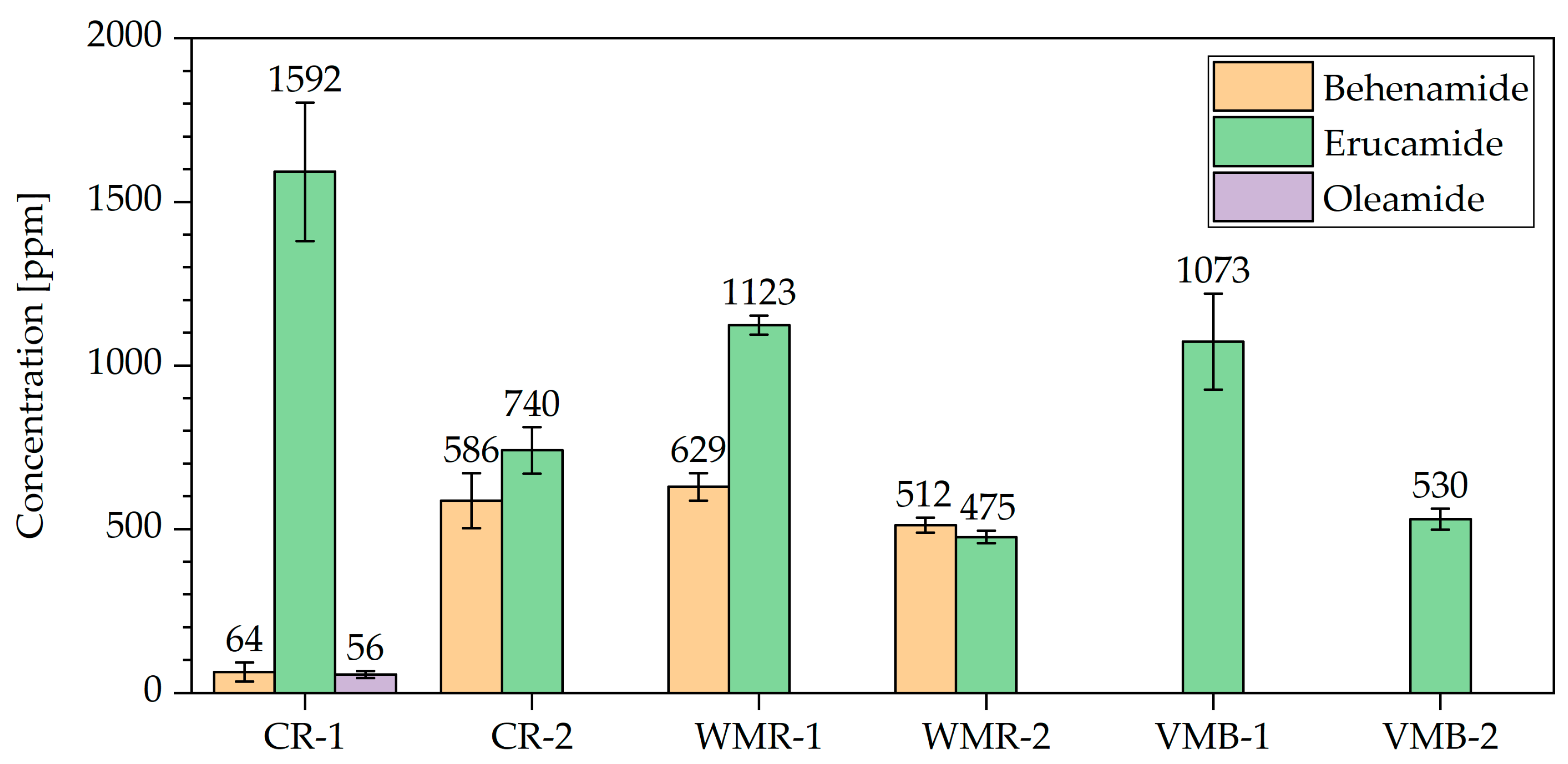
| Sample Acronym | Sample Type | Origin | Composition | Remarks |
|---|---|---|---|---|
| CR-1 | commercial recyclate | post-consumer PET bottles | mainly post-consumer polyolefin screw caps | example of a commercially available regranulate |
| CR-2 | commercial regrind | post-consumer PET bottles | post-consumer polyolefin screw caps | example of commercially available regrind |
| WMR-1 | waste model recyclate | post-consumer PET bottles | mix of shredded screw caps and bottle labels | illustrates the impact of labels of PET bottles on recyclate characteristics |
| WMR-2 | waste model recyclate | PET bottles, PE-HD bottles, composite beverage packaging | self-collected caps of used PET bottles, PE-HD bottles, and composite beverage packaging | illustrates the impact of caps of diverse packaging articles on recyclate characteristics |
| VMB-1 | virgin model blend | virgin polymer resin from | 50% PE-HD1 45% PE-HD2 2.5% PP1 2.5% PP2 | simulates a recyclate with slip agent and low-level PP contamination |
| VMB-2 | virgin model blend | virgin polymer resin from | 25% PE-HD1 25% PE-HD2 25% PP1 25% PP2 | simulates a recyclate with slip agent, anti-statics, and high PP fraction |
| Sample | TC [°C] 1 | ∆HC [J/g] 2 | TM [°C] 3 | ∆HM [J/g] 4 |
|---|---|---|---|---|
| CR-1 | 117.2, 125.2 | 72.4, 25.0 | 131.4, 162.8 | 92.7, 25.8 |
| CR-2 | 116.3 | 175.4, 0 | 131.4, 159.1 | 196.7, 1.9 |
| WMR-1 | 116.5, 123.8 | 118.6, 7.6 | 131.6, 161.3 | 153.1, 12.7 |
| WMR-2 | 116.3, 125.5 | 176.4, 1.1 | 131.3, 162.1 | 186.4, 2.6 |
| VMB-1 | 115.6, 121.4 | 170.5, 0.7 | 130.7, 157.0 | 192.1, 2.5 |
| VMB-2 | 115.9, 122.7 | 77.0, 31.2 | 131.0, 158.1 | 95.2, 25.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gall, M.; Schweighuber, A.; Buchberger, W.; W. Lang, R. Plastic Bottle Cap Recycling—Characterization of Recyclate Composition and Opportunities for Design for Circularity. Sustainability 2020, 12, 10378. https://doi.org/10.3390/su122410378
Gall M, Schweighuber A, Buchberger W, W. Lang R. Plastic Bottle Cap Recycling—Characterization of Recyclate Composition and Opportunities for Design for Circularity. Sustainability. 2020; 12(24):10378. https://doi.org/10.3390/su122410378
Chicago/Turabian StyleGall, Markus, Andrea Schweighuber, Wolfgang Buchberger, and Reinhold W. Lang. 2020. "Plastic Bottle Cap Recycling—Characterization of Recyclate Composition and Opportunities for Design for Circularity" Sustainability 12, no. 24: 10378. https://doi.org/10.3390/su122410378
APA StyleGall, M., Schweighuber, A., Buchberger, W., & W. Lang, R. (2020). Plastic Bottle Cap Recycling—Characterization of Recyclate Composition and Opportunities for Design for Circularity. Sustainability, 12(24), 10378. https://doi.org/10.3390/su122410378





