New Environmentally Friendly Acid System for Iron Sulfide Scale Removal
Abstract
:1. Introduction
Iron Sulfide Scale Removal
2. Materials and Experimental
2.1. Materials
2.2. Experimental Work
2.2.1. Solubility Test
2.2.2. Corrosion Test
- An acid volume of 350 mL was prepared.
- The weight, thickness, and curvature radius of the steel coupon were measured.
- The steel coupon was hanged in the high-pressure high-temperature aging cell.
- A teflon liner that can be placed inside the aging cell was filled with the 350 mL acid volume.
- The steel coupon was hanged in the teflon liner and ensured to be completely submerged in the fluid.
- The teflon liner was placed inside the aging cell and the aging cell cap was closed.
- A pressure of 3.447 MPa was applied from the top valve of the aging cell using nitrogen gas.
- The aging cell was placed inside the oven for 6 h under a temperature of 125 °C.
- After 6 h, the aging cell was taken out from the oven and waited until it cooled, then the pressure was vented and the cell was opened.
- The measurements in step 2 were recorded again after the interaction with the fluid.
- The corrosion rate was calculated.
3. Results and Discussion
4. Conclusions
- NEFAS (75 wt.%) achieved iron sulfide solubility of (83 g/L) after 6 h.
- NEFAS (75 wt.%) outperformed GLDA (20 wt.%), HDC-3, and HCl (15 wt.%) for iron sulfide scale removal.
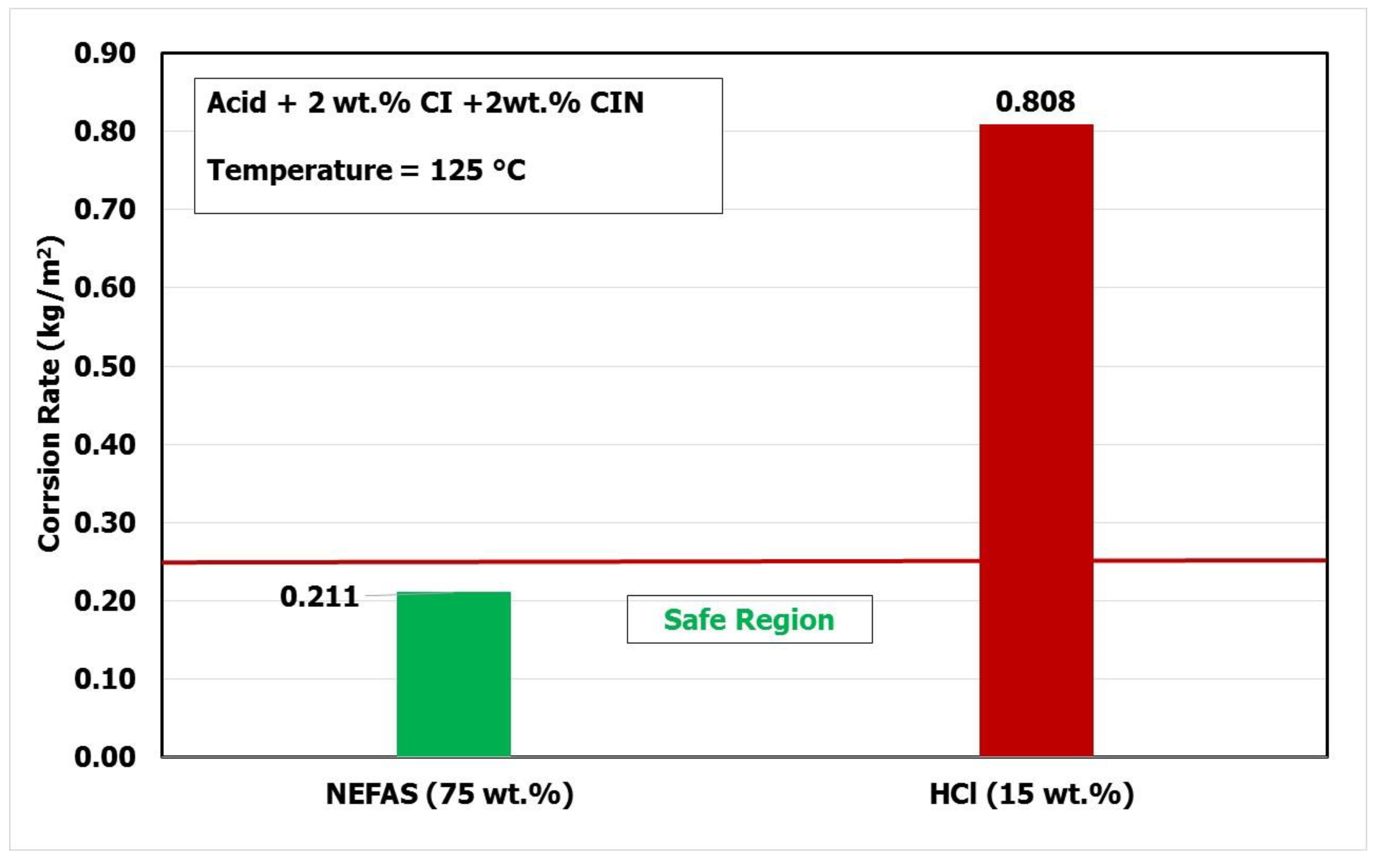
- The corrosion rate for the NEFAS (75 wt.%) was 0.211 kg/m2 at 125 °C, which is lower than the corrosion rate accepted by the oil and gas industry after adding 2 wt.% corrosion inhibitor and 2 wt.% corrosion intensifier.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasnick, M.A.; Engen, R.J. Iron Sulfide Scaling and Associated Corrosion in Saudi Arabian Khuff Gas Wells. Presenced at the SPE Middle East Oil Technical Conference and Exhibition, Manama, Bahrain, 11–14 March 1989. SPE-280-MS. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Fadhel, B.A.; Al-Humaidan, A.Y.; Frenier, W.W.; Hill, D. An Experimental Study of Removing Iron Sulfide Scale from Well Tubulars. Presenced at the International Symposium on Oilfield Scale, Aberdeen, UK, 26–27 January 2000. SPE-60205-MS. [Google Scholar] [CrossRef]
- Chen, T.; Montgomerie, H.; Chen, P.; Hagen, T.H.; Kegg, S.J. Development of Environmental Friendly Iron Sulfide Inhibitors for Field Application. Presenced at the International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 20–22 April 2009. SPE-121456-MS. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Rosser, H.R.; Hopkins, J.A. Simulation of Injection Water Supply Wells in Central Arabia. Society of Petroleum Engineers. Presenced at the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 13–16 October 1996. SPE-36181-MS. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-Humaidan, A.Y.; Mohamed, S.K.; Al-Salman, A.M. Iron Sulfide Formation in Water Supply Wells With Gas Lift. Presenced at the the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. SPE-65028-MS. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-Humaidan, A.Y. Iron Sulfide Scale: Formation, Removal, and Prevention. Presenced at the International Symposium on Oilfield Scale, Aberdeen, UK, 30–31 January 2001. SPE-68315-MS. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R.; Kleinitz, W.; Widdel, F. Sulfate-reducing Bacteria and Their Activities in Oil Production. J. Pet. Technol. 1987, 39, 97–106. [Google Scholar] [CrossRef]
- Cusack, F.; McKinley, V.L.; Lappin-Scott, H.M.; Brown, D.R.; Clementz, D.M.; Costerton, J.W. Diagnosis and Removal of Microbial/Fines Plugging in Water Injection Wells. Presenced at the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 27–30 September 1987. SPE-16907-MS. [Google Scholar] [CrossRef]
- Taylor, K.C.; Nasr-El-Din, H.A.; Al-Alawi, M.J. Systematic Study of Iron Control Chemicals Used During Well Stimulation. SPE J. 1999, 4, 19–24. [Google Scholar] [CrossRef]
- Wang, Q.; Ajwad, H.; Shafai, T.; Lynn, J.D. Iron Sulfide Scale Dissolvers: How Effective Are They? Presenced at the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 19–22 May 2013. SPE-168063-MS. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.; Mahmoud, M.; Sultan, A.S.; Saad, M.A. Oilfield scale formation and chemical removal: A review. J. Pet. Sci. Eng. 2018, 171, 127–139. [Google Scholar] [CrossRef]
- Seto, C.J.; Beliveau, D.A. Reservoir Souring in the Caroline Field. Presenced at the SPE/CERI Gas Technology Symposium, Calgary, AB, Canada, 3–5 April 2000. SPE-59778-MS. [Google Scholar] [CrossRef]
- Hall, B.E.; Dill, W.R. Iron Control Additives for Limestone and Sandstone Acidizing of Sweet and Sour Wells. Presenced at the SPE Formation Damage Control Symposium, Bakersfield, CA, USA, 8–9 February 1988. SPE-17157-MS. [Google Scholar] [CrossRef]
- Crowe, C.W. Evaluation of Agents for Preventing Precipitation of Ferric Hydroxide from Spent Treating Acid. J. Pet. Technol. 1958, 37, 691–695. [Google Scholar] [CrossRef]
- Crowe, C.W. Prevention of Undesirable Precipitates from Acid Treating Fluids. Presenced at the International Meeting on Petroleum Engineering, Beijing, China, 17–20 March 1986. SPE-14090-MS. [Google Scholar] [CrossRef]
- Sherik, A.M.; Zaidi, S.R.; Tuzan, E.V.; Perez, J.P. Black powder in gas transmission systems. Presenced at the NACE International Source CORROSION 2, New Orleans, LA, USA, 16–20 March 2008. NACE-08415. [Google Scholar]
- Ford, W.G.F.; Walker, M.L.; Halterman, M.P.; Parker, D.L.; Brawley, D.G.; Fulton, R.G. Removing a Typical Iron Sulfide Scale: The Scientific Approach. Presenced at the SPE Rocky Mountain Regional Meeting, Casper, WY, USA, 18–21 May 1992. SPE-24327-MS. [Google Scholar] [CrossRef]
- Leal Jauregui, J.A.; Solares, J.R.; Nasr-El-Din, H.A.; Franco, C.A.; Garzon, F.O.; Al-Marri, H.M.; Al-Aqeel, S.A.; Izquierdo, G.A. A Systematic Approach to Remove Iron Sulphide Scale: A Case History. Society of Petroleum Engineers. Presenced at the Middle East Oil and Gas Show and Conference, Manama, Bahrain, 11–14 March 2007. SPE-105607-MS. [Google Scholar] [CrossRef]
- Lawson, M.B. Method for Removing Iron Sulfide Scale From Metal Surfaces. U.S. Patent No. 4,351,673, 28 September 1982. [Google Scholar]
- Nasr-El-Din, H.A.; Al-Humaidan, A.Y.; Fadhel, B.A.; Saleh, R. Effect of Acid Additives on the Efficiency of Dissolving Iron Sulfide Scale. Presenced at the NACE International, Orlando, FL, USA, 26–31 March 2000. NACE-00439. [Google Scholar]
- Miller, R. Iron Sulfide Clean-Up Composition and Method. U.S. Patent No. 6,887,840, 3 May 2005. [Google Scholar]
- Frenier, W.W.; Coffey, M.D.; Huffnes, J.D.; Smith, D.C. Method and Composition for Removing Sulfide-Containing Scale from Metal Surfaces. U.S. Patent No. 4,220,550, 2 September 1980. [Google Scholar]
- Kelland, M.A. Production Chemicals for the Oil and gas Industry; CRC Press Taylor & Francis Group: Manchester, UK; Boca Raton FL, USA, 2014. [Google Scholar]
- Mahmoud, M.A.; Ba Geri, B.; Kamal, M.S.; Hussien, I.A. Removal of Pyrite and Different Types of Iron Sulfide Scales in Oil and Gas Wells without H2S Generation. Presenced at the International Petroleum Technology Conference, Doha, Qatar, 7–9 December 2015. IPTC-18279-MS. [Google Scholar] [CrossRef]
- Mahmoud, M.; Hussein, I.A.; Sultan, A.; Saad, M.A.; Buijs, W.; Vlugt, T.J. Development of efficient formulation for the removal of iron sulphide scale in sour production wells. Can. J. Chem. Eng. 2018, 96, 2526–2533. [Google Scholar] [CrossRef]
- Abou Bakr, M.; Ibrahim, A.F.; Nasr-El-Din, H.; Abd El-Hay, A.; Amin, E. Removal of Iron Sulphide Scale with a New Formulation: A Field Application in a Sandstone Reservoir in Egypt. Presenced at the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 24–26 September 2018. [Google Scholar] [CrossRef]
- Zaid, G.H.; Wolf, B.A. Compositions and Methods for Controlling Downhole suLfide Deposits. U.S. Patent 6,774,090, 10 August 2004. [Google Scholar]
- Jorda, R.M. Aqualin Biocide in Injection Waters. Presenced at the SPE Production Research Symposium, Tulsa, OK, USA, 12–13 April 1962. SPE-280-MS. [Google Scholar] [CrossRef]
- Salma, T. Cost Effective Removal of Iron Sulfide and Hydrogen Sulfide from Water Using Acrolein. Society of Petroleum Engineers. Presenced at the Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 21–23 March 2000. SPE-59708-MS. [Google Scholar] [CrossRef]
- Wang, H.T.; Hu, Y.; Tong, D.; Huang, J.; Gu, L.; Wu, X.R.; Chung, F.L.; Li, G.M.; Tang, M.S. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J. Biol. Chem. 2012, 287, 12379–12386. [Google Scholar] [CrossRef] [PubMed]
- Nasr-El-Din, H.A.; Chesson, J.B.; Al-Mohammed, A.M. A New Chemical Treatment to Remove Multiple Damages in a Water Supply Well. Presenced at the European Formation Damage Conference, Sheveningen, The Netherlands, 25–27 May 2005. SPE-95001-MS. [Google Scholar] [CrossRef]
- Rincon, P.R.; McKee, J.P.; Tarazon, C.E.; Guevara, L.A. Biocide Stimulation in Oil Wells for Downhole Corrosion Control and Increasing Production. Presenced at the International Symposium on Oilfield Corrosion, Aberdeen, UK, 28 May 2004. SPE-87562-MS. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, S.; Badairy, H.; Shafai, T.; Jeshi, Y.; Chen, T.; Chang, F.F. Laboratory assessment of tetrakis (hydroxymethyl) phosphonium sulfate as dissolver for scales formed in sour gas wells. Int. J. Corros. Scale Inhib. 2015, 4, 235–254. [Google Scholar] [CrossRef]
- Talbot, R.E.; Grech, J.M. Formulation for Corrosion and Scale Inhibition. International (PCT) Patent Application WO/2005/040050, 6 May 2005. [Google Scholar]
- Onawole, A.T.; Hussein, I.A.; Saad, M.A.; Mahmoud, M.; Ahmed, M.E.; Nimir, H.I. Effect of pH on acidic and basic chelating agents used in the removal of iron sulfide scales: A computational study. J. Pet. Sci. Eng. 2019, 178, 649–654. [Google Scholar] [CrossRef]
- Hafiz, T.; Hoegerl, M.; AlSuwaij, A.; Almathami, A. Synthetic Iron Sulfide Scale and Polymeric Scale Dissolvers. Presenced at the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 6–9 March 2017. [Google Scholar] [CrossRef]
- McCafferty, J.F.; Tate, E.W.; Williams, D.A. Field Performance in the Practical Application of Chlorine Dioxide as a Stimulation Enhancement Fluid. SPE Prod. Facil. 1993, 8, 9–14. [Google Scholar] [CrossRef]
- Romaine, J.; Strawser, T.G.; Knippers, M.L. Application of Chlorine Dioxide as an Oilfield Facilities Treatment Fluid. SPE Prod. Facil. 1996, 11, 18–21. [Google Scholar] [CrossRef]
- Aljeban, N.; Chen, T.; Balharth, S. Kinetics Study of Iron Sulfide Scale Dissolution. Society of Petroleum Engineers. Presenced at the Abu Dhabi International Petroleum Exhibition and Conference, Dhabi, United Arab Emirates, 12–15 November 2018. SPE-192675-MS. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Q.; Chang, F.; Aljeaban, N. Recent Development and Remaining Challenges of Iron Sulfide Scale Mitigation in Sour Gas Wells. Presenced at the International Petroleum Technology Conference, Beijing, China, 26–28 March 2019. IPTC-19315-MS. [Google Scholar] [CrossRef]
- Ramanathan, R.; Nasr-El-Din, H. Improving the Dissolution of Iron Sulfide by Blending Chelating Agents and its Synergists. Presenced at the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 18–21 March 2019. SPE-195128-MS. [Google Scholar] [CrossRef]
- Ko, S.; Wang, X.; Kan, A.T.; Tomson, M.B. Identification of Novel Chemicals for Iron Sulfide Scale Control and Understanding of Scale Controlling Mechanism. Presenced at the SPE International Conference on Oilfield Chemistry, Galveston, TX, USA, 8–9 April 2019. SPE-193550-MS. [Google Scholar] [CrossRef]
- El Menjra, A.I.; Seyeux, A.; Mercier, D.; Beech, I.; Makama, Z.; Marcus, P. ToF-SIMS analysis of abiotic and biotic iron sulfide layers formed in aqueous conditions on iron surfaces. Appl. Surf. Sci. 2019, 484, 876–883. [Google Scholar] [CrossRef]
- Onawole, A.T.; Hussein, I.A.; Sultan, A.; Abdel-Azeim, S.; Mahmoud, M.; Saad, M.A. Molecular and electronic structure elucidation of Fe2+/Fe3+ complexed chelators used in iron sulphide scale removal in oil and gas wells. Can. J. Chem. Eng. 2019, 97, 2021–2027. [Google Scholar] [CrossRef]
- Ramanathan, R.; Nasr-El-Din, H. November. Evaluation of Chelating Agents for Iron Sulfide FeS Scale Removal. Presenced at the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 11–14 November 2019. SPE-197891-MS. [Google Scholar] [CrossRef]
- Ahmed, M.; Onawole, A.; Hussien, I.; Saad, M.; Mahmoud, M.; Nimir, H. March. Effect of pH on Dissolution of Iron Sulfide Scales Using THPS. Presenced at the SPE International Conference on Oilfield Chemistry, Galveston, TX, USA, 8–9 April 2019. SPE-193573-MS. [Google Scholar] [CrossRef]
- Bageri, B.S.; Mahmoud, M.A.; Shawabkeh, R.A.; Al-Mutairi, S.H.; Abdulraheem, A. Filter Cake Porosity and Permeability Profile Along the Horizontal Well and Their Impact on Filter Cake Removal. Presenced at the International Petroleum Technology Conference, Doha, Qatar, 6–9 December 2015. IPTC-18465-MS. [Google Scholar] [CrossRef]
- Bageri, B.S.; Mahmoud, M.A.; Shawabkeh, R.A.; Al-Mutairi, S.H.; Abdulraheem, A. Toward a Complete Removal of Barite (Barium Sulfate Barium Sulfate BaSO4) Scale Using Chelating Agents and Catalysts. Arab. J. Sci. Eng. 2017, 42, 1667–1674. [Google Scholar] [CrossRef]
- Elkatatny, S. New Formulation for Iron Sulfide Scale Removal. Presenced at the Middle East Oil and Gas Show and Conference, Manama, Bahrain, 6–9 March 2017. SPE-183914-MS. [Google Scholar] [CrossRef]
- Syafii, I.; Pandya, N.; Sabhapondit, A.; Hajj, H.E. High-Temperature Acidizing: Advantages of Inhibitor-Intensifier Synergy. Presenced at the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 7–10 November 2016. SPE-183034-MS. [Google Scholar] [CrossRef]
- Brezinski, M.M. New Environmental Options for Corrosion Inhibitor Intensifiers. Presenced at the SPE/EPA Exploration and Production Environmental Conference, Austin, TX, USA, 1–3 March 1999. SPE-52707-MS. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Nasr-El-Din, H.A.; De Wolf, C.; LePage, J.N. Stimulation of Carbonate Reservoirs Using GLDA (Chelating Agent) Solutions. Presenced at the SPE 132286-MS presented at the Trinidad and Tobago Energy Resources Conference, Port of Spain, Trinidad and Tobago, 27–30 June 2010. [Google Scholar] [CrossRef]
- Mostofizadeh, B.; Economides, M.J. Optimum Injection Rate from Radial Acidizing Experiments. Presenced at the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 25–28 September 1994. SPE-28547-MS. [Google Scholar]






| Component | Chemical Formula | Percentage, % |
|---|---|---|
| Pyrrhotite | Fe7S8 | 55 |
| Troilite | FeS | 6 |
| Mackinawite | FeS | 1 |
| Pyrite | FeS2 | 8 |
| Hibbingite | Fe (OH)3Cl | 2 |
| Siderite | FeCO3 | 3 |
| Geothite | a-FeOOH | 1 |
| Akaganeite | β-FeOOH | 3 |
| Calcite | CaCO3 | 21 |
| Fluid Property | 100 wt.% NEFAS | 75 wt.% NEFAS |
|---|---|---|
| Density (gm/cm3) | 1.04 | 1.07 |
| Viscosity (cP) | 1.46 | 1.58 |
| Surface Tension (mN/m) | 42.95 | 31.56 |
| pH | −1.29 | 0.04 |
| Removal Fluid | Time, (hr) | Solubility, (g/L) | Solubility, (%) |
|---|---|---|---|
| HDC-3 | 24 | 18 | 18 |
| GLDA (20 wt.%) | 24 | 65 | 65 |
| NEFAS (75 wt.%) | 24 | 82 | 82 |
| HCl (15 wt.%) | 24 | 84 | 84 |
| NEFAS (100 wt.%) | 6 | 82 | 82 |
| NEFAS (75 wt.%) | 6 | 83 | 83 |
| HCl (20 wt.%) | 6 | 83 | 83 |
| HCl (15 wt.%) | 6 | 84 | 84 |
| Acid | Coupon Weight, (g) | Coupon Thickness, (mm) | Corrosion Rate, (kg/m2) | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| 75% NEFAS + 2% C.I. + 2% Int. | 12.345 | 11.992 | 2.464 | 2.413 | 0.211 |
| 15% HCL + 2% C.I. + 2% Int. | 12.373 | 11.000 | 2.464 | 2.261 | 0.808 |
| 15% HCL | 12.118 | 2.697 | 2.464 | 1.245 | 0.244 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamal, H.; Abdelgawad, K.; Elkatatny, S. New Environmentally Friendly Acid System for Iron Sulfide Scale Removal. Sustainability 2019, 11, 6727. https://doi.org/10.3390/su11236727
Gamal H, Abdelgawad K, Elkatatny S. New Environmentally Friendly Acid System for Iron Sulfide Scale Removal. Sustainability. 2019; 11(23):6727. https://doi.org/10.3390/su11236727
Chicago/Turabian StyleGamal, Hany, Khaled Abdelgawad, and Salaheldin Elkatatny. 2019. "New Environmentally Friendly Acid System for Iron Sulfide Scale Removal" Sustainability 11, no. 23: 6727. https://doi.org/10.3390/su11236727
APA StyleGamal, H., Abdelgawad, K., & Elkatatny, S. (2019). New Environmentally Friendly Acid System for Iron Sulfide Scale Removal. Sustainability, 11(23), 6727. https://doi.org/10.3390/su11236727







