Comparative Study on the Adsorption Capacities of the Three Black Phosphorus-Based Materials for Methylene Blue in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BP-Based Materials
2.1.1. Preparation of BP
2.1.2. Preparation of BPR
2.1.3. Preparation of BPRS
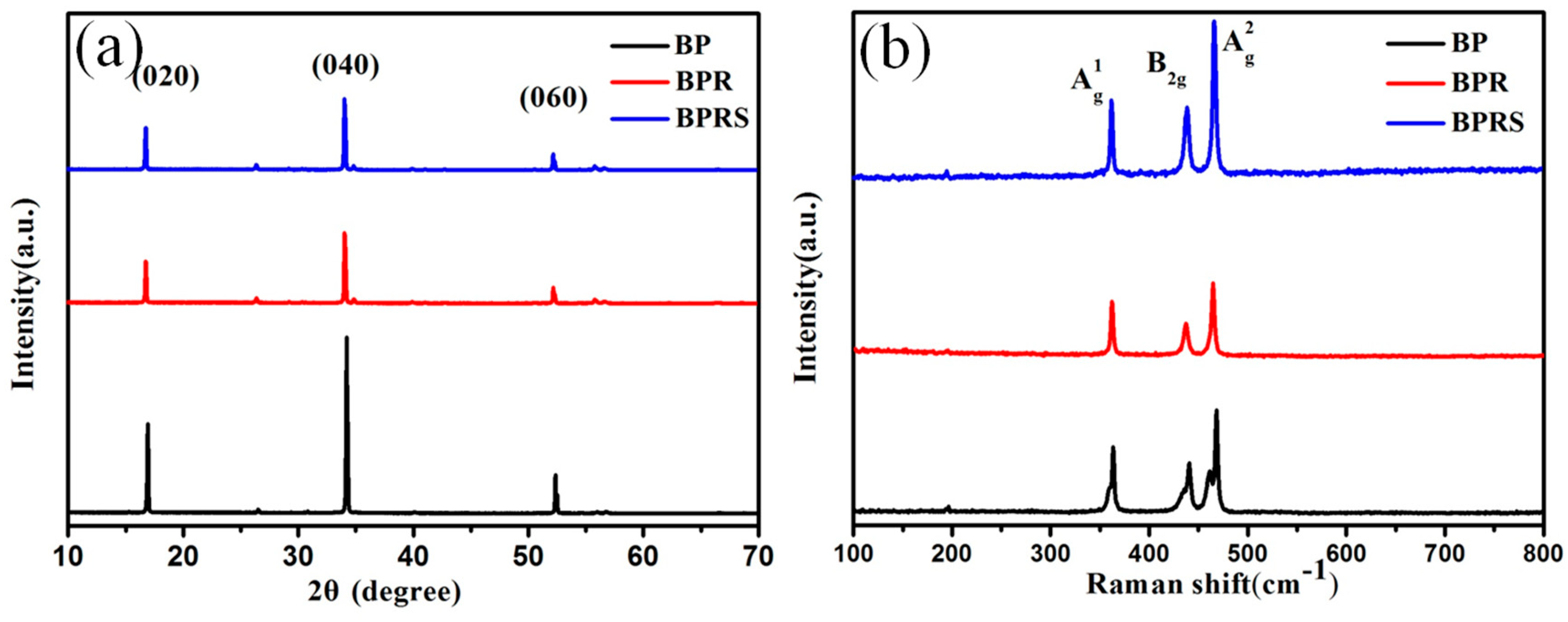
2.2. Characterization
2.3. Adsorption for MB
2.4. Data Analysis
3. Results and Discussions
3.1. Characterization
3.2. Adsorption for MB
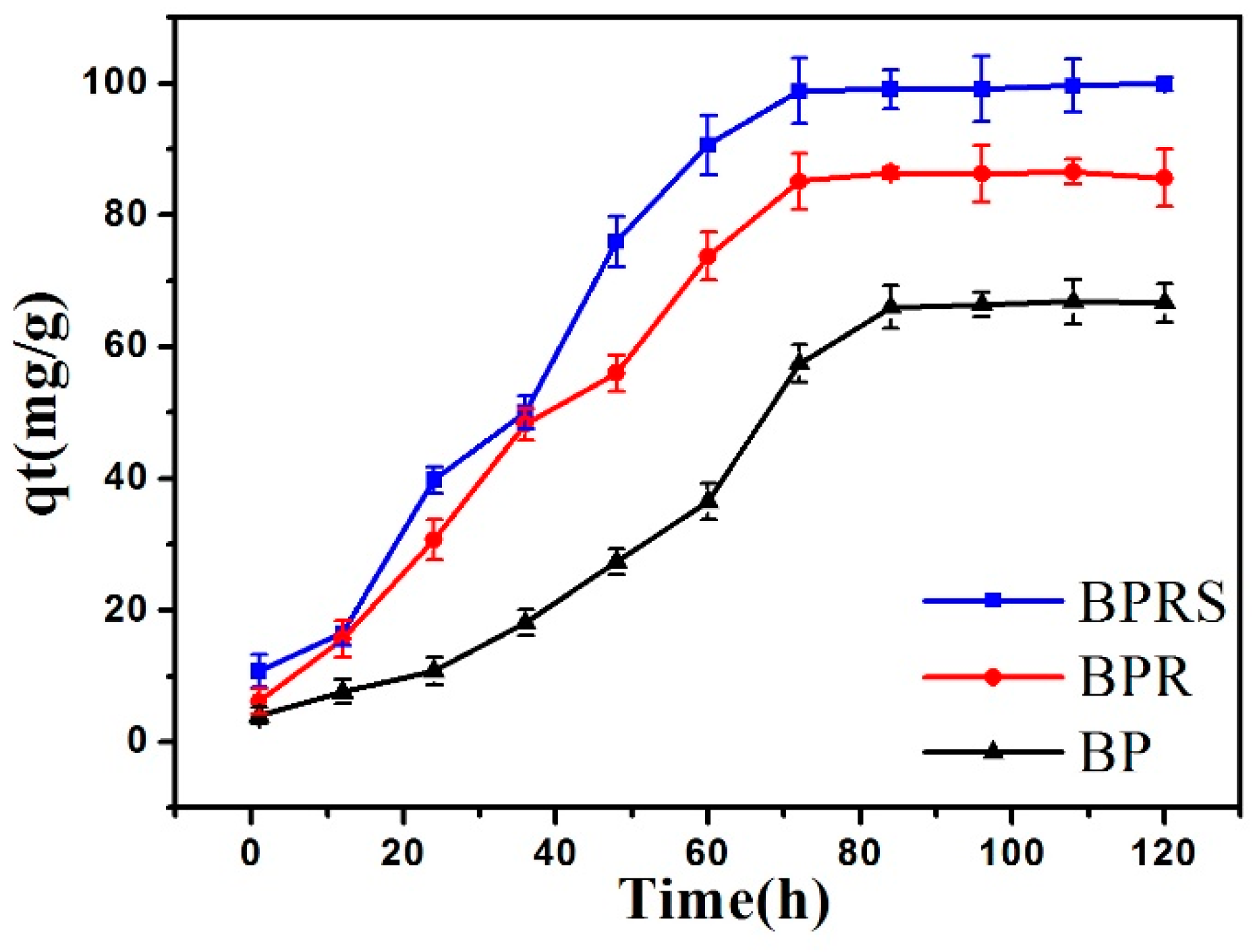
3.2.1. Adsorption Equilibrium Time
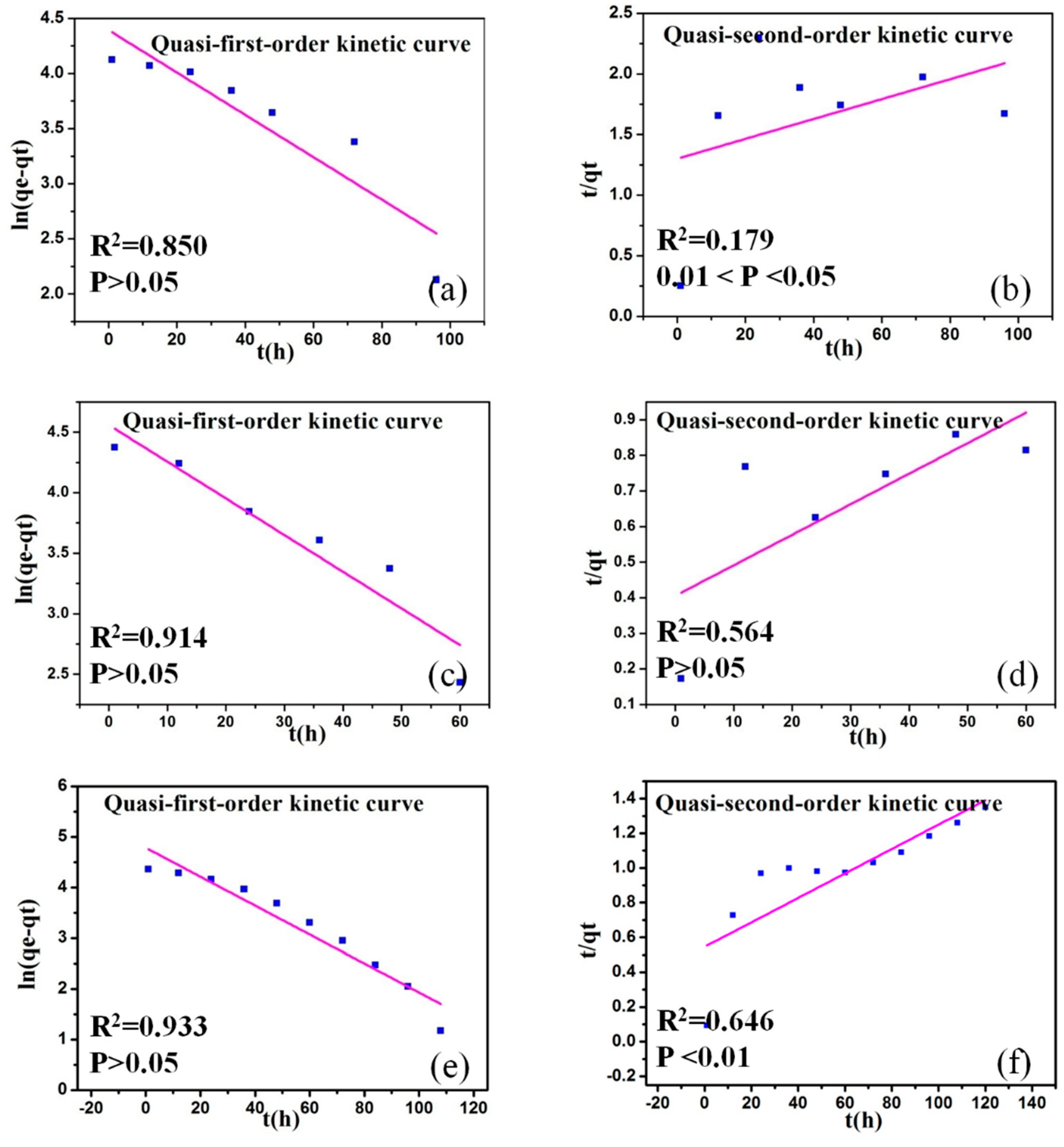
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Isotherms and Thermodynamics
3.2.4. Desorption
3.2.5. Influence Factors
3.2.6. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghaedi, M.; Hajjati, S.; Mahmudi, Z.; Tyagi, I.; Agarwal, S.; Maity, A.; Gupta, V.K. Modeling of competitive ultrasonic assisted removal of the dyes-methylene blue and safranin-O using Fe3O4 nanoparticles. Chem. Eng. J. 2015, 268, 28–37. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Environ. Prot. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Atarod, M.; Nasrollahzadeh, M.; Sajadi, S.M. Euphorbia heterophylla leaf extract mediated green synthesis of Ag/TiO2 nanocomposite and investigation of its excellent catalytic activity for reduction of variety of dyes in water. J. Colloid Interface Sci. 2016, 462, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.L.; Zhang, X.F.; Tang, Y.S.; Su, K.H.; Kong, J. Hierarchically porous silicon-carbon-nitrogen hybrid materials towards highly efficient and selective adsorption of organic dyes. Sci. Rep. 2015, 5, 7910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Jin, W.; Zhao, Y.P.; Zhang, G.S.; Zhang, W. Enhanced catalytic degradation of methylene blue by alpha-Fe2O3/graphene oxide via heterogeneous photo-fenton reactions. Appl. Catal. B Environ. 2017, 206, 642–652. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Goudarzi, A.; Raiabi, M. Response surface methodology approach for optimization of simultaneous dye and metal ion ultrasound-assisted adsorption onto Mn doped Fe3O4-NPs loaded on AC: Kinetic and isothermal studies. Dalton Trans. 2015, 44, 14707–14723. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Xin, M.; Zhang, X.; Wang, Q.; Deng, J. Adsorption of organic pollutants by modified chitosan microspheres. Environ. Sci. Technol. 2008, 31, 27–29. [Google Scholar]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Song, Z.W.; Li, T.; Yi, H.Y.; Qiu, J.; Zhang, D.; Chen, D.F. Adsorption mechanism of aerobic granular sludge to organic pollutants. Earth Sci. Ed. 2017, 47, 868–873. [Google Scholar]
- Wang, J.; Wu, S.S.; Zhao, W.B.; Lian, P.C.; Wang, Y.M. Research progress on adsorption of organic pollutant by graphene-based nanomaterials. Mater. Rev. 2014, 28, 42–45. [Google Scholar]
- Zhao, M.; Qian, H.L.; Niu, X.Y.; Wang, W.; Guan, L.; Sha, J.; Wang, Y.W. Growth mechanism and enhanced yield of black phosphorus microribbons. Cryst. Growth Des. 2016, 16, 1096–1103. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Chen, Q.; Tong, Y.L.; Wang, J.L. Light-induced ambient degradation of few-layer black phosphorus: Mechanism and protection. Angew. Chem. Int. Ed. 2016, 55, 11437–11441. [Google Scholar] [CrossRef] [PubMed]
- Abellan, G.; Wild, S.; Lloret, V.; Scheuschner, N.; Gillen, R.; Mundloch, U.; Maultzsch, J.; Varela, M.; Hauke, F.; Hirsch, A. Fundamental insights into the degradation and stabilization of thin layer black phosphorus. J. Am. Chem. Soc. 2017, 139, 10432–10440. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.Y.; Liu, G.; Zhang, J.; Liu, M.H. Black phosphorus nanostructures: Recent advances in hybridization, doping and functionalization. Chem. Soc. Rev. 2017, 46, 3492–3509. [Google Scholar] [CrossRef]
- Xia, F.N.; Wang, H.; Jia, Y.C. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014, 5, 4458. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.N.; Zhang, H.; Lu, S.B.; Wang, Z.T.; Tang, S.Y.; Shao, J.D.; Sun, Z.B.; Xie, H.H.; Wang, H.Y.; Yu, X.F.; et al. From black phosphorus to phosphorene: Basic solvent exfoliation, evolution of Raman scattering, and applications to ultrafast photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Sun, C.X.; Wen, L.; Zeng, J.F.; Wang, Y.; Sun, Q.; Deng, L.J.; Zhao, C.J.; Li, Z. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials 2016, 91, 81–89. [Google Scholar] [CrossRef]
- Chen, P.F.; Li, N.; Chen, X.Z.; Ong, W.J.; Zhao, X.J. The rising star of 2D black phosphorus beyond graphene: Synthesis, properties and electronic applications. 2D Mater. 2018, 5, 014002. [Google Scholar] [CrossRef]
- Wang, Z.W.; Qiu, J.J.; Wang, X.S.; Zhang, Z.P. Two-dimensional light-emitting materials: Preparation, properties and applications. Chem. Soc. Rev. 2018, 47, 6128–6174. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.H.; Ou, H.H.; Abdullah, A.M.; Chen, Y.L.; Wang, X.C. Black phosphorus and polymeric carbon nitride heterostructure for photoinduced molecular oxygen activation. Adv. Funct. Mater. 2018, 28, 1705407. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.M.; Liu, Z.D.; Tan, C.L.; Luo, Z.M.; Li, H.; Lin, J.D.; Sun, L.Q.; Chen, W.; Xu, Z.C.; et al. Black phosphorus quantum dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.L.F.; de Matos, C.J.S.; Ho, Y.W.; Peixoto, H.; de Oliveira, R.E.P.; Wu, H.Y.; Castro Neto, A.H.C.; Viana-Gomes, J. Resonantly increased optical frequency conversion in atomically thin black phosphorus. Adv. Mater. 2016, 28, 10693–10700. [Google Scholar] [CrossRef] [PubMed]
- Gamage, S.; Fali, A.; Aghamiri, N.; Yang, L.; Ye, P.D.; Abate, Y. Reliable passivation of black phosphorus by thin hybrid coating. Nanotechnology 2017, 28, 265201. [Google Scholar] [CrossRef] [PubMed]
- Walia, S.; Balendhran, S.; Ahmed, T.; Singh, M.; El-Badawi, C.; Brennan, M.D.; Weerathunge, P.; Karim, M.N. Ambient protection of few-layer black phosphorus via sequestration of reactive oxygen species. Adv. Mater. 2017, 29, 29. [Google Scholar] [CrossRef]
- Chen, W.S.; Jiang, O.Y.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.P.; Liu, Z.J.; Han, Y.J.; Wang, L.Q. Black phosphrus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv. Mater. 2017, 29, 201603864. [Google Scholar]
- Liu, G.; Tsai, H.I.; Zeng, X.W.; Qi, J.Y.; Luo, M.M.; Wang, X.S.; Mei, L.; Deng, W.B. Black phosphorus nanosheets-based stable drug delivery system via drug-self-stabilization for combined photothermal and chemo cancer therapy. Chem. Eng. J. 2019, 375, 121917. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, W.; Pan, B.; Zhang, Q.R.; Zhang, X.J.; Zhang, S.Y.; Xing, B.S. Wrinkle-induced high sorption makes few-layered black phosphorus a superior adsorbent for ionic organic compounds. Environ. Sci. Nano 2018, 5, 1454–1465. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, X.B.; Yu, X.H.; Zeng, X.W.; Xiao, Q.L.; Zhang, X.D.; Ji, X.Y.; Wang, X.S.; Shi, J.J.; Zhang, H.; et al. Black phosphorus nanosheets as a robust delivery platform for cancer theranostics. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Zhang, Z.C.; He, D.Y.; Liu, H.Y.; Ren, M.; Zhang, Y.N.; Qu, J.; Lu, N.; Guan, J.N.; Yuan, X. Synthesis of graphene/black phosphorus hybrid with highly stable P-C bond towards the enhancement of photocatalytic activity. Environ. Pollut. 2019, 245, 950–956. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wang, H.Y.; Huang, H.; Xiao, Q.L.; Xu, Y.H.; Guo, Z.N.; Xie, H.H.; Shao, J.D.; Sun, Z.B.; Han, W.J.; et al. Surface coordination of black phosphorus for robust air and water stability. Angew. Chem. Int. Ed. 2016, 55, 5003–5007. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.S.; Berner, N.C.; Boland, C.; Lee, K.; Harvey, A. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015, 6, 8563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Dong, N.N.; Tao, H.C.; Yan, C.; Huang, J.W.; Liu, T.F.; Robertson, A.W.; Texter, J.; Wang, J.; Sun, Z.Y. Exfoliation of stable 2D black phosphorus for device fabrication. Chem. Mater. 2017, 29, 6445–6456. [Google Scholar] [CrossRef]
- Yasaei, P.; Kumar, B.; Foroozan, T.; Wang, C.H.; Asadi, M.; Tuschel, D.; Indacochea, J.E.; Klie, R.F.; Salehi-Khojin, A. High-quality black phosphorus atomic layers by liquid-phase exfoliation. Adv. Mater. 2015, 27, 1887–1892. [Google Scholar] [CrossRef]
- Zhang, T.M.; Wan, Y.Y.; Xie, H.Y.; Mu, Y.; Du, P.W.; Wang, D.; Wu, X.J.; Ji, H.X.; Wan, L.J. Degradation chemistry and stabilization of exfoliated few-layer black phosphorus in water. J. Am. Chem. Soc. 2018, 140, 7561–7567. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, B.L. Sulfonated graphene nanosheets as a superb adsorbent for various environmental pollutants in water. Environ. Sci. Technol. 2015, 49, 7364–7372. [Google Scholar] [CrossRef]
- Ryder, C.R.; Wood, J.D.; Wells, S.A.; Yang, Y.; Jariwala, D.; Marks, T.J.; Schatz, G.C.; Hersam, M.C. Covalent functionalization and passivation of exfoliated black phosphorus via aryl diazonium chemistry. Nat. Chem. 2016, 8, 597–602. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Kinetic models for the sorption of dye from aqueous solutions by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Hu, C.C. Comparisons of pore properties and adsorption performance of KOH-activated and steam-activated carbons. Micrporous Mesoporous Mater. 2005, 80, 95–106. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Huang, Y.F.; Farooq, M.U.; Lai, S.X.; Feng, X.S.; Sampranpiboon, P.; Wang, X.D.; Huang, W. Model fitting of sorption kinetics data: Misapplications overlooked and their rectifications. AIChE J. 2018, 64, 1793. [Google Scholar] [CrossRef]
- Liu, Y. Is the free energy change of adsorption correctly calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Mukherjee, S.; Gao, J.; Sun, H.; Liu, Z.B.; Ma, X.L.; Gupta, T.; Singh, C.V.; Ren, W.C.; et al. Phosphorene as a polysulfide immobilizer and catalyst in high-performance lithium-sulfur batteries. Adv. Mater. 2017, 29, 4. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gomez, A.; Vicarelli, L.; Prada, E.; Island, J.O.; Narasimha-Acharya, K.L.; Blanter, S.I.; Groenendijk, D.J.; Buscema, M. Isolation and characterization of few-layer black phosphorus. 2D Mater. 2014, 1, 025001. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.F.; Tomanek, D.; Ye, P.D. Phosphorene: An unexplored 2D semiconductor with a high hole mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef]
- Zhu, M.S.; Osakada, Y.; Kim, S.; Fujitsuka, M.; Majima, T. Black phosphorus: A promising two dimensional visible andnear-infrared-activated photocatalyst for hydrogen evolution. Appl. Catal. B Environ. 2017, 217, 285–292. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A.; Perez-Ramirez, J. Mechanism of hierarchical porosity development in MFI zeolites by desilication: The role of aluminium as a pore-directing agent. Chem. A Eur. J. 2005, 11, 4983–4994. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef]
- Tripathy, A.; Srinivasan, V.; Singh, T.N. A comparative study on pore size distribution of different Indian shale gas reservoirs for gas production and potential CO2 sequestration. Energy Fuels 2018, 32, 3322–3334. [Google Scholar] [CrossRef]
- Fang, N.J.; Guo, J.X.; Shu, S.; Luo, H.D.; Chu, Y.H.; Li, J.J. Enhancement of low-temperature activity and sulfur resistance of Fe0.3Mn0.5Zr0.2 catalyst for NO removal by NH3-SCR. Chem. Eng. J. 2017, 325, 114–123. [Google Scholar] [CrossRef]
- Nieminen, M.; Niinist, L.; Lappalainen, R. Determination of P/Al ratio in phosphorus-doped aluminium oxide thin films by XRF, RBS and FTIR. Microchim. Acta 1995, 119, 13–22. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Pinto, R.B.; Lima, E.C.; Calvete, T.; Amavisca, C.V.; Royer, B.; Cunha, M.L.; Fernandes, T.H.M.; Pinto, I.S. Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination 2011, 269, 92–103. [Google Scholar] [CrossRef]
- Wu, J.G.; Shi, N.; Xu, X.H.; Xu, G.X. Fourier-Transform Infrared (FTIR) studies on the hydration of extractant anions in microemulsions. Infrared Phys. 1984, 24, 245–248. [Google Scholar] [CrossRef]
- Ai, L.H.; Zhang, C.Y.; Chen, Z.L. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Song, L.; Wang, Y.Q.; Zhang, X.F.; Hao, D.D.; Feng, Y.; Yao, J.F. Lightweight UiO-66/cellulose aerogels constructed through self-crosslinking strategy for adsorption applications. Chem. Eng. J. 2019, 371, 138–144. [Google Scholar] [CrossRef]
- Zou, W.H.; Han, P.; Lia, Y.L.; Liu, X.; He, X.T.; Han, R.P. Equilibrium, kinetic and mechanism study for the adsorption of neutral red onto rice husk. Desalin. Water Treat. 2009, 12, 210–218. [Google Scholar] [CrossRef]
- Liu, T.H.; Li, Y.H.; Du, Q.J.; Sun, J.K.; Jiao, Y.Q.; Yang, G.M.; Wang, Z.H.; Xia, Y.Z.; Zhang, W.; Wang, K.L.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Tang, L.; Yang, G.D.; Zeng, G.M.; Cai, Y.; Li, S.S.; Zhou, Y.Y.; Pang, Y.; Liu, Y.Y.; Zhang, Y.; Luna, B. Synergistic effect of iron doped ordered mesoporous carbon on adsorption-coupled reduction of hexavalent chromium and the relative mechanism study. Chem. Eng. J. 2014, 239, 114–122. [Google Scholar] [CrossRef]
- Li, Q.Y.; Wang, W.C.; Liu, Y.H.; Lian, B.L.; Zhu, Q.C.; Yao, L.P.; Liu, T.Y. The biological characteristics of a novel camptothecin–artesunate conjugate. Bioorganic Med. Chem. Lett. 2015, 25, 148–152. [Google Scholar] [CrossRef]
- Cui, L.M.; Hu, L.H.; Guo, X.Y.; Zhang, Y.K.; Wang, Y.G.; Wei, Q.; Du, B. Kinetic, isotherm and thermodynamic investigations of Cu2+ adsorption onto magnesium hydroxyapatite/ferroferric oxide nano-composites with easy magnetic separation assistance. J. Mol. Liq. 2014, 198, 157–163. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Khan-Bangash, F.; Alam, S. Adsorption of brilliant blue R on biotic precursor based carbon. J. Chem. Soc. Pakistan 2007, 29, 401–414. [Google Scholar]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef] [PubMed]
- Maryam, A.T.; Toraj, M. Nickel ions removal from water by two different morphologies of induced CNTs in mullite pore channels as adsorptive membrane. Ceram. Int. 2015, 41, 5464–5472. [Google Scholar]
- Darvish, G.M.; Alinezhad, H.; Soleymani, E. Adsorption of TCDD molecule onto CNTs and BNNTs: Ab initio van der Waals density-functional study. Physica E 2015, 67, 105–111. [Google Scholar] [CrossRef]
- Rosenzweig, S.; Sorial, G.A.; Sahle, D.E.; McAvoy, D.C. Optimizing the physical-chemical properties of carbon nanotubes(CNT) and graphene nanoplatelets (GNP) on Cu(II) adsorption. J. Hazard. Mater. 2014, 279, 410–417. [Google Scholar] [CrossRef]
- Kumar, K.V.; Ramamurthi, V.; Sivanesan, S. Modeling the mechanism involved during the sorption of methylene blue onto fly ash. J. Colloid Interface Sci. 2005, 284, 14–21. [Google Scholar] [CrossRef]
- Zou, W.H.; Bai, H.J.; Gao, S.P.; Li, K. Characterization of modified sawdust, kinetic and equilibrium study about methylene blue adsorption in batch mode. Korean J. Chem. Eng. 2013, 30, 111–122. [Google Scholar] [CrossRef]
- Aydin, H.; Bulut, Y.; Yerlikaya, C. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J. Environ. Manag. 2008, 87, 37–45. [Google Scholar] [CrossRef]
- Gao, B.L.; Yang, Y.; Wang, J.; Zhang, Y. Preparation and adsorption characteristic of polymeric microsphere with strong adsorbability for creatinine. J. Biochem. Mol. Toxicol. 2008, 22, 166–174. [Google Scholar] [CrossRef]
- Mukerjee, P.; Moroi, Y. Cell for isoextraction studies and determination of some acid dissociation constant. Anal. Chem. 1993, 50, 1589–1591. [Google Scholar] [CrossRef]
- Kang, J.; Wells, S.A.; Wood, J.D.; Lee, J.H.; Liu, X.L.; Ryder, C.R.; Zhu, J.; Guest, J.R.; Husko, C.A.; Hersam, M.C. Stable aqueous dispersions of optically and electronically active phosphorene. Proc. Natl. Acad. Sci. USA 2006, 113, 11688–11693. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.J.; Hong, Y.Z.; Cao, F.; Li, L.; Li, J.B. Preparation of high-surface-area activated carbon from coconut shell fibers. Carbon 2010, 48, 3005. [Google Scholar] [CrossRef]
- Fei, R.X.; Yang, L. Strain-engineering the anisotropic electrical conductance of few-layer black phosphorus. Nano Lett. 2014, 14, 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Rodin, A.S.; Carvalho, A.; Castro Neto, A.H. Strain-induced gap modification in black phosphorus. Phys. Rev. Lett. 2014, 112, 176801. [Google Scholar] [CrossRef]
- Ofomaja, A.E. Kinetic study and sorption mechanism of methylene blue and methyl violet onto mansonia (Mansonia altissima) wood sawdust. Chem. Eng. J. 2008, 143, 85–95. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, R.Q.; Deng, S.B.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res. 2009, 43, 1150–1158. [Google Scholar] [CrossRef]
- Yan, H.; Tao, X.; Yang, Z.; Li, K.; Yang, H.; Li, A.M.; Cheng, R.S. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J. Hazard. Mater. 2014, 268, 191–198. [Google Scholar] [CrossRef]
- Ferrero, F. Dye removal by low cost adsorbents: Hazelnut shells in comparison with wood sawdust. J. Hazard. Mater. 2007, 142, 144–152. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Gunasekar, V.; Ponnusami, V. Kinetics, equilibrium, and thermodynamic studies on adsorption of methylene blue by carbonized plant leaf powder. J. Chem. 2013, 13, 1–6. [Google Scholar] [CrossRef]
- Weng, C.H.; Pan, Y.F. Adsorption characteristics of methylene blue from aqueous solution by sludge ash. Colloids Surf. A Physicochem. Eng. Asp. 2006, 274, 154–162. [Google Scholar] [CrossRef]
- Wang, S.B.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Otero, M. Kinetic and equilibrium modelling of the methylene blue removal from solution by adsorbent materials produced from sewage sludges. Biochem. Eng. J. 2003, 15, 59–68. [Google Scholar] [CrossRef]
- Gucek, A.; Sener, S.; Bilgen, S.; Mazmanci, M.A. Adsorption and kinetic studies of cationic and anionic dyes on pyrophyllite from aqueous solutions. J. Colloid Interface Sci. 2005, 286, 53–60. [Google Scholar] [CrossRef]
- El Sikaily, A. Removal of methylene blue from aqueous solution by marine green alga Ulva lactuca. Chem. Ecol. 2006, 22, 149–157. [Google Scholar] [CrossRef]








| Sample | SSA (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| BP | 6.78 | 0.0303 | 13.99 |
| BPR | 6.96 | 0.0319 | 12.75 |
| BPRS | 7.72 | 0.0451 | 12.81 |
| Adsorbent | Quasi-First-Order Kinetic | Quasi-Second-Order Kinetic | ||||
|---|---|---|---|---|---|---|
| q1 (mg·g−1) | k1 (h−1) | R2 | q2 (mg·g−1) | k2 (g·(mg·h)−1) | R2 | |
| BP | 80.891 | 0.044 | 0.850 | 121.951 | 0.517·10−4 | 0.179 |
| BPR | 95.488 | 0.070 | 0.914 | 116.279 | 0.182·10−3 | 0.564 |
| BPRS | 120.217 | 0.066 | 0.933 | 136.986 | 0.991·10−4 | 0.646 |
| Adsorbent | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qm (mg·g−1) | kL (L·g−1) | R2 | Kf ((mg·g−1)·(mg·L−1)−n) | n | R2 | |
| BP | 84.034 | 7.933 | 0.996 | 62.110 | 3.384 | 0.895 |
| BPR | 91.743 | 21.800 | 0.999 | 78.367 | 2.843 | 0.871 |
| BPRS | 140.845 | 11.833 | 0.981 | 240.760 | 1.719 | 0.964 |
| Adsorbent | ΔH0 (KJ·mol−1) | ΔS0 (KJ·mol−1) | ΔG0 (KJ·mol−1) | ||
|---|---|---|---|---|---|
| 298.15 K | 308.15 K | 318.15 K | |||
| BP | 35.983 | 0.140 | −5.743 | −6.716 | −8.551 |
| BPR | 68.229 | 0.255 | −7.855 | −9.850 | −12.971 |
| BPRS | 68.289 | 0.269 | −12.022 | −14.064 | −17.424 |
| Adsorbent | qm (mg·g−1) | SSA (m2·g−1) | Ref. |
|---|---|---|---|
| Walnut sawdust | 59.17 | 13.30 | [80] |
| Rice husk | 40.59 | - | [81] |
| Plant leaf powder | 61.22 | - | [82] |
| Sludge ash | 7.99 | 3.70 | [83] |
| Red mud | 2.490 | 21.00 | [84] |
| Sewage sludge | 114.90 | - | [85] |
| Pyrophyllite | 70.42 | 7.03 | [86] |
| Green alga | 40.20 | - | [87] |
| GO | 623.00 | 261.00 | [36] |
| rGO | 136.00 | 399.00 | [36] |
| GS | 906.00 | 616.00 | [36] |
| Activated carbon | 495.00 | 2032.00 | [74] |
| BP | 84.03 | 6.78 | This study |
| BPR | 91.74 | 6.96 | This study |
| BPRS | 140.85 | 7.72 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Z.; He, D.; Yang, H.; Jin, D.; Qu, J.; Zhang, Y. Comparative Study on the Adsorption Capacities of the Three Black Phosphorus-Based Materials for Methylene Blue in Water. Sustainability 2020, 12, 8335. https://doi.org/10.3390/su12208335
Wang J, Zhang Z, He D, Yang H, Jin D, Qu J, Zhang Y. Comparative Study on the Adsorption Capacities of the Three Black Phosphorus-Based Materials for Methylene Blue in Water. Sustainability. 2020; 12(20):8335. https://doi.org/10.3390/su12208335
Chicago/Turabian StyleWang, Juanhong, Zhaocheng Zhang, Dongyang He, Hao Yang, Dexin Jin, Jiao Qu, and Yanan Zhang. 2020. "Comparative Study on the Adsorption Capacities of the Three Black Phosphorus-Based Materials for Methylene Blue in Water" Sustainability 12, no. 20: 8335. https://doi.org/10.3390/su12208335
APA StyleWang, J., Zhang, Z., He, D., Yang, H., Jin, D., Qu, J., & Zhang, Y. (2020). Comparative Study on the Adsorption Capacities of the Three Black Phosphorus-Based Materials for Methylene Blue in Water. Sustainability, 12(20), 8335. https://doi.org/10.3390/su12208335







