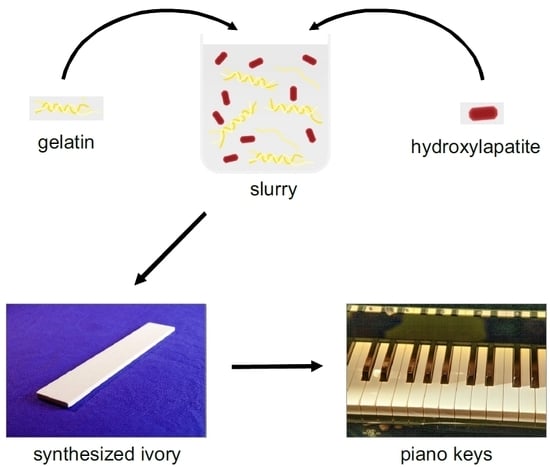
Bio-Inspired Synthetic Ivory as a Sustainable Material for Piano Keys
Abstract
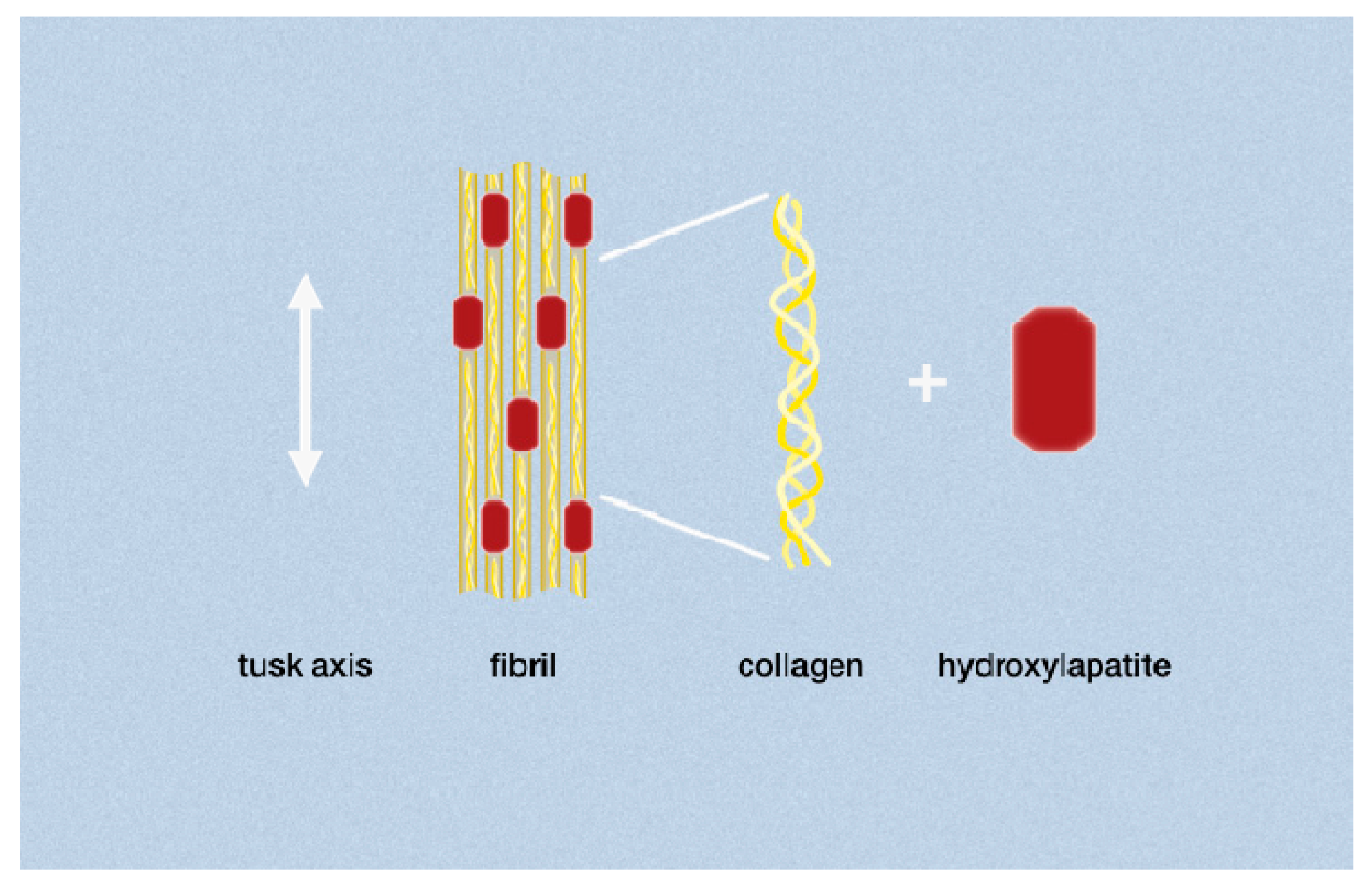
:1. Introduction
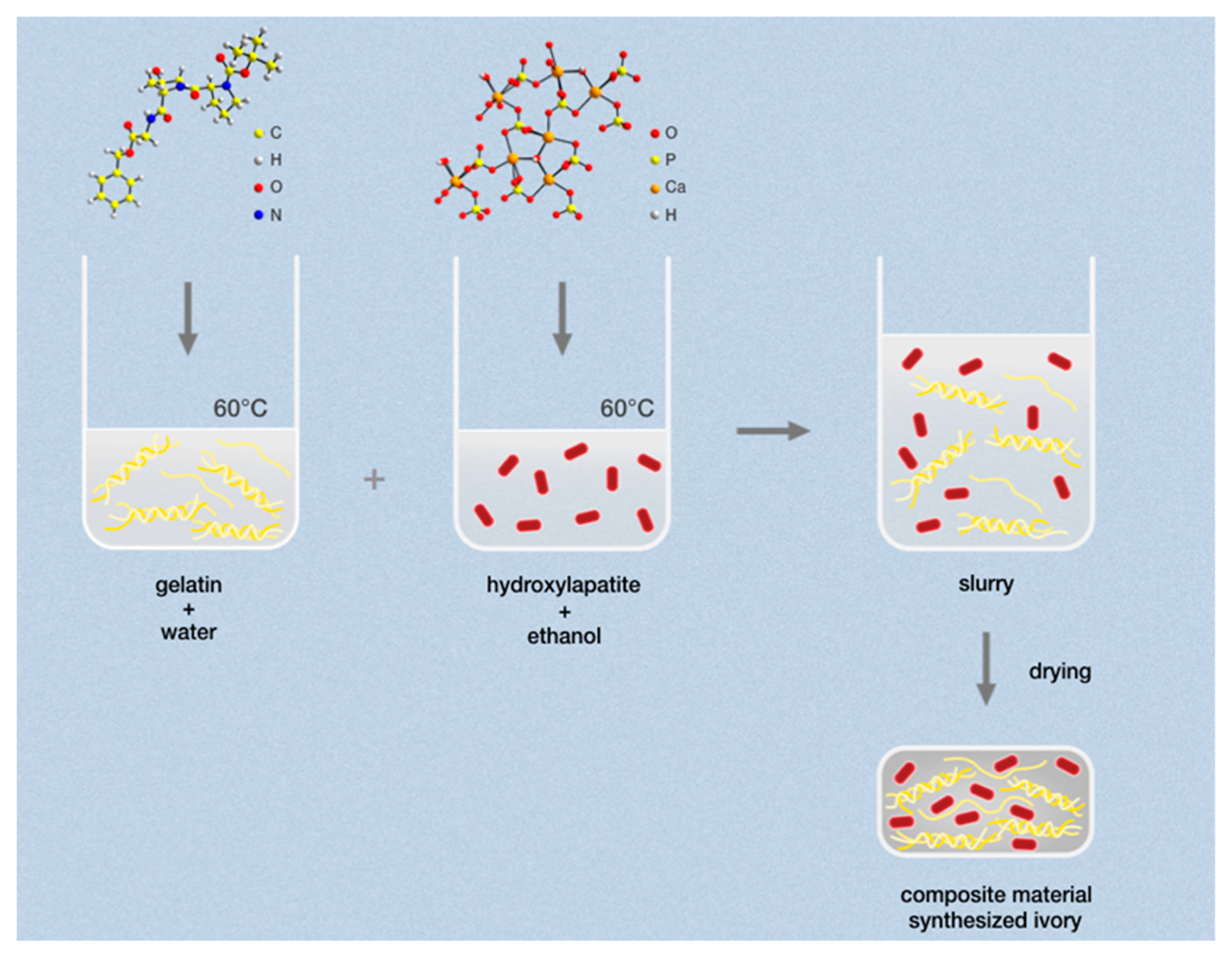
2. Materials and Methods
2.1. Preparation
2.2. Characterization
3. Results and Discussion
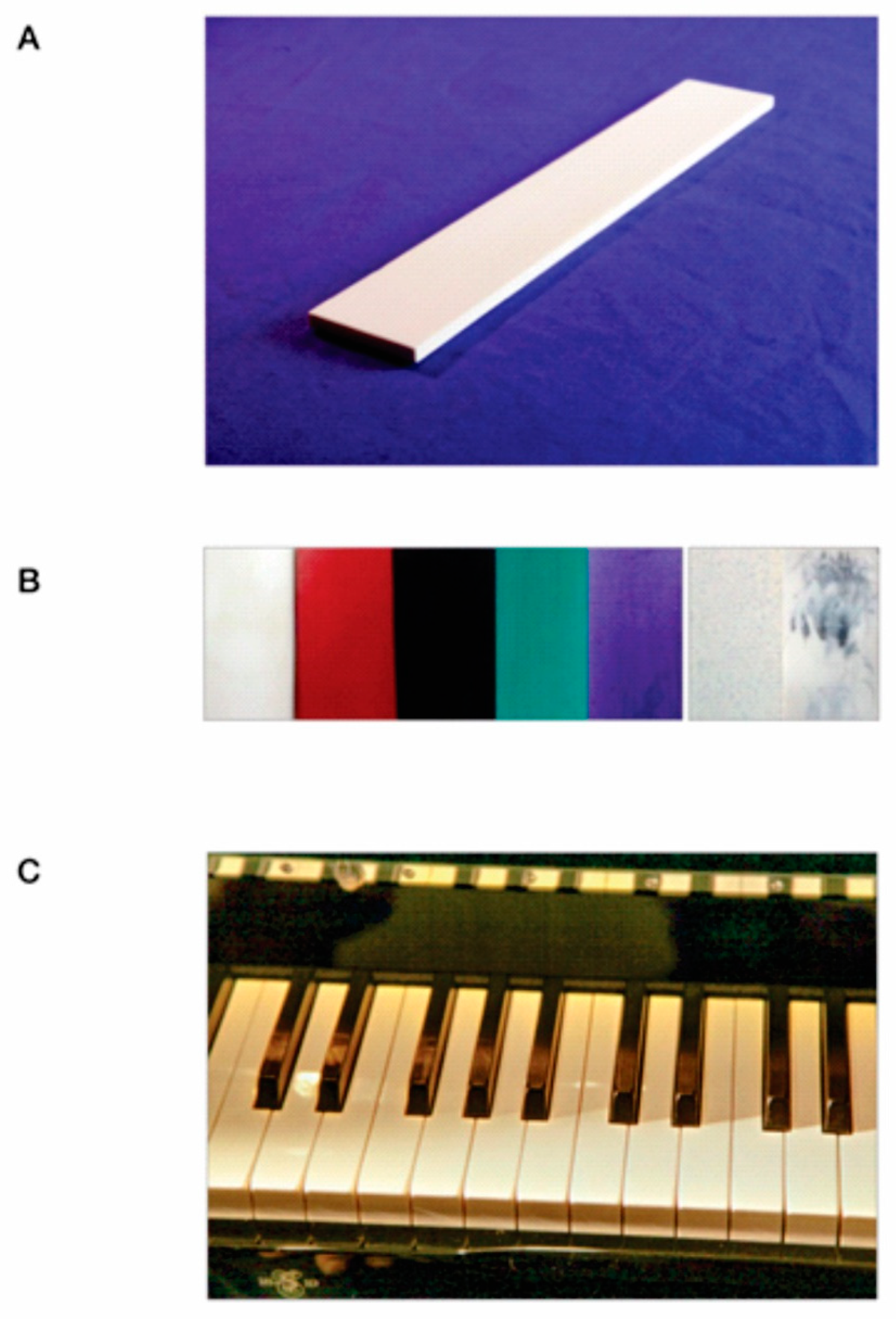
3.1. Development and Preparation
3.2. Properties of Synthesized vs. Natural Ivory
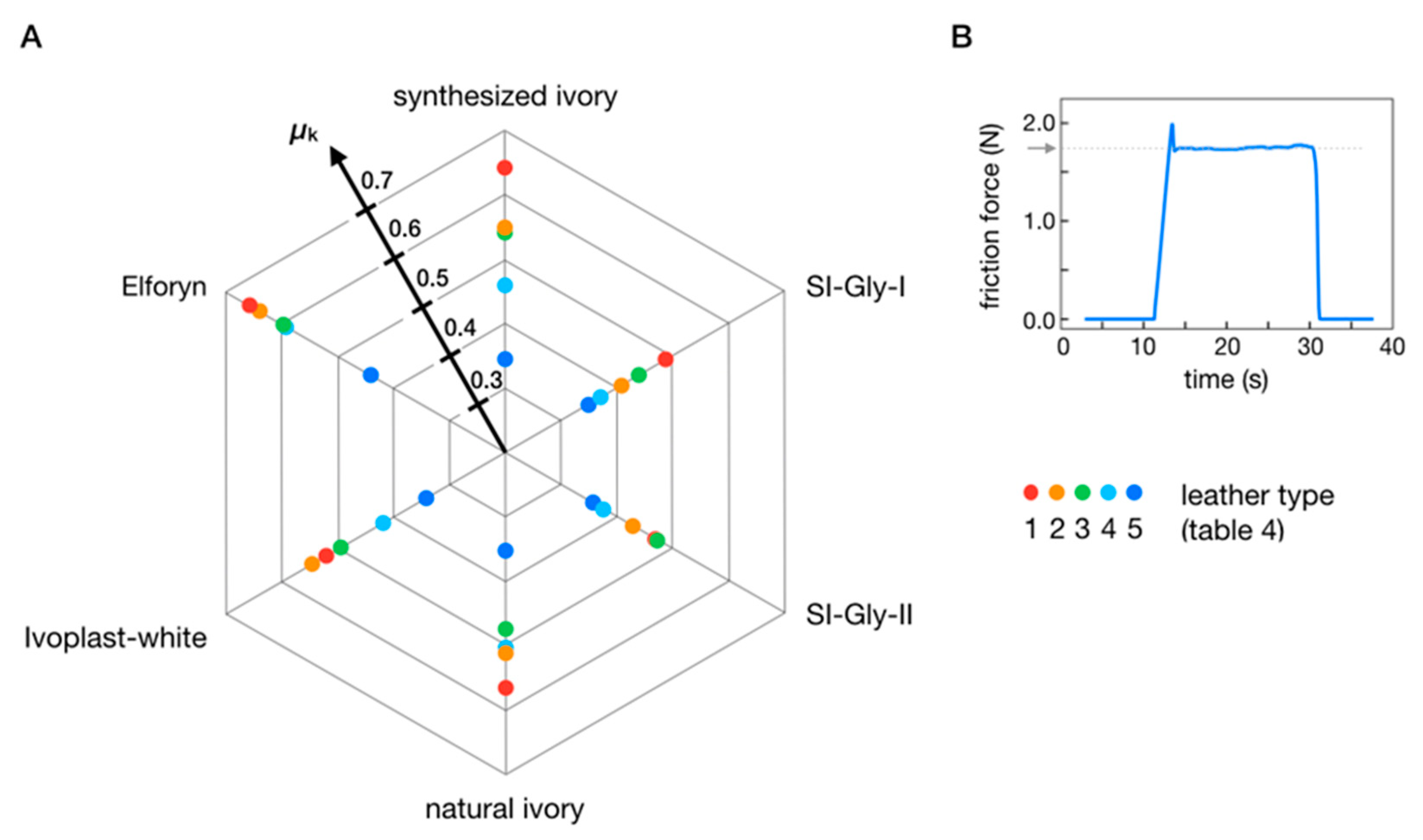
3.3. Requirements for Replacing Natural-Ivory Piano Keys
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chase, M.J.; Schlossberg, S.; Griffin, C.R.; Bouche, P.J.C.; Djene, S.W.; Elkan, P.W.; Ferreira, S.; Grossman, F.; Kohi, E.M.; Landen, K.; et al. Continent-wide survey reveals massive decline in African savannah elephants. PeerJ 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Biggs, D.; Holden, M.H.; Braczkowski, A.; Cook, C.N.; Milner-Gulland, E.J.; Phelps, J.; Scholes, R.J.; Smith, R.J.; Underwood, F.M.; Adams, V.M.; et al. Breaking the deadlock on ivory. Science 2017, 358, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Wittemyer, G.; Northrup, J.M.; Blanc, J.; Douglas-Hamilton, I.; Omondi, P.; Burnham, K.P. Illegal killing for ivory drives global decline in African elephants. Proc. Natl. Acad. Sci. USA 2014, 111, 13117–13121. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, E.O.; Mann, M.-J. Identification Guide for Ivory and Ivory Substitutes; World Wildlife Fund and The Conservation Foundation Press: Ashland, OR, USA, 1991; pp. 1–38. [Google Scholar]
- Muller, K.; Reiche, I. Differentiation of archaeological ivory and bone materials by micro-PIXE/PIGE with emphasis on two Upper Palaeolithic key sites: Abri Pataud and Isturitz, France. J. Archaeol. Sci. 2011, 38, 3234–3243. [Google Scholar] [CrossRef]
- Chu, Y.H.; Meyers, M.A.; Wang, B.; Yang, W.; Jung, J.Y.; Coimbra, C.F.M. A Sustainable Substitute for Ivory: The Jarina Seed from the Amazon. Sci. Rep. 2015, 5, 10. [Google Scholar] [CrossRef]
- Vollrath, F.; Mi, R.X.; Shah, D.U. Ivory as an Important Model Bio-composite. Curator Mus. J. 2018, 61, 95–110. [Google Scholar] [CrossRef]
- Braun, D. Polymer research before Hermann Staudinger the Long Road to Macromolecule. Chem. Unserer Zeit 2012, 46, 310–320. [Google Scholar] [CrossRef]
- Feldman, D. Polymer history. Des. Monomers Polym. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Germershaus, O.; Luhmann, T.; Rybak, J.C.; Ritzer, J.; Meinel, L. Application of natural and semi-synthetic polymers for the delivery of sensitive drugs. Int. Mater. Rev. 2015, 60, 101–130. [Google Scholar] [CrossRef]
- Eder, M.; Amini, S.; Fratzl, P. Biological composites-complex structures for functional diversity. Science 2018, 362, 543–547. [Google Scholar] [CrossRef]
- Su, X.W.; Cui, F.Z. Hierarchical structure of ivory: From nanometer to centimeter. Mater. Sci. Eng. C 1999, 7, 19–29. [Google Scholar] [CrossRef]
- Jantou-Morris, V.; Horton, M.A.; McComb, D.W. The nano-morphological relationships between apatite crystals and collagen fibrils in ivory dentine. Biomaterials 2010, 31, 5275–5286. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Goyal, S.P.; Khanna, P.P.; Mukherjee, P.K.; Sukumar, R. Using morphometric and analytical techniques to characterize elephant ivory. Forensic Sci. Int. 2006, 162, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Buddhachat, K.; Thitaram, C.; Brown, J.L.; Klinhom, S.; Bansiddhi, P.; Penchart, K.; Ouitavon, K.; Sriaksorn, K.; Pa-in, C.; Kanchanasaka, B.; et al. Use of handheld X-ray fluorescence as a non-invasive method to distinguish between Asian and African elephant tusks. Sci. Rep. 2016, 6, 11. [Google Scholar] [CrossRef]
- Alberic, M.; Dean, M.N.; Gourrier, A.; Wagermaier, W.; Dunlop, J.W.C.; Staude, A.; Fratzl, P.; Reiche, I. Relation between the Macroscopic Pattern of Elephant Ivory and Its Three-Dimensional Micro-Tubular Network. PLoS ONE 2017, 12, e0166671. [Google Scholar] [CrossRef]
- Alberic, M.; Gourrier, A.; Wagermaier, W.; Fratzl, P.; Reiche, I. The three-dimensional arrangement of the mineralized collagen fibers in elephant ivory and its relation to mechanical and optical properties. Acta Biomater. 2018, 72, 342–351. [Google Scholar] [CrossRef]
- Seidel, R.; Gourrier, A.; Kerschnitzki, M.; Burghammer, M.; Fratzl, P.; Gupta, H.S.; Wagermaier, W. Synchrotron 3D SAXS analysis of bone nanostructure. In Bioinspired Biomimetic and Nanobiomaterials; Ice Publishing: Westminister, UK, 2012; Volume 1, pp. 123–131. [Google Scholar]
- Busch, S.; Schwarz, U.; Kniep, R. Morphogenesis and structure of human teeth in relation to biomimetically grown fluorapatite-gelatine composites. Chem. Mater. 2001, 13, 3260–3271. [Google Scholar] [CrossRef]
- Srot, V.; Bussmann, B.; Salzberger, U.; Koch, C.T.; van Aken, P.A. Linking Microstructure and Nanochemistry in Human Dental Tissues. Microsc. Microanal. 2012, 18, 509–523. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.G.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Dunlop, J.W.C.; Fratzl, P. Biological Composites. In Annual Review of Materials Research; Clarke, D.R., Ruhle, M., Zok, F., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 40, pp. 1–24. [Google Scholar]
- Tlatlik, H.; Simon, P.; Kawska, A.; Zahn, D.; Kniep, R. Biomimetic fluorapatite-gelatine nanocomposites: Pre-structuring of gelatine matrices by ion impregnation and its effect on form development. Angew. Chem. Int. Ed. Engl. 2006, 45, 1905–1910. [Google Scholar] [CrossRef]
- Simon, P.; Rosseeva, E.; Buder, J.; Carrillo-Cabrera, W.; Kniep, R. Embryonic States of Fluorapatite-Gelatine Nanocomposites and Their Intrinsic Electric-Field-Driven Morphogenesis: The Missng Link on the Way from Atomistic Simulations to Pattern Formation on the Mesoscale. Adv. Funct. Mater. 2009, 19, 3596–3603. [Google Scholar] [CrossRef]
- Wang, Y.; Azais, T.; Robin, M.; Vallee, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Bleek, K.; Taubert, A. New developments in polymer-controlled, bio-inspired calcium phosphate mineralization from aqueous solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, S.; Al-Jawad, M.; Pantano, M.F.; Tejeda-Montes, E.; Mehta, K.; Jamal, H.; Agarwal, S.; Shuturminska, K.; Rice, A.; Tarakina, N.V.; et al. Protein disorder-order interplay to guide the growth of hierarchical mineralized structures. Nat. Commun. 2018, 9, 2145. [Google Scholar] [CrossRef] [PubMed]
- Alberic, M.; Gourrier, A.; Muller, K.; Zizak, I.; Wagermaier, W.; Fratzl, P.; Reiche, I. Early diagenesis of elephant tusk in marine environment. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 416, 120–132. [Google Scholar] [CrossRef]
- Kollmann, T.; Simon, P.; Carrillo-Cabrera, W.; Braunbarth, C.; Poth, T.; Rosseeva, E.V.; Kniep, R. Calcium Phosphate-Gelatin Nanocomposites: Bulk Preparation (Shape- and Phase-Control), Characterization, and Application as Dentine Repair Material. Chem. Mater. 2010, 22, 5137–5153. [Google Scholar] [CrossRef]
- Chang, M.C.; Douglas, W.H.; Tanaka, J. Organic-inorganic interaction and the growth mechanism of hydroxyapatite crystals in gelatin matrices between 37 and 80 degrees C. J. Mater. Sci. Mater. Med. 2006, 17, 387–396. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef]
- Coelho, A.A. General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS GmbH Press: Karlsruhe, Germany, 2009. [Google Scholar]
- MacKenzie, K.J.D.; Smith, M.E. Multinuclear Solid-State NMR of Inorganic Materials; Elsevier Press: Oxford, UK, 2002. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Doi, M.; Imori, K.; Sakaguchi, N.; Asano, A. Boc-Pro-Hyp-Gly-OBzl and Boc-Ala-Hyp-Gly-OBzl, two repeating triplets found in collagen. Acta Cryst. Sect. C Cryst. Struct. Commun. 2006, 62, O577–O580. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization. Front. Biosci. 2012, 3, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Jantou, V.; Turmaine, M.; West, G.D.; Horton, M.A.; McComb, D.W. Focused ion beam milling and ultramicrotomy of mineralised ivory dentine for analytical transmission electron microscopy. Micron 2009, 40, 495–501. [Google Scholar] [CrossRef]
- Cui, F.Z.; Wen, H.B.; Zhang, H.B.; Li, H.D.; Liu, D.C. Anisotropic indentation morphology and hardness of Natural Ivory. Mater. Sci. Eng. C 1994, 2, 87–91. [Google Scholar] [CrossRef]
- Rajaram, A. Tensile properties and fracture of ivory. J. Mater. Sci. Lett. 1986, 5, 1077–1080. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; Samarasekera, C.J.; White, M.A. Elephant ivory: A low thermal conductivity, high strength nanocomposite. J. Mater. Res. 2006, 21, 287–292. [Google Scholar] [CrossRef]
- Bracco, S.; Brajkovic, A.; Comotti, A.; Rolandi, V. Characterization of Elephant and Mammoth Ivory by Solid State NMR. Periodico di Mineralogia 2013, 82, 239–250. [Google Scholar]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatin Handbook: Theory and Industrial Practice; Wiley-VCH Verlag GmbH & Co. KGaA Press: Weinheim, Germany, 2007. [Google Scholar]
- Banerjee, A.; Bortolaso, G.; Hofmeister, W.; Petrovic-Prelevic, I.; Kiewisch, B. Investigation of quality of commercial mammoth ivory by means of X-ray Powder Diffraction (Rietveld method) and FTIR Spectroscopy. In Ivory and Species Conservation, Proceedings of the INCENTIVS–Meetings (2004–2007); Bundesamt für Naturschutz Skripten: Bonn, Germany, 2008; p. 51. [Google Scholar]
- Thouless, C.R.; Dublin, H.T.; Blanc, J.J.; Skinner, D.P.; Daniel, T.E.; Taylor, R.D.; Maisels, F.; Frederick, H.L.; Bouché, P. African Elephant Status Report 2016: An Update from the African Elephant Database; Occasional Paper Series of the IUCN Species Survival Commission Press: Nairobi, Kenya, 2016; pp. 1–309. [Google Scholar]

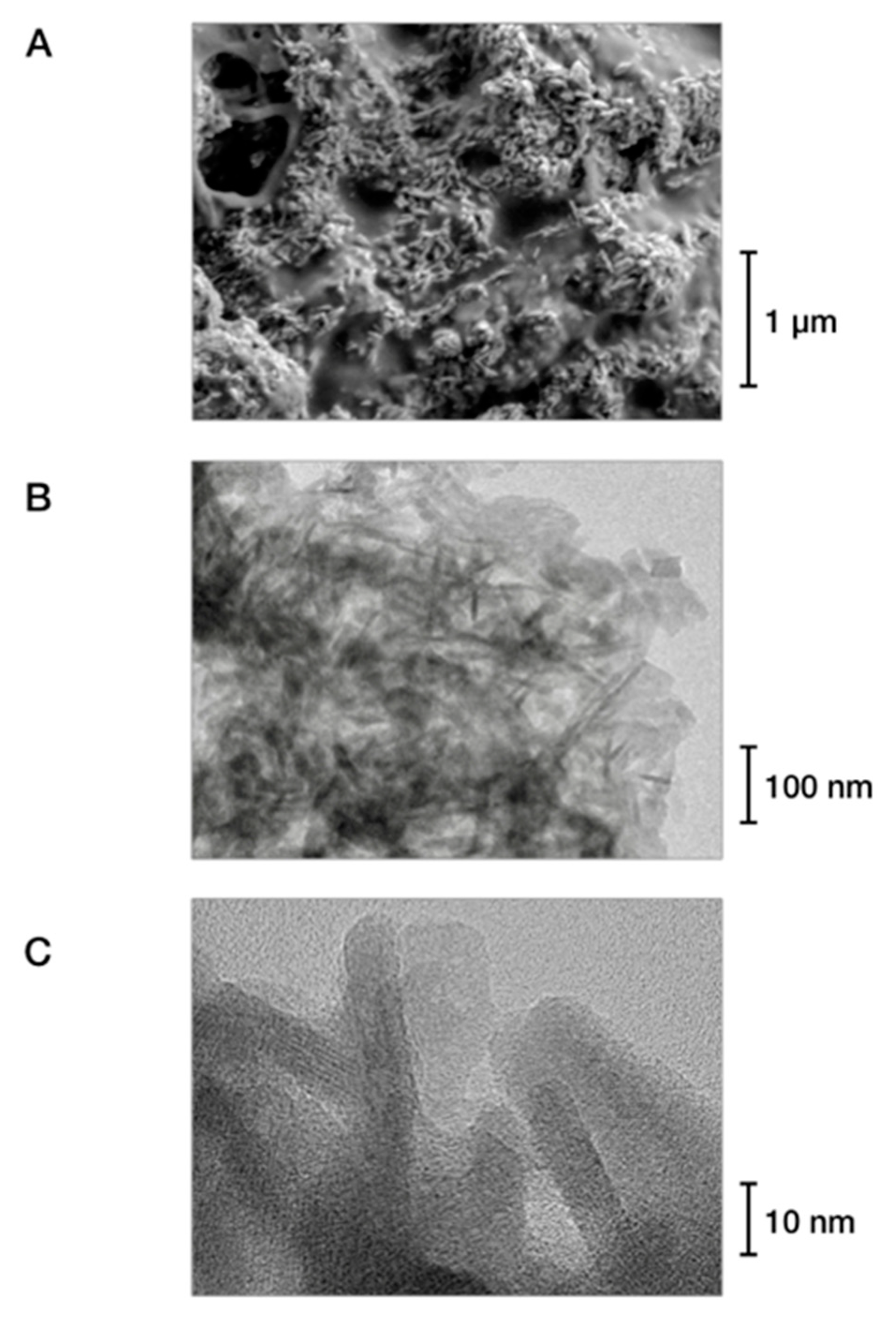
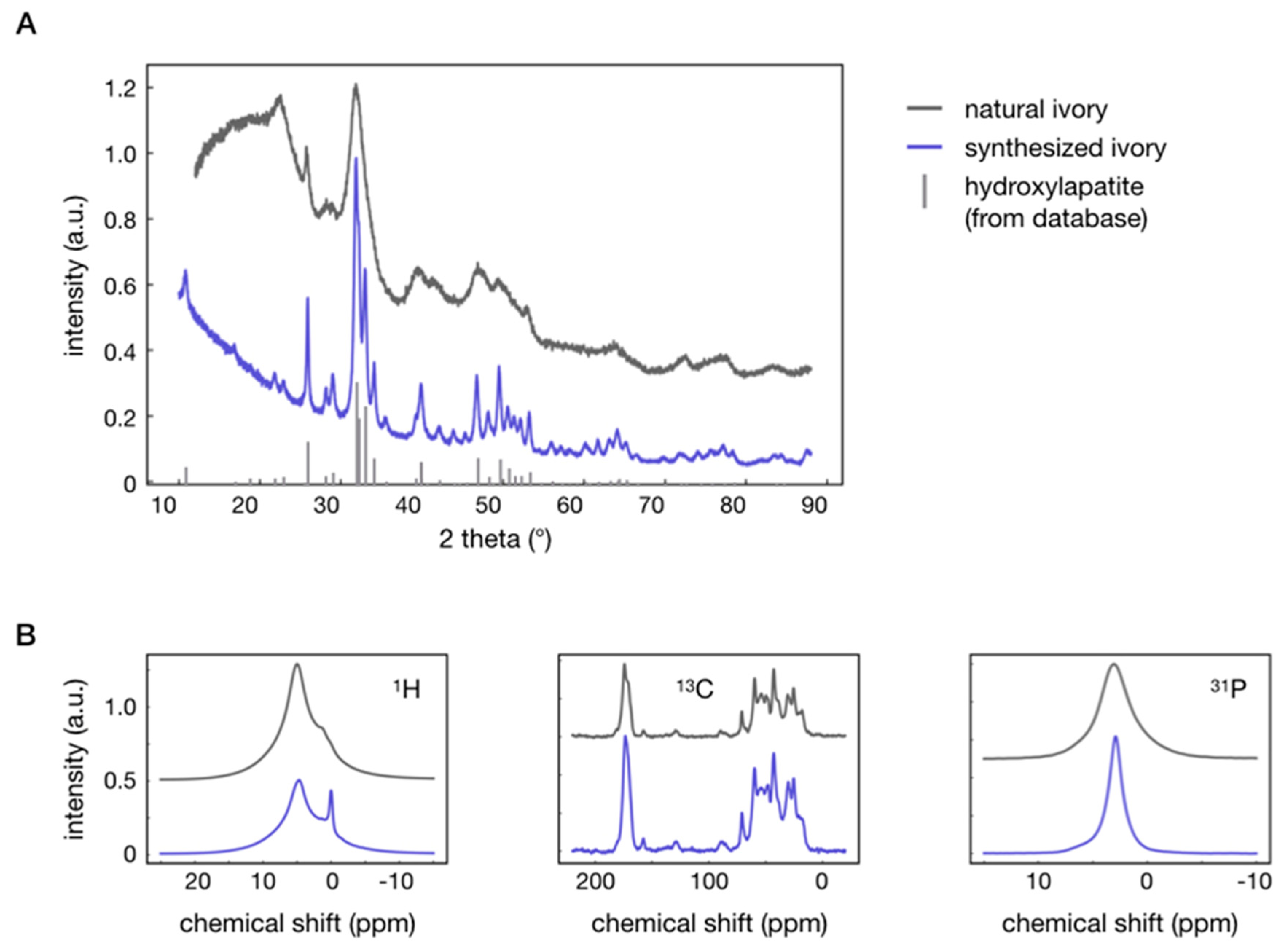
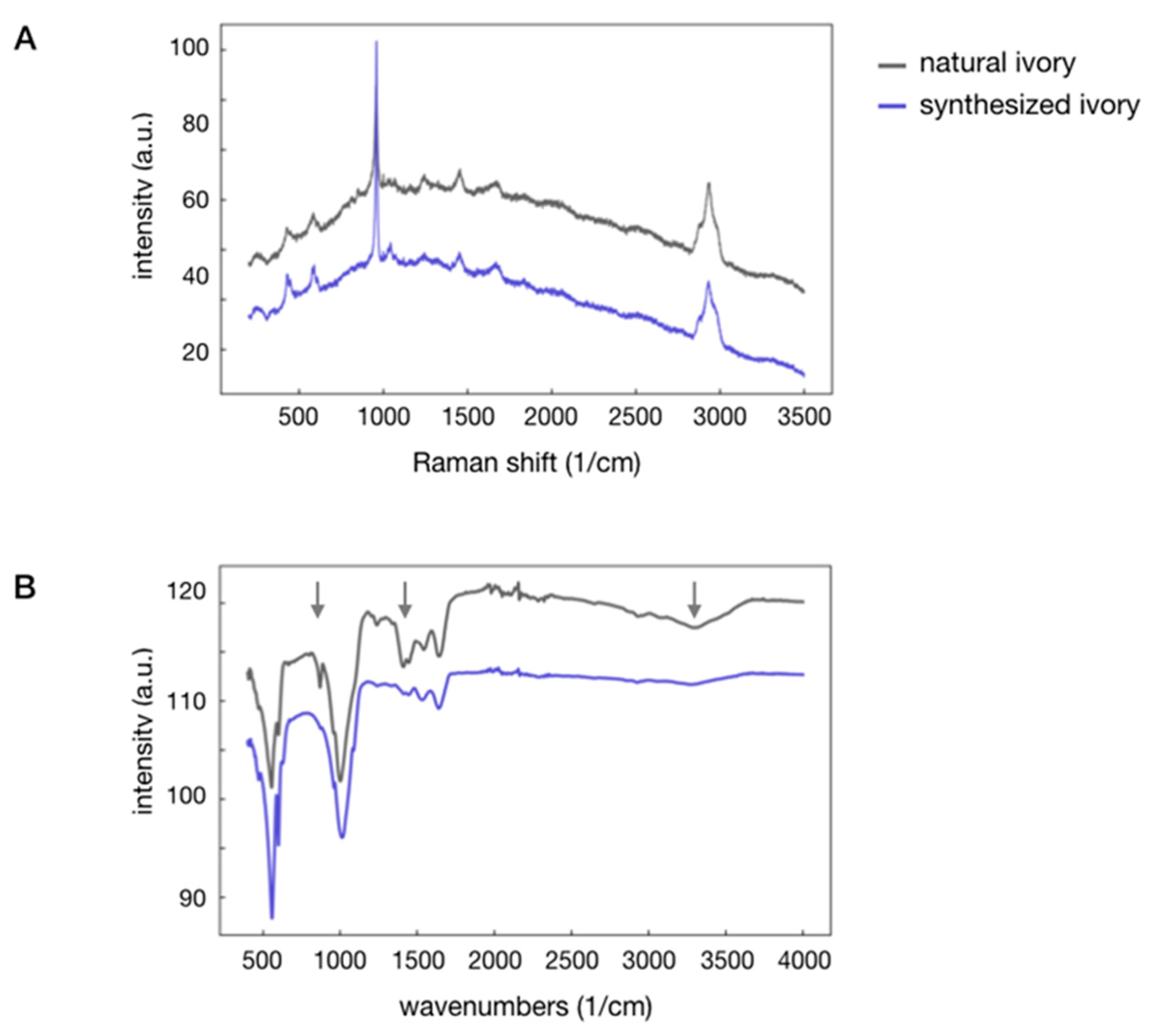
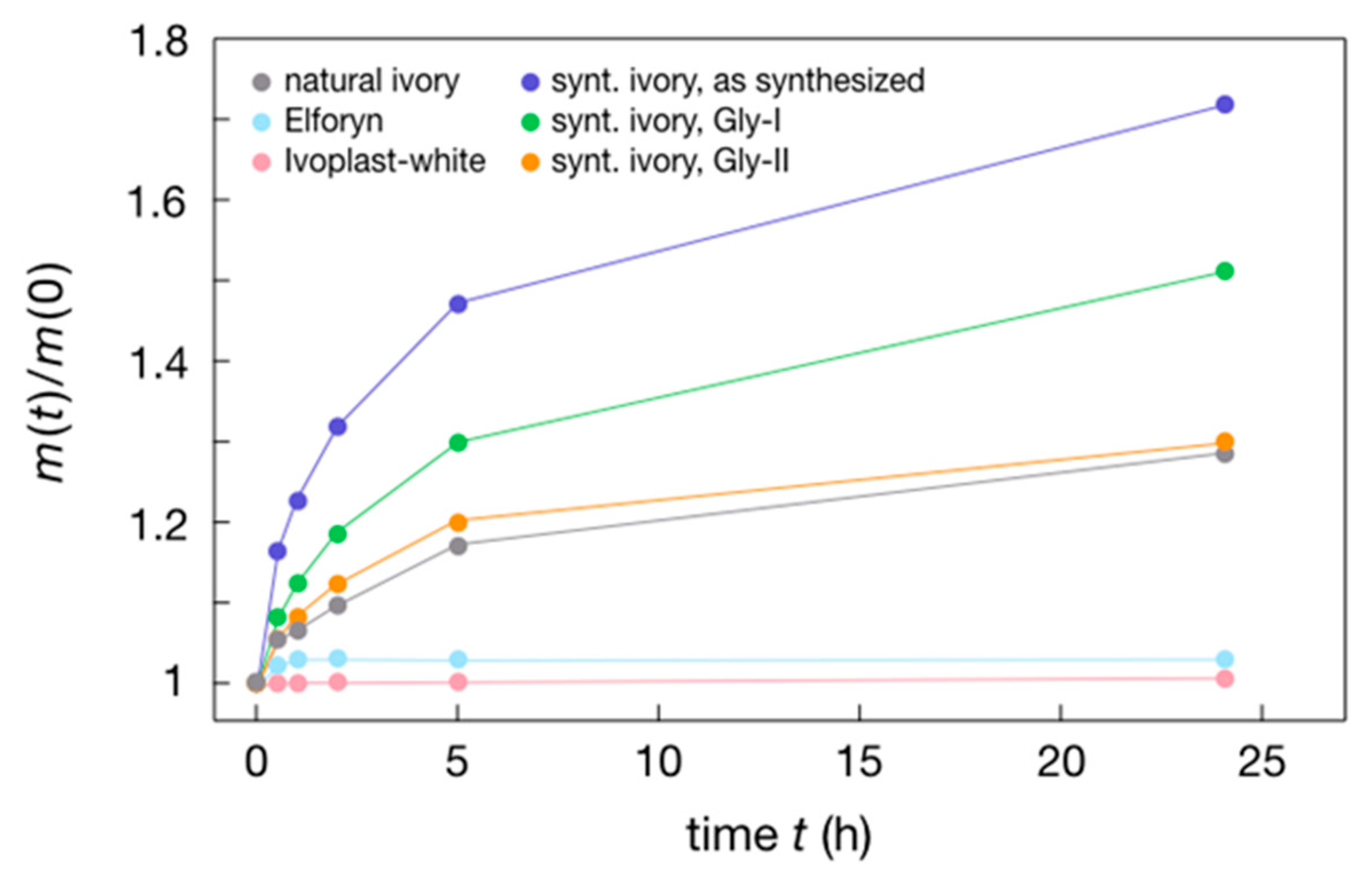
| Sample | Hardness (GPa) | Young’s Modulus (GPa) | Thermal Conductivity (W/K·m) at RT | Density (g/cm3) |
|---|---|---|---|---|
| Synthesized ivory | 0.4 ± 0.1 | 13 ± 2 | 0.83 ± 0.02 | 1.6 ± 0.1 |
| Natural ivory | 0.36 [38] | 12.5 [39] | 0.34–0.5 [40] | 1.70 [39,40] |
| Sample | a (Å) | c (Å) | λ (Å) |
|---|---|---|---|
| Synthesized ivory | 9.4202 ± 0.0004 | 6.8897 ± 0.0003 | 220 ± 1 |
| Natural ivory | 9.415 ± 0.006 | 6.876 ± 0.005 | 60 ± 1 |
| Hydroxylapatite | 9.4222 ± 0.0001 | 6.8812 ± 0.0001 | 1030 |
| IR Frequency (cm−1) | Raman Shift (cm−1) | ||||
|---|---|---|---|---|---|
| Synthesized Ivory | Natural Ivory | Assignments | Synthesized Ivory | Natural Ivory | Assignments |
| 471 | 470 | δ PO4 | 428/448 | 429 | δ PO4 |
| 557 | 555 | δ PO4 | 578/589 | 584 | δ PO4 |
| 599 | 600 | δ PO4 | |||
| 876 | 871 | ν CO3 | |||
| 961 | 960 | ν PO4 | 959 | 960 | ν PO4 |
| 1014 | 1002 | ν PO4 | 1001 | 1000 | ν CC (ar) |
| 1239 | 1240 | δ NH | 1043 | 1043/1067 | ν PO4, ν CO3 |
| 1413 | 1413 | ν CO3 | 1245 | 1246 | δ NH |
| 1445 | 1446 | ν CO3 | 1452 | 1457 | δ NH, δ CH2 |
| 1534 | 1544 | δ NH | 1672 | 1676 | ν C = O, δ NH |
| 1640 | 1645 | δ NH | |||
| ≈3300 | ν OH (H2O) | 2877/2933 | 2880/2937 | ν CH2, ν CH3 | |
| Leather | Surface | Leather Type | Origin | Thickness (mm) |
|---|---|---|---|---|
| 1 | smooth | Nappa | Cow | 1.0–1.2 |
| 2 | rough | Nappa | Cow | 1.2–1.4 |
| 3 | smooth | Nappa | Lamb | 0.6–0.7 |
| 4 | rough | Nubuck | Cow | 1.0–1.2 |
| 5 | smooth | Nubuck | Cow | 1.0–1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, D.; Parks, S.C.; Mannhart, J. Bio-Inspired Synthetic Ivory as a Sustainable Material for Piano Keys. Sustainability 2019, 11, 6538. https://doi.org/10.3390/su11236538
Fischer D, Parks SC, Mannhart J. Bio-Inspired Synthetic Ivory as a Sustainable Material for Piano Keys. Sustainability. 2019; 11(23):6538. https://doi.org/10.3390/su11236538
Chicago/Turabian StyleFischer, Dieter, Sarah C. Parks, and Jochen Mannhart. 2019. "Bio-Inspired Synthetic Ivory as a Sustainable Material for Piano Keys" Sustainability 11, no. 23: 6538. https://doi.org/10.3390/su11236538