Effects of Land Use Change from Natural Forest to Livestock on Soil C, N and P Dynamics along a Rainfall Gradient in Mexico
Abstract
1. Introduction
2. Material and Methods
2.1. Study Areas
2.2. Soil Sampling and Analysis
2.3. Statistical Analyses
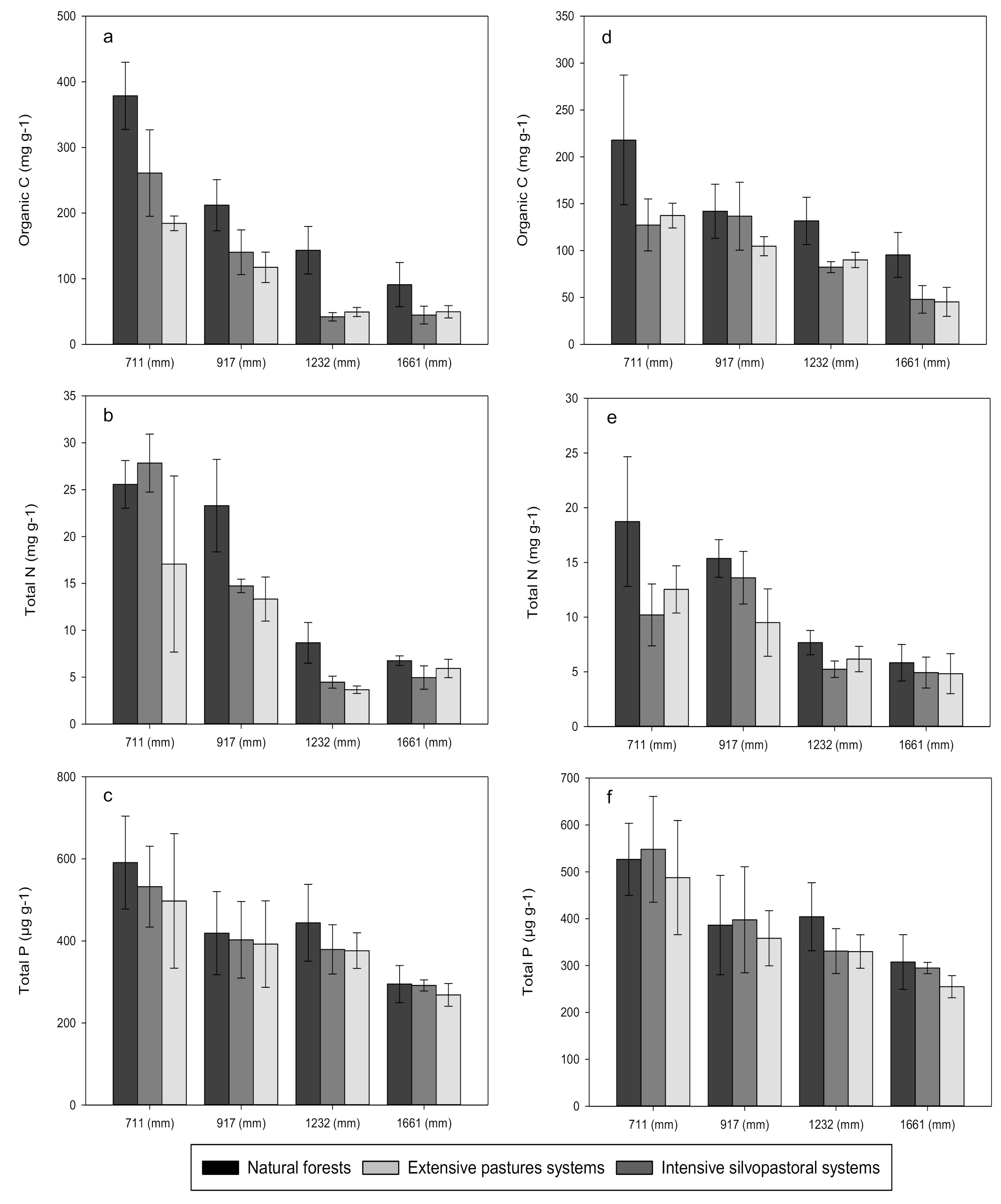
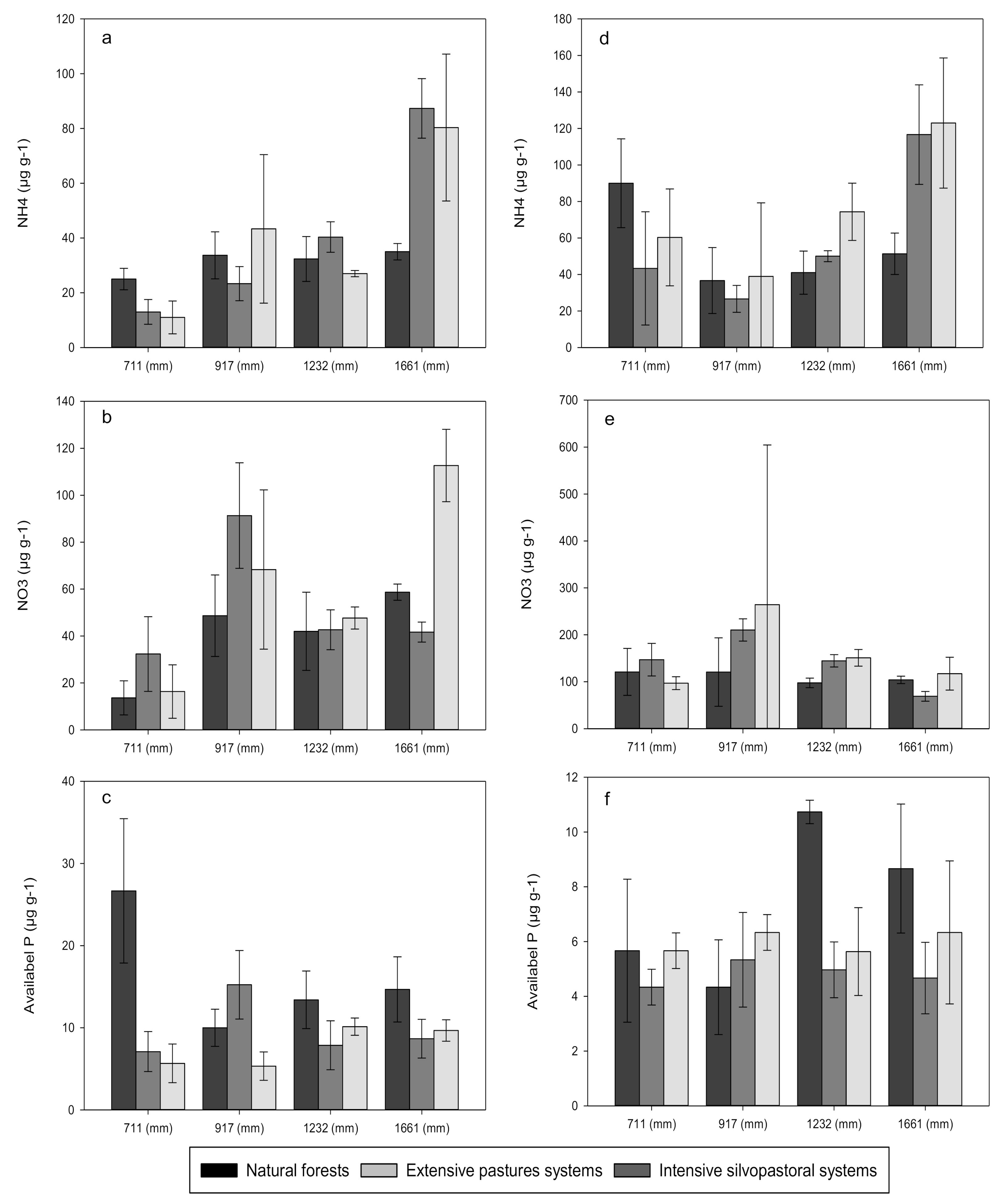
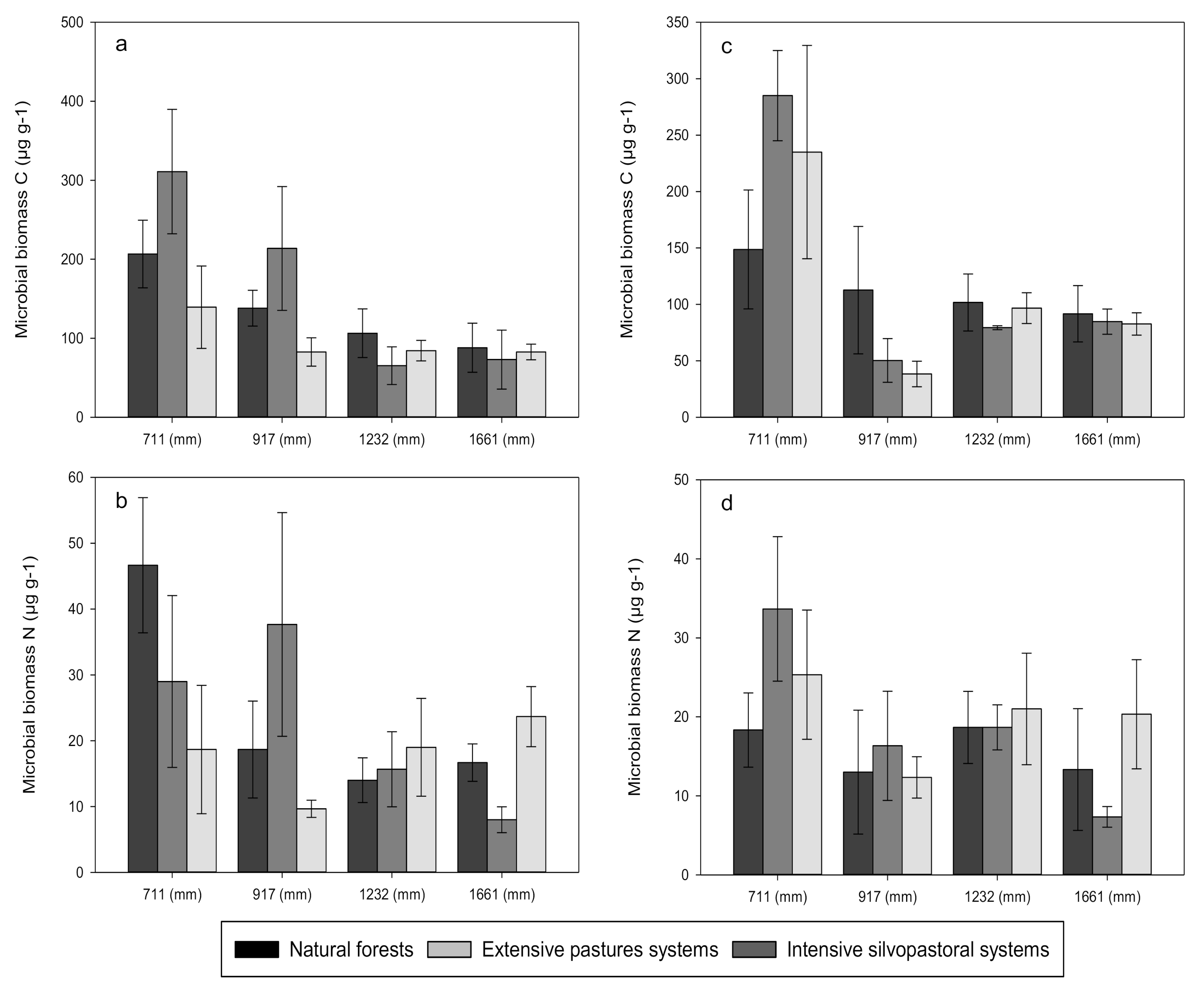
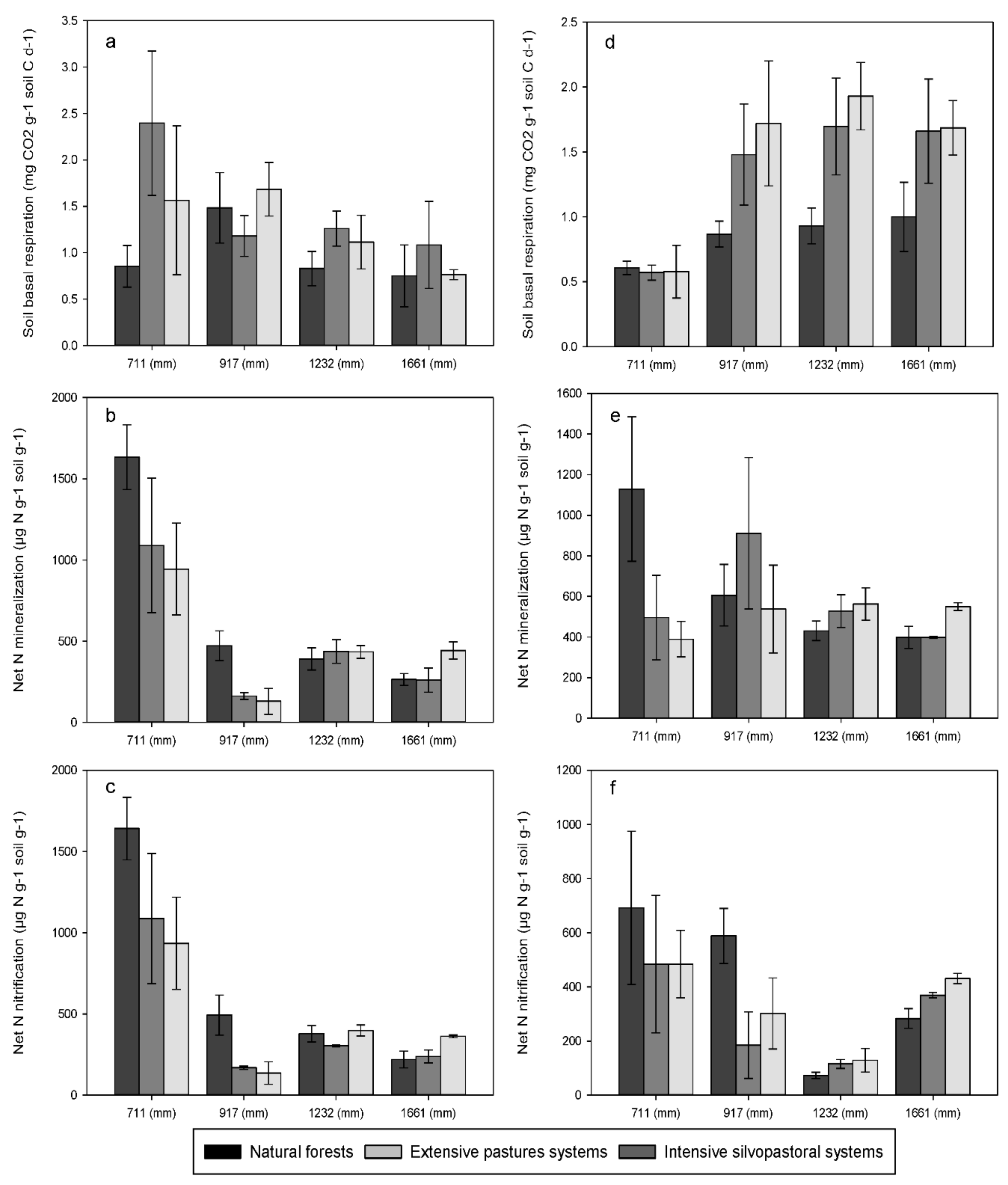
3. Results
3.1. Carbon, Nitrogen and Phosphorus Concentrations
3.2. Soil Basal Respiration and Net Nitrogen Transformations
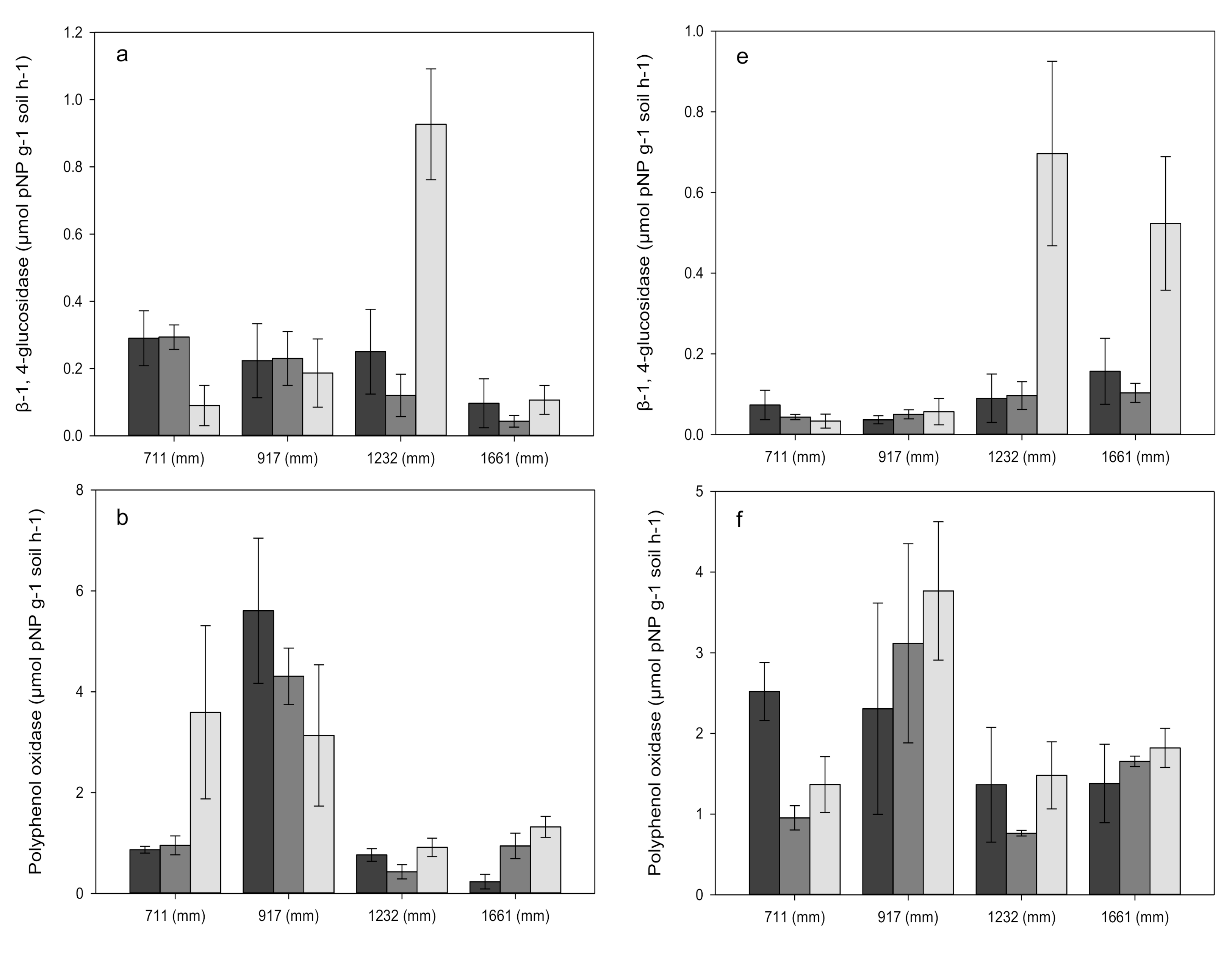
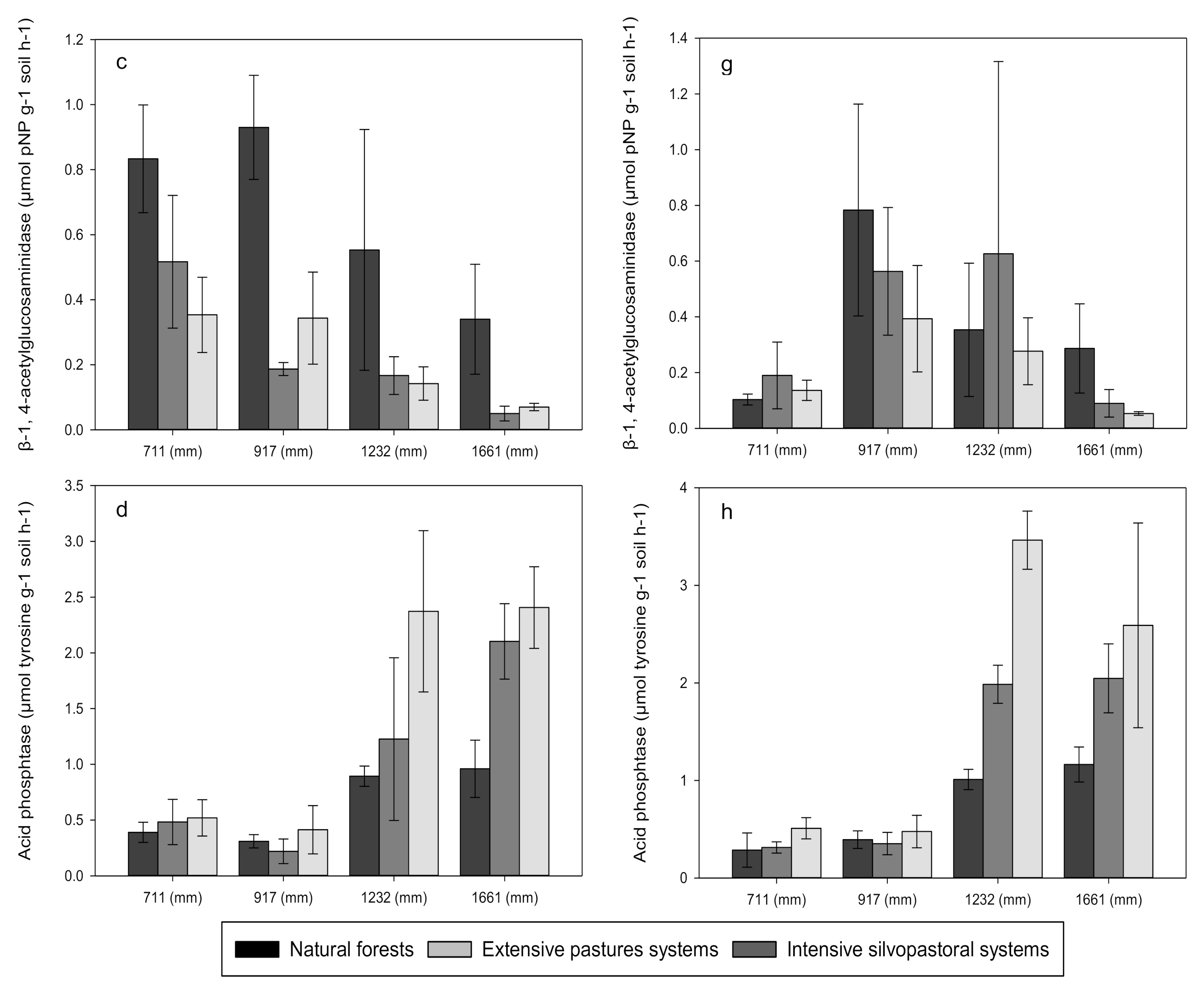
3.3. Enzyme Activities
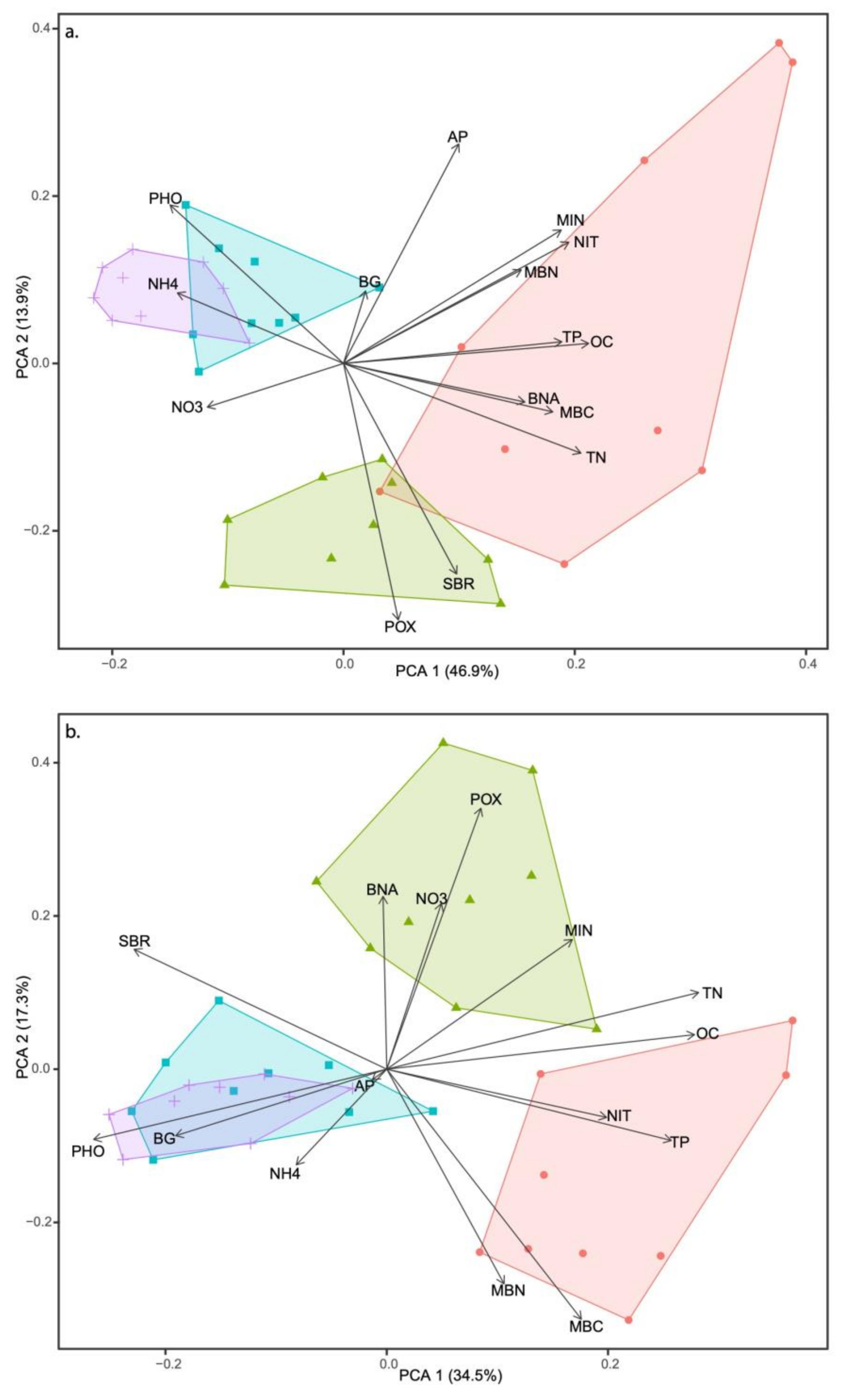
3.4. Multivariate Analysis and Soil Metric Relationships
4. Discussion
4.1. Effects of Forest Conversion on Soil Carbon, Nitrogen and Phosphorus Dynamics
4.2. Effects of Rainfall Regime on Soil Carbon, Nitrogen and Phosphorus Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cias, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 465–570. [Google Scholar]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2015, 5, 81–91. [Google Scholar] [CrossRef]
- Quéré, C.L.; Andrew, R.J.; Canadell, J.G.; Sitch, S.; Korsbakken, J.I.; Peters, G.P.; Manning, A.C.; Boden, T.A.; Tans, P.P.; Houghton, R.A.; et al. Global carbon budget 2016. Earth Syst. Sci. Data 2016, 8, 605–649. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the World’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Carvalhais, N.; Forkel, M.; Khomik, M.; Bellarby, J.; Jung, M.; Migliavacca, M.; Μu, M.; Saatchi, S.; Santoro, M.; Thurner, M.; et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014, 514, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; Houghton, R.A.; Marland, G.; Hackler, J.; Boden, T.A.; Conway, T.J.; Canadell, J.G.; Raupach, M.R.; Ciais, P.; Le Quéré, C. Update on CO2 emissions. Nat. Geosci. 2010, 3, 811–812. [Google Scholar] [CrossRef]
- Houghton, R.A.; Nassikas, A.A. Global and regional fluxes of carbon from land use and land cover change 1850–2015. Glob. Biogeochem. Cycles 2017, 31, 1–17. [Google Scholar] [CrossRef]
- Veldkamp, E.; Schmidt, M.; Powers, J.S.; Corre, M.D. Deforestation and reforestation impacts on soils in the tropics. Nat. Rev. Earth Environ. 2020. [Google Scholar] [CrossRef]
- Graesser, J.; Aide, T.M.; Grau, H.R.; Ramankutty, N. Cropland/pastureland dynamics and the slowdown of deforestation in Latin America. Environ. Res. Lett. 2015, 10, 034017. [Google Scholar] [CrossRef]
- DeSy, V.; Herold, M.; Achard, F.; Avitabile, V.; Baccini, A.; Carter, S.; Clevers, J.G.; Lindquist, E.; Pereira, M.; Verchot, L. Tropical deforestation drivers and associated carbon emission factors derived from remote sensing data. Environ. Res. Lett. 2019, 14, 094022. [Google Scholar] [CrossRef]
- Chadwick, R.; Good, P.; Martin, G.; Rowell, D.P. Large rainfall changes consistently projected over substantial areas of tropical land. Nat. Clim. Chang. 2016, 6, 177–181. [Google Scholar] [CrossRef]
- Townsend, A.R.; Cleveland, C.C.; Houlton, B.Z.; Leck, C.; White, J.W. Multi-element regulation of the tropical forest carbon cycle. Front. Ecol. Environ. 2011, 9, 9–17. [Google Scholar] [CrossRef]
- Townsend, A.R.; Asner, G.P.; Cleveland, C.C. The biogeochemical heterogeneity of tropical forests. Trends Ecol. Evol. 2008, 23, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, J.B.; Sanford, R.L., Jr.; Cummings, D.L.; Salcedo, I.; Sampaio, E.V.S.B. Biomass and nutrient dynamics associated with slash fires in Neotropical dry forests. Ecology 1993, 74, 140–151. [Google Scholar] [CrossRef]
- Allen, K.; Dupuy, J.M.; Gei, M.G.; Hulshof, C.; Medvigy, D.; Pizano, C.; Salgado-Negret, B.; Smith, C.M.; Trierweiler, A.; van Bloem, S.J. Will seasonally dry tropical forests be sensitive or resistant to future changes in rainfall regimes? Environ. Res. Lett. 2017, 12, 094002. [Google Scholar] [CrossRef]
- Singh, J.S.; Chaturvedi, R.K. Tropical Dry Deciduous Forest: Research Trends and Emerging Features; Springer: New York, NY, USA, 2017. [Google Scholar]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Asner, G.P.; Archer, S.R. Livestock and the Global Carbon Cycle. In Livestock in a Changing Landscape: Drivers, Consequences and Responses; Steinfeld, H., Mooney, H.A., Schneider, F., Neville, L.E., Eds.; Island Press: Washington, DC, USA, 2010; pp. 69–82. [Google Scholar]
- Galloway, J.; Dentener, F.; Burke, M.; Dumont, E.; Bouwman, A.F.; Kohn, R.A.; Mooney, H.A.; Seitzinger, S.; Kroeze, C. The Impact of Animal Production Systems on the Nitrogen Cycle. In Livestock in a Changing Landscape: Drivers, Consequences and Responses; Steinfeld, H., Mooney, H.A., Schneider, F., Neville, L.E., Eds.; Island Press: Washington, DC, USA, 2010; pp. 83–95. [Google Scholar]
- Bhandari, K.B.; West, C.P.; Acosta-Martínez, V.; Cotton, J.; Cano, A. Soil health indicators as affected by diverse forage species and mixtures in semi-arid pastures. Appl. Soil Ecol. 2018, 132, 179–186. [Google Scholar] [CrossRef]
- Campo, J.; García-Oliva, F.; Navarrete-Segueda, A.; Siebe, C. Stocks and dynamics of organic carbon in tropical forest ecosystems of Mexico. Terra Latinoamericana 2016, 34, 31–38. [Google Scholar]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Change Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Mendoza-Ponce, A.; Corona-Núñez, R.; Kraxner, F.; LeDuc, S.; Patrizio, P. Identifying effects of land use cover changes and climate change on terrestrial ecosystems and carbon stocks in Mexico. Glob. Environ. Change 2018, 56, 12–23. [Google Scholar] [CrossRef]
- Galeana-Pizaña, J.M.; Couturier, S.; Monsivais-Huertero, A. Assessing food security and environmental protection in Mexico with a GIS-based Food Environmental Efficiency index. Land Use Pol. 2018, 76, 442–454. [Google Scholar] [CrossRef]
- Rivera Huerta, A.; Güereca, L.P.; Rubio Lozano, M.S. Environmental impact of beef production in Mexico through life cycle assessment. Resour. Conserv. Recycl. 2016, 109, 44–53. [Google Scholar] [CrossRef]
- Rivera Huerta, A.; Rubio Lozano, M.S.; Padilla-Rivera, A.; Güereca, L.P. Social sustainability assessment in livestock production: A social life cycle assessment approach. Sustainability 2019, 11, 4419. [Google Scholar] [CrossRef]
- Tello, J.; Garcillán, P.P.; Ezcurra, E. How dietary transition changed land use in Mexico. Ambio 2020. [Google Scholar] [CrossRef] [PubMed]
- Dirzo, R.; García, M.C. Rates of deforestation in Los Tuxtlas, a neotropical area in Southeast Mexico. Conserv. Biol. 1992, 6, 84–90. [Google Scholar] [CrossRef]
- Bray, D.B.; Klepeis, P. Deforestation, forest transitions, and institutions for sustainability in Southeastern Mexico, 1900–2000. Environ. Hist. 2005, 11, 195–223. [Google Scholar] [CrossRef]
- Balvanera, P.; Cotler, H.; Oropeza, O.A.; Aguilar Contreras, A.; Aguilera Peña, M.; Aluja, M.; Andrade Cetto, A.; Arroyo Quiroz, I.; Ashworth, L.; Astier, M.; et al. State and Trends of Ecosystem Services. In Natural Capital of Mexico, vol II.; Sarukhán, J., Dirzo, R., González, R., March, I.J., Eds.; CONABIO: Mexico City, Mexico, 2009; pp. 185–245. (In Spanish) [Google Scholar]
- Reid, R.S.; Bedelian, C.; Said, M.Y.; Kruska, R.L.; Mauricio, R.M.; Castel, V.; Olson, J.; Thornton, P.K. Global Livestock Impacts on Biodiversity. In Livestock in a Changing Landscape: Drivers, Consequences and Responses; Steinfeld, H., Mooney, H.A., Schneider, F., Neville, L.E., Eds.; Island Press: Washington, DC, USA, 2010; pp. 111–137. [Google Scholar]
- Broom, D.M.; Galindo, F.A.; Murgueitio, E. Sustainable, efficient livestock production with high biodiversity and good welfare for animals. Proc. R. Soc. Biol. Sci. 2013, 280, 2013–2025. [Google Scholar] [CrossRef]
- Nahed-Toral, J.; Valdivieso-Pérez, A.; Aguilar-Jiménez, R.; Cámara-Cordova, J.; Grande-Cano, D. Silvopastoral systems with traditional management in southeastern Mexico: A prototype of livestock agroforestry for cleaner production. J. Clean. Prod. 2013, 57, 266–279. [Google Scholar] [CrossRef]
- Williams, D.R.; Alvarado, F.; Green, R.E.; Manica, A.; Phalan, B.; Balmford, A. Land-use strategies to balance livestockproduction, biodiversity conservation and carbon storage in Yucatan, Mexico. Glob. Chang. Biol. 2017, 23, 5260–5272. [Google Scholar] [CrossRef]
- Havlik, P.; Valin, H.; Herrero, M.; Obersteiner, M.; Schmid, E.; Rufino, M.C.; Mosnier, A.; Thornton, P.K.; Böttcher, H.; Conant, R.T.; et al. Climate change mitigation through livestock system transitions. Proc. Nat. Acad. Sci. USA 2014, 111, 3709–3714. [Google Scholar] [CrossRef]
- Murgueitio, E.; Chará, J.; Barahona, R.; Cuartas, C.; Naranjo, J. Los sistemas silvopastoriles intensivos (SSPi), herramienta de mitigación y adaptación al cambio climático. Trop. Subtrop. Agroecosys. 2014, 17, 501–507. [Google Scholar]
- Murgueitio, E.; Calle, Z.; Uribe, F.; Calle, A.; Solorio, B. Native trees and shrubs for the productive rehabilitation of tropical cattle ranching lands. For. Ecol. Manag. 2011, 261, 1654–1663. [Google Scholar] [CrossRef]
- Holdridge, L.R.; Grenke, W.C.; Hatheway, W.H.; Liang, T.; Tosi, J.A. Forest Environment in Tropical Life Zones: A Pilot Study; Pergamon Press: New York, NY, USA, 1971. [Google Scholar]
- García, E. Modificación al Sistema de Clasificación Climática de Koeppen; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2004. [Google Scholar]
- Middleton, N.; Thomas, D. World Atlas of Desertification; Edward Arnold: London, UK, 1997. [Google Scholar]
- Mooney, H.A.; Bullock, S.H.; Medina, E. Introduction. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 1–8. [Google Scholar]
- Flores-Guido, J.S.; Durán, R.; Ortíz, J.J. Comunidades Vegetales Terrestres. In Biodiversidad y Desarrollo Humano en Yucatán; Durán, R., Méndez, M., Eds.; CICY, PPD-FMAM, CONABIO, SEDUMA: Merida, Mexico, 2010; pp. 125–129. [Google Scholar]
- Gómez-Pompa, A.; Krömer, T.; Castro-Cortéz, R. Atlas de la Flora de Veracruz. Un Patrimonio Natural en Peligro; Gobierno del Estado de Veracruz: Veracruz, Mexico, 2010. [Google Scholar]
- Burgos-Hernández, M.; Castillo-Campos, G.; Vergara-Tenorio, M. Potentially useful flora from the tropical rainforest in central Veracruz, Mexico: Considerations for their conservation. Acta Botánica Mexicana 2014, 109, 55–77. [Google Scholar] [CrossRef]
- INEGI. Actualización del Marco Censal Agropecuario. 2016. Available online: http://www.beta.inegi.org.mx/proyectos/agro/amca/ (accessed on 24 February 2020).
- Porder, S.; Johnson, A.H.; Xing, H.X.; Brocard, G.; Goldsmith, S.; Pett-Ridge, J. Linking geomorphology, weathering and cation availability in the Luquillo Mountains of Puerto Rico. Geoderma 2015, 249, 100–110. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-size Analysis. In Methods of Soil Analysis. Part 1; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Jenkinson, D.S. The Determination of Microbial Biomass Carbon and Nitrogen in Soil. In Advances in Nitrogen Cycling in Agricultural Ecosystems; Wilson, J.R., Ed.; CAB International: Wallingford, UK, 1988; pp. 368–386. [Google Scholar]
- Anderson, J.P.E. Soil Respiration. In Methods of Soil Analysis: Chemical and Microbiological Properties, Part II; Page, A.L., Miller, R.M., Kenny, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 831–871. [Google Scholar]
- Robertson, G.; Wedin, D.; Groffman, P.; Blair, J.; Holland, E.; Nadelhoffer, K.; Harris, D.F. Soil Carbon and Nitrogen Availability: Nitrogen Mineralization, Nitrification, and Soil Respiration Potentials. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Coleman, D.C., Bledsoe, C.S., Sollins, P., Eds.; Oxford University Press: New York, NY, USA, 1999; pp. 258–271. [Google Scholar]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 468, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Tyler, H.L.; Millar, J.J. Determination of microbial extracellular enzyme activity in waters, soils and sediments using high throughput microplate assays. J. Vis. Exp. 2013, 80, e50399. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 28 July 2020).
- Guo, L.B.; Gifford, M. Soil carbon stocks and land use change: A meta-analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Yang, Y.S.; Guo, J.; Chen, G.; Xie, J.; Gao, R.; Li, Z.; Jin, Z. Carbon and nitrogen pools in Chinese fir and evergreen broadleaved forests and changes associated with felling and burning in mid-subtropical China. Forest Ecol. Manag. 2005, 216, 216–226. [Google Scholar] [CrossRef]
- Wang, Y.P.; Houlton, B.Z. Nitrogen constraints on terrestrial carbon uptake: Implications for the global carbon-climate feedback. Geophys. Res. Lett. 2009, 36, L24403. [Google Scholar] [CrossRef]
- Campo, J.; Merino, A. Variations in soil carbon sequestration and their determinants along a precipitation gradient in seasonally dry tropical forest ecosystems. Glob. Chang. Biol. 2016, 22, 1942–1956. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, R.M.; Hidalgo, C.; Payán, F.; Etchevers, J.D.; Campo, J. Precipitation influences on active fractions of soil organic matter in seasonally dry tropical forests of the Yucatan: Regional and seasonal patterns. Eur. J. For. Res. 2013, 132, 667–677. [Google Scholar] [CrossRef]
- Bejarano, M.; Crosby, M.M.; Parra, V.; Etchevers, J.D.; Campo, J. Precipitation regime and nitrogen addition effects on leaf litter decomposition in tropical dry forests. Biotropica 2014, 46, 415–424. [Google Scholar] [CrossRef]
- Hertel, D.; Harteveld, M.A.; Leuschner, C. Conversion of a tropical forest into agroforest alters the fine root-related carbon flux to the soil. Soil Biol. Biochem. 2009, 41, 481–490. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Ladd, J.N. Microbial Biomass in Soil: Measurement and Turnover. In Soil Biochemistry; Paul, E.A., Ladd, J.N., Eds.; Marcel Dekker: New York, NY, USA, 1981; pp. 415–471. [Google Scholar]
- Falloon, P.D.; Smith, P. Modelling refractory soil organic matter. Biol. Fertil. Soils 2000, 30, 388–398. [Google Scholar]
- Loveland, P.; Webb, J. Is there a critical level of organic matter in the agricultural soils of temperate regions: A review. Soil Tillage Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Fontaine, S.; Bardoux, G.; Abbadie, L.; Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 2004, 7, 314–320. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Moscatelli, M.C.; De Angelis, P.; Grego, S. Labile substrates quality as the main driving force of microbial mineralization activity in a poplar plantation soil under elevated CO2 and nitrogen fertilization. Sci. Total Environ. 2006, 372, 256–265. [Google Scholar] [CrossRef]
- Angers, D.A.; Eriksen-Hamel, N.S. Full-inversion tillage and organic carbon distribution in soil profiles: A meta-analysis. Soil Sci. Soc. Am. J. 2008, 72, 1370–1374. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Jat, M.L.; Gerard, B.G.; Palm, C.A.; Sanchez, P.A.; Cassman, K.G. Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 2014, 4, 678–683. [Google Scholar] [CrossRef]
- Ludwig, M.; Achtenhagen, J.; Miltner, A.; Eckhardt, K.-U.; Leinweber, P.; Emmerling, C.; Thiele-Bruhn, S. Microbial contribution to SOM quantity and quality in density fractions of temperate arable soils. Soil Biol. Biochem. 2015, 81, 311–322. [Google Scholar] [CrossRef]
- Bloor, J.M.G. Additive effect of dung amendment and plant species identity on soil processes and soil inorganic nitrogen in grass monocultures. Plant Soil 2015, 396, 189–200. [Google Scholar] [CrossRef]
- West, T.O.; Post, W.M. Soil organic carbon sequestration rates by tillage and crop rotation: A global data analysis. Soil Sci. Soc. Am. J. 2002, 66, 1930–1946. [Google Scholar] [CrossRef]
- Campo, J.; Vázquez-Yanes, C. Effects of nutrient limitation on aboveground carbon dynamics during tropical dry forest regeneration in Yucatán, Mexico. Ecosystems 2004, 7, 311–319. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Belnap, J.; Findlay, S.G.; Shah, J.J.F.; Hill, B.H.; Kuehn, K.A.; Kuske, C.R.; Litvak, M.E.; Martinez, N.G.; Moorhead, D.L.; et al. Extracellular enzyme kinetics scale with resource availability. Biogeochemistry 2014, 121, 287–304. [Google Scholar] [CrossRef]
- Vitousek, P. Nutrient Cycling and Limitation: Hawai’i as a Model. System; Princeton University Press: Princeton, NJ, USA, 2004. [Google Scholar]
- Ochoa-Hueso, R.; Arca, V.; Delgado-Baquerizo, M.; Hamonts, K.; Piñeiro, J.; Serrano-Grijalva, L.; Shawyer, J.; Power, S.A. Links between soil microbial communities, functioning, and plant nutrition under altered rainfall in Australian grassland. Ecol. Monogr. 2020. [Google Scholar] [CrossRef]
- Burke, I.C.; Yonker, C.M.; Parton, W.J.; Cole, C.V.; Flach, K.; Schimel, D.S. Texture, climate, and cultivation effects on soil organic matter content in U.S. grassland soils. Soil Sci. Soc. Am. J. 1989, 53, 800–805. [Google Scholar] [CrossRef]
- Jasso-Flores, I.; Galicia, L.; Chávez-Vergara, B.; Merino, A.; Tapia-Torres, Y.; García-Oliva, F. Soil organic matter dynamics and microbial metabolism along an altitudinal gradient in Highland tropical forests. Sci. Total Environ. 2020, 741, 140143. [Google Scholar] [CrossRef]
- Conde, C.; Estrada, F.; Martínez, B.; Sánchez, O.; Gay, C. Regional climate change scenarios for Mexico. Atmósfera 2011, 24, 125–140. [Google Scholar]
- Finzi, A.C.; Austin, A.T.; Cleland, E.E.; Frey, S.D.; Houlton, B.Z.; Wallenstein, M.D. Responses and feedbacks of coupled biogeochemical cycles to climate change: Examples from terrestrial ecosystems. Front. Ecol. Environ. 2011, 9, 61–67. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; García-Gómez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, K.B.; Longing, S.D.; West, C.P. Soil microbial communities in corn fields treated with atoxigenic Aspergillus flavus. Soil Syst. 2020, 4, 35. [Google Scholar] [CrossRef]
- Singh, J.S.; Raghubanshi, A.S.; Singh, R.S.; Srivastava, S.C. Microbial biomass acts as a source of plant nutrients in dry tropical forests and savanna. Nature 1989, 338, 499–500. [Google Scholar] [CrossRef]
- Campo, J.; Jaramillo, V.J.; Maass, J.M. Pulses of soil phosphorus availability in a tropical dry forest: Effects of seasonality and level of wetting. Oecologia 1998, 115, 167–172. [Google Scholar] [CrossRef]
- Gei, M.G.; Powers, J.S. Nutrient Cycling in Tropical Dry Forests. In Tropical Dry Forests in the Americas; Sánchez-Asofeifa, A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 141–156. [Google Scholar]
- Krasilnikov, P.; Gutiérrez-Castorena, M.C.; Ahrens, R.J.; Cruz-Gaistardo, C.O.; Sedov, S.; Solleiro-Rebolledo, E. The Soils of Mexico; Springer: New York, NY, USA, 2013. [Google Scholar]
- Tobón, W.; Urquiza-Haass, T.; Koleff, P.; Schröter, M.; Ortega-Álvarez, R.; Campo, J.; Lindig-Cisneros, R.; Sarukhán, J.; Bonn, A. Restoration planning using a multi-criteria approach to guide Aichi targets in a megadiverse country. Conserv. Biol. 2017, 31, 1086–1097. [Google Scholar] [CrossRef]
- Instituto Nacional de Ecologia y Cambio Climático. Inventario Nacional de Gases y Compuestos de Efecto Invernadero. Available online: https://www.gob.mx/inecc/acciones-y-programas/inventario-nacional-de-emisiones-de-gases-y-compuestos-de-efecto-invernadero (accessed on 5 October 2020).
| Location | 20°51′ N, 89°37′ W | 21°08′ N, 88°09′ W | 19°24′ N, 96°22′ W | 20°01′ N, 97°06′ W |
|---|---|---|---|---|
| Altitude (m) | 10 | 20 | 14 | 121 |
| Mean annual temperature (°C) 2 | 25.7 | 25.3 | 25.4 | 24.4 |
| Mean annual rainfall (mm year−1) 2 | 711 | 917 | 1232 | 1661 |
| Potential evapotranspiration (mm year−1) 1 | 1719 | 1677 | 1561 | 1215 |
| Aridity index 2 | 0.41 (semiarid) | 0.55 (dry subhumid) | 0.79 (humid) | 1.37 (humid) |
| Water stress months | October–May | November–May | October–May | January–April |
| Mean month rainfall in water stress months | ||||
| (mm month−1) 3 | 27 | 53 | 29 | 80 |
| Soil bulk density (g cm−3) | 0.8 | 0.8 | 0.9 | 1.1 |
| Soil clay content (%) | 12.1 | 12.5 | 15.4 | 15.5 |
| Soil pH (H2O) | 8.0 | 7.6 | 7.4 | 5.1 |
| Metric | Source of Variation | ||
|---|---|---|---|
| Site | Season | Land Use | |
| F | |||
| Organic C (OC) | 34.6 *** | 0.595 NS | 8.45 ** |
| Total N (TN) | 50.5 *** | 1.33 NS | 6.47 ** |
| NH4 | 23.0 *** | 13.9 *** | 0.626 NS |
| NO3 | 4.36 NS | 59.8 *** | 1.55 NS |
| Total P (TP) | 43.0 *** | 0.676 NS | 2.61 NS |
| Available P (AP) | 0.565 NS | 29.9 *** | 6.48 ** |
| Microbial biomass C (MBC) | 32.0 *** | 1.14 NS | 3.44 NS |
| Microbial biomass N (MBN) | 16.4 *** | 0.860 NS | 0.042 NS |
| Soil basal respiration (SBR) | 2.95 * | 0.124 NS | 6.76 ** |
| Net N mineralization (MIN) | 17.4 *** | 4.20 * | 1.13 NS |
| Net nitrification (NIT) | 32.9 *** | 4.15 * | 1.05 NS |
| ß-1, 4-glucosidase (BG) | 8.06 * | 8.94 ** | 3.36 NS |
| Polyphenol oxidase (POX) | 37.0 *** | 2.85 NS | 2.21 NS |
| ß-1, 4-N-acetylglucosaminidase (BNA) | 10.1 ** | 0.851 NS | 4.69 * |
| Acid phosphatase (PHO) | 70.1 *** | 0.305 NS | 6.84 * |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalue | 7.045 | 2.091 | 1.440 |
| Cumulative percent variation | 46.9 | 60.8 | 70.4 |
| Eigenvectors | |||
| Organic C (OC) | 0.94 | 0.06 | 0.14 |
| Total N (TN) | 0.91 | −0.26 | 0.12 |
| NH4 | −0.64 | 0.20 | 0.12 |
| NO3 | −0.52 | −0.13 | 0.58 |
| Total P (TP) | 0.84 | 0.06 | −0.14 |
| Available P (AP) | 0.44 | 0.63 | 0.49 |
| Microbial biomass C (MBC) | 0.80 | −0.14 | 0.001 |
| Microbial biomass N (MBN) | 0.68 | 0.27 | 0.31 |
| Soil basal respiration (SBR) | 0.43 | −0.61 | −0.41 |
| Net N mineralization (MIN) | 0.84 | 0.39 | −0.15 |
| Net nitrification (NIT) | 0.87 | 0.35 | −0.13 |
| ß-1, 4-glucosidase (BG) | 0.08 | 0.21 | −0.43 |
| Polyphenol oxidase (POX) | 0.21 | −0.74 | 0.34 |
| ß-1, 4-N-acetylglucosaminidase (BNA) | 0.70 | −0.11 | 0.17 |
| Acid phosphatase (PHO) | −0.67 | 0.46 | −0.29 |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalue | 5.174 | 2.601 | 2.025 |
| Cumulative percent variation | 34.4 | 51.7 | 65.2 |
| Eigenvectors | |||
| Organic C (OC) | 0.89 | 0.10 | 0.11 |
| Total N (TN) | 0.90 | 0.23 | 0.22 |
| NH4 | −0.26 | −0.28 | 0.74 |
| NO3 | 0.16 | 0.49 | 0.22 |
| Total P (TP) | 0.82 | −0.21 | −0.04 |
| Available P (AP) | −0.04 | −0.03 | −0.14 |
| Microbial biomass C (MBC) | 0.56 | −0.74 | 0.01 |
| Microbial biomass N (MBN) | 0.34 | −0.63 | 0.03 |
| Soil basal respiration (SBR) | −0.72 | 0.35 | 0.30 |
| Net N mineralization (MIN) | 0.53 | 0.38 | 0.58 |
| Net nitrification (NIT) | 0.63 | −0.14 | 0.46 |
| ß-1, 4-glucosidase (BG) | −0.61 | −0.20 | 0.51 |
| Polyphenol oxidase (POX) | 0.27 | 0.77 | 0.29 |
| ß-1, 4-N-acetylglucosaminidase (BNA) | −0.01 | 0.51 | −0.50 |
| Acid phosphatase (PHO) | −0.84 | −0.21 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa, D.; Ortega-Fernández, P.; Abbruzzini, T.F.; Rivero-Villlar, A.; Galindo, F.; Chavez-Vergara, B.; Etchevers, J.D.; Campo, J. Effects of Land Use Change from Natural Forest to Livestock on Soil C, N and P Dynamics along a Rainfall Gradient in Mexico. Sustainability 2020, 12, 8656. https://doi.org/10.3390/su12208656
Figueroa D, Ortega-Fernández P, Abbruzzini TF, Rivero-Villlar A, Galindo F, Chavez-Vergara B, Etchevers JD, Campo J. Effects of Land Use Change from Natural Forest to Livestock on Soil C, N and P Dynamics along a Rainfall Gradient in Mexico. Sustainability. 2020; 12(20):8656. https://doi.org/10.3390/su12208656
Chicago/Turabian StyleFigueroa, Daniela, Patricia Ortega-Fernández, Thalita F. Abbruzzini, Anaitzi Rivero-Villlar, Francisco Galindo, Bruno Chavez-Vergara, Jorge D. Etchevers, and Julio Campo. 2020. "Effects of Land Use Change from Natural Forest to Livestock on Soil C, N and P Dynamics along a Rainfall Gradient in Mexico" Sustainability 12, no. 20: 8656. https://doi.org/10.3390/su12208656
APA StyleFigueroa, D., Ortega-Fernández, P., Abbruzzini, T. F., Rivero-Villlar, A., Galindo, F., Chavez-Vergara, B., Etchevers, J. D., & Campo, J. (2020). Effects of Land Use Change from Natural Forest to Livestock on Soil C, N and P Dynamics along a Rainfall Gradient in Mexico. Sustainability, 12(20), 8656. https://doi.org/10.3390/su12208656







