Assessing Genetic Diversity and Population Structure of Kalmia latifolia L. in the Eastern United States: An Essential Step towards Breeding for Adaptability to Southeastern Environmental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Collection
2.2. ISSR Procedure
2.3. Data Analysis
3. Results
3.1. ISSR Polymorphism
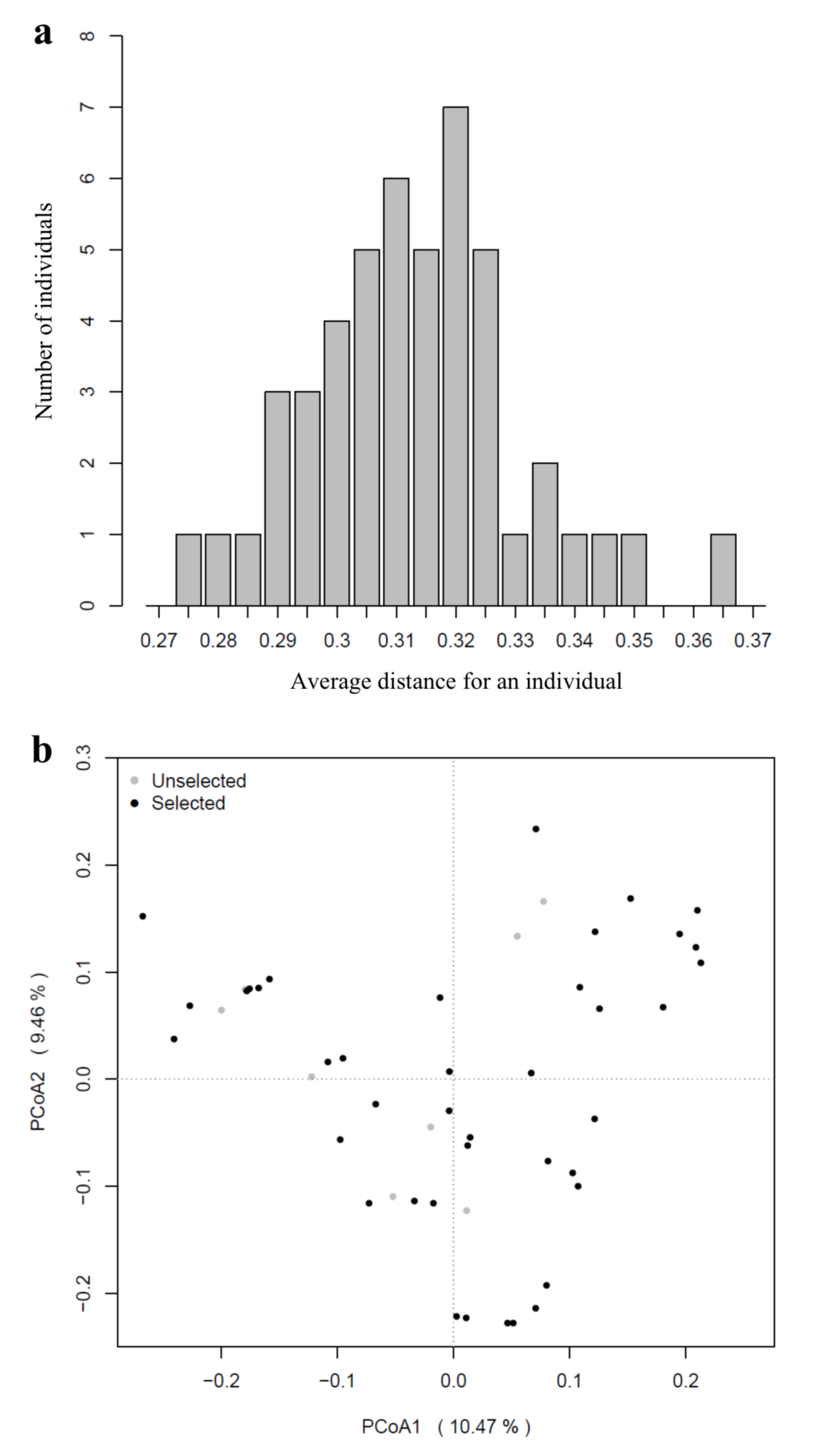
3.2. Genetic Diversity
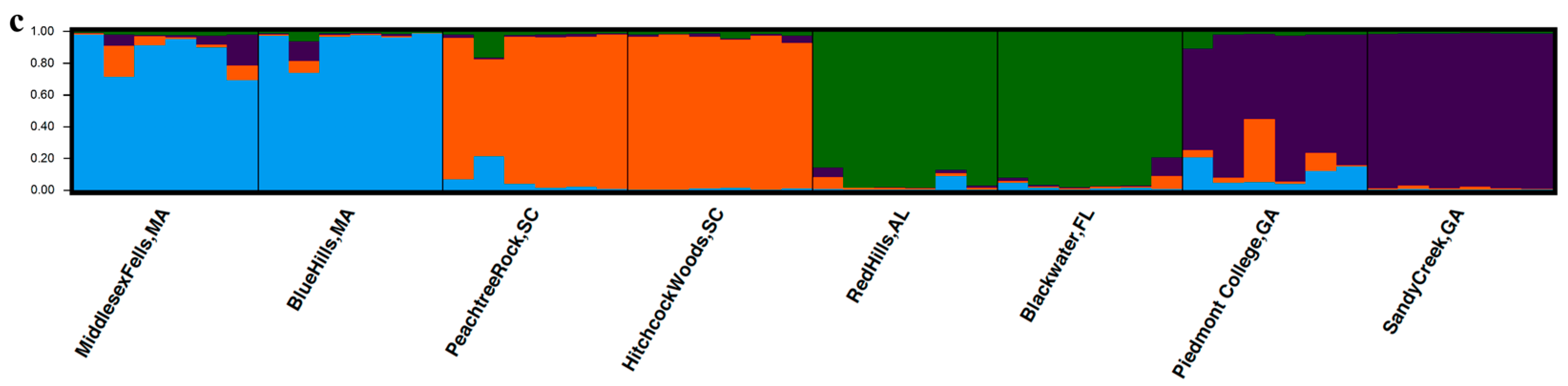
3.3. Population Structure
4. Discussion
4.1. Effectiveness of ISSR in K. latifolia
4.2. Genetic Diversity and Differentiation of K. latifolia
4.3. Population Structure of K. latifolia
4.4. Conservation and Breeding Strategy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaynes, R.A. Kalmia: The Laurel Book; Timber Press: Portland, OR, USA, 1988. [Google Scholar]
- Dirr, M.A. Manual of Woody Landscape Plants, 6th ed.; Stipes Publishing: Champaign, IL, USA, 2009. [Google Scholar]
- Li, H.; Chappell, M.; Zhang, D. Evaluation of twenty-one mountain laurel cultivars for container and landscape performance in the southeastern United States. HortTechnology 2018, 28, 867–874. [Google Scholar] [CrossRef]
- Engels, J.M.M.; Arora, R.K.; Guarino, L. An introduction to plant germplasm exploration and collecting: Planning, methods, and procedures, follow-up. In Collecting Plant Genetic Diversity: Technical Guidelines; Guarino, L., Rao, V.R., Reid, R., Eds.; CABI: Oxfordshire, UK, 1995; pp. 21–63. [Google Scholar]
- Li, H.; Zhang, D.; Chappell, M. Utilization of Kalmia latifolia L. germplasm as ornamentals. Acta Hort. 2017, 1185, 241–246. [Google Scholar] [CrossRef]
- Brown, A.H.D. Core collections: A practical approach to genetic resource management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- Jaynes, R.A. Self-incompatibility and inbreeding depression in three laurel species. Proc. Am. Soc. Hort. Sci. 1968, 93, 618–622. [Google Scholar]
- Chappell, M.; Robacker, C.; Jenkins, T.M. Genetic diversity of seven deciduous azalea species (Rhododendron spp. section Pentanthera) native to the eastern United States. J. Am. Soc. Hort. Sci. 2008, 133, 374–382. [Google Scholar] [CrossRef]
- De Keyser, E.; Scariot, V.; Kobayashi, N.; Handa, T.; De Riek, J. Azalea phylogeny reconstructed by means of molecular techniques. In Protocols for In Vitro Propagation of Ornamental Plants. Methods in Molecular Biology (Methods and Protocols); Jain, S., Ochatt, S., Eds.; Humana Press: New York, NY, USA, 2010; pp. 349–364. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, M.; Zhao, W.; Fan, R.; Luo, S.; Chen, X. Genetic diversity analysis of Rhododendron aureum Georgi (Ericaceae) located on Changbai Mountain using ISSR and RAPD markers. Plant Syst. Evol. 2012, 298, 921–930. [Google Scholar] [CrossRef]
- Boraks, A.; Broders, K.D. Population genetic diversity of the rare hardwood butternut (Juglans cinerea) in the northeastern USA. Tree Genet. Genomes 2016, 12, 43. [Google Scholar] [CrossRef]
- Cardoso, R.; Ruas, C.F.; Giacomin, R.M.; Ruas, P.M.; Ruas, E.A.; Barbieri, R.L.; Rodrigues, R.; Goncalves, L.S.A. Genetic variability in Brazilian Capsicum baccatum germplasm collection assessed by morphological fruit traits and AFLP markers. PLoS ONE 2018, 13, e0196468. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Weng, Y.; Liu, S.; Hao, Z.; Sheng, Y.; Guan, L.; Shi, J.; Chen, J. Genetic diversity and differentiation of relict plant Liriodendron populations based on 29 novel EST-SSR markers. Forests 2019, 10, 334. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Barreneche, T.; Mattioni, C.; Villani, F.; Diaz-Hernandez, M.B.; Martin, L.M.; Martin, A. Database of European chestnut cultivars and definition of a core collection using simple sequence repeats. Tree Genet. Genomes 2017, 13, 114. [Google Scholar] [CrossRef]
- Nagaoka, T.; Ogihara, Y. Applicability of inter-simple sequence repeat polymorphisms in wheat for use as DNA markers in comparison to RFLP and RAPD markers. Theor. Appl. Genet. 1997, 94, 597–602. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Z.; Wu, C.; Ding, G.; Cao, G.; Ruan, S.; Lin, S. Genetic diversity and variation of Chinese fir from Fujian province and Taiwan, China, based on ISSR markers. PLoS ONE 2017, 12, e0175571. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, F.; Yeh, K.; Chen, J. ISSR analysis of genetic diversity and structure of plum varieties cultivated in southern China. Biology 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Laakili, A.; Belkadi, B.; Medraoui, L.; Alami, M.; Yatrib, C.; Pakhrou, Q.; Makhloufi, M.; El Antry, S.; Laamarti, A.; Filali-Maltouf, A. Diversity and spatial genetic structure of natural Moroccan Quercus susber L. assessed by ISSR markers for conservation. Physiol. Mol. Biol. Plants 2018, 24, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Al Salameen, F.; Habibi, M.; Kumar, V.; Al Amad, S.; Dashti, J.; Talebi, L.; Al Doaij, B. Genetic diversity and population structure of Haloxylon salicornicum moq. in Kuwait by ISSR markers. PLoS ONE 2018, 13, e0207369. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, X.; Zhang, Y. Genetic relationship of nine hardy Rhododendrons by ISSR markers. Acta Agric. Bor-Occidentalis Sin. 2010, 19, 89–92. [Google Scholar]
- Zheng, Y.; He, T.; Chen, L.; Chen, L.; Rong, J.; Zheng, Y. ISSR analysis on the cultivars of Rhododendron hybridum. J. Fujian Coll. For. 2011, 40, 271–275. [Google Scholar]
- POPGENE Version 1.32; the user-friendly shareware for population genetic analysis, Molecular Biology and Biotechnology Centre, University of Alberta, Edmonton, Canada. Available online: https://sites.ualberta.ca/~fyeh/popgene.html (accessed on 23 November 2017).
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar]
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Yang, M.; Fu, Y. AveDissR: An R function for assessing genetic distinctness and genetic redundancy. Appl. Plant Sci. 2017, 5, apps.1700018. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hong, Z.; Xu, D.; Jia, H.; Zhang, N.; Liu, X.; Yang, Z.; Lu, M. Genetic diversity of the endangered Dalbergia odorifera revealed by SSR markers. Forests 2019, 10, 225. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc: Microcomputer Programs for Numerical Taxonomy and Multivariate Analysis. Am. Stat. 1987, 41, 330. [Google Scholar] [CrossRef]
- Garriga, M.; Parra, P.A.; Caligari, P.D.S.; Retamales, J.B.; Carrasco, B.A.; Lobos, G.A.; García-Gonzáles, R. Application of inter-simple sequence repeats relative to simple sequence repeats as a molecular marker system for indexing blueberry cultivars. Can. J. Plant Sci. 2013, 93, 913–921. [Google Scholar] [CrossRef]
- Hamrick, J.L.; Godt, M.J.W.; Sherman-Broyles, S.L. Factors influencing levels of genetic diversity in woody plant species. New Forests 1992, 6, 95–124. [Google Scholar] [CrossRef]
- Nagy, E.S.; Strong, L.; Galloway, L.F. Contribution of delayed autonomous selfing to reproductive success in mountain laurel, Kalmia latifolia (Ericaceae). Am. Midl. Nat. 1999, 142, 39–46. [Google Scholar] [CrossRef]
- Hamrick, J.L.; Godt, M.J.W. Effects of life history traits on genetic diversity in plant species. Phil. Trans. R. Soc. Lond B 1996, 351, 1291–1298. [Google Scholar] [CrossRef]
- Nybom, H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 2004, 13, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wuyun, T.; Li, T.; Liu, H.; Jiang, Z.; Zhu, X.; Du, H.; Bai, Y. Genetic diversity and population structure of Prunus mira (Koehne) from the Tibet plateau in China and recommended conservation strategies. PLoS ONE 2017, 12, e0188685. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.A.; Knapp, E.E.; Guerrant, E.O. An Introduction to Restoration Genetics. Available online: ttps://cdn.ymaws.com/www.ser.org/resource/resmgr/custompages/publications/ser_publications/ser_restoration_genetics.pdf (accessed on 21 July 2020).
- Godt, M.J.W.; Johnson, B.R.; Hamrick, J.L. Genetic diversity and population size in four rare southern Appalachian plant species. Conserv. Biol. 1996, 10, 796–805. [Google Scholar] [CrossRef]
- Ling, X.; Li, X.; Wang, X.; Yang, J.; Lv, K.; Xiong, Z.; Chen, F.; Huang, C. Genetic diversity and population structure of Distylium chinense revealed by ISSR and SRAP analysis in the Three Gorges Reservoir Region of the Yangtze River, China. Glob. Ecol. Conserv. 2020, 21, e00805. [Google Scholar] [CrossRef]
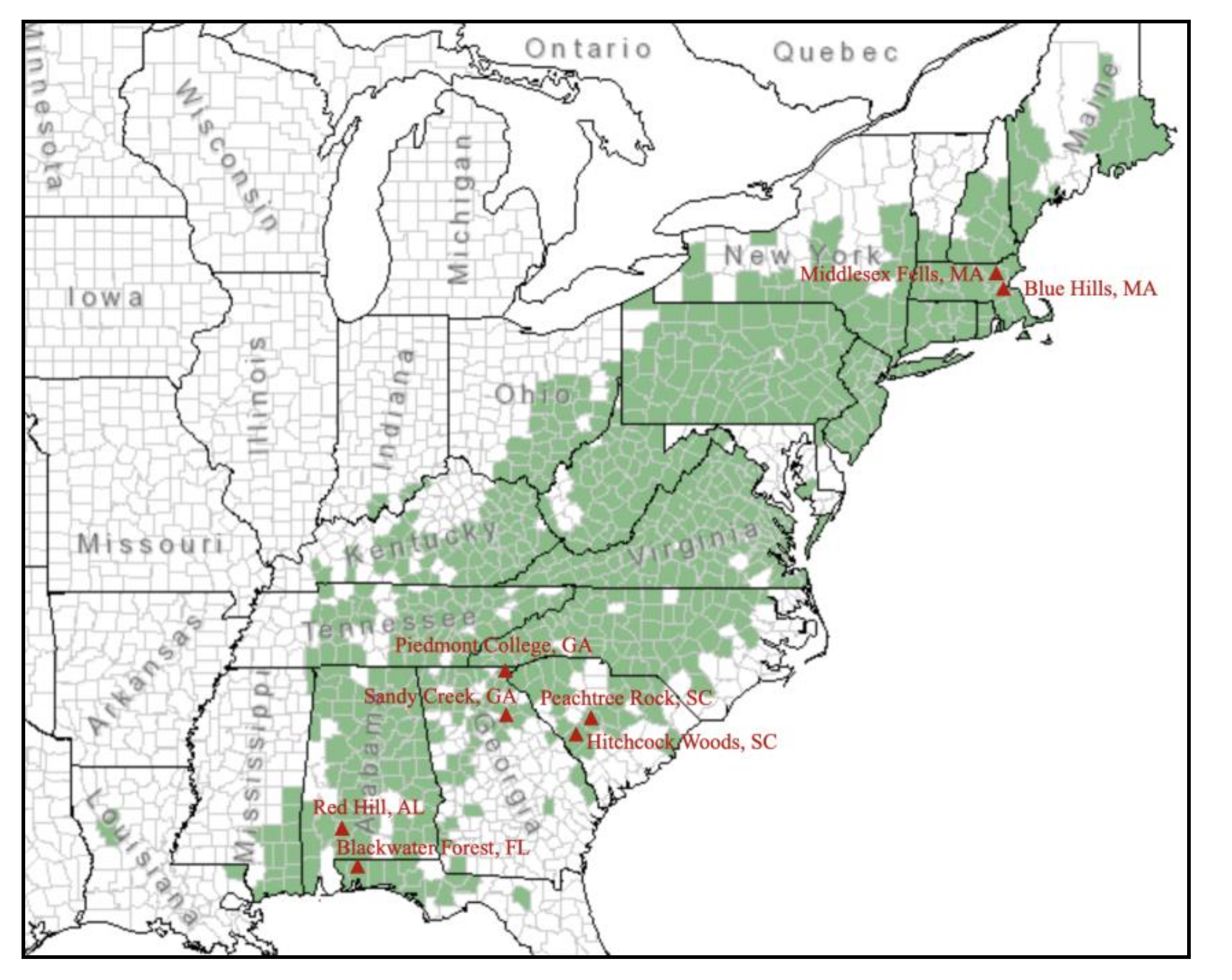



| Population | Location | Latitude | Longitude | Sample nos. |
|---|---|---|---|---|
| Middlesex Fells (MF) | Middlesex, Massachusetts | 42°26′51″ N | 71°5′26″ W | MA1-6 |
| Blue Hills (BH) | Norfolk, Massachusetts | 42°12′55″ N | 71°3′3″ W | MA7-12 |
| Peachtree Rock (PR) | Lexington, South Carolina | 33°49′47″ N | 81°11′57″ W | SC1-6 |
| Hitchcock Woods (HW) | Aiken, South Carolina | 33°33′9″ N | 81°44′33″ W | SC7-12 |
| Red Hill (RH) | Monroe, Alabama | 31°44′17″ N | 87°21′30″ W | AL1-6 |
| Blackwater Forest (BF) | Santa Rosa, Florida | 30°47′23″ N | 86°47′33″ W | FL1-6 |
| Piedmont College (PC) | Rabun, Georgia | 34°51′50″ N | 83°23′16″ W | GA1-6 |
| Sandy Creek (SC) | Clarke, Georgia | 34°0′58″ N | 83°22′32″ W | GA7-12 |
| ISSR Primers | Sequence (5’ – 3’) | Total Number of Bands | Number of Polymorphic Bands | PPB |
|---|---|---|---|---|
| UBC808 | (AG)8C | 14 | 13 | 92.86% |
| UBC814 | (CT)8A | 10 | 8 | 80% |
| UBC835 | (AG)8YC | 13 | 12 | 92.31% |
| UBC836 | (AG)8YA | 16 | 13 | 81.25% |
| UBC841 | (GA)8YC | 13 | 11 | 84.62% |
| UBC856 | (AC)8YA | 15 | 14 | 93.33% |
| UBC864 | (ATG)6 | 19 | 19 | 100% |
| UBC873 | (GACA)4 | 16 | 15 | 93.75% |
| Total | 116 | 105 | 90.52% |
| Population | Na | Noa | Nea | h | I | PPB |
|---|---|---|---|---|---|---|
| MF | 81 | 1.4569 | 1.3130 | 0.1801 | 0.2648 | 45.69 |
| BH | 78 | 1.4052 | 1.2485 | 0.1485 | 0.2224 | 40.52 |
| PR | 89 | 1.5517 | 1.3812 | 0.2174 | 0.3194 | 55.17 |
| HW | 90 | 1.5086 | 1.3362 | 0.1949 | 0.2884 | 50.86 |
| RH | 87 | 1.5086 | 1.3239 | 0.1906 | 0.2837 | 50.86 |
| BF | 94 | 1.5431 | 1.3357 | 0.2007 | 0.3000 | 54.31 |
| PC | 97 | 1.5603 | 1.3881 | 0.2213 | 0.3249 | 56.03 |
| SC | 90 | 1.4741 | 1.2981 | 0.1762 | 0.2629 | 47.41 |
| Total | 116 | 1.9052 | 1.5191 | 0.3089 | 0.4654 | 90.52 |
| Population | HT | HS | GST |
|---|---|---|---|
| Massachusetts group (MF and BH) | 0.2059 | 0.1643 | 0.2023 |
| South Carolina group (PR and HW) | 0.2523 | 0.2062 | 0.1827 |
| Southernmost group (RH and BF) | 0.2391 | 0.1956 | 0.1818 |
| Georgia group (PC and SC) | 0.2474 | 0.1988 | 0.1965 |
| Total | 0.3089 | 0.1912 | 0.3809 |
| Population | MF | BH | PR | HW | RH | BF | PC | SC |
|---|---|---|---|---|---|---|---|---|
| MF | ||||||||
| BH | 0.2023 | |||||||
| PR | 0.2095 | 0.2554 | ||||||
| HW | 0.2699 | 0.3252 | 0.1827 | |||||
| RH | 0.2925 | 0.3426 | 0.2420 | 0.2452 | ||||
| BF | 0.2656 | 0.3177 | 0.2392 | 0.2761 | 0.1818 | |||
| PC | 0.2049 | 0.2431 | 0.2083 | 0.2347 | 0.2675 | 0.2356 | ||
| SC | 0.3155 | 0.3586 | 0.2541 | 0.2733 | 0.3142 | 0.3039 | 0.1965 |
| Source of Variation | d.f. | Sum of Squares | Variance Component | Percentage of Variance | P |
|---|---|---|---|---|---|
| Among populations | 7 | 327.542 | 5.58 | 29.54 | <0.001 |
| Within populations | 40 | 532.333 | 13.31 | 70.46 | <0.001 |
| Total | 47 | 859.875 | 18.89 |
| Population | MF | BH | PR | HW | RH | BF | PC | SC |
|---|---|---|---|---|---|---|---|---|
| MF | 29.45 | 1533.70 | 1594.86 | 2158.12 | 2203.19 | 1593.25 | 1667.28 | |
| BH | 0.0868 | 1528.87 | 1590.03 | 2153.30 | 2198.36 | 1588.42 | 1662.45 | |
| PR | 0.1178 | 0.1451 | 64.86 | 708.11 | 711.33 | 271.98 | 238.18 | |
| HW | 0.1659 | 0.2033 | 0.0994 | 645.35 | 646.95 | 251.06 | 191.51 | |
| RH | 0.1875 | 0.2201 | 0.1550 | 0.1466 | 147.74 | 597.07 | 534.30 | |
| BF | 0.1647 | 0.1993 | 0.1574 | 0.1857 | 0.0920 | 626.03 | 550.39 | |
| PC | 0.1151 | 0.1354 | 0.1341 | 0.1514 | 0.1859 | 0.1554 | 114.91 | |
| SC | 0.2031 | 0.2263 | 0.1597 | 0.1670 | 0.2098 | 0.2117 | 0.1061 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chappell, M.; Zhang, D. Assessing Genetic Diversity and Population Structure of Kalmia latifolia L. in the Eastern United States: An Essential Step towards Breeding for Adaptability to Southeastern Environmental Conditions. Sustainability 2020, 12, 8284. https://doi.org/10.3390/su12198284
Li H, Chappell M, Zhang D. Assessing Genetic Diversity and Population Structure of Kalmia latifolia L. in the Eastern United States: An Essential Step towards Breeding for Adaptability to Southeastern Environmental Conditions. Sustainability. 2020; 12(19):8284. https://doi.org/10.3390/su12198284
Chicago/Turabian StyleLi, He, Matthew Chappell, and Donglin Zhang. 2020. "Assessing Genetic Diversity and Population Structure of Kalmia latifolia L. in the Eastern United States: An Essential Step towards Breeding for Adaptability to Southeastern Environmental Conditions" Sustainability 12, no. 19: 8284. https://doi.org/10.3390/su12198284
APA StyleLi, H., Chappell, M., & Zhang, D. (2020). Assessing Genetic Diversity and Population Structure of Kalmia latifolia L. in the Eastern United States: An Essential Step towards Breeding for Adaptability to Southeastern Environmental Conditions. Sustainability, 12(19), 8284. https://doi.org/10.3390/su12198284





