Iron Sulfide Minerals as Potential Active Capping Materials for Mercury-Contaminated Sediment Remediation: A Minireview
Abstract
1. Mercury Risk and Global Management Efforts
2. In Situ Remediation and the Need for Alternative Mechanisms
3. Iron Sulfide Minerals: Potential Alternatives
3.1. Introduction of Iron Sulfide Minerals
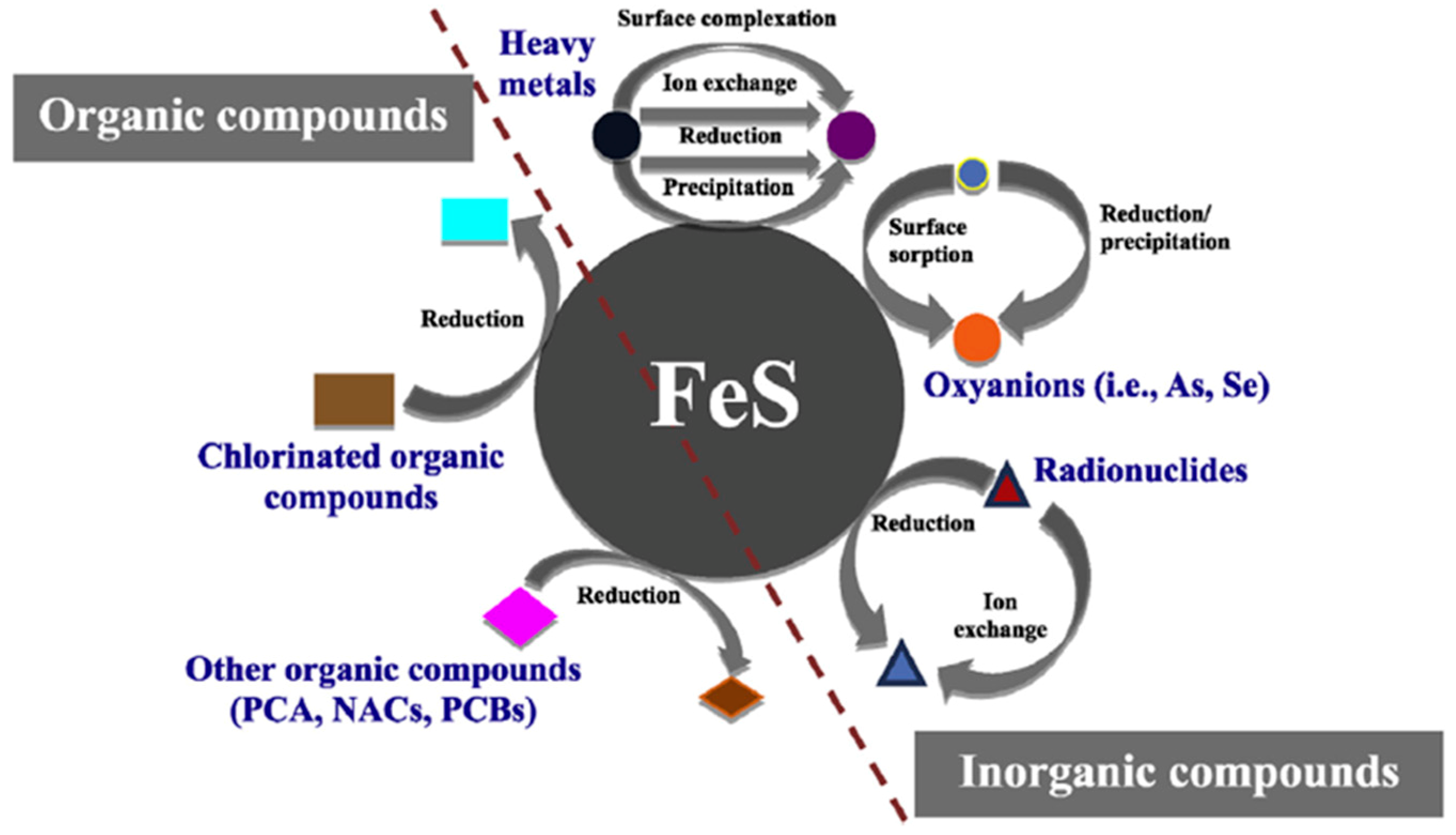
3.2. Hg Sequestration Mechanisms by Iron Sulfides
3.3. Hg Reduction by Aqueous Fe2+
3.4. Advantages of Using Iron Sulfides
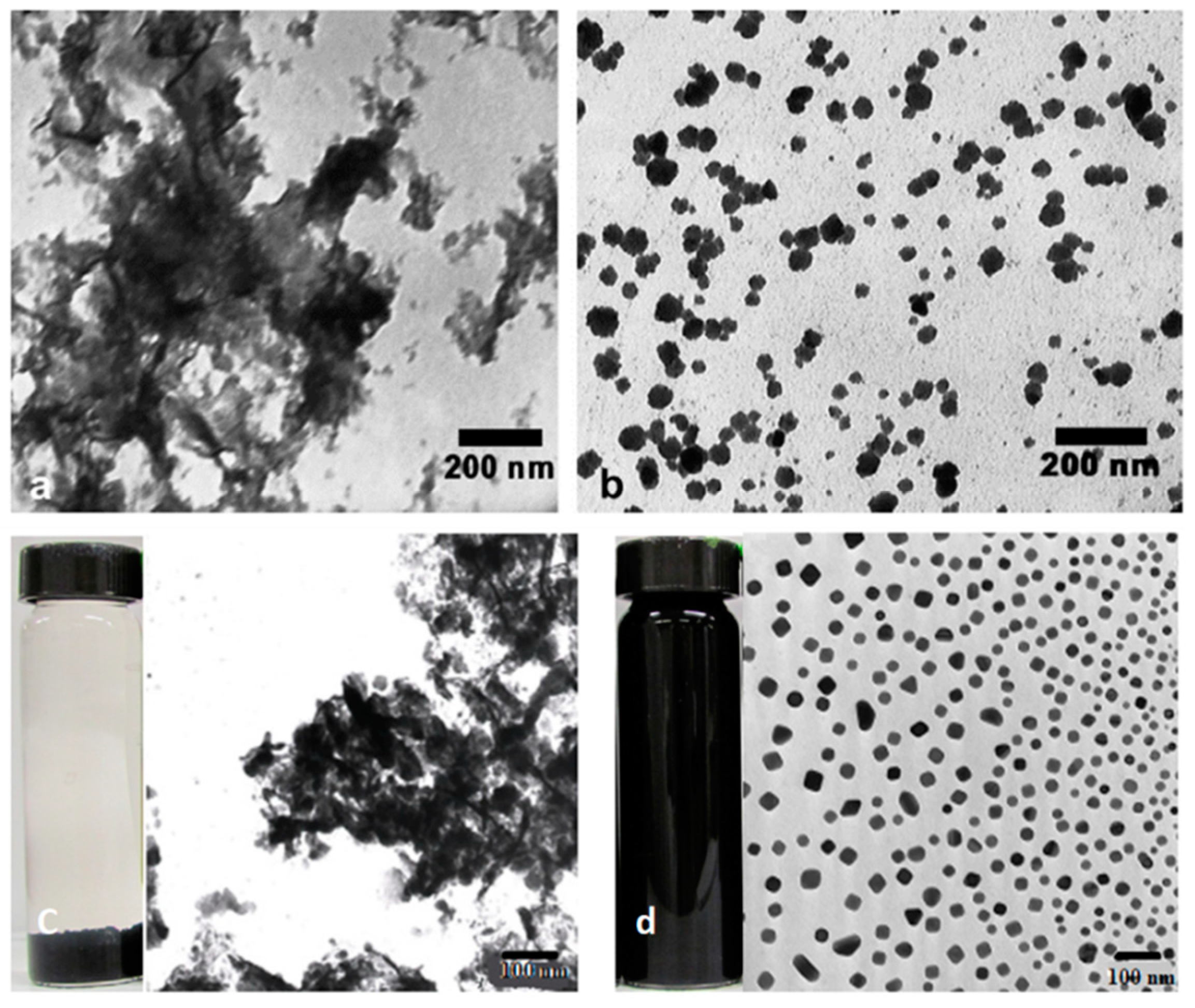
3.5. Material Engineering
4. Conclusions
Funding
Conflicts of Interest
References
- Hsiao, H.W.; Ullrich, S.M.; Tanton, T.W. Burdens of mercury in residents of Temirtau, Kazakhstan I: Hair mercury concentrations and factors of elevated hair mercury levels. Sci. Total Environ. 2011, 409, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Mutter, J.; Naumann, J.; Sadaghiani, C.; Walach, H.; Drasch, G. Amalgam studies: Disregarding basic principles of mercury toxicity. Int. J. Hyg. Environ. Health 2004, 207, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.; James, K.; Levy, L. Is low-level environmental mercury exposure of concern to human health? Sci. Total Environ. 2009, 408, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.H. A review of the studies of the cardiovascular health effects of methylmercury with consideration of their suitability for risk assessment. Environ. Res. 2005, 98, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, H.T.; Kinsner-Ovaskainen, A.; Coecke, S.; Hartung, T.; Bal-Price, A.K. mRNA expression is a relevant tool to identify developmental neurotoxicants using an in vitro approach. J. Toxicol. Sci. 2009, 113, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, X.; Anderson, C.W.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221, 1–18. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Yu, J.G.; Yue, B.Y.; Wu, X.W.; Liu, Q.; Jiao, F.P.X.; Jiang, Y.; Chen, X.Q. Removal of mercury by adsorption: A review. Environ. Sci. Pollut. Res. Int. 2016, 23, 5056–5076. [Google Scholar] [CrossRef]
- Paruchuri, Y.; Siuniak, A.; Johnson, N.; Levin, E.; Mitchell, K.; Goodrich, J.M.; Renne, E.P.; Basu, N. Occupational and environmental mercury exposure among small-scale gold miners in the Talensi–Nabdam District of Ghana’s Upper East region. Sci. Total Environ. 2010, 408, 6079–6085. [Google Scholar] [CrossRef]
- Amos, H.M.; Jacob, D.J.; Kocman, D.; Horowitz, H.M.; Zhang, Y.; Dutkiewicz, S.; Horvat, M.; Corbitt, E.S.; Krabbenhoft, D.P.; Sunderland, E.M. Global biogeochemical implications of mercury discharges from rivers and sediment burial. Environ. Sci. Technol. 2014, 48, 9514–9522. [Google Scholar] [CrossRef]
- Kocman, D.; Wilson, S.J.; Amos, H.M.; Telmer, K.H.; Steenhuisen, F.; Sunderland, E.M.; Mason, R.P.; Outridge, P.; Horvat, M. Toward an assessment of the global inventory of present-day mercury releases to freshwater environments. Int. J. Environ. Res. Public Health 2017, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Horowitz, H.M.; Jacob, D.J.; Lu, Z.; Levin, L.; Ter Schure, A.F.H.; Sunderland, E.M. Total mercury released to the environment by human activities. Environ. Sci. Technol. 2017, 51, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Batley, G.; Stahl, R.; Babut, M.; Bott, T.; Clark, J.; Field, L.; Ho, K.; Mount, D.; Swartz, R.; Tessier, A. Scientific Underpinnings of Sediment Quality Guidelines. In Use of Sediment Quality Guidelines and Related Tools for the Assessment of Contaminated Sediments; SETAC Press: Pensacola, FL, USA, 2005; pp. 39–120. [Google Scholar]
- Sunderland, E.M.; Gobas, F.A.P.C.; Heyes, A.; Branfireun, B.A.; Bayer, A.K.; Cranston, R.E.; Parsons, M.B. Speciation and bioavailability of mercury in well-mixed estuarine sediments. Mar. Chem. 2004, 90, 91–105. [Google Scholar] [CrossRef]
- Mason, R.P.; Kim, E.H.; Cornwell, J.; Heyes, D. An examination of the factors influencing the flux of mercury, methylmercury and other constituents from estuarine sediment. Mar. Chem. 2006, 102, 96–110. [Google Scholar] [CrossRef]
- Clarke, T.L.; Lesht, B.; Young, R.A.; Swift, D.J.P.; Freeland, G.L. Sediment resuspension by surface-wave action—An examination of possible mechanisms. Mar. Geol. 1982, 49, 43–59. [Google Scholar] [CrossRef]
- Lund-Hansen, L.C.; Petersson, M.; Nurjaya, W. Vertical sediment fluxes and wave-induced sediment resuspension in a shallow-water coastal lagoon. Estuaries 1999, 22, 39–46. [Google Scholar] [CrossRef]
- Morgan, B.; Rate, A.W.; Burton, E.D. Water chemistry and nutrient release during the resuspension of FeS-rich sediments in a eutrophic estuarine system. Sci. Total Environ. 2012, 432, 47–56. [Google Scholar] [CrossRef]
- Josefsson, S.; Leonardsson, K.; Gunnarsson, J.S.; Wiberg, K. Bioturbation-driven release of buried PCBs and PBDEs from different depths in contaminated sediments. Environ. Sci. Technol. 2010, 44, 7456–7464. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, M. Interactions between mercury and dissolved organic matter—A review. Chemosphere 2004, 55, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.T.; Han, Y.J.; Chen, C.Y.; Evers, D.C.; Lambert, K.F.; Holsen, T.M.; Kamman, N.C.; Munson, R.K. Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. Bioscience 2007, 57, 17–28. [Google Scholar] [CrossRef]
- Kraepiel, A.M.; Keller, K.; Chin, H.B.; Malcolm, E.G.; Morel, F.M. Sources and variations of mercury in tuna. Environ. Sci. Technol. 2003, 37, 5551–5558. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Q.; Lu, X.; Fang, F.; Wang, Y. Distribution and speciation of mercury in the peat bog of Xiaoxing’an Mountain, northeastern China. Environ. Pollut. 2003, 124, 39–46. [Google Scholar] [CrossRef]
- Loseto, L.L.; Siciliano, S.D.; Lean, D.R. Methylmercury production in High Arctic wetlands. Environ. Toxicol. Chem. 2004, 23, 17–23. [Google Scholar] [CrossRef]
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the aquatic environment: A review of factors affecting methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Efroymson, R.; Suter, G.; Sample, B.; Jones, D. Preliminary Remediation Goals for Ecological Endpoints; Oak Ridge National Lab.: Oak Ridge, TN, USA, 1996.
- Shamsijazeyi, H.; Kaghazchi, T. Simultaneous activation/sulfurization method for production of sulfurized activated carbons: Characterization and Hg(II) adsorption capacity. Water Sci. Technol. 2014, 69, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Shabbir, M.; Abdullah, M.; Shah, S.; McKay, G. Sorption of cadmium from aqueous solution by surfactant-modified carbon adsorbents. Chem. Eng. 2009, 148, 365–370. [Google Scholar] [CrossRef]
- Wajima, T.; Murakami, K.; Kato, T.; Sugawara, K. Heavy metal removal from aqueous solution using carbonaceous K2 S-impregnated adsorbent. J. Environ. Sci. 2009, 21, 1730–1734. [Google Scholar] [CrossRef]
- Kupryianchyk, D.; Rakowska, M.I.; Reible, D.; Harmsen, J.; Cornelissen, G.; van Veggel, M.; Hale, S.E.; Grotenhuis, T.; Koelmans, A.A. Positioning activated carbon amendment technologies in a novel framework for sediment management. Integr. Environ. Assess. Manag. 2015, 11, 221–234. [Google Scholar] [CrossRef]
- Martins, M.; Costa, P.M.; Raimundo, J.; Vale, C.; Ferreira, A.M.; Costa, M.H. Impact of remobilized contaminants in Mytilus edulis during dredging operations in a harbour area: Bioaccumulation and biomarker responses. Ecotoxicol. Environ. Saf. 2012, 85, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, G.; Pedron, F.; Grifoni, M.; Barbafieri, M.; Rosellini, I.; Pezzarossa, B. Soil remediation technologies towards green remediation strategies. Int. J. Environ. Chem. Ecol. Geol. Geophys. Eng. 2016, 10, 654–658. [Google Scholar]
- Zhang, C.; Zhu, M.Y.; Zeng, G.M.; Yu, Z.G.; Cui, F.; Yang, Z.Z.; Shen, L.Q. Active capping technology: A new environmental remediation of contaminated sediment. Environ. Sci. Pollut. Res. Int. 2016, 23, 4370–4386. [Google Scholar] [CrossRef] [PubMed]
- Reible, D.D. Processes, Assessment and Remediation of Contaminated Sediments; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Zimmerman, J.R.; Werner, D.; Ghosh, U.; Millward, R.N.; Bridges, T.S.; Luthy, R.G. Effects of dose and particle size on activated carbon treatment to sequester polychlorinated biphenyls and polycyclic aromatic hydrocarbons in marine sediments. Environ. Toxicol. Chem. 2005, 24, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Cho, Y.M.; Luthy, R.G. In situ sequestration of hydrophobic organic contaminants in sediments under stagnant contact with activated carbon. 1. Column studies. Environ. Sci. Technol. 2014, 48, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- McLeod, P.B.; van den Heuvel-Greve, M.J.; Luoma, S.N.; Luthy, R.G. Biological uptake of polychlorinated biphenyls by Macoma balthica from sediment amended with activated carbon. Environ. Toxicol. Chem. 2007, 26, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, J.E.; Werner, D.; Luthy, R.G. Activated carbon amendment as a treatment for residual DDT in sediment from a superfund site in San Francisco Bay, Richmond, California, USA. Environ. Toxicol. Chem. 2007, 26, 2143–2150. [Google Scholar] [CrossRef]
- Sun, X.L.; Ghosh, U. PCB bioavailability control in Lumbriculus variegatus through different modes of activated carbon addition to sediments. Environ. Sci. Technol. 2007, 41, 4774–4780. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Tomaszewski, J.E.; Luthy, R.G.; Werner, D. Sorption of dichlorodiphenyltrichloroethane (DDT) and its metabolites by activated carbon in clean water and sediment slurries. Water Res. 2009, 43, 4336–4346. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Werner, D. Modeling the mass transfer of hydrophobic organic pollutants in briefly and continuously mixed sediment after amendment with activated carbon. Environ. Sci. Technol. 2010, 44, 3381–3387. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M.L.; Croteau, M.N.l.; Luoma, S.N.; Luthy, R.G. Measurement and modeling of polychlorinated biphenyl bioaccumulation from sediment for the marine polychaete Neanthes arenaceodentata and response to sorbent amendment. Environ. Sci. Technol. 2009, 44, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Millward, R.N.; Bridges, T.S.; Ghosh, U.; Zimmerman, J.R.; Luthy, R.G. Addition of activated carbon to sediments to reduce PCB bioaccumulation by a polychaete (Neanthes arenaceodentata) and an amphipod (Leptocheirus plumulosus). Environ. Sci. Technol. 2005, 39, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Brandli, R.C.; Hartnik, T.; Henriksen, T.; Cornelissen, G. Sorption of native polyaromatic hydrocarbons (PAH) to black carbon and amended activated carbon in soil. Chemosphere 2008, 73, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Patmont, C.R.; Ghosh, U.; LaRosa, P.; Menzie, C.A.; Luthy, R.G.; Greenberg, M.S.; Cornelissen, G.; Eek, E.; Collins, J.; Hull, J.; et al. In situ sediment treatment using activated carbon: A demonstrated sediment cleanup technology. Integr. Environ. Assess. Manag. 2015, 11, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Eyles, J.L.; Yupanqui, C.; Beckingham, B.; Riedel, G.; Gilmour, C.; Ghosh, U. Evaluation of biochars and activated carbons for in situ remediation of sediments impacted with organics, mercury, and methylmercury. Environ. Sci. Technol. 2013, 47, 13721–13729. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Riedel, G.S.; Riedel, G.; Kwon, S.; Landis, R.; Brown, S.S.; Menzie, C.A.; Ghosh, U. Activated carbon mitigates mercury and methylmercury bioavailability in contaminated sediments. Environ. Sci. Technol. 2013, 47, 13001–13010. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.S.; Huntington, T.G.; Marvin-DiPasquale, M.C.; Amirbahman, A. Mercury remediation in wetland sediment using zero-valent iron and granular activated carbon. Environ. Pollut. 2016, 212, 366–373. [Google Scholar] [CrossRef]
- Hadi, P.; To, M.H.; Hui, C.W.; Lin, C.S.; McKay, G. Aqueous mercury adsorption by activated carbons. Water Res. 2015, 73, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; He, J.; Gao, Y.; Wu, H.; Zhu, X. Cosorption of phenanthrene and mercury(II) from aqueous solution by soybean stalk-based biochar. J. Agric. Food Chem. 2011, 59, 12116–12123. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Chen, C.; Ch’ng, B.L.; Wang, Y.L.; Hsi, H.C. Using raw and sulfur-impregnated activated carbon as active cap for leaching inhibition of mercury and methylmercury from contaminated sediment. J. Hazard. Mater. 2018, 354, 116–124. [Google Scholar] [CrossRef]
- Wharton, M.J.; Atkins, B.; Charnock, J.M.; Livens, F.R.; Pattrick, R.A.D.; Collison, D. An X-ray absorption spectroscopy study of the coprecipitation of Tc and Re with mackinawite (FeS). Appl. Geochem. 2000, 15, 347–354. [Google Scholar] [CrossRef]
- Wolthers, M.; Van der Gaast, S.J.; Rickard, D. The structure of disordered mackinawite. Am. Miner. 2003, 88, 2007–2015. [Google Scholar] [CrossRef]
- Morse, J.W.; Arakaki, T. Adsorption and coprecipitation of divalent metals with mackinawite (FeS). Geochim. Cosmochim. Acta 1993, 57, 3635–3640. [Google Scholar] [CrossRef]
- Ito, D.; Miura, K.; Ichimura, T.; Ihara, I.; Watanabe, T. Removal of As, Cd, Hg and Pb ions from solution by adsorption with bacterially-produced magnetic iron sulfide particles using high gradient magnetic separation. IEEE Trans. Appl. Supercond. 2004, 14, 1551–1553. [Google Scholar] [CrossRef]
- Özverdi, A.; Erdem, M. Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J. Hazard. Mater. 2006, 137, 626–632. [Google Scholar] [CrossRef]
- Watson, J.H.P.; Ellwood, D.C.; Deng, Q.X.; Mikhalovsky, S.; Hayter, C.E.; Evans, J. Heavy-metal adsorption on bacterially produced FeS. Miner. Eng. 1995, 8, 1097–1108. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Zhao, D. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Valsaraj, K.T.; Devai, I.; DeLaune, R.D. Immobilization of aqueous Hg(II) by mackinawite (FeS). J. Hazard. Mater. 2008, 157, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Jean, G.E.; Bancroft, G.M. Heavy metal adsorption by sulphide mineral surfaces. Geochim. Cosmochim. Acta 1986, 50, 1455–1463. [Google Scholar] [CrossRef]
- Behra, P.; Bonnissel-Gissinger, P.; Alnot, M.; Revel, R.; Ehrhardt, J.J. XPS and XAS study of the sorption of Hg(II) onto pyrite. Langmuir 2001, 17, 3970–3979. [Google Scholar] [CrossRef]
- Han, D.S.; Orillano, M.; Khodary, A.; Duan, Y.; Batchelor, B.; Abdel-Wahab, A. Reactive iron sulfide (FeS)-supported ultrafiltration for removal of mercury (Hg(II)) from water. Water Res. 2014, 53, 310–321. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Y.; Xiong, Z.; Zhao, D. Immobilization of mercury by carboxymethyl cellulose stabilized iron sulfide nanoparticles: Reaction mechanisms and effects of stabilizer and water chemistry. Environ. Sci. Technol. 2014, 48, 3986–3994. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, D.; Zhou, J.; Zhou, X.; Lou, Z.; Baig, S.A.; Xu, X. Adsorption of mercury (II) from aqueous solutions using FeS and pyrite: A comparative study. Chemosphere 2017, 185, 452–461. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, J.H.; Hayes, K.F. Characterization of synthetic nanocrystalline mackinawite: Crystal structure, particle size, and specific surface area. Geochim. Cosmochim. Acta 2008, 72, 493–505. [Google Scholar] [CrossRef]
- Skyllberg, U.; Drott, A. Competition between disordered iron sulfide and natural organic matter associated thiols for mercury (II): An EXAFS study. Environ. Sci. Technol. 2010, 44, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Paquette, K.; Helz, G. Solubility of cinnabar (Red HgS) and implications for mercury speciation in sulfidic waters. Water Air Soil Pollut. 1995, 80, 1053–1056. [Google Scholar] [CrossRef]
- Benoit, J.M.; Gilmour, C.C.; Mason, R.P.; Heyes, A. Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ. Sci. Technol. 1999, 33, 951–957. [Google Scholar] [CrossRef]
- Barnett, M.O.; Turner, R.R.; Singer, P.C. Oxidative dissolution of metacinnabar (β-HgS) by dissolved oxygen. Appl. Geochem. 2001, 16, 1499–1512. [Google Scholar] [CrossRef]
- Beldowski, J.; Pempkowiak, J. Horizontal and vertical variabilities of mercury concentration and speciation in sediments of the Gdansk Basin, Southern Baltic Sea. Chemosphere 2003, 52, 645–654. [Google Scholar] [CrossRef]
- Taylor, L.; Finger, L. Structural refinement and composition of mackinawite. Carnegie Inst. Wash. Geophys. Lab. Ann. Rep. 1970, 69, 318–322. [Google Scholar]
- Jeong, H.Y.; Klaue, B.; Blum, J.D.; Hayes, K.F. Sorption of mercuric ion by synthetic nanocrystalline mackinawite (FeS). Environ. Sci. Technol. 2007, 41, 7699–7705. [Google Scholar] [CrossRef]
- Deonarine, A.; Hsu-Kim, H. Precipitation of mercuric sulfide nanoparticles in NOM-containing water: Implications for the natural environment. Environ. Sci. Technol. 2009, 43, 2368–2373. [Google Scholar] [CrossRef]
- Slowey, A.J. Rate of formation and dissolution of mercury sulfide nanoparticles: The dual role of natural organic matter. Geochim. Cosmochim. Acta 2010, 74, 4693–4708. [Google Scholar] [CrossRef]
- Graham, A.M.; Aiken, G.R.; Gilmour, C.C. Dissolved organic matter enhances microbial mercury methylation under sulfidic conditions. Environ. Sci. Technol. 2012, 46, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kim, B.; Levard, C.; Reinsch, B.C.; Lowry, G.V.; Deshusses, M.A.; Hsu-Kim, H. Methylation of mercury by bacteria exposed to dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ. Sci. Technol. 2012, 46, 6950–6958. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, Y.; Xiong, Z.; Kaback, D.; Zhao, D. Immobilization of mercury in field soil and sediment using carboxymethyl cellulose stabilized iron sulfide nanoparticles. Nanotechnology 2012, 23, 294007. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; He, F.; Zhao, D.; Barnett, M.O. Immobilization of mercury in sediment using stabilized iron sulfide nanoparticles. Water Res. 2009, 43, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lou, Z.M.; Yu, J.B.; Zhou, X.X.; Lv, D.; Zhou, J.S.; Baig, S.A.; Xu, X.H. Immobilization of mercury (II) from aqueous solution using Al2O3-supported nanoscale FeS. Chem. Eng. 2017, 323, 483–491. [Google Scholar] [CrossRef]
- Bone, S.E.; Bargar, J.R.; Sposito, G. Mackinawite (FeS) reduces mercury(II) under sulfidic conditions. Environ. Sci. Technol. 2014, 48, 10681–10689. [Google Scholar] [CrossRef] [PubMed]
- Barringer, J.L.; Szabo, Z.; Schneider, D.; Atkinson, W.D.; Gallagher, R.A. Mercury in ground water, septage, leach-field effluent, and soils in residential areas, New Jersey coastal plain. Sci. Total Environ. 2006, 361, 144–162. [Google Scholar] [CrossRef]
- Lamborg, C.H.; Kent, D.B.; Swarr, G.J.; Munson, K.M.; Kading, T.; O’Connor, A.E.; Fairchild, G.M.; Leblanc, D.R.; Wiatrowski, H.A. Mercury speciation and mobilization in a wastewater-contaminated groundwater plume. Environ. Sci. Technol. 2013, 47, 13239–13249. [Google Scholar] [CrossRef]
- Wiatrowski, H.A.; Das, S.; Kukkadapu, R.; Ilton, E.S.; Barkay, T.; Yee, N. Reduction of Hg(II) to Hg(0) by magnetite. Environ. Sci. Technol. 2009, 43, 5307–5313. [Google Scholar] [CrossRef] [PubMed]
- Charlet, L.; Bosbach, D.; Peretyashko, T. Natural attenuation of TCE, As, Hg linked to the heterogeneous oxidation of Fe(II): An AFM study. Chem. Geol. 2002, 190, 303–319. [Google Scholar] [CrossRef]
- Amirbahman, A.; Kent, D.B.; Curtis, G.P.; Marvin-Dipasquale, M.C. Kinetics of homogeneous and surface-catalyzed mercury(II) reduction by iron(II). Environ. Sci. Technol. 2013, 47, 7204–7213. [Google Scholar] [CrossRef] [PubMed]
- Wiatrowski, H.A.; Ward, P.M.; Barkay, T. Novel reduction of mercury (II) by mercury-sensitive dissimilatory metal reducing bacteria. Environ. Sci. Technol. 2006, 40, 6690–6696. [Google Scholar] [CrossRef] [PubMed]
- Kritee, K.; Blum, J.D.; Barkay, T. Mercury stable isotope fractionation during reduction of Hg(II) by different microbial pathways. Environ. Sci. Technol. 2008, 42, 9171–9177. [Google Scholar] [CrossRef] [PubMed]
- Bouffard, A.; Amyot, M. Importance of elemental mercury in lake sediments. Chemosphere 2009, 74, 1098–1103. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Sun, K.; Hayes, K.F. Microscopic and spectroscopic characterization of Hg(II) immobilization by mackinawite (FeS). Environ. Sci. Technol. 2010, 44, 7476–7483. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.W.; Luther, G.W. Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochim. Cosmochim. Acta 1999, 63, 3373–3378. [Google Scholar] [CrossRef]
- Janssen, E.M.; Beckingham, B.A. Biological responses to activated carbon amendments in sediment remediation. Environ. Sci. Technol. 2013, 47, 7595–7607. [Google Scholar] [CrossRef]
- Boszke, L.; Kowalski, A.; Astel, A.; Baranski, A.; Gworek, B.; Siepak, J. Mercury mobility and bioavailability in soil from contaminated area. Environ. Geol. 2008, 55, 1075–1087. [Google Scholar] [CrossRef]
- Wolfenden, S.; Charnock, J.M.; Hilton, J.; Livens, F.R.; Vaughan, D.J. Sulfide species as a sink for mercury in lake sediments. Environ. Sci. Technol. 2005, 39, 6644–6648. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.S.; Horne, A.J.; Sedlak, D.L. Reduction of net mercury methylation by iron in Desulfobulbus propionicus (1pr3) cultures: Implications for engineered wetlands. Environ. Sci. Technol. 2003, 37, 3018–3023. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.S.; Sedlak, D.L. Decrease in net mercury methylation rates following iron amendment to anoxic wetland sediment slurries. Environ. Sci. Technol. 2005, 39, 2564–2570. [Google Scholar] [CrossRef] [PubMed]
| Type of Adsorbent | Nano-CMC-FeS | Nano-CMC-FeS | FeS/Al2O3 | Nano-Fes, Pyrite | Nano-FeS | Nano-FeS | Nano-FeS |
|---|---|---|---|---|---|---|---|
| Hg concentration range | 21.96, 193.04, and 100.53 mg/kg Hg sediment | 177 mg/kg Hg sediment | 0–80 mg/L | 1–100 mg/L | 100, 200, and 250 mg/L | 0.01–1 mM | 0.05–20 mM |
| Hg to sorbent ratio (w/w) | 0.011–0.11, 4.26 × 10−3–8.47× 10−3 0.018–0.036 | 0.038–0.5 | 0–10 | 0.06–0.6 (FeS) 0.2–20 (pyrite) | 0.2, 0.25, 0.5 | 50–4000 | 4.4 × 10−4–3.5 |
| Sorption condition | pH = 7.0; T = 20 °C; t = 20 h; on rotator 30 rpm; 0.1 M NaNO3 | pH = 7.0; T = 22 °C; t = 1 week; on rotator 30 rpm | pH = 6.0; T = 30 °C; t = 24 h; on shaker 180 rpm | pH = 7.0; T = 30 °C; t = 24 h; on shaker 180 rpm | pH = 8; t = 10~144 min; rotated | pH = 5.6; T = not stated; t = 24 h; on shaker; anoxic with constant N2 purging | pH = 4~11; T = 25 °C; t = 48 h [Cl]T = 0.2 M; on shaker |
| Removal efficiency of Hg | Qmax = 2866.6 mg/g79–96% removal in batch contacted with Hg sediment. | Distribution coefficient = 8.93 × 106 Hg(aq) decrease > 73% in all batches contacted with Hg sediment, with the maximum reduction of 97% (Hg/sorbent = 0.5). | Qmax = 891 mg/g (theoretical) Qmax = 313 mg/g (observed) >97% removal in 24 h and >95% removal in 30 d test. | Qmax = 762 mg/g (FeS) Qmax = 9.9 mg/g (pyrite) | KD = 1.98 × 106 More than 99% removal | Qmax = 1700 mg/g Hg removal >99% when Hg/FeS is smaller than 1000 at [Hg]0 = 1 mM | Qmax = 88 mg/g Removal of Hg was higher than 99% when Hg/FeS is smaller than 0.05 |
| Reference | [63] | [78] | [79] | [64] | [62] | [59] | [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, Y.; Hsi, H.-C. Iron Sulfide Minerals as Potential Active Capping Materials for Mercury-Contaminated Sediment Remediation: A Minireview. Sustainability 2019, 11, 1747. https://doi.org/10.3390/su11061747
Ting Y, Hsi H-C. Iron Sulfide Minerals as Potential Active Capping Materials for Mercury-Contaminated Sediment Remediation: A Minireview. Sustainability. 2019; 11(6):1747. https://doi.org/10.3390/su11061747
Chicago/Turabian StyleTing, Yu, and Hsing-Cheng Hsi. 2019. "Iron Sulfide Minerals as Potential Active Capping Materials for Mercury-Contaminated Sediment Remediation: A Minireview" Sustainability 11, no. 6: 1747. https://doi.org/10.3390/su11061747
APA StyleTing, Y., & Hsi, H.-C. (2019). Iron Sulfide Minerals as Potential Active Capping Materials for Mercury-Contaminated Sediment Remediation: A Minireview. Sustainability, 11(6), 1747. https://doi.org/10.3390/su11061747





