Deforestation and Subsequent Cultivation of Nutrient Poor Soils of Miombo Woodlands of Tanzania: Long Term Effect on Maize Yield and Soil Nutrients
Abstract
1. Introduction
2. Materials and Methods
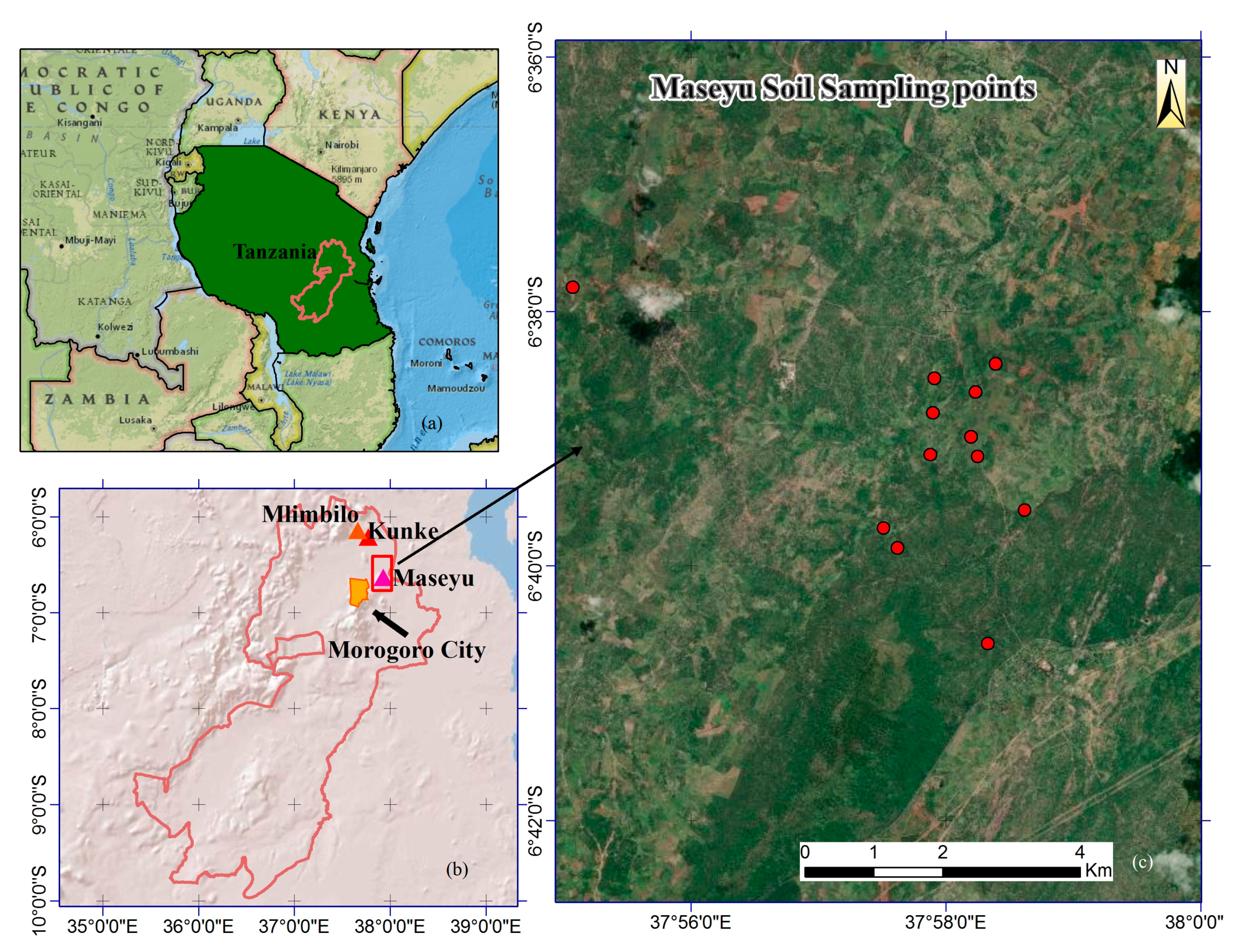
2.1. Study Sites
2.2. Farming Systems
2.3. Data Collection
2.3.1. Socioeconomic Survey
2.3.2. Soil Sample Collection and Analysis
2.4. Data Analysis
2.4.1. Socioeconomic Data
2.4.2. Soil Data
3. Results
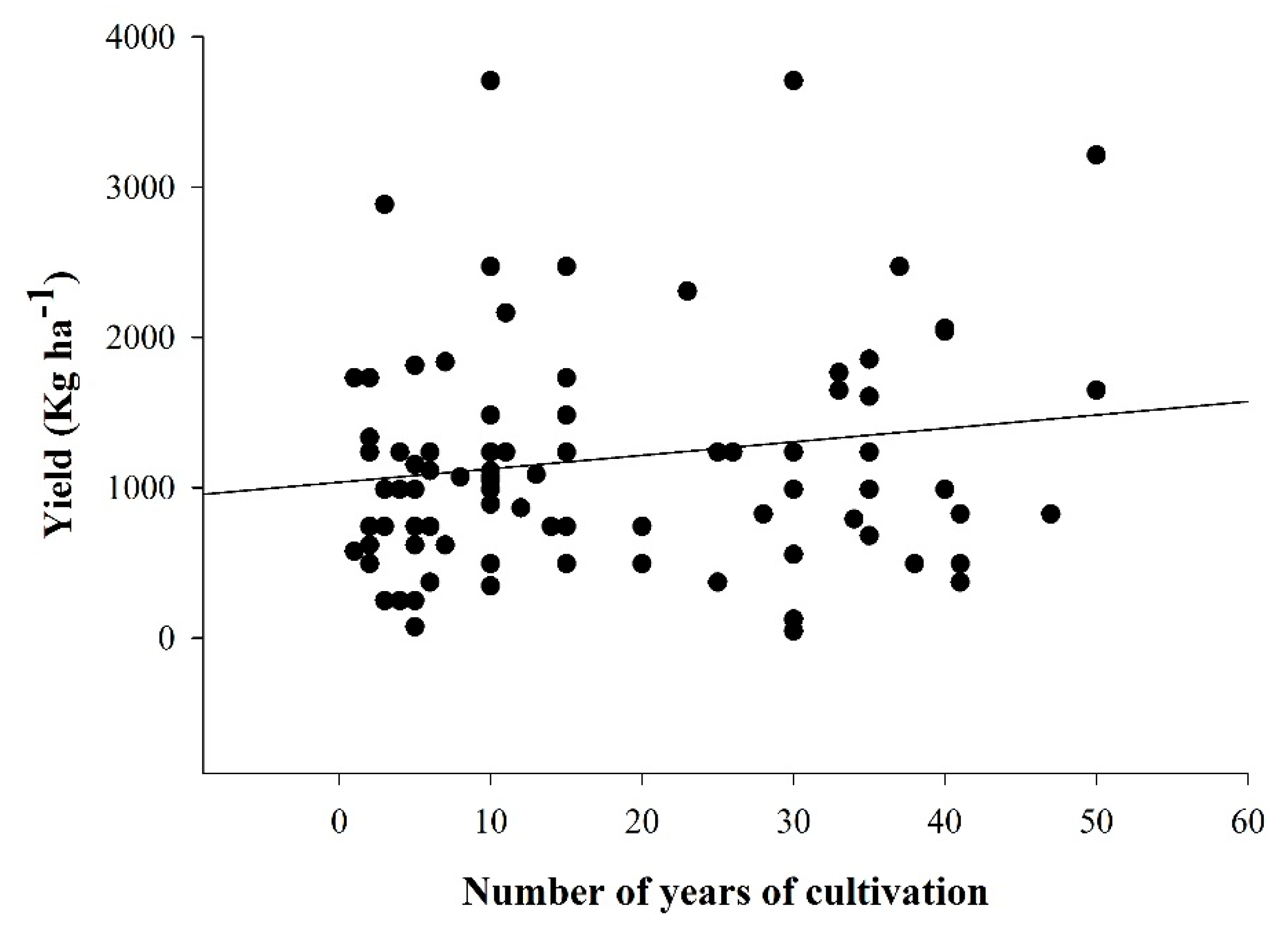
3.1. Maize Yield and Cultivation Period
3.2. Soil Properties Responses to Land-Use Conversion and Continuous Cultivation
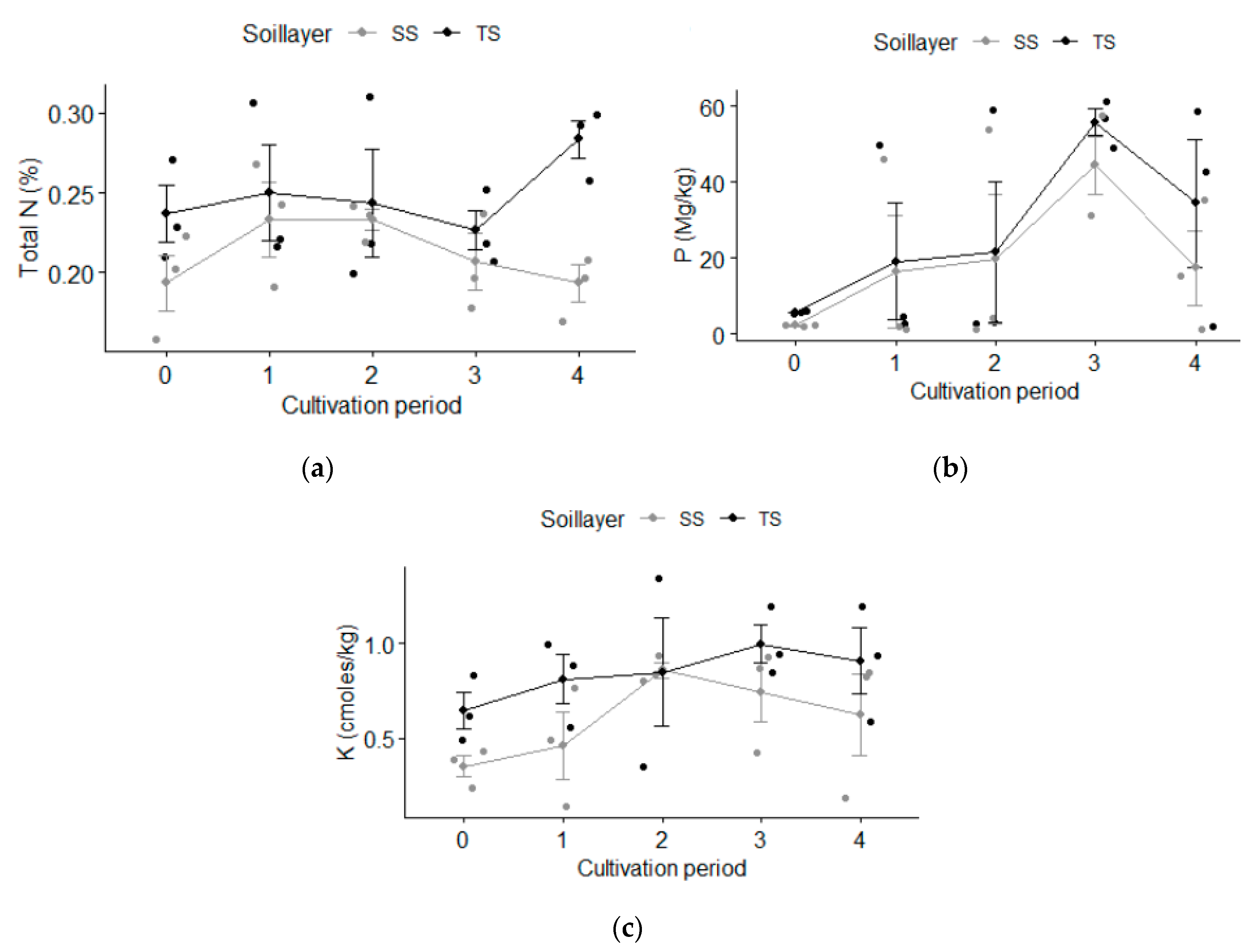
3.2.1. Major Plant Nutrients (N, P and K)
3.2.2. Soil Bulk Density (BD)
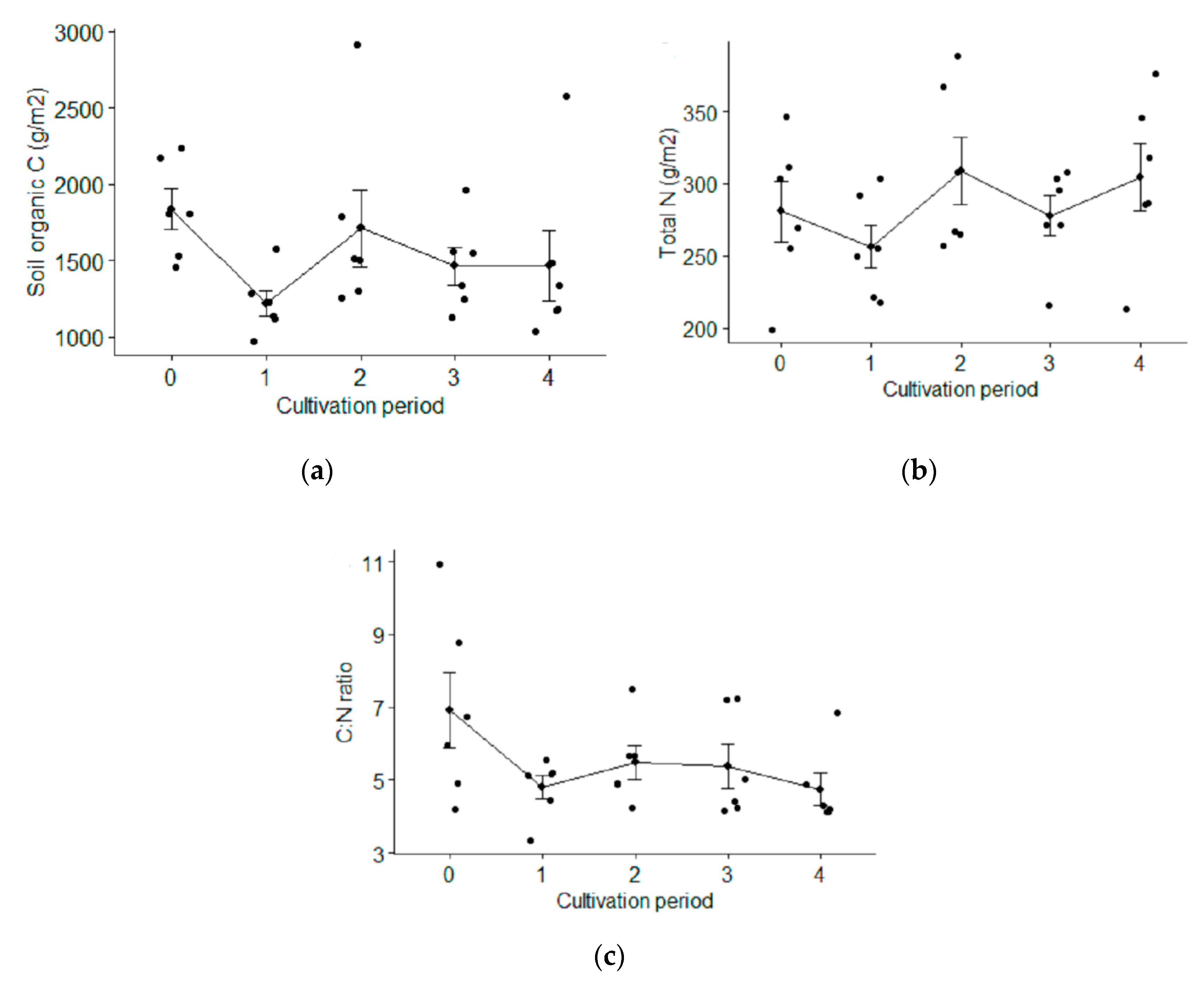
3.2.3. Soil Organic Carbon (SOC) Concentration
3.2.4. Soil Organic Carbon (SOC) and Total Nitrogen (Total N) Stocks
3.2.5. Carbon to Nitrogen Ration (C:N Ratio)
4. Discussion
4.1. Maize Yield and Number of Years of Cultivation
4.2. Soil Chemical and Physical Properties Responses to Continuous Cultivation
4.2.1. Major Plant Nutrients (N, P and K)
4.2.2. Bulk Density (BD)
4.2.3. Soil Organic Carbon (SOC)
4.2.4. Soil Organic Carbon (SOC) and Total Nitrogen Stocks (Total N)
4.2.5. Carbon to Nitrogen Ration (C:N Ratio)
4.3. Implications of the Soil Analysis Results for Maize Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arunrat, N.; Pumijumnong, N.; Sereenonchai, S.; Chareonwong, U. Factors Controlling Soil Organic Carbon Sequestration of Highland Agricultural Areas in the Mae Chaem Basin, Northern Thailand. Agronomy 2020, 10, 305. [Google Scholar] [CrossRef]
- Sonneveld, B.G.J.S.; Keyzer, M.A.; Ndiaye, D. Quantifying the impact of land degradation on crop production: The case of Senega. Solid Earth 2016, 7, 93–103. [Google Scholar] [CrossRef]
- ELD Initiative. The Rewards of Investing in Sustainable Land Management. Interim Report for the Economics of Land Degradation Initiative: A Global Strategy for Sustainable Land Management, The Economics of Land Degradation (ELD) Initiative. Available online: https://www.eld-initiative.org/fileadmin/pdf/ELD-Interim_Report_web.pdf (accessed on 18 May 2020).
- Yin, H.; Pflugmacher, D.; Kennedy, R.E.; Sulla-Menashe, D.; Hostert, P. Mapping annual land use and land cover changes using MODIS time series. IEEE J. Sel. Top. Appl. 2014, 7, 3421–3427. [Google Scholar] [CrossRef]
- Jew, E.K.K.; Dougill, A.J.; Sallu, S.M.; O’Connell, J.; Benton, T.G. Miombo woodland under threat: Consequences for tree diversity and carbon storage. Ecol. Manag. 2016, 361, 144–153. [Google Scholar] [CrossRef]
- Abdallah, J.M.; Monela, G.G. Overview of Miombo Woodlands in Tanzania. Work. Pap. Finn. For. Res. Inst. 2007, 50, 9–23. [Google Scholar]
- NAFORMA. Forest Resources in Tanzania; Ministry of Natural Resources and Tourism, Forestry and Beekeeping Division: Dar es Salaam, Tanzania, 2014. [Google Scholar]
- Lupala, Z.J.; Lusambo, L.P.; Ngaga, Y.M.; Makatta, A.A. The Land Use and Cover Change in Miombo Woodlands under Community Based Forest Management and Its Implication to Climate Change Mitigation: A Case of Southern Highlands of Tanzania. Int. J. Res. 2015. [Google Scholar] [CrossRef]
- Angelsen, A.; Shitindi, E.F.K.; Aarrestad, J. Why do farmers expand their land into forests? Theories and evidence from Tanzania. Environ. Dev. Econ. 1999, 4, 313–331. [Google Scholar] [CrossRef]
- Luoga, E.J.; Witkowski, E.T.F.; Balkwill, K. Land Cover and Use Changes in Relation to the Institutional Framework and Tenure of Land and Resources in Eastern Tanzania Miombo Woodlands. Environ. Dev. Sustain. 2005, 7, 71–93. [Google Scholar] [CrossRef]
- Lusambo, L.; Monela, G.; Katani, J. Socio-economic Analysis of Land Use Factors Causing Degradation and Deforestation of miombo woodlands in Kilosa district, Tanzania. Tanzan. J. For. Nat. Conserv. 2008, 76, 28–39. [Google Scholar]
- Nduwamungu, J.; Bloesch, U.; Munishi, P.T.K.; Hagedorn, F.; Lulu, K. Recent land cover and use changes in miombo woodlands of eastern Tanzania. Tanzan. J. For. Nat. Conserv. 2008, 78, 50–59. [Google Scholar]
- Kissinger, G.; Herold, M.; Sy, V.D. Drivers of Deforestation and Forest Degradation: A Synthesis Report for REDD+ Policymakers; Lexeme Consulting: Vancouver, BC, Canada, 2012. [Google Scholar]
- Koellner, T.; Sell, J.; Gähwiler, M.; Scholz, R.W. Assessment of the management of organizations supplying ecosystem services from tropical forests. Glob. Environ. Chang. 2008, 18, 746–757. [Google Scholar] [CrossRef]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable intensification in agriculture: Premises and policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil quality changes under continuous cropping for seventeen seasons of an Alfisol in western Nigeria. Land Degrad. Dev. 1998, 9, 259–274. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric. Ecosyst. Environ. 2000, 79, 9–16. [Google Scholar] [CrossRef]
- Zingore, S.; Manyame, C.; Nyamugafata, P.; Giller, K. Long-term changes in organic matter of woodland soils cleared for arable cropping in Zimbabwe. Eur. J. Soil Sci. 2005, 56, 727–736. [Google Scholar] [CrossRef]
- Yimer, F.; Ledin, S.; Abdelkadir, A. Changes in soil organic carbon and total nitrogen contents inthree adjacent land use types in the Bale Mountains, south-eastern highlands of Ethiopia. For. Ecol. Manag. 2007, 242, 337–342. [Google Scholar] [CrossRef]
- Abegaz, A.; van Keulen, H. Modeling soil nutrient dynamics under alternative farm management practices in the Northern Highlands of Ethiopia. Soil Tillage Res. 2009, 103, 203–215. [Google Scholar] [CrossRef]
- Murty, D.; Kirschbaum, M.U.; Mcmurtrie, R.E.; Mcgilvray, H. Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Glob. Chang. Biol. 2002, 8, 105–123. [Google Scholar] [CrossRef]
- Haileslassie, A.; Priess, J.; Veldkamp, E.; Teketay, D.; Lesschen, J.P. Assessment of soil nutrient depletion and its spatial variability on smallholders’ mixed farming systems in Ethiopia using partial versus full nutrient balances. Agric. Ecosyst. Environ. 2005, 108, 1–16. [Google Scholar] [CrossRef]
- Hati, K.M.; Swarup, A.; Singh, D.; Misra, A.K.; Ghosh, P.K. Long-term continuous cropping, fertilisation, and manuring effects on physical properties and organic carbon content of a sandy loam soil. Soil Res. 2006, 44, 487–495. [Google Scholar] [CrossRef]
- Lemenih, M.; Karltun, E.; Olsson, M. Assessing soil chemical and physical property responses to deforestation and subsequent cultivation in smallholders farming system in Ethiopia. Agric. Ecosyst. Environ. 2005, 105, 373–386. [Google Scholar] [CrossRef]
- MRDO. Land Use Plan for Kunke, Maseyu, and Mlimbilo Villages; Morogoro Rural District Office: Morogoro, Tanzania, 2006. [Google Scholar]
- Msanya, B.M.; Kimaro, D.N.; Shayo-ngowi, A.J. Soils of Kitulanghalo Forest Reserve Area, Morogoro District, Tanzania; Department of Soil Science, Faculty of Agriculture, Sokoine University of Agriculture: Morogoro, Tanzania, 1995. [Google Scholar]
- Msanya, B.M.; Kaaya, A.K.; Araki, S.; Otsuka, H.; Nyadzi, G.I. Pedological characterstics, general fertility and classification of some benchmark soils of Morogoro district, Tanzania. Afr. J. Sci. Technol. 2003, 4, 11. [Google Scholar]
- Araya, M.M.; Hofstad, O. Monetary incentives to avoid deforestation under the Reducing emissions from deforestation and degradation (REDD+) climate change mitigation scheme in Tanzania. Mitig. Adapt. Strateg. Glob. Chang. 2016, 21, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.K.R. An Introduction to Agroforestry; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Blake, G.R. Bulk Density 1. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Black, C.A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 374–390. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties; Page, A.L., et al., Eds.; ASA Monograph Number 9; ASA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable Cations. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Day, P.R. Particle Fractionation and Particle-Size Analysis. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Black, C.A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareffff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kimaro, A.; Timmer, V.; Mugasha, A.; Chamshama, S.O.; Kimaro, D. Nutrient use efficiency and biomass production of tree species for rotational woodlot systems in semi-arid Morogoro, Tanzania. Agrofor. Syst. 2007, 71, 175–184. [Google Scholar] [CrossRef]
- Walker, S.M.; Desanker, P.V. The impact of land use on soil carbon in Miombo Woodlands of Malawi. For. Ecol. Manag. 2004, 203, 345–360. [Google Scholar] [CrossRef]
- Haile, S.G.; Nair, P.; Nair, V.D. Carbon storage of different soil-size fractions in Florida silvopastoral systems. J. Environ. Qual. 2008, 37, 1789–1797. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Soil organic carbon and total nitrogen stocks under different land uses in a semi-arid watershed in Tigray, Northern Ethiopia. Agric. Ecosyst. Environ. 2014, 188, 256–263. [Google Scholar] [CrossRef]
- Mtambanengwe, F.; Kirchmann, H. Litter from a tropical savanna woodland (Miombo): Chemical composition and C and N mineralization. Soil Biol. Biochem. 1995, 27, 1639–1651. [Google Scholar] [CrossRef]
- Detwiler, R.P. Land use change and the global carbon cycle: The role of tropical soils. Biogeochemistry 1986, 2, 67–93. [Google Scholar] [CrossRef]
- Davidson, E.A.; Ackerman, I.L. Changes in soil carbon inventories following cultivation of previously untilled soils. Biogeochemistry 1986, 20, 161. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.W.B. Particle-size Fractions and their Use in Studies of Soil Organic Matter: II. Cultivation Effects on Organic Matter Composition in Size Fractions1. Soil Sci. Soc. Am. J. 1983, 47, 509–514. [Google Scholar] [CrossRef]
| Variables | Description | Variable Type | Number of Factors (Levels) | Effect |
|---|---|---|---|---|
| Response variable | ||||
| Yield (Yie) | Total yield harvested (kg ha−1) | Continuous | ||
| Explanatory variables | ||||
| Village (Vil) | An administrative unit | Factor | 3 factors | Random |
| Sex | Sex of the household head | Factor | 2 factors | Fixed |
| Age | Age of the household head | Continuous | Fixed | |
| Family size (FL) | Family member above 10 years old are considered in the analysis | Continuous | Fixed | |
| Education (Ed) | Number of schooling years completed by the household head | Factor | 3 factors | Fixed |
| Number of plots (NP) | Number of plots managed by the household | Continuous | Fixed | |
| Ownership (OS) | If the farm manager has user right to the plot or rents from others | Factor | 2 factors | Fixed |
| Plot size (PS) | Total area of a given plot | Continuous | Fixed | |
| Distance from household (DHH) | Time required to reach to the plot from the user’s house | Continuous | Fixed | |
| Cultivation period (CY) | The time period since the land was being used for crop cultivation | Factor | 4 factors | Fixed |
| Distance from the nearest forest (DF) | Distance in minutes walking to the particular plot from the nearest forest | Continuous | Fixed | |
| Soli type (ST) | Soil texture based on the farm managers description | Factor | 4 factors | Fixed |
| Land quality (LQ) | Land quality in terms of fertility according to the farm managers | Factor | 3 factors | Fixed |
| Slop (Slo) | The degree to which the plot is exposed to erosion (slop of the farmland) | Factor | 3 factors | Fixed |
| Degree of weed infestation (DW) | The degree to which the plot is affected by weed | Factor | 4 factors | Fixed |
| Seed input (SI) | Amount of seed used per hectare | Continuous | Fixed | |
| Labor input (LI) | Man-days employed per hectare of land | Continuous | Fixed |
| Source of Variation | df | SS | MS | F | Pr (>F) |
|---|---|---|---|---|---|
| Sex | 1 | 1,254,948 | 1,254,948 | 2.209 | 0.141 |
| Age | 1 | 18,745 | 18,745 | 0.033 | 0.856 |
| FL | 1 | 221,637 | 221,637 | 0.390 | 0.534 |
| Ed | 2 | 610,983 | 305,492 | 0.538 | 0.586 |
| NP | 1 | 362,213 | 362,213 | 0.637 | 0.427 |
| DHH | 1 | 7,641,817 | 7,641,817 | 13.448 | <0.000 *** |
| CY | 1 | 287,360 | 287,360 | 0.506 | 0.479 |
| OS | 1 | 585,152 | 585,152 | 1.030 | 0.313 |
| DF | 1 | 8750 | 8750 | 0.015 | 0.901 |
| ST | 3 | 2,201,571 | 733,857 | 1.291 | 0.284 |
| LQ | 2 | 4,492,679 | 2,246,340 | 3.953 | <0.05 * |
| Slo | 3 | 554,817 | 184,939 | 0.325 | 0.807 |
| DW | 3 | 251,357 | 83,786 | 0.147 | 0.931 |
| PS | 1 | 221,993 | 221,993 | 0.391 | 0.534 |
| SI | 1 | 30,956 | 30,956 | 0.054 | 0.816 |
| LI | 1 | 889139 | 889139 | 1.565 | 0.215 |
| Soil Parameter | Depth (cm) | Cultivation Period | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1–10 | 11–20 | 21–30 | >30 | |||
| Total N (%) | 0–10 | 0.24 (±0.02) | 0.25 (±0.03) | 0.24 (±0.03) | 0.23 (±0.01) | 0.28 (±0.01) | |
| 10–20 | 0.19 (±0.02) | 0.23 (±0.02) | 0.23 (±0.01) | 0.21 (±0.02) | 0.19 (±0.01) | ||
| P (mg kg−1) | 0–10 | 5.64 (±0.16) | 19.15 (±15.98) | 21.61 (±18.65) | 55.71 (±3.50) | 34.36 (±16.81) | |
| 10–20 | 2.20 (±0.12) | 16.38 (±14.83) | 19.68 (±17.16) | 44.38 (±7.63) | 17.31 (±9.86) | ||
| K exchangeable (cmoles kg−1) | 0–10 | 0.65 (±0.10) | 0.81 (±0.13) | 0.85 (±0.28) | 1.00 (±0.10) | 0.91 (±0.17) | |
| 10–20 | 0.35 (±0.06) | 0.46 (±0.18) | 0.86 (±0.04) | 0.74 (±0.16) | 0.62(±0.22) | ||
| pH | 0–10 | 5.79 (±0.28) | 5.62 (±0.28) | 5.58 (±0.44) | 5.72 (±0.12) | 5.73 (±0.36) | |
| 10–20 | 5.56 (±0.26) | 5.38 (±0.24) | 5.14 (±0.06) | 5.67 (±0.17) | 5.64 (±0.07) | ||
| SOC (%) | 0–10 | 1.47 (±0.28) | 1.24 (±0.18) | 1.52 (±0.41) | 1.24 (±0.18) | 1.47 (±0.29) | |
| 10–20 | 1.40 (±0.23) | 1.06 (±0.10) | 1.14 (±0.07) | 1.08 (±0.20) | 0.83 (±0.01) | ||
| BD (g cm−3) | 0–10 | 1.28 (±0.10) | 0.99 (±0.10) | 1.25 (±0.02) | 1.29 (±0.09) | 1.22 (±0.02) | * |
| 10–20 | 1.34 (±0.11) | 1.17 (±0.05) | 1.34 (±0.10) | 1.28 (±0.12) | 1.35 (±0.05) | ||
| Clay (%) | 0–10 | 14.10 (±1.32) | 15.93 (±2.03) | 23.43 (±2.80) | 15.77 (±3.09) | 15.07 (±1.35) | * |
| 10–20 | 18.27 (±1.48) | 15.93 (±2.85) | 22.27 (±2.90) | 15.60 (±0.58) | 19.77 (±4.05) | ||
| Cultivation Period | Depth (cm) | SOC (gm−2) | Total N (gm−2) | C:N Ratio |
|---|---|---|---|---|
| 0 | 0–10 | 1835 (±227) | 302 (±27) | 6.31 (±1.33) |
| 10–20 | 1837 (±187) | 260 (±33) | 7.52 (±1.79) | |
| 1–10 | 0–10 | 1181 (±52) | 241 (±12) | 4.92 (±0.23) |
| 10–20 | 1260 (±175) | 272 (±26) | 4.70 (±0.69) | |
| 11–20 | 0–10 | 1892 (±517) | 303 (±43) | 6.03 (±0.77) |
| 10–20 | 1538 (±140) | 314 (±29) | 4.93 (±0.41) | |
| 21–30 | 0–10 | 1589 (±208) | 292 (±11) | 5.50 (±0.90) |
| 10–20 | 1340 (±124) | 264 (±25) | 5.26 (±0.98) | |
| >30 | 0–10 | 1802 (±390) | 347 (±17) | 5.12 (±0.87) |
| 10–20 | 1131 (±48) | 262 (±24) | 4.37 (±0.25) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rannestad, M.M.; Gessesse, T.A. Deforestation and Subsequent Cultivation of Nutrient Poor Soils of Miombo Woodlands of Tanzania: Long Term Effect on Maize Yield and Soil Nutrients. Sustainability 2020, 12, 4113. https://doi.org/10.3390/su12104113
Rannestad MM, Gessesse TA. Deforestation and Subsequent Cultivation of Nutrient Poor Soils of Miombo Woodlands of Tanzania: Long Term Effect on Maize Yield and Soil Nutrients. Sustainability. 2020; 12(10):4113. https://doi.org/10.3390/su12104113
Chicago/Turabian StyleRannestad, Meley Mekonen, and Tigist Araya Gessesse. 2020. "Deforestation and Subsequent Cultivation of Nutrient Poor Soils of Miombo Woodlands of Tanzania: Long Term Effect on Maize Yield and Soil Nutrients" Sustainability 12, no. 10: 4113. https://doi.org/10.3390/su12104113
APA StyleRannestad, M. M., & Gessesse, T. A. (2020). Deforestation and Subsequent Cultivation of Nutrient Poor Soils of Miombo Woodlands of Tanzania: Long Term Effect on Maize Yield and Soil Nutrients. Sustainability, 12(10), 4113. https://doi.org/10.3390/su12104113





